Abstract
Reticular dysgenesis is a rare inherited immunodeficiency characterized by the lack of blood monocytes and neutrophils and low lymphocyte counts, contrasting with normal red blood cell counts and normal or decreased platelet counts. Whether dendritic cells or macrophages, both of which derive primarily from blood monocytes, are affected in this condition remains unknown. We studied 7 patients with reticular dysgenesis. Macrophages were present in normal numbers in the dermis and in the atrophic lymphoid tissues of these patients, proving that at least some subsets of macrophages can differentiate despite very low monocyte counts. By contrast, Langerhans cells, which are CD1a-positive epidermal dendritic cells, were absent in all (n = 5) patients before bone marrow transplantation. After bone marrow transplantation, Langerhans cells were present (n = 2), suggesting that the defect is not related to keratinocyte dysfunction. A split chimeric reconstitution, characterized by the presence of autologous blood monocytes able to differentiate in vitro into CD1a-positive dendritic cells, was observed in a patient who underwent successful engraftment. These results suggest that an intrinsic cell defect is unlikely and that a bone marrow-derived factor may be defective in reticular dysgenesis; it may be responsible for the Langerhans cell defect but not involved in macrophage differentiation.
Dendritic cells (DC) have a crucial function in the initiation of the antigen-specific immune response. They have the unique properties of actively internalizing particles at their immature stage in most nonlymphoid tissues, of migrating toward the T cell areas of secondary lymphoid organs, and of maturing and becoming able to activate naive T cells.1,2 Langerhans cells (LC) are immature DC of the malpighian and respiratory epithelium. They have been particularly well studied because of their specific localization within the epidermis, mucosa, and bronchi. They exhibit a characteristic dendritic morphology, and they express markers such as CD1a, major histocompatibility class II proteins, S100 proteins,3-5 and Birbeck granules. Human CD34+hematopoietic progenitors6 and CD14+ blood monocytes7 can differentiate in vitro into LC. In vivo, allogenic human LC are detectable as early as 2 weeks after allogenic bone marrow transplantation (BMT).8 The study of human or animal primary immunodeficiencies should contribute to a better understanding of in vivo LC differentiation. Although transforming growth factor (TGF)-β1 has been demonstrated in the mouse to be required for in vivo differentiation of LC,9 10 no human disease has been associated with impaired LC differentiation.
We have recently shown that some children with severe combined immunodeficiency disease may lack LC.11 In the current study, we investigated 7 patients with reticular dysgenesis (RD) before or after allogeneic BMT to determine whether macrophages and LC are affected by this condition. RD is a rare inherited condition characterized by an absence of blood neutrophils and monocytes, low lymphocyte counts, normal red blood cell counts, and normal or low platelet counts.12 We report that a selective absence of LC within the epidermis is a constant feature of this condition. Before BMT, macrophages were detected in these patients, but LC were not. After BMT, hematopoietic reconstitution was associated with the presence of LC in the epidermis, even though 1 patient had a split chimeric hematopoietic reconstitution with autologous blood monocytes.
Patients and methods
Patients
An extensive bibliographic search was performed through Medline, Embase, and Biosis databases. Twenty-five reports of children with reticular dysgenesis, since the first reported case in 1959,13 were retrieved from the indexed literature.11,14-31 The authors of each paper were repeatedly contacted to obtain histologic slides of the published reports. Some RD cases were also reported in a series concerning children who underwent transplantation.32 33 For 23 patients with RD, we obtained information concerning the availability of histologic samples. For 10 patients, histologic samples were not, or were no longer, available, whereas for 6 patients various tissue samples, but not skin samples, were available. Finally, 7 children with RD (approximately one quarter of the published case reports) were available for the study of Langerhans cells.
