Abstract
Thrombin is primarily known for its role in homeostasis and thrombosis. However, this enzyme also plays important roles in wound healing and pathologic situations such as inflammation and tumorigenesis. Among the molecules stimulated by thrombin in these latter processes are the stress response proteins, chemokines. Chemokines are also known for their roles in inflammatory responses and tumor development. These correlative observations strongly suggest that chemokines may be mediators of some of thrombin's functions in these processes. Elucidation of the molecular mechanisms of stimulation of chemokines by thrombin may help to unravel the ways in which their expression can be modulated. Up-regulation of the chemokine 9E3/cCAF by thrombin occurs via its proteolytically activated receptor with subsequent transactivation of the epidermal growth factor receptor tyrosine kinase. This study shows that stimulation by thrombin very rapidly activates this chemokine at the transcriptional level, that 2 Elk1 binding elements located between −534 and −483 bp of the promoter are major thrombin response elements, that activation occurs via the Elk1 transcription factor, and that the latter is directly activated by MEK1/ERK2. The common occurrence of Elk1 binding domains in the promoters of immediate early response genes suggests that it may be characteristically involved in gene activation by stress-inducing agents.
Introduction
Thrombin is a multifunctional serine protease known primarily for its role in homeostasis and thrombosis. However, this enzyme also has an important role in the stress responses that occur during inflammation, wound healing, and tumor development.1-3 Thrombin can stimulate fibroblasts, endothelial cells, and leukocytes to initiate signal transduction mechanisms that regulate the expression of genes important in stress responses during these traumatic and pathologic processes.4,5 Signaling mechanisms stimulated by thrombin involve its 7 transmembrane receptors, trimeric G proteins, and primarily serine/threonine (Ser/Thr) kinases.6,7 However, tyrosine (Tyr) kinases are also important in some of the signaling events triggered after thrombin receptor activation.8-12
Among the molecules stimulated by thrombin after wounding and during tumorigenesis are chemokines, small molecular weight cytokines known primarily for their function in leukocyte trafficking and physiology.13-15 Chemokines can be stimulated in response to injury (be it a wound or a tumor), are immediate early stress-response genes that initiate the immune response (eg, chemotaxis for leukocytes), and perform functions involved in the formation of the repair tissue and of tumor stroma (eg, angiogenesis). Understanding regulation of thrombin-stimulated chemokine expression during wound healing and tumor development may contribute to finding ways of modulating these processes.
Thrombin is the most potent natural activator of the chemokine chicken chemotactic and angiogenic factor (cCAF),12,16 which is the product of the 9E3 gene. Henceforth we will refer to this chemokine as 9E3/cCAF. In addition to stimulating 9E3/cCAF, thrombin also stimulates the expression of human chemokines, such as interleukin-8 (IL-8),17 melanoma growth stimulatory activity (MGSA),18,19 and monocyte chemoattractant protein 1 (MCP-1)20 in a manner similar to that of 9E3/cCAF. Furthermore, this chemokine is more homologous to IL-8 than is any other chemokine, human or otherwise, and also is highly homologous to MGSA/groα and β-thromboglobulin.
In vivo, 9E3/cCAF is highly expressed shortly after injury; expression remains high during the inflammatory phase of healing and declines to a lower level, but still elevated, during formation of the repair tissue. In addition, it is also highly expressed in the stroma of tumors.21,22 In these tissues, 9E3/cCAF is up-regulated primarily in fibroblasts present in areas where interstitial collagen21,22 and tenascin23 are abundant. Studies in the chorioallantoic membrane (CAM) show that this chemokine is chemotactic for monocyte/macrophages and lymphocytes and is angiogenic,5 6 functions that are important in responses to the trauma inflicted by both wounding and tumors.
