Abstract
CD40 ligand (CD40L) is essential for the initiation of antigen-specific T-cell responses. This study is based on the hypothesis that dendritic cells (DCs) genetically modified ex vivo to express CD40L will enhance in vivo presentation of tumor antigen to the cellular immune system with consequent induction of antitumor immunity to suppress tumor growth. To examine this concept, subcutaneous murine tumors were injected with bone marrow-derived DCs that had been modified in vitro with an adenovirus (Ad) vector expressing murine CD40L (AdmCD40L). In B16 (H-2b, melanoma) and CT26 (H-2d, colon cancer) murine models, intratumoral injection of 2 × 106 AdmCD40L-modified DCs (CD40L-DCs) to established (day 8) subcutaneous tumors resulted in sustained tumor regression and survival advantage. This antitumor effect was sustained when the number of CD40L-DCs were reduced 10-fold to 2 × 105. Analysis of spleens from CD40L-DC–treated animals demonstrated that CD40L-DCs injected into the subcutaneous CT26 flank tumors migrated to the spleen, resulting in activation of immune-relevant processes. Consistent with this concept, intratumoral administration of CD40L-DCs elicited tumor-specific cytotoxic T-lymphocyte responses, and the transfer of spleen cells from CD40L-DC–treated mice efficiently protected naive mice against a subsequent tumor challenge. In a distant 2-tumor model of metastatic disease, an untreated B16 tumor in the right flank regressed in parallel with a left B16 tumor treated with direct injection of CD40L-DCs. These results support the concept that genetic modification of DCs with a recombinant CD40L adenovirus vector may be a useful strategy for directly activating DCs for cancer immunotherapy.
Although many tumors have antigens recognizable by the immune system, the ability of tumors to escape a functional immune system suggests that the immune mechanism is often insufficient to effectively overtake the potential of many tumors to grow. In an attempt to bolster antitumor immunity, this study focuses on a strategy to enhance the ability of the adaptive immune system to recognize the tumor cells as foreign by activating dendritic cells (DCs) by the genetic strategy of modifying them to express CD40 ligand (CD40L), a ligand normally found on the surface of activated CD4+ T cells that play a central role in activating DCs as antigen-presenting cells (APCs).1-3
CD40L is a 33-kd type II membrane protein that is preferentially expressed on activated CD4+ T cells.2,4-6 The receptor for CD40L, CD40 (40 kd), is expressed on APCs, including DCs, B cells, activated macrophages, and follicular DCs.2 DCs are professional APCs specialized in sampling antigen throughout the body, migrating to lymphoid organs, and presenting antigen to naive T cells.1 Stimuli from CD4+ T-helper cells via the CD40/CD40L interaction is essential in bringing the DCs to a state in which they can autonomously trigger antigen-specific T-cell responses.7-9 In this context, the CD40L on antigen-stimulated CD4+ T-helper cells activates DCs, with up-regulation of T-cell costimulatory molecules such as B7 and intercellular adhesion molecule (ICAM)-1, and consequent direct stimulation of CD8+ T-killer cells.10,11 As part of the activation of DCs, the CD40L-CD40 interaction induces the production of cytokines that favor the development of a T-helper 1 (Th1) response.10-12
On the basis of the hypothesis that augmentation of APC activation will augment antigen-specific immune responses against a tumor, and with the knowledge that CD40L (normally expressed on activated CD4+T cells) can trigger activation of APCs via the CD40 receptor on DCs, we and others have used gene transfer of the coding sequence of CD40L to tumor cells, leukemia cells, or fibroblasts, or intradermally as a strategy to activate APCs.13-20 In this study, with the knowledge that the triggering of CD40 with anti-CD40 antibodies on DCs will substitute for the requirement of activated CD4+ T cells in activating DCs,7-9 we have extended the concept of using gene transfer to activate the DCs directly by hypothesizing that, by genetically modifying DCs to express CD40L, the DC would be able to activate itself and thus enhance the function of DCs within a tumor to augment antitumor immune responses. With this background, this study evaluates the concept of suppressing the growth of preexisting tumors by direct administration of syngenic bone marrow-derived DCs that have been genetically modified with the CD40L coding sequence in vitro. To accomplish this, we have used an E1− adenovirus (Ad) gene vector (AdmCD40L) to transfer the murine CD40L complementary DNA (cDNA) to DCs, and then injected the modified DCs into preexisting tumors in syngenic hosts. The data demonstrate that direct administration of AdmCD40L-modified DCs will elicit specific antitumor immunity and inhibit the growth of preexisting tumors mediated by tumor-specific systemic immune processes.
Materials and methods
Mice
Female C57Bl/6 (H-2b), Balb/c (H-2d) mice, and CD4 knockout (CD4−/−) mice that had been backcrossed to the C57Bl/6 background for 8 generations, 6 to 8 weeks old, were purchased from the Jackson Laboratories (Bar Harbor, ME), and housed under specific pathogen-free conditions.
Cell culture
CT26 is an undifferentiated colon adenocarcinoma cell line (H-2d) originally derived by intrarectal injections ofN-nitroso-N-methylurethane in a female Balb/c mouse (provided by N. P. Restifo, National Cancer Institute, Bethesda, MD).21 The SVBalb fibroblast cell line is also syngenic to Balb/c mice (provided by L. Gooding, Emory University, Atlanta, GA).22 C3 is a cell line originally derived by transfecting C57Bl/6 mouse embryonal fibroblasts (H-2b) with a plasmid containing the entire genome of the human papilloma virus type 16 (provided by C. J. M. Melief, University Hospital Leiden, The Netherlands).23 The CL7 fibroblast cell line (H-2d), the B16 murine melanoma cell line (H-2b), and Lewis lung carcinoma (LLC) cell line (H-2b) were obtained from the American Type Culture Collection (Manassas, VA). The CT26 and C3 cell lines were maintained in complete RPMI-1640 media (10% fetal bovine serum, 2 mmol/L L-glutamine, 100 μg/mL streptomycin, and 100 U/mL penicillin). DCs were generated from mouse bone marrow precursors in complete RPMI-1640 media with recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF) (100 U/mL; Sigma, St Louis, MO) and recombinant murine interleukin-4 (IL-4; 2 ng/mL; R & D Systems, Minneapolis, MN), as described previously.24 By flow cytometric analysis, more than 70% of DCs generated in this way displayed the characteristic DC surface markers CD11c and MHC class II, with less than 7% contaminating T cells (not shown). All other cell lines were maintained in complete DMEM media.
Adenovirus vectors
The replication-deficient adenovirus vectors used in this study are based on human Ad5 genome with E1 and E3 deletions. The AdmCD40L vector and the AdNull control vector express the murine CD40L cDNA and no transgene, respectively, under the control of the cytomegalovirus (CMV) early/immediate promoter/enhancer.18,25 Propagation, purification, and titration of the adenovirus vectors were as previously described.26 27
Flow cytometry
To quantify expression of surface molecules on DCs, DCs from Balb/c mice were transduced with AdmCD40L or AdNull at a multiplicity of infection (moi) 40 for 4 hours, and cultured at 2 × 106 or 2 × 105 cells/mL. After incubation for 72 hours, 4 × 105 DCs were stained for 30 minutes at 4°C with fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (mAb) to CD86 (B7-2, GL1), CD80 (B7-1, 16-10A1), CD54 (ICAM-1, 3E2), CD48 (BCM-1, HM48-1), CD40 (HM40-3), or CD25 (IL-2 receptor α chain, 7D4), and phycoerythrin (PE)-conjugated anti-mCD40L mAb MR1 (Pharmingen, San Diego, CA). Isotype-matched antibodies served as FITC- or PE-conjugated controls (Pharmingen). Stained cells were analyzed in an Elite flow cytometer (Coulter, Hialeah, FL). For coexpression analysis, an FL1 (fluorescence 1, 530 nm for FITC)/FL2 (fluorescence 2, 585 nm for PE) (FL-1/FL-2) contour plot was used to calculate the percentage of positive cells.