Diagnoses of reticular dysgenesis in the patients included in the study were established at the following pediatric centers: Clinica Infantil Vall d'Hebron, Barcelona, Spain (patient 1); Department of Pediatrics, Ulm University, Germany (patients 2-4); Unité d'Immunologie et d'Hématologie Pédriatrique, Necker–Enfants Malades Hospital, Paris, France (patient 5); Department of Pediatrics, Division of Bone Marrow Transplantation, University of California, San Francisco, CA (patient 6); and Departments of Immunology and Pediatrics, Hospital de la Santa Creu i Sant Paul, Barcelona, Spain (patient 7). Clinical and biologic data of the 7 patients were published in the corresponding case reports. Briefly, all patients had severe lymphopenia and no or fewer than 0.4 × 109/L neutrophils at 1 week of age. Red blood cell counts were normal, and platelet counts were either normal or decreased. In all patients, bone marrow analysis on smears or necropsy samples showed a maturation arrest of the myeloid lineage. Because the diagnosis of RD is difficult, the diagnosis of severe combined immunodeficiency associated with neutropenia34 cannot be excluded in all patients.
One patient with RD was recently reported to have a split chimeric reconstitution after allogeneic transplantation of his sister's bone marrow cells. The patient's monocytes and CD5+B-lymphocytes were shown to be autologous, whereas his T-lymphocytes were of donor origin.31 Blood and skin samples from this patient (2 years after transplantation) and from his sister were obtained, in accordance with local ethics committee, and were used to study LC/DC differentiation in vitro.
Histology and immunohistochemistry
Altogether 9 skin samples from 7 children with RD were available for the study. These samples were taken from biopsies (n = 5) or necropsies (n = 4) performed before (n = 5) or after (n = 4) allogeneic BMT. The same pathologist reviewed the histology of all tissue samples. Retrospective diagnosis of graft-versus-host disease was performed according to histology, clinical symptoms, and clinical evolution.
Immunohistochemistry was performed on 4-μm thick sections of paraffin-embedded, frozen samples or cell preparations. Primary antibodies were mouse anti-CD1a (IOT6 and O10; Immunotech, Marseille, France), anti-HLA-DR (DAKO, Copenhagen, Denmark; L243, Becton Dickinson, Bedford, MA), anti-CD68 (KP1 and KiM7; DAKO), and rabbit polyclonal anti-S100 protein (DAKO). The sections were stained with the use of a 3-step avidin–biotin kit according to the manufacturer's instructions (DAKO). Negative controls were obtained by omission of the first antibody or by its replacement by an isotype-matched, irrelevant mouse monoclonal antibody. Internal positive controls of LC-negative samples were obtained by the staining of HLA-DR–positive histiocytes or S100 protein-positive nerve bundles within the dermis. For CD1a, positive controls were obtained by the simultaneous staining of skin samples of children without immunodeficiency. Langerhans cells within the epidermis of the patients was compared with those of age-matched controls, as described.8 Immunohistochemical data of 1 of the 8 skin samples were previously reported.11
In vitro differentiation of CD14+ blood monocytes
Patient and donor blood samples were transmitted by mail. Differentiation of DC from human blood monocytes was performed as described.7 Briefly, CD14+ monocytes were isolated from blood mononuclear cells by negative magnetic depletion using hapten-conjugated CD3, CD7, CD19, CD45RA, and anti-IgE antibodies and a magnetic cell separator, routinely resulting in 95% or greater purity of CD14+ cells. CD14+ monocytes were then cultured for 6 days in 250 ng/mL granulocyte macrophage–colony-stimulating factor (GM-CSF; Sandoz AG, Bale, Switzerland), 100 mg/mL IL-4 (Genzyme, Cambridge, MA), and 10 ng/mL TGF-β1 (R&D Systems, Minneapolis, MN).