Because chemokines perform a variety of crucial functions in vivo, it is important to determine the molecular mechanisms involved in their activation so that their expression can be modulated. Thrombin stimulates 9E3/cCAF expression in primary normal fibroblasts independently of its mitogenic activities.12 After activation, the G-protein–coupled receptor for thrombin transactivates the epidermal growth factor receptor (EGFR) leading to phosphorylation and activation of the EGFR and of Src tyrosine kinases.12 The work presented here furthers these studies by showing that the signaling pathways downstream from the EGFR involve the activation of the mitogenic-activated protein (MAP) kinase cascade despite the fact that stimulation of 9E3/cCAF by thrombin occurs independently of mitogenesis. Furthermore, thrombin activates the Elk1 transcription factor, and these events are critical for regulation of9E3/cCAF gene expression. These results advance our knowledge of the mechanisms activated by the interactions of thrombin with its receptor, which lead to stimulation of immediate early stress-response genes.
Materials and methods
Reagents
Bovine thrombin (9 U; Sigma, St Louis, MO) was reconstituted in water; PD98059 and SB203580 (Calbiochem, San Diego, CA) were reconstituted in dimethyl sulfoxide (DMSO). Primary antibodies: polyclonal antibodies to Elk1 (New England Biolab, Beverly, MA), Ser383 phosphor-Elk1 (New England Biolab), SAP1a (Santa Cruz, Santa Cruz, CA), Ser136 phosphor-Bad (New England Biolab), and cCAF16; monoclonal antibodies phospho-p44/42 MAP kinase (Thr202/Tyr204) E10 (New England Biolab), and anti-MAP kinase 2/ERK2, clone 1B3B9 (Upstate Biotech, New York, NY). Secondary antibodies: goat antirabbit or antimouse conjugated to horseradish peroxidase (HRP) for use of the enhanced chemiluminescence (ECL) detection system (Amersham, Piscataway, NJ). Gal4-based expression systems and the reporter system containing 7 AP-1 consensus binding elements were purchased from Stratagene.
Cell culture
Cultures of primary fibroblasts were prepared from 10-day-old chicken embryos as previously described.24 Secondary cultures were prepared by trypsinizing and plating the primary cells in modified 199 media12 at a density of 3.5 × 105/35 mm or 3.0 × 106/100-mm plates and incubated for 24 hours before transfection.
Northern blots and immunoblots
These procedures were described previously in detail.12
Promoter cloning and luciferase reporter construction
Using polymerase chain reaction (PCR) a 1.5- kb DNA fragment from the immediate 5′ upstream region of the 9E3 gene (−1503 to +32) was amplified. Chicken genomic DNA served as template and 2 oligonucleotides (5′ primer:-GGATGAATGGCATTTCAGTG-CAC-; 3′primer: -TCGACACTAGAGAGGACAGTCTCCT-) were used for the PCR reactions. The 1.5-kb PCR product was cloned using the TA cloning system pCR2.1 (Invitrogen, Carlsbad, CA) and sequenced with a Li-Cor sequencer using M13 reverse (700 nm) and forward (800 nm) primers labeled with color markers. The 1.5-kb PCR product was then subcloned into the pGL3 basic vector (Promega, Madison, WI). The 5′ deletions of this promoter were obtained using restriction enzyme digests and re-ligations of this vector (p1503), resulting in constructs with 66, 218, 470, 683, and 1116 bp upstream from the initiation site. Other constructs within the p683 were prepared by PCR with the following primers: (1) p542 (−542 to +32 bp), 5′-GGCAAAA-TGCAGGAATTGTTTGCAC-; 3′-TCGACACTAGAGA-GGACAGTCTCCT-); (2) p495 (−495 to +28 bp), 5′-ATCAGGATGCTTTTAATACTGCACCCT-; 3′-GTGATCTCTCCTGTCAGAGGAT-); and (3) pmElk1 (−495 to +28 bp with the mutation on the Elk1 conserved binding element, 5′-ATacGcgTGCTTTTAATACTGCACCCT-; 3′-GTGATCTCTCCTGTCAGAGGAT-). These 3 constructs were cloned into pGL3 basic vector through KpnI and SmaI sites. All PCR products were sequenced and compared for fidelity to the published sequence.25 To generate the mutated AP-1 binding element (TGACTCAT, was changed into gGcCTtAT; pmAP-1), we used the p683 construct and the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). The mutation was verified by endonuclease mapping and confirmed by sequencing. All pGL3/9E3 promoter subclones were prepared using an Endotoxin-Free Maxiprep Kit (Qiagen, Valencia, CA).