Cytokines
To demonstrate the function of the AdmCD40L to confer to DCs the ability to express functional mCD40L, DCs from Balb/c mice were transduced with AdmCD40L, AdNull, or phosphate-buffered saline (PBS) alone (ie, no transduction) at a moi 40 for 4 hours, and plated on 24-well plates at 5 × 106 cells/mL. Where indicated, transduced DCs were incubated with anti-mCD40L mAb MR1 (10 μg/mL; Pharmingen) or the same amount of the control hamster IgG (Pharmingen), or were placed at several concentrations, 5 × 106, 1 × 106, 5 × 105, and 1 × 105 cells/mL. After incubation for 72 hours, 37°C, the supernatant (400 μL) was harvested and centrifuged to remove debris. The levels of murine IL-12 or macrophage inflammatory protein 1α (MIP-1α) released into the culture medium were assessed by enzyme-linked immunosorbent assay (ELISA), using the mouse IL-12 p40 or MIP-1α immunoassay (R & D Systems), respectively.
Tumor therapy model
Tumor cells (5 × 105 B16 or 2 × 105 CT26) were injected subcutaneously in the right flank of mice. On day 8, tumors of the mice were inoculated with 100 μL of DCs or CL7 cells, infected or mock-infected with the AdmCD40L or AdNull vector (moi 40, 24 hours). The size of each tumor was assessed 3 times weekly and recorded as the average tumor area (mm2) ± standard error by measuring the largest perpendicular diameters. When animals became moribund or the tumors reached 15 mm in diameter, the mice were killed and this was recorded as the date of death for survival studies. For some studies, where indicated, mice were challenged in both flanks with tumor cells: 5 × 105 B16 in the right flank, and 5 × 105 B16 or LLC in the left flank.
Tumor-specific cytotoxic T lymphocytes
To assess the ability of AdmCD40L-modified DCs to induce tumor-specific cytotoxic T lymphocytes (CTL), 10 days after the treatment (intratumoral injection of AdmCD40L-modified DCs to 8-day established tumors), splenocytes were isolated from 2 mice, pooled, and restimulated for 5 days at 3 × 106 or 4 × 106 cells/mL with 106 cells/mL CT26 or B16 cells treated with 100 μg/mL mitomycin C (Sigma). After restimulation, viable cells were collected and tested in a51Cr-release assay for their ability to lyse CT26 or B16 cells. Where indicated, antibodies to H-2d or H-2b (Pharmingen) were included at a final concentration of 10 μg/mL. The percentage of specific 51Cr release was calculated as 100 × ([experimental release − spontaneous release]/[maximal release − spontaneous release]).
Adoptive transfer of splenocytes
Ten days after the treatment of day 8 tumor-bearing mice, the spleens were removed from 11 to 15 mice, pooled, and a single cell suspension prepared by mechanical dissociation. Splenocytes (5 × 107 cells per mouse) were administered into recipient animals via tail vein. After 7 days, recipient animals were challenged by subcutaneous injection in the right flank with 2 × 105 CT26 cells or 5 × 105B16 cells (day 0). Survival was then recorded as described above.
Reverse transcriptase-polymerase chain reaction
To demonstrate expression of CD40L mediated by the AdmCD40L vector, total cellular RNA was extracted from infected cells in vitro or spleens by the guanidinium thiocyanate-phenol chloroform extraction method.28 Synthesis for cDNA from total cellular RNA (1 μg) was performed using the Advantage RT-for-PCR kit (Clontech, Palo Alto, CA). One tenth of the cDNA was amplified with primers specific for either vector-derived mCD40L or the control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts: for mCD40L, 5′-TGCCAAGAGTGACGTGTCCA-3′ and 5′-TCAGAGTTTGAGTAAGCCAAAAGA-3′; for the control GAPDH, 5′-ATGGTGAAGGTCGGTGTGAACGGA-3′ and 5′-TTACTCCTTGGAGGCCATGTAGGC-3′. Amplification conditions were 94°C, 2 minutes, and then 30 cycles of 94°C, 1 minute; 55°C, 1 minute; and 68°C, 1 minute, followed by 68°C, 7 minutes. For detection of vector-derived mCD40L mRNA in spleens, 2% of the amplified product was used as a template for nested polymerase chain reaction (PCR). Nested PCR was conducted for 25 cycles with the same thermal cycling profile as above using inner primers specific for vector-derived mCD40L transcripts: 5′-CCTAATACGACTCACTATAC-3′ and 5′-CTCTGTGGATCACTTGGCTT-3′. Each PCR was carried out in 50 μL, and 5 μL of PCR products were resolved on a 1% agarose gel.
Immunohistochemistry
To examine the ability of AdmCD40L-modified DCs to elicit immune-related process in vivo, spleens or tumors from treated animals were assessed by immunohistochemistry for cells expressing interleukin-2 receptor α chain (IL-2Rα), an activation marker of T lymphocytes, or CD4 and CD8, respectively. After frozen sections (6 μm in thickness) were fixed in cold acetone, the samples were treated with 10 μg/mL antimouse CD25 mAb (IL-2Rα; Pharmingen), 2.5 μm/mL antimouse CD8a mAb (Pharmingen), or antimouse CD4 mAb (Pharmingen), 30 minutes, and then stained with 10 μg/mL goat antirat IgG Oregon Green antibody (Molecular Probes, Eugene, OR) for 30 minutes. Sections were assessed by counting the number of positive cells in 10 random high power fields (hpf; magnification ×600).
Statistical analysis
All data are reported as mean ± standard error. Statistical comparison was made using Fisher's exact method, and a value ofP < .05 was accepted as indicating significance. Survival evaluation was carried out using Kaplan-Meier analysis.
Results
Cell surface phenotype of AdmCD40L-modified dendritic cells
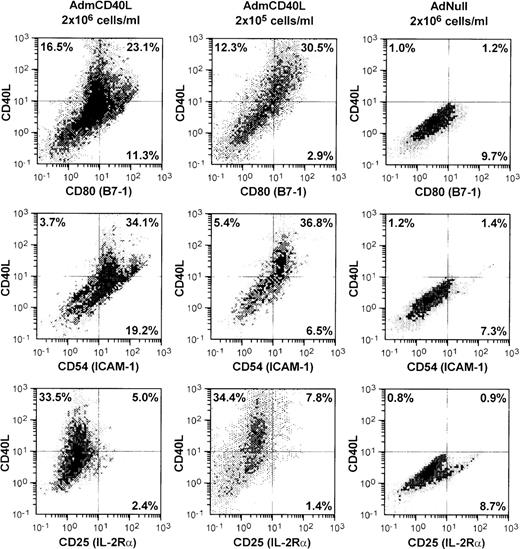
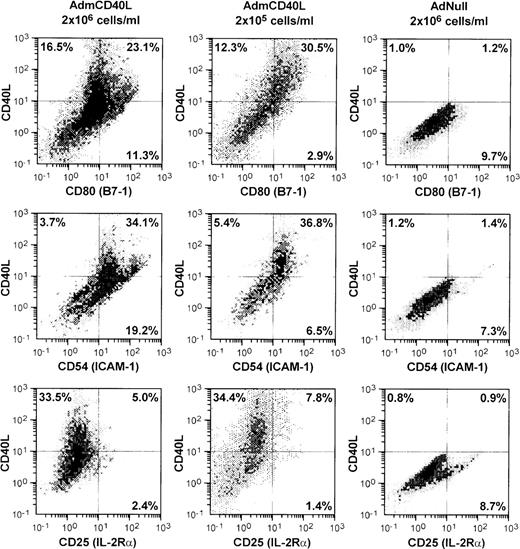
AdmCD40L infection of DCs enhanced expression of CD40L and other DC-lymphocyte costimulatory molecules (Figure1). AdmCD40L-modified DCs cultured at the standard density (2 × 106 cells/mL) had a 3- to 6-fold increase in the number of CD80 (B7-1) and CD54 (ICAM-1) expressing DCs compared with AdNull-infected DCs. When DCs were cultured at a lower density (2 × 105 cells/mL) after AdmCD40L modification, DCs expressed CD80+ and CD54+, but at a slightly decreased level compared with DCs cultured at a higher density, suggesting that at least some of the DC activation was via a bystander mechanism. In this regard, despite the similar percentage of CD40L-expressing DCs cultured at a higher or lower density, the lower-density culture was associated with a 3- to 4-fold decrease in the percentage of DCs expressing CD80+CD40L− (11.3% vs 2.9%) or CD54+CD40L− (19.2% vs 6.5%). DCs cultured at a lower density expressed no less CD80+CD40L+ or CD54+CD40L+ than DCs cultured at a higher density. Other surface molecules CD86 (B7-2) and CD48 (the mouse homologue of LFA-3) expression was also augmented in the higher density cultures by AdmCD40L modification (CD86+: AdNull 2.8% vs AdmCD40L 27.8%; CD48+: AdNull 17.6% vs AdmCD40L 27.1%; not shown), but CD40L-transduced DCs displayed lower frequency expression of CD40, the receptor for CD40L (AdNull 72% vs AdmCD40L 45%; not shown). CD25 (IL-2Rα), an activation marker of lymphocytes and macrophages was expressed on only a small percentage of AdmCD40L-modified DCs. A similar proportion of cultures infected with the control vector AdNull expressed CD25. The percentages of cells expressing CD25 were less than 10% and were independent of AdmCD40L-mediated CD40L expression, indicating that there were minimal numbers of macrophages and lymphocytes contaminating the DC cultures.