Phenotypes of monocyte-derived DC were assessed by flow cytometry analysis. Briefly, 3 × 105 cells were incubated for 15 minutes at 4°C in phosphate-buffered saline, 2% human AB serum, and 0.01 mol/L NaN3, with CD14–phycoerythrin (Leu-M3; Becton Dickinson) and CD1a–fluorescein isothiocyanate (FITC) (BL1; Immunotech) monoclonal antibodies at the appropriate concentration or with control isotype-matched irrelevant monoclonal antibodies at the same concentration. After they were washed, cells were analyzed with a FACScalibur (Becton Dickinson) using CellQuest software (Becton Dickinson).
Fluorescence in situ hybridization
Dual-color fluorescence in situ hybridization (FISH) was performed with a biotinylated (peri)centromeric α satellite DNA probe specific for chromosome X (Oncor, Gaithersburg, MD) and a probe specific for chromosome Y heterochromatin. This chromosome Y-specific probe was obtained by polymerase chain reaction amplification of human male genomic DNA with the following oligonucleotides: 5′ TCC ACT TTA TTC CAG GCC TGT CC 3′ and 5′ TTG AAT GGA ATG GGA ACG AAT GG 3′. It was labeled with digoxigenin-11-dUTP by nick-translation. The X-biotinylated and Y-digoxigenin–labeled probes were revealed by Texas Red stain and FITC fluorochromes, respectively. Slides were counter-stained with DAPI, mounted with an anti-fading medium (Vector, Burlingame, CA), viewed using a Zeiss Axiophot fluorescence microscope (Carl Weiss SA, Le Pecq, France), and analyzed with the Applied Imaging system (BioScience Centre, Newcastle upon Tyne, UK). On each slide, the proportion of XY and XX cells was determined by counting 100 cells.
Results
Detection of macrophages
In contrast with monocyte defects, macrophages—identified as histiocytes expressing CD68—were detected in the dermis of 5 skin samples from 4 children before BMT (Figure1A). Some of these histiocytes were also positive for HLA-DR. The amounts of macrophages within the dermis were similar to those in control patients. Macrophages were also detected within the sinuses of atrophic lymph nodes of 2 patients who did not undergo BMT (Figure 1B,C). They were also present within the cortex of lymph nodes, the red pulp of the spleen, the thymus, and several nonlymphoid organs, including the pancreas and the parathyroid and adrenal glands. In addition, macrophages were present within the dermis of the 4 patients who underwent transplantation, whatever their hematologic status.
Immunohistochemical detection of macrophages with CD68 in patients who did not undergo transplantation but who had reticular dysgenesis.
Macrophages were detected within the dermis (A; original magnification, ×100) and within the subcapsular sinuses of the atrophic lymph nodes (B; original magnification, ×100 [left] and ×400 [right]).
Immunohistochemical detection of macrophages with CD68 in patients who did not undergo transplantation but who had reticular dysgenesis.
Macrophages were detected within the dermis (A; original magnification, ×100) and within the subcapsular sinuses of the atrophic lymph nodes (B; original magnification, ×100 [left] and ×400 [right]).
Detection of Langerhans cells
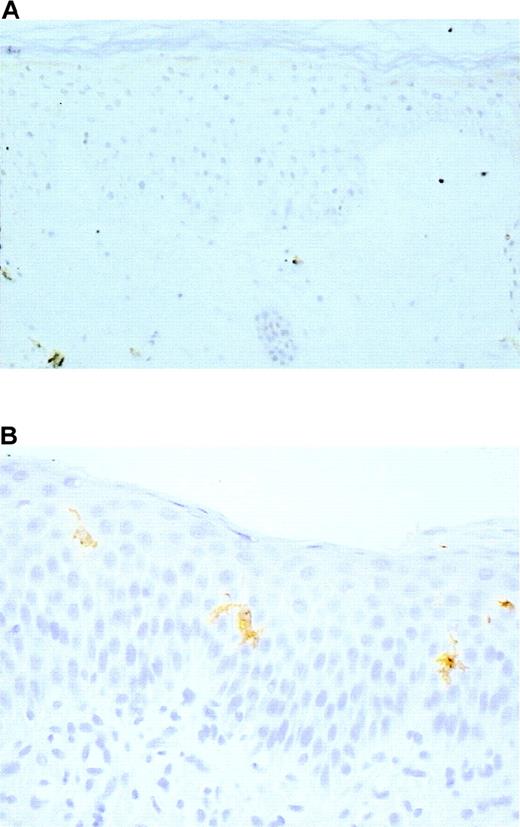
Identification of LC in skin sections was based on 3 criteria: localization within the epidermis, dendritic morphology, and expression of at least 1 of the 3 following markers: CD1a, HLA-DR, and S100 protein. Langerhans cells were undetectable in the 5 skin samples of the 4 patients before BMT (Figure 2A). However, LC of normal morphology and expressing CD1a, HLA-DR, and S100 protein (Figure 2B) were present in 2 of 4 samples from patients assessed after BMT (Table1). The 2 patients who did not have LC had no evidence of bone marrow engraftment at the time of biopsy.