Transient transfection, thrombin activation, and cell lysate collection
The calcium phosphate precipitation method without glycerol shock was used for the transient transfections. DNA concentrations were determined using spectrophotometry and confirmed by ethidium bromide staining of agarose gels. Primary fibroblasts were transiently transfected with a total of 6 μg DNA. Cotransfections always included 2 μg of the PCH110 vector (Pharmacia) containing lac-Z as internal transfection control and, when necessary, pcDNA 3.1 vector or calf thymus DNA was added to bring the total amount of DNA transfected to 6 μg. For all experiments, transfected primary fibroblasts were incubated in the modified 199 media for 36 hours and then stimulated with thrombin for 3 hours prior to lysis. For inhibition experiments, the fibroblasts were incubated with inhibitors for 30 minutes before thrombin stimulation. Cell extracts were prepared with reporter lysis buffer according to the protocol provided by the manufacturer (Promega) and stored in a freezer at −70°C.
Luciferase and β-galactosidase assay
Cell extracts were assayed using a luminometer with an automated injection device (Monolight 2010, Analytical Luminescence Laboratory) using the luciferase assay system (Promega). The frozen samples were used for the β-galactosidase assay (Promega). A minimum of triplicate samples for each experiment was performed and the data were expressed as mean light units of luminescence per unit β-galactosidase activity.
Electrophoretic mobility shift assays
For electrophoretic mobility shift assays (EMSAs) fibroblasts transfected with the pCMV3.1 (Invitrogen) or pCMV-Elk1 expression vector26 were treated with thrombin for 1 hour before extraction. The methods to prepare the nuclear extracts have been described previously.27 Protein concentration was determined with the D-C protein assay kit (BioRad). Briefly, EMSA buffer (50 mmol/L HEPES pH 7.9, 10 mmol/L MgCl2, 0.2 mol/L KCl, 0.5 mmol/L EGTA, 5 mmol/L DTT, 20% Ficoll) containing 10 μg extracted nuclear protein and 2 ng γ-32P-adenosine triphosphate-labeled probe containing the conserved Elk1 binding element located between −493 and −483 bp (5′-TTTGCAAAATGCAGGAATTGTTTTCACAGT-3′) was used. As a control, mutated oligonucleotide (5′-TTTGCAAAATaCgatAATTGTTTTCACAGT-3′) was labeled and used for EMSA. Unlabeled probe (50 ng) was used for the competition assay and 2 to 3 μg anti-SAP1a (Santa Cruz), anti-Elk1 (New England Biolab), or antiphospho-Elk1 (Ser383) antibody (New England Biolab) was added for the supershift assay. For peptide competition experiments, GST-Elk1310-428 vector was used to produce the protein as previously described.27 GST-P-Elk1307-428 (Ser 383 phosphorylated C-terminus of Elk1) was purchased from New England Biolab. It was concentrated and sodium dodecyl sulfate (SDS) washed away with 2.5% Triton X-100 before use. All samples were assayed in 5% nondenaturing polyacrylamide gel electrophoresis (PAGE) (acrymide:bis as 60:1).