Accessory molecules expressed by AdmCD40L-modified DCs.
Bone marrow-derived DCs were transduced with AdmCD40L or AdNull at moi 40 for 4 hours, and cultured at 2 × 106 or 2 × 105 cells/mL for 72 hours. The modified DCs were then analyzed for surface coexpression of CD40L with CD80 (B7-1), CD54 (ICAM-1), or CD25 (IL-2 receptor α-chain) by 2-color flow cytometry. The percentage of cells in each quadrant is listed.
Accessory molecules expressed by AdmCD40L-modified DCs.
Bone marrow-derived DCs were transduced with AdmCD40L or AdNull at moi 40 for 4 hours, and cultured at 2 × 106 or 2 × 105 cells/mL for 72 hours. The modified DCs were then analyzed for surface coexpression of CD40L with CD80 (B7-1), CD54 (ICAM-1), or CD25 (IL-2 receptor α-chain) by 2-color flow cytometry. The percentage of cells in each quadrant is listed.
Cytokine production of AdmCD40L-modified dendritic cells
ELISA analyses confirmed that Ad vector-mediated transfer of the CD40L cDNA to DCs induced the DCs to secrete cytokines (not shown). Because stimulation of CD40 on DCs is known to induce IL-12 and MIP-1α expression,10-12 the AdmCD40L-transduced DCs cultures were assessed for IL-12 and MIP-1α in the supernatant. Infection of DCs by AdmCD40L stimulated the production of IL-12 (P < .0001), whereas AdNull-infection did not (P > .9). For AdmCD40L-transduced DCs, the amount of IL-12 secretion per cell was not affected by decreasing the cell concentration of DCs culture after CD40L transduction, even to 105 cells/mL (not shown). AdmCD40L, but not AdNull control infection of DCs, also induced MIP-1α secretion (P < .0001). Both IL-12 and MIP-1α secretion were inhibited by addition of anti-mCD40L mAb MR1 compared with control IgG (IL-12, P < .0001; MIP-1α, P < .01), suggesting that the AdmCD40L vector directed CD40L expression on the DC surface was responsible for stimulating the DCs to secrete IL-12 and MIP-1α.
Antitumor effects of mCD40L-modified dendritic cells
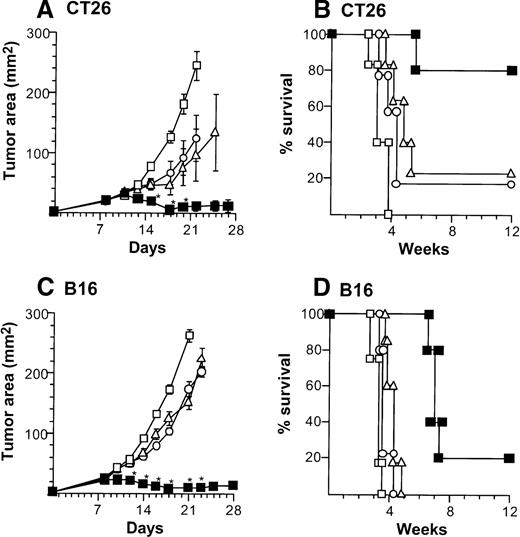
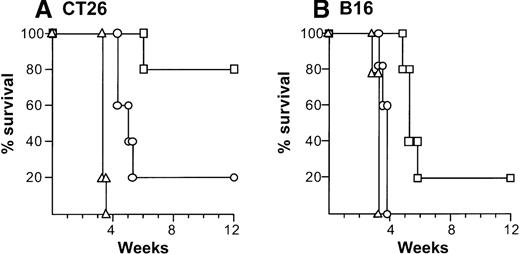
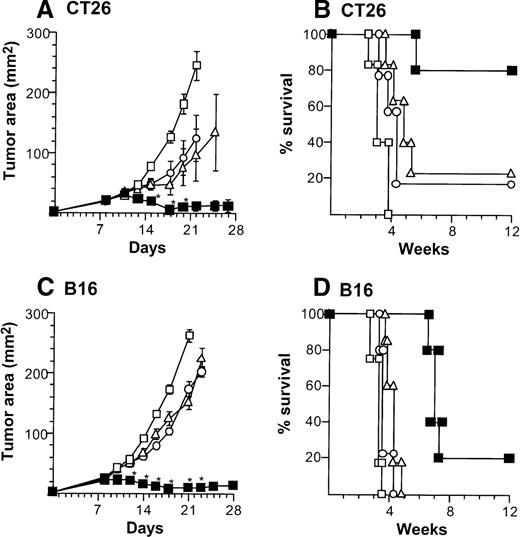
Treatment of CT26-bearing Balb/c mice (H-2d) with 2 × 106 CD40L-DCs induced significant inhibition of tumor growth (P < .05 to all other groups) at time points 15 to 20 days, and resulted in the long-term survival in most mice (Kaplan-Meier analysis, P < .05 to all other groups; Figure2A,B). Administration of 2 × 106 AdNull- or mock-infected DCs also had some beneficial effect compared with no treatment (tumor size days 15 to 22,P < .05; survival, P < .05). In the B16 tumor model in C57Bl/6 mice (H-2b), tumor growth was suppressed significantly by 2 × 106 CD40L-DCs treatment compared with that of all other control groups (days 12 to 23,P < .005), resulting in survival advantage (P < .005; Figure 2C,D). To a lesser extent, the B16 tumor size treated with 2 × 106 AdNull- or mock-infected DCs was also suppressed significantly compared with the tumors without any treatment (tumor area days 14 to 21,P < .005; survival, P < .05).
Suppression of growth of preexisting tumors and survival of mice with preexisting tumors by intratumoral administration of AdmCD40L-modified DCs.
(A) Tumor growth, CT26 tumors, Balb/c mice. Tumor cells (2 × 105) were implanted subcutaneously in the midflank. On day 8, tumor-bearing mice were treated by intratumoral injection of 2 × 106 bone marrow DCs modified by in vitro infection with AdmCD40L (▪), AdNull (□), or PBS alone (Δ) (vector moi 40, 24 hours). (B) Survival, CT26 tumors, Balb/c mice. Shown is the survival of animals in panel A. (C) Tumor growth, B16 tumors, C57Bl/6 mice. The study was similar to that in panel A, but 5 × 105 tumor cells were used. (D) Survival, B16 tumors, C57Bl/6 mice. Similar to B, using mice in panel C. For panels A and C, the size of each tumor was assessed 3 times per week, and is reported as the average tumor area (mm2) ± standard error of n = 5 per group. Asterisks in panels A and C indicate significant differences at 95% confidence limits between AdmCD40L and all other surviving groups; on days 22 and 25 in panel A, AdmCD40L-infected DCs had no significant effect compared with mock-infected DCs. For panels B and D, survival was recorded as the percentage of animals in each group (Kaplan-Meier analysis, for all panels, P < .05 AdmCD40L compared with all other groups). In all panels, controls included tumor-bearing mice without any treatment (‘).
Suppression of growth of preexisting tumors and survival of mice with preexisting tumors by intratumoral administration of AdmCD40L-modified DCs.