Immunohistochemical detection of Langerhans cells in the epidermis of patients with reticular dysgenesis.
Langerhans cells were absent before bone marrow transplantation (A), S100 protein, original magnification ×100, but they were present after allogeneic bone marrow engraftment (B). CD1a, original magnification, ×400.
Immunohistochemical detection of Langerhans cells in the epidermis of patients with reticular dysgenesis.
Langerhans cells were absent before bone marrow transplantation (A), S100 protein, original magnification ×100, but they were present after allogeneic bone marrow engraftment (B). CD1a, original magnification, ×400.
In vitro differentiation of dendritic cells
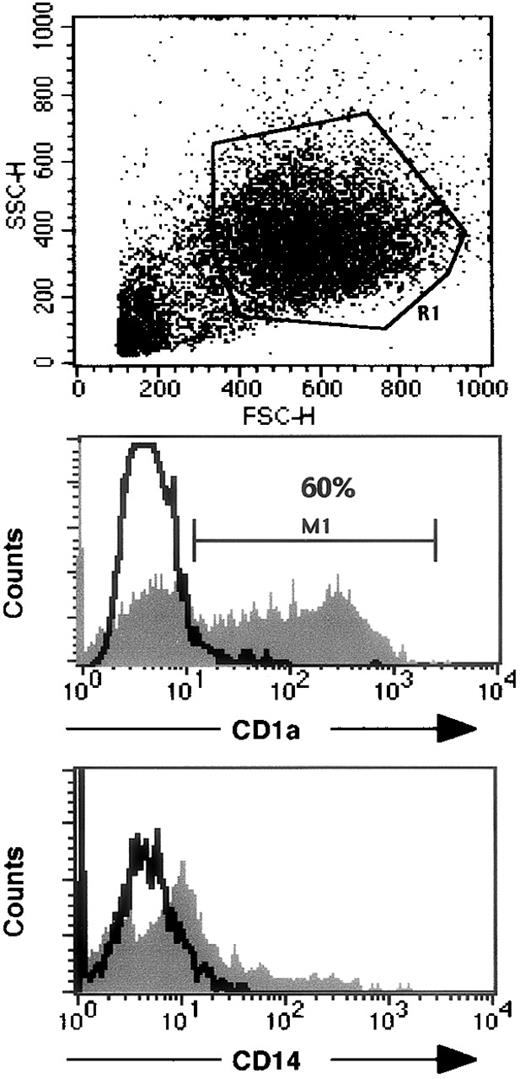
In a patient who underwent successful BMT, evidence of a split chimeric reconstitution has been reported31 because his T cells originated from his sister, but his B cells and monocytes were of autologous origin. FISH detection of chromosomes X and Y was performed on the patient's blood cells. Most (71%) of the purified CD14+ blood monocytes from this patient were autologous (Figure 3), whereas most (80%) of his lymphocytes originated from the donor. These purified CD14+monocytes were induced to differentiate in vitro into monocyte-derived DC in the presence of GM-CSF (100 ng/mL), IL-4 (10 ng/mL), and TGF-β1 (10 ng/mL). After a 6-day culture, most of the cells expressed very low to negative CD14, and 60% were CD1a positive (Figure4). Most (92%) of these DC generated in vitro were XY (Figure 3). As controls, blood cells obtained from the patient's sister and in vitro-generated CD1a-positive DC were all XX (not shown). These results suggest that the impairment of LC differentiation was an extrinsic cell defect. Unfortunately, though FISH was performed on the skin sample of this patient, it was not possible to distinguish unambiguously the putative Y chromosome-positive LC from the Y chromosome-positive surrounding autologous keratinocytes.