Results
Transcription activation of the 9E3/cCAFgene
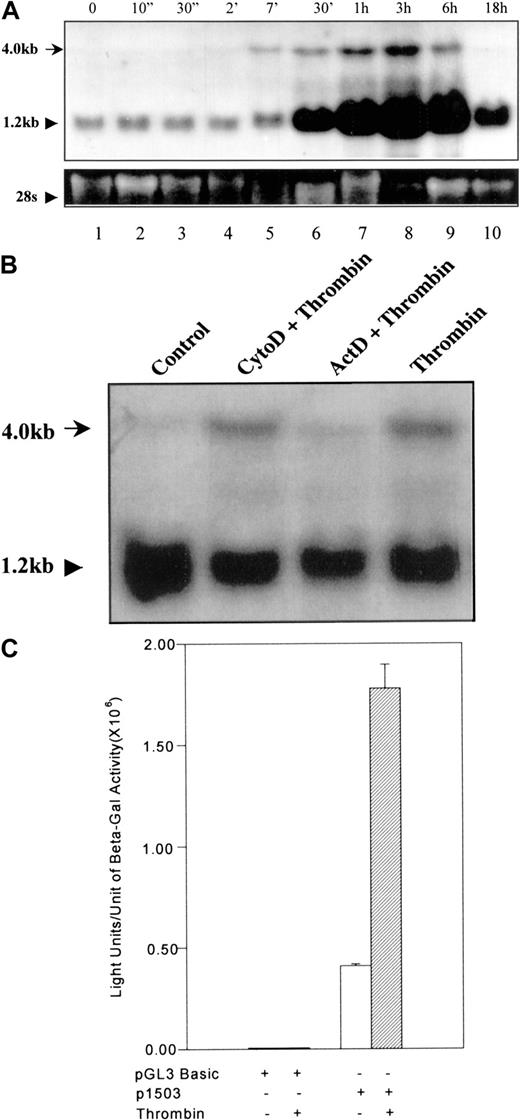
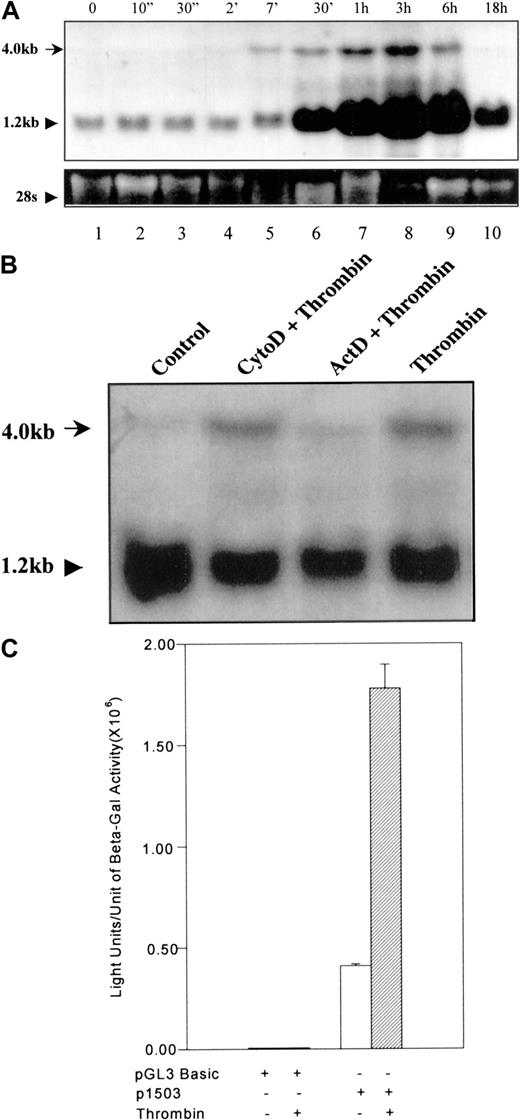
To determine the gene activation pattern of 9E3/cCAF after thrombin stimulation, we treated primary fibroblasts with thrombin for varying periods of time and collected samples for RNA analysis. Seven minutes after exposure to thrombin, a 4.0-kb RNA band corresponding to the full-length gene (Kaiser and Hughes, published in GeneBank; see legend for Figure 1) was visible by Northern blot analysis (Figure 1A, lane 5). This RNA species increased to a maximum at 3 hours and declined slowly thereafter, returning to basal levels by 18 hours (Figure 1A, lanes 6-10). Shortly after the appearance of this band, we also observed a marked increase in the level of the processed (1.2 kb) 9E3/cCAF messenger RNA (mRNA; Figure1A, lane 6). Figure 1B shows that when cells were treated with thrombin in the presence of the transcription inhibitor actinomycin D, the 4.0-kb band was not observed. Cells treated with cytochalasin D, an inhibitor that does not affect transcription but rather inhibits actin cystoskeleton functions, did not eliminate the 4.0-kb band, indicating that the effect of actinomycin D was not due to general disruption of cellular functions. These results show that stimulation of the9E3/cCAF gene by thrombin is very rapid and suggest that this activation occurs first at the transcriptional, then at the posttranscriptional level. To further verify that activation of this gene by thrombin occurs at the transcriptional level, we cloned the 9E3/cCAF promoter (−1503 bp to +32 bp25) into a reporter construct containing the firefly luciferase gene. When this construct (p1503) was transfected into primary fibroblasts, expression of luciferase was greatly stimulated by thrombin (4-5-fold; Figure 1C). To determine whether this promoter contains the recently published thrombin response region that consists of a repeat of a CCACCC element in an ABBA configuration,28 we used the Transcription Element Search Software (TESS assay).29 Although no such thrombin response region was found in p1503, a number of potentially functional transcription factor binding elements known to be important in the regulation of chemokine gene expression and in gene activation by thrombin were identified (Figure2).30-34
Transcription activation of 9E3/cCAF by thrombin.