(A) Tumor growth, CT26 tumors, Balb/c mice. Tumor cells (2 × 105) were implanted subcutaneously in the midflank. On day 8, tumor-bearing mice were treated by intratumoral injection of 2 × 106 bone marrow DCs modified by in vitro infection with AdmCD40L (▪), AdNull (□), or PBS alone (Δ) (vector moi 40, 24 hours). (B) Survival, CT26 tumors, Balb/c mice. Shown is the survival of animals in panel A. (C) Tumor growth, B16 tumors, C57Bl/6 mice. The study was similar to that in panel A, but 5 × 105 tumor cells were used. (D) Survival, B16 tumors, C57Bl/6 mice. Similar to B, using mice in panel C. For panels A and C, the size of each tumor was assessed 3 times per week, and is reported as the average tumor area (mm2) ± standard error of n = 5 per group. Asterisks in panels A and C indicate significant differences at 95% confidence limits between AdmCD40L and all other surviving groups; on days 22 and 25 in panel A, AdmCD40L-infected DCs had no significant effect compared with mock-infected DCs. For panels B and D, survival was recorded as the percentage of animals in each group (Kaplan-Meier analysis, for all panels, P < .05 AdmCD40L compared with all other groups). In all panels, controls included tumor-bearing mice without any treatment (‘).
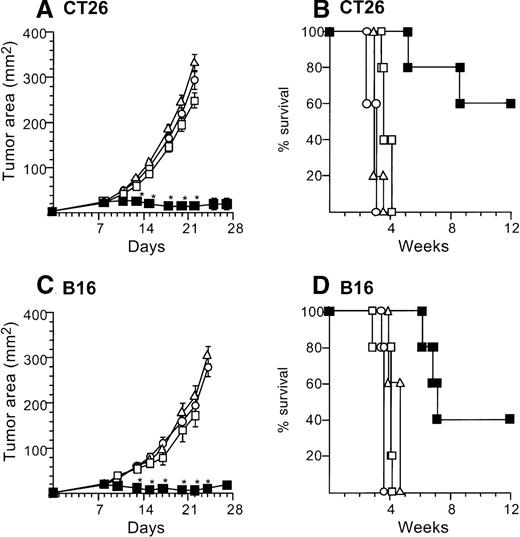
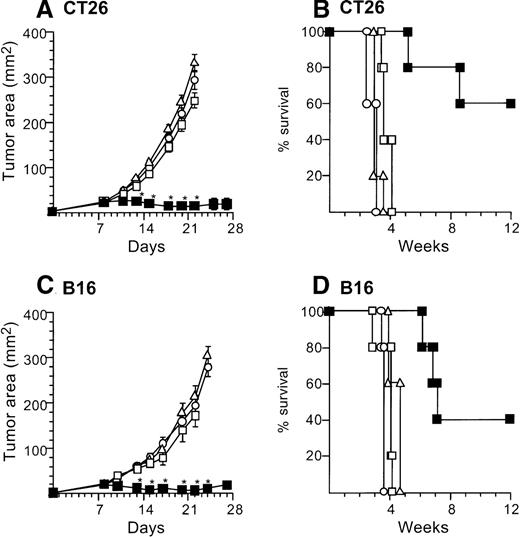
Marked tumor suppression was also observed with administration to the tumors of one tenth the numbers (2 × 105) of AdmCD40L-infected DCs. In this context, 2 × 105 AdmCD40L-DCssuppressed the growth of established tumors (both CT26 and B16) when injected intratumorally, but AdNull- or mock-infected DCs did not (Figure 3). Balb/c mice bearing CT26 8-day established tumors were treated by direct injection with 2 × 105 AdmCD40L-transduced DCs. This therapy significantly inhibited tumor growth days 13 to 22 (P < .0001) and survival at 12 weeks in 60% (P < .005), in contrast with AdNull- or mock-infected DCs as well as no treatment (Figure 3A,B). Intratumoral injection with AdNull- or mock-infected DCs had no therapeutic effect on CT26-bearing mice days 11 to 22 (P > .7). Similar results were achieved in the B16 established tumors. The growth of B16 tumors injected with AdmCD40L-modified DCs was suppressed significantly over days 13 to 24 (P < .0005) with enhanced survival (P < .005), whereas tumors injected with AdNull- or mock-infected DCs grew in a similar fashion to naive tumors from days 10 to 22 (P > .2) and did not have enhanced survival (P > .2; Figure 3C,D). This antitumor effect of 2 × 105 AdmCD40L-modified DCs against B16 tumors was also observed in CD4 knockout mice over days 10 to 18 (P < .005 compared with tumor-bearing wild-type mice without any treatment; not shown).
Treatment of established tumors with intratumoral administration of reduced numbers of AdmCD40L-modified DCs.
The study was carried out in a fashion parallel to that in Figure 2, but with administration of 10% the number of AdmCD40L-modified DCs. (A) Growth of CT26 tumors in Balb/c mice. Balb/c mice were injected in the flank with 2 × 105 CT26 tumor cells as described in Figure 2A (day 0). On day 8, the tumor-bearing mice received intratumor injections of 2 × 105 DCs that had been transduced in vitro with AdmCD40L (▪), AdNull (□), or PBS alone (Δ) at moi 40 for 24 hours. (B) Survival, CT26 tumors, Balb/c mice. Shown is the survival of animals in panel A. (C) Growth of B16 tumors in C57Bl/6 mice. The study was identical to that described in panel A, except for the different tumor type and its number; ie, 5 × 105 B16 cells were used as described in Figure2C. (D). Survival, B16 tumors, C57Bl/6 mice. Similar to B, using mice in panel C. For panels A and C, the size of each tumor was assessed 3 times per week, and is reported as the average tumor area (mm2) ± standard error of n = 5 mice per group. Asterisks indicate significant differences at 95% confidence limits between AdmCD40L-transduced DCs and all other surviving groups. For panels B and D, survival was recorded as the percentage of animals in each group (Kaplan-Meier analysis, for both panels, P < .05 AdmCD40L compared with all other groups). In all panels, controls included tumor-bearing mice without any treatment (□).
Treatment of established tumors with intratumoral administration of reduced numbers of AdmCD40L-modified DCs.
The study was carried out in a fashion parallel to that in Figure 2, but with administration of 10% the number of AdmCD40L-modified DCs. (A) Growth of CT26 tumors in Balb/c mice. Balb/c mice were injected in the flank with 2 × 105 CT26 tumor cells as described in Figure 2A (day 0). On day 8, the tumor-bearing mice received intratumor injections of 2 × 105 DCs that had been transduced in vitro with AdmCD40L (▪), AdNull (□), or PBS alone (Δ) at moi 40 for 24 hours. (B) Survival, CT26 tumors, Balb/c mice. Shown is the survival of animals in panel A. (C) Growth of B16 tumors in C57Bl/6 mice. The study was identical to that described in panel A, except for the different tumor type and its number; ie, 5 × 105 B16 cells were used as described in Figure2C. (D). Survival, B16 tumors, C57Bl/6 mice. Similar to B, using mice in panel C. For panels A and C, the size of each tumor was assessed 3 times per week, and is reported as the average tumor area (mm2) ± standard error of n = 5 mice per group. Asterisks indicate significant differences at 95% confidence limits between AdmCD40L-transduced DCs and all other surviving groups. For panels B and D, survival was recorded as the percentage of animals in each group (Kaplan-Meier analysis, for both panels, P < .05 AdmCD40L compared with all other groups). In all panels, controls included tumor-bearing mice without any treatment (□).