Dual color FISH with probes for chromosomes X (red spot) and Y (green spot) of cells from a patient with reticular dysgenesis and chimeric hematologic reconstitution.
Most purified CD14+ blood monocytes (A) and of in vitro–generated dendritic cells (B) were autologous (XY).
Dual color FISH with probes for chromosomes X (red spot) and Y (green spot) of cells from a patient with reticular dysgenesis and chimeric hematologic reconstitution.
Most purified CD14+ blood monocytes (A) and of in vitro–generated dendritic cells (B) were autologous (XY).
Flow cytometry analysis of monocyte-derived DC from a patient with reticular dysgenesis and chimeric hematologic reconstitution.
The upper panel indicates FSC and SSC characteristics of DC cultured as described in the “Patients and methods” section. Expression of CD1a and CD14 on gated viable cells (R1) are represented on the middle and lower panels, respectively. Open histograms represent isotypic controls, and filled histograms represent specific staining. 104 events were analyzed with a FACScalibur using CellQuest software.
Flow cytometry analysis of monocyte-derived DC from a patient with reticular dysgenesis and chimeric hematologic reconstitution.
The upper panel indicates FSC and SSC characteristics of DC cultured as described in the “Patients and methods” section. Expression of CD1a and CD14 on gated viable cells (R1) are represented on the middle and lower panels, respectively. Open histograms represent isotypic controls, and filled histograms represent specific staining. 104 events were analyzed with a FACScalibur using CellQuest software.
Discussion
Reticular dysgenesis is an extremely rare primary immunodeficiency that accounts for 1% to 3% of severe combined immunodeficiencies.26,30,32 The myeloid lineage of patients with RD has a characteristic maturation arrest at the stage of promyelocyte.18 The mechanism responsible for the leukocyte maturation defect in RD is still unknown. Because rare mature blood leukocytes are detectable, the primary defect is supposed to interfere with the normal growth or survival of leukocytes rather than with the initiation of stem cell differentiation.21Patients with RD can be cured by BMT, indicating that RD results from a primary defect of hematopoietic cells. The lack of blood leukocytes and LC may be caused by either a direct or an indirect (ie, intrinsic or extrinsic) mechanism. The split chimeric reconstitution of 1 patient after BMT argues for an extrinsic cell defect.
Human monocytes can differentiate in vitro either into macrophages35 and osteoclasts36 or into DC subsets,37 including LC,7 depending on the cytokines added to the medium. Patients with RD have very low blood monocyte counts. However, these patients do not have osteopetrosis, suggesting that a normal differentiation of monocyte derived-osteoclasts occurs in RD. Osteopetrotic op/op mice, which lack M-CSF, also have dramatically reduced numbers of blood monocytes. These mice lack subsets of macrophages, such as osteoclasts, splenic marginal zone metallophils, and lymph node subcapsular sinus macrophages, and they have reduced densities of Langerhans cells within the epidermis.38 We detected numerous macrophages within the lymph node subcapsular sinuses of patients with RD and within many other organs. These results show that the mechanism of LC defect in RD does not interfere with the differentiation of M-CSF–dependent macrophages.