Antitumor cytotoxic T lymphocyte induced by intratumoral injection with mCD40L-transduced dendritic cells
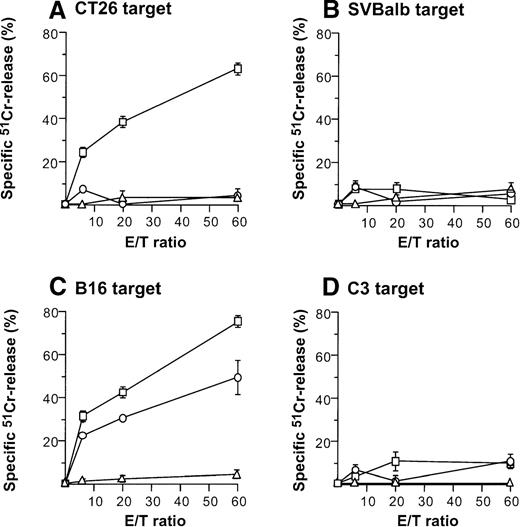
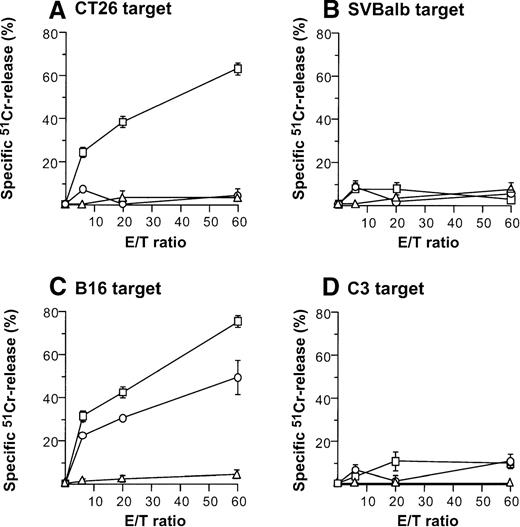
Direct injection of AdmCD40L-modified DCs to CT26 or B16 tumors elicited tumor-specific CTL activity (Figure4). Balb/c mice bearing CT26 tumors were intratumorally inoculated with AdmCD40L- or AdNull-modified DCs, or were not inoculated. Effector cells were generated from splenocytes obtained 10 days after the inoculation by culture with mitomycin C-treated CT26 cells. Cells from only mCD40L-DC–treated mice exhibited specific lysis of CT26 target cells (Figure 4A). This cytotoxicity was inhibited by addition of the relevant MHC class I antibody (H-2d), but not by addition of isotype-matched irrelevant class I antibody (H-2b) (H-2d: 85%-96% inhibition; H-2b: 11%-24% inhibition; not shown). No apparent lysis was observed against irrelevant but syngenic fibroblast SVBalb cells (Figure 4B). C57Bl/6 mice treated with mCD40L-transduced DCs exhibited a strong B16-specific splenic CTL response (Figures4C,D). Controls for this analysis included lymphocytes obtained from tumor-bearing mice injected with AdNull-transduced DCs or without any treatment. Splenocytes were restimulated with B16 cells as described above, and the resulting effector cells were evaluated for cytolytic activity against B16 and C3 cells. Injection with AdNull-transduced DCs induced minimal CTL with reactivity against B16 cells, but AdmCD40L transduction markedly enhanced this specific cytolytic activity. No specific lysis with effector cells in the B16 model was observed against C3 cells.
Induction of tumor-specific cytotoxic T cells after intratumoral administration of AdmCD40L-modified DCs.
(A,B) CT26 tumors. DCs purified from bone marrow were transduced in vitro with AdmCD40L (▪) or AdNull (□) at moi of 40 for 24 hours, and 2 × 105-modified DCs were administered intratumorally to day 8 established CT26 tumors in Balb/c mice. Controls included tumor-bearing mice without any treatment (Δ). Ten days after treatment, splenocytes were isolated and restimulated in vitro for 5 days with mitomycin C-treated CT26 cells and assayed for cytolytic function against 51Cr-labeled CT26 targets (panel A) or SVBalb targets (panel B). (C,D) B16 tumors. The study was identical to that described in panel A, except for the different tumor type. Ten days after administration of transduced DCs, the splenocytes were isolated, restimulated with mitomycin C-treated B16 cells, and assayed as in A,B. Shown are data for 51Cr-labeled B16 targets (panel C) and C3 targets (panel D). All panels, results are presented as the mean ± standard error (n = 3/data point).
Induction of tumor-specific cytotoxic T cells after intratumoral administration of AdmCD40L-modified DCs.
(A,B) CT26 tumors. DCs purified from bone marrow were transduced in vitro with AdmCD40L (▪) or AdNull (□) at moi of 40 for 24 hours, and 2 × 105-modified DCs were administered intratumorally to day 8 established CT26 tumors in Balb/c mice. Controls included tumor-bearing mice without any treatment (Δ). Ten days after treatment, splenocytes were isolated and restimulated in vitro for 5 days with mitomycin C-treated CT26 cells and assayed for cytolytic function against 51Cr-labeled CT26 targets (panel A) or SVBalb targets (panel B). (C,D) B16 tumors. The study was identical to that described in panel A, except for the different tumor type. Ten days after administration of transduced DCs, the splenocytes were isolated, restimulated with mitomycin C-treated B16 cells, and assayed as in A,B. Shown are data for 51Cr-labeled B16 targets (panel C) and C3 targets (panel D). All panels, results are presented as the mean ± standard error (n = 3/data point).
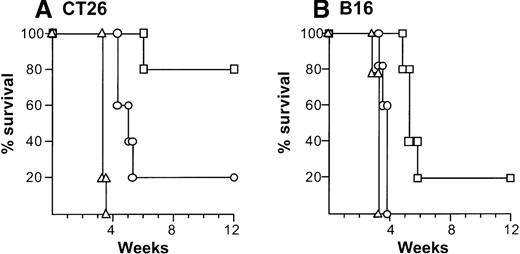
The relevance of the in vitro specific cytolysis to in vivo function was demonstrated by showing that adoptive transfer of splenocytes protected against a subsequent challenge with the identical tumor cells (Figure 5). In this context, adoptive transfer of 5 × 107 splenocytes isolated 10 days after intratumoral administration of AdmCD40L- or AdNull-transduced DCs mediated 80% or 20% protection against a CT26 challenge over a 12-week period, respectively (AdmCD40L-DCs compared with naive control,P < .005; AdmCD40L-DCs compared with AdNull-DCs,P < .05; Figure 5A). Splenocytes from B16 tumor-bearing mice treated with AdmCD40L-transduced DCs provided 20% protection (compared with naive, P < .005; Figure 5B). In contrast, transfer of lymphocytes isolated from mice injected with AdNull-modified DCs mediated only a minor enhancement in survival compared with no infusion of splenocytes (P < .05; AdmCD40L-DCs vs AdNull-DCs, P < .0005).
Ability of splenocytes from tumor-bearing mice treated with AdmCD40L-modified DCs to transfer tumor-specific immunity.
(A) Splenocytes from mice with CT26 tumors treated with AdmCD40L-infected DCs. The 8-day established subcutaneous CT26 tumors in Balb/c mice were inoculated with 2 × 105 DCs modified with AdmCD40L (▪) or AdNull (□) at moi of 40 for 24 hours. Ten days after administration, splenocytes were transferred intravenously to naive recipients (5 × 107 cells per mouse). Controls included mice without any infusion of splenocytes (Δ). Recipient mice were challenged 7 days later with subcutaneous administration of 2 × 105 CT26 cells (day 0). (B) Splenocytes from C57Bl/6 mice with B16 tumors. The study was similar to that in panel A, but B16 established tumors in C57Bl/6 mice were inoculated with modified DCs and recipient mice were challenged with 5 × 105 B16 cells. In both panels, survival was recorded as the percentage of surviving animals (Kaplan-Meir analysis,P < .05 AdmCD40L compared with all other groups).
Ability of splenocytes from tumor-bearing mice treated with AdmCD40L-modified DCs to transfer tumor-specific immunity.
(A) Splenocytes from mice with CT26 tumors treated with AdmCD40L-infected DCs. The 8-day established subcutaneous CT26 tumors in Balb/c mice were inoculated with 2 × 105 DCs modified with AdmCD40L (▪) or AdNull (□) at moi of 40 for 24 hours. Ten days after administration, splenocytes were transferred intravenously to naive recipients (5 × 107 cells per mouse). Controls included mice without any infusion of splenocytes (Δ). Recipient mice were challenged 7 days later with subcutaneous administration of 2 × 105 CT26 cells (day 0). (B) Splenocytes from C57Bl/6 mice with B16 tumors. The study was similar to that in panel A, but B16 established tumors in C57Bl/6 mice were inoculated with modified DCs and recipient mice were challenged with 5 × 105 B16 cells. In both panels, survival was recorded as the percentage of surviving animals (Kaplan-Meir analysis,P < .05 AdmCD40L compared with all other groups).
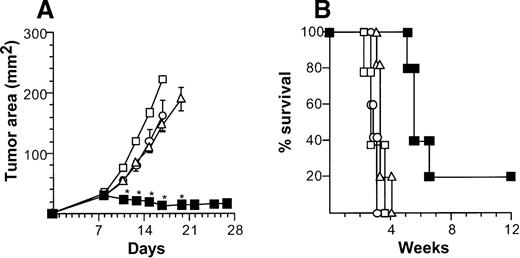
Antitumor CTL activity was also associated with a systemic therapeutic effect in a distant 2-tumor model of metastatic disease (Figure6). Mice bearing bilateral B16 flank tumors were treated by intratumoral injection to the left tumor with CD40L-transduced DCs. This therapy actively suppressed growth of the untreated right tumor as well as the treated tumor, with 20% of the mice alive at the end of the experiment on day 84, with no detectable tumors in both flanks. In contrast, tumors grew progressively and were eventually lethal in control mice treated with AdNull-infected DCs or without any therapy. As a further control, administration of CD40L-transduced DCs to a left Lewis lung carcinoma tumor had no beneficial effect on inhibiting B16 tumor growth in the right flank, confirming the tumor specificity of the antitumor effect.
Treatment of contralateral tumors with intratumoral administration of AdmCD40L-modified DCs.
(A) Tumor growth. C57Bl/6 mice were injected in both flanks with 5 × 105 B16 tumor cells (day 0). On day 8, the bilateral tumor-bearing mice intratumorally received 2 × 105 DCs that had been modified in vitro with AdmCD40L (▪), or AdNull (○) in the tumors in the left flank. Controls included bilateral tumor-bearing mice without any treatment (Δ). A parallel group of control mice were injected in the left Lewis lung carcinoma tumors with AdmCD40L-transduced DCs (treatment for a different tumor type than the right B16 tumors; ▪). The size of right tumors is reported as at the average tumor area (mm2) ± standard error of n = 5 per group. Asterisks indicate significant differences at 95% confidence limits between AdmCD40L-transduced DCs in syngenic tumors and all other surviving groups. (B) Survival. The survival of animals in panel A was recorded as the percentage of animals in each group (Kaplan-Meir analysis, P < .05 AdmCD40L-transduced DCs for syngenic tumors compared with all other groups).
Treatment of contralateral tumors with intratumoral administration of AdmCD40L-modified DCs.
(A) Tumor growth. C57Bl/6 mice were injected in both flanks with 5 × 105 B16 tumor cells (day 0). On day 8, the bilateral tumor-bearing mice intratumorally received 2 × 105 DCs that had been modified in vitro with AdmCD40L (▪), or AdNull (○) in the tumors in the left flank. Controls included bilateral tumor-bearing mice without any treatment (Δ). A parallel group of control mice were injected in the left Lewis lung carcinoma tumors with AdmCD40L-transduced DCs (treatment for a different tumor type than the right B16 tumors; ▪). The size of right tumors is reported as at the average tumor area (mm2) ± standard error of n = 5 per group. Asterisks indicate significant differences at 95% confidence limits between AdmCD40L-transduced DCs in syngenic tumors and all other surviving groups. (B) Survival. The survival of animals in panel A was recorded as the percentage of animals in each group (Kaplan-Meir analysis, P < .05 AdmCD40L-transduced DCs for syngenic tumors compared with all other groups).
Immunohistochemical analysis demonstrated a marked intratumoral CD8+ T-cell infiltration (58 CD8+ T cells/10 hpf) and to a lesser extent of CD4+ T cells (12 CD4+ T cells/10 hpf) in CT26 tumors treated with 2 × 105 AdmCD40L-modified DCs 3 days after treatment, but a minimal infiltration in those treated with AdNull-modified DCs (14 CD8+/10 hpf, 10 CD4+/10 hpf) or without any treatment (17 CD8+/10 hpf, 14 CD4+/10 hpf; not shown).
Spleen analyses
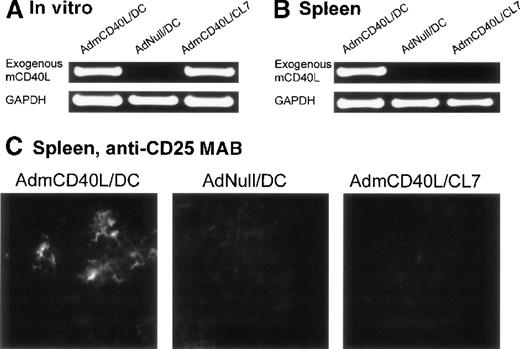
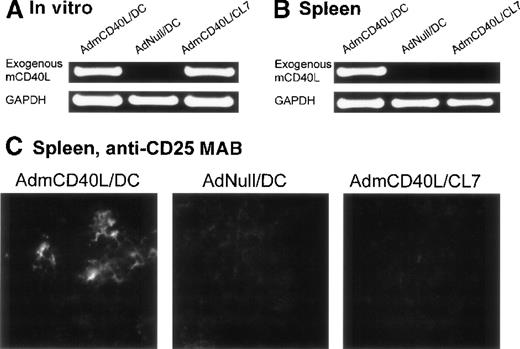
In the spleens from the mice treated with CD40L-DCs, AdmCD40L-derived mCD40L messenger RNA (mRNA) was detected by reverse transcriptase-polymerase chain reaction (RT-PCR) analysis, and the splenocytes expressed the activation marker CD25 by immunocytochemical analysis (Figure 7). Expression of CD40L mRNA driven by AdmCD40L-modified DCs was first confirmed in vitro by RT-PCR analysis (Figure 7A). As expected, an 877-base pair (bp) fragment corresponding to the exogenous mCD40L cDNA was amplified from total cellular RNA from both AdmCD40L-transduced DCs and control CL7 cells at a similar intensity. The intactness of the RNA was confirmed by amplification of GAPDH cDNA. Importantly, nested RT-PCR analysis demonstrated the expression of exogenous mCD40L transcripts in the spleens from CD40L-DC treated mice, but no expression in the spleens from mice that had been intratumorally injected with AdNull-infected DCs or AdmCD40L-modified CL7 cells (Figure 7B). These data suggested that the AdmCD40L-modified DCs injected into the flank tumors migrated to the spleens in vivo. With the use of immunocytochemical analyses, an assessment of spleens from mice injected with AdmCD40L-modified DCs demonstrated cells expressing CD25 (IL-2 receptor α chain; 39 positive cells per 10 hpf; Figure 7C). In contrast, control spleens from mice injected with AdNull-modified DCs or AdmCD40L-modified CL7 had minimal CD25 expression (9 or 6 positive cells per 10 hpf, respectively).
Demonstration of the ability of intratumoral administration of AdmCD40L-transduced DCs to traffic to the spleen and induce T-cell activation.
The expression of CD40L mRNA by the AdmCD40L-modified DCs (panel A) was used to tract the genetically modified DCs, and immunocytochemistry for the IL-2 receptor α chain used to identify activated T cells in the local milieu. (A) AdmCD40L-mediated CD40L expression assessed by RT-PCR in DCs or control CL7 cells transduced with AdmCD40L. The RT-generated cDNA from DCs transduced with AdmCD40L or AdNull, or CL7 cells transduced with AdmCD40L was amplified with primers for exogenous mCD40L or control GAPDH mRNA. PCR products were resolved on a 1% agarose gel and stained with ethidium bromide. (B) Detection of DCs expressing AdmCD40L-mediated CD40L by nested RT-PCR in spleens from mice treated with intratumoral administration of AdmCD40L-transduced DCs. Balb/c mice were injected subcutaneously in the right midflank with 2 × 105 CT26 cells (day 0). On day 8, tumor-bearing mice were treated by intratumoral injection of 2 × 106 AdmCD40L- or AdNull-transduced DCs or AdmCD40L-transduced CL7 cells. On day 11, the RT-generated cDNA from the spleens was amplified with outer and inner primer pairs for exogenous mCD40L by nested PCR. PCR products were resolved on a 1% agarose gel and stained with ethidium bromide. PCR for the control GAPDH mRNA is shown in the lower part of the panel. (C) Immunohistochemical evaluation of spleens in panel B for CD25 (IL-2 receptor α chain). On day 11, spleens were dissected, and frozen spleen sections were stained using 10 μg/mL rat antimouse CD25 mAb, followed by the visualization with 10 μg/mL antirat IgG Oregon Green antibody. Specific immunoreactivity showed the presence of CD25-expressing cells in spleens from mice treated with AdmCD40L-modified DCs, whereas only a low level of tissue autofluorescence was evident in control spleens (AdNull/DCs and AdmCD40L/CL7).