No LC were detected in 5 skin samples from 4 patients with RD before BMT. Langerhans cells were also absent in skin samples from 2 patients who underwent BMT but not bone marrow reconstitution. Thus, the absence of LC is part of the phenotype of this rare inherited condition. The only animal model described thus far that lacks LC is the model with TGF-β1 deficiency.9,10 These mice have no LC, but they have increased circulating blood monocytes and macrophages and normal lymphocyte counts.39 They also have epidermal hyperplasia. The TGF-β–deficient hematopoietic mouse stem cells can differentiate in LC in vitro and when transplanted in normal mice.10 In vitro, TGF-β has been shown to be necessary for the differentiation of human LC.7 As do TGF-β–deficient mice, patients with RD exhibit a deficiency in LC but do have macrophages. However, they lack blood monocytes, have low lymphocyte counts, and do not have epidermal hyperplasia. Thus, TGF-β is unlikely to be responsible for the LC defect observed in patients with RD.
Langerhans cells were detected in the epidermis of 2 patients after BMT. The LC defect is thus not related to a keratinocyte dysfunction. One patient with RD was shown to have a split chimeric blood reconstitution, with mostly autologous monocytes,31 proving that the defect in blood monocytes in RD is an extrinsic cell defect. A cell-autonomous defect of a gene responsible for abnormal myeloid and lymphoid development, such as PU.1,40 41 is thus unlikely. Patients' monocytes were shown to differentiate in vitro into CD1a-positive immature DC, most of which were of host origin, as shown by FISH. This indicates that, at least in vitro, monocytes were able to give rise to immature DC. However, definitive proof that this is also the case for LC in the skin is unavailable because FISH did not allow us to distinguish LC from the Y chromosome-positive surrounding autologous keratinocytes.
In conclusion, our data show that patients with RD have a deficiency of LC that can be corrected by BMT. These data suggest that the LC defect is an extrinsic cell defect resulting from the lack of a bone marrow–derived factor required for LC, but not for macrophage, differentiation.
Acknowledgments
We warmly thank Drs A. Ferster and J. Pardo for providing histologic samples. We thank all the pathologists, immunologists, and pediatricians who gave us information concerning the availability of histologic samples from the published reports of reticular dysgenesis, particularly Drs R.H. Buckley, M.D. Cooper, D. Gitlin, R.J. Haas, R. Jaffe, H.P.W. Kozakewich, R.J. Lewinsky, R.C. McCoy, and S.V. Pizzo. We also thank M. Ortin–Serrano for expert technical assistance.
Supported by grants from the G.R.I.P.
Reprints:Jean-François Emile, Service d'Anatomie Pathologique, Hôpital Paul Brousse BP200, F-94804 Villejuif Cedex, France; e-mail: jean-francois.emile@pbr.ap-hop-paris.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. Immunohistochemical detection of macrophages with CD68 in patients who did not undergo transplantation but who had reticular dysgenesis. / Macrophages were detected within the dermis (A; original magnification, ×100) and within the subcapsular sinuses of the atrophic lymph nodes (B; original magnification, ×100 [left] and ×400 [right]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/1/10.1182_blood.v96.1.58/4/m_bloo0132001z.jpeg?Expires=1763985758&Signature=FZ0kh9ew8O8q4VCWChl2TSKL5f1EDNNeXLl6Ts69ZYSaZbsH~PdtxtCbc7PECxK~Au88vPfmTCu8dtQWfvxGR5Xt6itWRa~wBGHkWy8dlTeUwHOaAnIGY7yMzV5tvExnuiMGumAizbOE7jsVYDi7HK3H8A9JoBMSAeA-z5ttT~7GeGEsBs5PKtjjDsQXBnrKrTqftnxDQ1k8VbNRW9wn0TfepY6f1hAO69nNhaLBZ18RpBR7oIjwPl7-H5SDeHRnaawT0rPk4C2BtimNAT1LGbGVfz-ejLGylE8El7gt9UiGWqGPH41ojt0k7T68G6EW5LQbrYYNIHDo1gH5moi4Ng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)