Demonstration of the ability of intratumoral administration of AdmCD40L-transduced DCs to traffic to the spleen and induce T-cell activation.
The expression of CD40L mRNA by the AdmCD40L-modified DCs (panel A) was used to tract the genetically modified DCs, and immunocytochemistry for the IL-2 receptor α chain used to identify activated T cells in the local milieu. (A) AdmCD40L-mediated CD40L expression assessed by RT-PCR in DCs or control CL7 cells transduced with AdmCD40L. The RT-generated cDNA from DCs transduced with AdmCD40L or AdNull, or CL7 cells transduced with AdmCD40L was amplified with primers for exogenous mCD40L or control GAPDH mRNA. PCR products were resolved on a 1% agarose gel and stained with ethidium bromide. (B) Detection of DCs expressing AdmCD40L-mediated CD40L by nested RT-PCR in spleens from mice treated with intratumoral administration of AdmCD40L-transduced DCs. Balb/c mice were injected subcutaneously in the right midflank with 2 × 105 CT26 cells (day 0). On day 8, tumor-bearing mice were treated by intratumoral injection of 2 × 106 AdmCD40L- or AdNull-transduced DCs or AdmCD40L-transduced CL7 cells. On day 11, the RT-generated cDNA from the spleens was amplified with outer and inner primer pairs for exogenous mCD40L by nested PCR. PCR products were resolved on a 1% agarose gel and stained with ethidium bromide. PCR for the control GAPDH mRNA is shown in the lower part of the panel. (C) Immunohistochemical evaluation of spleens in panel B for CD25 (IL-2 receptor α chain). On day 11, spleens were dissected, and frozen spleen sections were stained using 10 μg/mL rat antimouse CD25 mAb, followed by the visualization with 10 μg/mL antirat IgG Oregon Green antibody. Specific immunoreactivity showed the presence of CD25-expressing cells in spleens from mice treated with AdmCD40L-modified DCs, whereas only a low level of tissue autofluorescence was evident in control spleens (AdNull/DCs and AdmCD40L/CL7).
No antitumor effect of AdmCD40L-modified fibroblasts
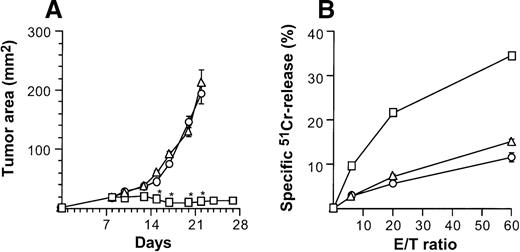
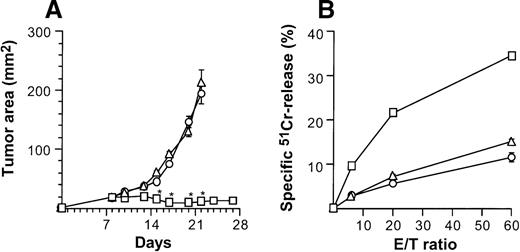
When injected intratumorally, CD40L-transduced DCs induced therapeutic tumor immunity, but CD40L-transduced fibroblasts did not (Figure 8). Growth of subcutaneous CT26 tumors was profoundly affected by 2 × 106AdmCD40L-modified DCs (Figure 2A), whereas tumors treated with 2 × 106 of CL7 fibroblasts that had been infected with AdmCD40L at the identical moi grew, as did the control group without any treatment (P > .3; Figure 8A). This antitumor effect in vivo correlated to tumor-specific CTL activity demonstrated by splenocytes from mice treated with the same regimen (Figure 8B). Animals bearing untreated CT26 tumors generated a 15% lysis of51Cr-labeled CT26 target cells at an effector/target (E/T) ratio of 60/1. This lytic activity was enhanced to 34% after CD40L-transduced DC treatment, but not after intratumoral injection of CD40L-transduced CL7 cells. Taken together with the adoptive transfer and spleen mRNA and immunohistochemical data (Figures5, 7, 8), these data suggest that optimal therapeutic immunity of CD40L-transduced DCs depends, at least in part, not on regional stimulation by CD40L expression in tumors, but on migration of activated DCs to the lymphoid tissues to stimulate tumor antigen-specific T cells.
Specificity of suppression of tumor growth after treatment with intratumoral administration of AdmCD40L-transduced DCs compared with AdmCD40L-modified fibroblasts.
(A) Tumor growth. Groups of Balb/c mice were challenged subcutaneously with 2 × 105 CT26 cells (day 0). On day 7, bone marrow DCs (□) or syngenic fibroblast CL7 cells (○) were transduced in vitro with AdmCD40L at moi of 40 for 24 hours. Mice bearing day 8 established tumors were given 2 × 106 modified cells intratumorally. Controls included tumor-bearing mice without any treatment (Δ). The size of each tumor was assessed 3 times per week, and is reported as the average tumor area (mm2) ± standard error (n = 5 per group). Asterisks indicate significant differences at 95% confidence limits between AdmCD40L-transduced DCs and all other groups. (B) Tumor-specific cytotoxic T lymphocyte activity. Ten days after treatment described for panel A, the splenocytes were isolated, restimulated, and assayed as in Figure 4A,B. Shown are data for 51Cr-labeled CT26 targets. Results are presented as the mean ± standard error (n = 3/data point). In all groups, lysis of control 51Cr-labeled SVBalb targets was within 8% (not shown).
Specificity of suppression of tumor growth after treatment with intratumoral administration of AdmCD40L-transduced DCs compared with AdmCD40L-modified fibroblasts.
(A) Tumor growth. Groups of Balb/c mice were challenged subcutaneously with 2 × 105 CT26 cells (day 0). On day 7, bone marrow DCs (□) or syngenic fibroblast CL7 cells (○) were transduced in vitro with AdmCD40L at moi of 40 for 24 hours. Mice bearing day 8 established tumors were given 2 × 106 modified cells intratumorally. Controls included tumor-bearing mice without any treatment (Δ). The size of each tumor was assessed 3 times per week, and is reported as the average tumor area (mm2) ± standard error (n = 5 per group). Asterisks indicate significant differences at 95% confidence limits between AdmCD40L-transduced DCs and all other groups. (B) Tumor-specific cytotoxic T lymphocyte activity. Ten days after treatment described for panel A, the splenocytes were isolated, restimulated, and assayed as in Figure 4A,B. Shown are data for 51Cr-labeled CT26 targets. Results are presented as the mean ± standard error (n = 3/data point). In all groups, lysis of control 51Cr-labeled SVBalb targets was within 8% (not shown).
Discussion
This study is based on the hypothesis that DCs that have been genetically modified with a recombinant adenovirus to express CD40L will be self-activated, and when placed in the milieu of a tumor, will initiate a tumor-specific cellular immune response that will suppress growth of the tumor and increase survival of the host. The data support this hypothesis. Intratumoral injection of AdmCD40L-modified syngenic DCs to 2 different types of murine tumors elicited therapeutic antitumor immunity that suppressed the growth of established tumors and enhanced survival. Administration of AdmCD40L-infected DCs elicited tumor-specific cytolytic T-lymphocyte responses that protected naive syngenic mice against a subsequent tumor challenge and impaired the growth of distant established tumors.
AdmCD40L-modified dendritic cells elicit antitumor immune responses
The observations in this study are consistent with the concept that DCs transduced to express CD40L efficiently initiates protective antitumor immunity. DCs are professional antigen-presenting cells that sample antigens in all body tissues and migrate to lymphoid organs where they present antigens to T cells.1 Several recent reports suggest that DCs cannot stimulate cytotoxic T cells directly unless they are first stimulated via CD40 on their surface.7-9 This is usually accomplished by CD40L expressed on CD4+ helper T cells.5,29 The T-helper–mediated CD40 triggering up-regulates adhesion and costimulatory molecule in the DCs, bringing the DCs to a state where they can autonomously stimulate a T-killer response.2 7-11This study extends this concept by demonstrating that genetic modification of DCs to express CD40L accomplishes the goal of activating DCs to induce functionally relevant cell-mediated adoptive immune responses such as suppression of tumor in an antigen-specific fashion. After adenovirus gene transfer of the CD40L cDNA to DCs to express CD40L, the transduced DCs most likely self-trigger CD40 and activate themselves to present tumor antigen to naive CD8+CTL. When administered to tumors, these genetically modified DCs likely capture tumor antigens and present them to naive CD8+ CTL, probably after migrating to lymphoid organs. Consistent with this hypothesis, CD40L-transduced DCs administration to tumors resulted in the up-regulation of IL-2Rα in spleen cells. Further evidence for this concept comes from the observation that exogenous CD40L mRNA expression originating from injected AdmCD40L-modified DCs was detected in the spleen of treated mice, suggesting the modified, activated DCs had migrated from the tumor to the spleen.
The triggering of CD40 on DCs leads to increased production of several inflammatory cytokines and chemokines, including interleukin-12 (IL-12) and macrophage inflammatory protein-1α (MIP-1α).10-12 IL-12, a cytokine that promotes the development of Th1 CD4+ T cells and the maturation of CTLs, likely plays a supportive role for generation of T-killer responses for tumor immunity.30 In contrast, MIP-1α, a chemokine known to preferentially induce the migration of CD8+ T cells, helps DCs to encounter and stimulate rare tumor antigen-specific CD8+ CTLs.31
The expression of CD40L is normally restricted almost exclusively to activated CD4+ helper T cells, and exquisitely regulated in concert with other receptor-ligand pairs within a specialized microenvironment.32,33 In this regard, studies with murine models have shown that inappropriate presence of the CD40L protein may have adverse consequences. Wiley et al34 showed that soluble CD40L protein administration to the murine airway induced pulmonary inflammation. Brown et al35 demonstrated that constitutive CD40L expression in bone marrow or thymic cells by a retroviral vector produced monoclonal T-lymphoblastic lymphomas of thymic origin. In this context, the use of genetic modification of DCs will have to be closely monitored for adverse effects. However, the use of Ad vectors to transfer to CD40L cDNA to DCs may have an advantage, in that expression by Ad in vivo is generally transient and locally restricted to the genetically modified cells without induction of systemic adverse effects.36-39
Because tumor-specific immunity relies on professional APC activated through CD40-CD40L interactions, genetic modification of tumor cells, lymphoma cells, or bystander fibroblasts to express CD40L has been evaluated in an attempt to stimulate APCs to induce tumor-specific cellular immunity. Kato et al17 and we18 have used adenovirus vectors to transfer CD40L cDNA to human lymphoma cells and murine tumor cells, respectively, and demonstrated that this genetic modification induced generation of specific CTLs. Grossmann et al15 and Couderc et al13 transduced murine tumor cells ex vivo with a retroviral construct containing the CD40L cDNA, and induced a systemic immune response capable of impeding tumor growth in vivo. Dilloo et al14 showed that triggering CD40 on murine lymphoblastic cells with retrovirus-mediated expression of CD40L on bystander fibroblasts enhanced the antigen-presenting potential of those lymphoma cells. Finally, Nakajima et al20 transfected the CD40L-expressing plasmid into murine mastocytoma cells, and investigated its potentiation in enhancing host APC functions. On the basis of this concept, Mendoza et al19 and Gurunathan et al16 have shown that intradermal or subcutaneous coinjection of a plasmid expressing CD40L with a plasmid expressing β-galactosidase DNA enhances cellular immune response to β-galactosidase as a model of a tumor antigen. As an alternative strategy, culture of DCs with recombinant soluble CD40L protein, fibroblasts, or hybridoma cells transfected with CD40L cDNA, or anti-CD40 antibody, induced APC functions of DCs.7-12,40This is associated with up-regulation of accessory molecules such as ICAM-1, B7-1, and B7-2, on CD40-triggered DCs, and high levels of production of cytokines such as IL-12, MIP-1α, IL-8, and TNF-α.10-12 Ridge et al,8 Bennett et al,7 and Schoenberger et al9 clearly illustrated that anti-CD40 antibody (acting as a surrogate of CD40L triggered CD40 on DCs) brings the DCs to a state in which they can autonomously present antigen to CD8+ killer T cells and induce antigen-specific CTL responses. Finally, Labeur et al40 has recently shown that DCs incubated with soluble CD40L protein became mature as evident by morphology, up-regulation of adhesion and costimulatory molecules, and high levels of IL-12 secretion, to promote antitumor immunity in vivo.
Dendritic cell–based cancer immunotherapy
With the use of methods for isolation and in vitro culture of murine and human DCs, a variety of strategies for cancer immunotherapy have been developed using DCs as vaccines.1,41-46 After tumor antigens are incubated with DCs ex vivo, these antigen-loaded DCs are then reinfused as vaccines. Several approaches for delivering tumor antigens to DCs have been evaluated, including whole tumor lysates, defined tumor antigenic peptides, tumor antigen-encoding DNA or RNA, recombinant viral vectors (adenovirus, poxvirus, vaccinia virus, and retrovirus), encoding tumor antigens, tumor-derived mRNA, coculture with tumor cells, or fusion with whole tumor cells.24,47-73Immunization using these antigen-pulsed DCs elicit host protective and therapeutic antitumor immunity associated with the induction of tumor reactive CD8+ T cells.46
In contrast to the strategy of loading DCs with tumor antigens ex vivo, the strategy in this study of using DCs genetically modified to express CD40L has several theoretical advantages of potential clinical interest. First, the mCD40L-modified DCs can directly interact with tumor antigens in vivo without the possible alteration or concern for alteration or loss of tumor antigen-associated RNA or peptides induced in vitro manipulation. Second, in vivo interaction between the mCD40L-modified DCs and tumor cells expressing the entire repertoire of tumor antigens should allow the host defense system to be stimulated against multiple tumor antigens. The involvement of oligoclonal effector subpopulations specific for diverse antigenic tumor epitopes may be advantageous for an optimal antitumor response. Third, intratumoral administration of the mCD40L-modified DCs does not depend on the prior identification of appropriate tumor antigens and is not limited to individuals expressing a particular corresponding MHC allele. In this context, although it is possible to use some defined tumor antigens in DCs adoptive transfer therapy, potential MHC-binding tumor-specific peptides remain unknown for most human tumors.46,74 This advantage is not unique to CD40L-modified DCs in that some tumor cell-based DC-pulsing approaches, such as unfractionated peptides eluted from tumor cells, tumor-cell–derived polyadenylated RNA, and coculture or fusion with tumor cells, do not also require prior knowledge of the relevant tumor antigenic peptides.47,52,56,58,64,72,73 Finally, CD40L expressed exogenously on DCs may protect modified DCs from both CD95-mediated and spontaneous apoptosis through self-CD40 triggering, as does recombinant soluble CD40L,75 resulting in long-term stimulation of host antitumor immunity. There, however, is no evidence that constitutive expression of CD40L on transduced DCs has similar effects on their survival as an appropriate extrinsic triggering of CD40. Definitive proof awaits further studies addressing this issue.
Acknowledgments
We thank Dr Tsuneyoshi Hamada, Division of Hematology-Oncology, for technical support; and N. Mohamed for help in preparing this manuscript.
Supported in part by NIH R01 CA75192; the Will Rogers Memorial Fund, Los Angeles, CA, and GenVec, Inc, Rockville, MD. M.A.S.M. was supported, in part, by NCI-P30-CA-08748 and the Gar Reichman Fund of the Cancer Research Institute, New York, NY.
Reprints:Institute of Genetic Medicine, 520 E 70th St, ST 505, New York, NY 10021; e-mail:geneticmedicine@mail.med.cornell.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.