(A) Northern blot analysis of cCAF mRNA in primary fibroblasts after thrombin stimulation. Total RNA was prepared using TRIzol reagent (GIBCO/BRL); RNA samples (20 μg each) were denatured in formamide-formaldehyde loading buffer containing ethidium bromide and separated on 1% formaldehyde-agarose gel. The blots were probed with a radiolabeled DNA probe prepared from the 9E3/cCAF complementary DNA (cDNA). The higher molecular weight RNA represents the full length of the gene (see accession no. AJ009800 or NID g4467411 in GeneBank). The bottom panel shows ethidium bromide staining of the 28S ribosomal RNA (rRNA). (B) Northern blot analysis of cCAF mRNA in primary fibroblasts after treatment with thrombin in the presence of the inhibitor of transcription actinomycin D (ActD) or the inhibitor of the actin cytoskeleton, cytochalasin D (Cyto D). The cells treated with Act D did not produce the higher molecular weight message, whereas those treated with Cyto D did. (C) Primary fibroblasts were cotransfected with 4 μg of the reporter plasmid containing the 1503-bp promoter region and 2 μg of PCH110 vector containing lac-Z as an internal transfection control. Thirty-six hours after transfection, the cells were treated in the presence or absence of thrombin for 3 hours and the extracts assayed for luciferase activity. Thrombin stimulation induced a significant increase in transcription. The background seen with the construct in absence of thrombin treatment is due to the stress generated by the transfection procedure. The data shown here are representative of several experiments performed under the same conditions but with different batches of primary fibroblasts. Bars represent SEMs of 3 samples per condition in the same batch of cells.
Transcription activation of 9E3/cCAF by thrombin.
(A) Northern blot analysis of cCAF mRNA in primary fibroblasts after thrombin stimulation. Total RNA was prepared using TRIzol reagent (GIBCO/BRL); RNA samples (20 μg each) were denatured in formamide-formaldehyde loading buffer containing ethidium bromide and separated on 1% formaldehyde-agarose gel. The blots were probed with a radiolabeled DNA probe prepared from the 9E3/cCAF complementary DNA (cDNA). The higher molecular weight RNA represents the full length of the gene (see accession no. AJ009800 or NID g4467411 in GeneBank). The bottom panel shows ethidium bromide staining of the 28S ribosomal RNA (rRNA). (B) Northern blot analysis of cCAF mRNA in primary fibroblasts after treatment with thrombin in the presence of the inhibitor of transcription actinomycin D (ActD) or the inhibitor of the actin cytoskeleton, cytochalasin D (Cyto D). The cells treated with Act D did not produce the higher molecular weight message, whereas those treated with Cyto D did. (C) Primary fibroblasts were cotransfected with 4 μg of the reporter plasmid containing the 1503-bp promoter region and 2 μg of PCH110 vector containing lac-Z as an internal transfection control. Thirty-six hours after transfection, the cells were treated in the presence or absence of thrombin for 3 hours and the extracts assayed for luciferase activity. Thrombin stimulation induced a significant increase in transcription. The background seen with the construct in absence of thrombin treatment is due to the stress generated by the transfection procedure. The data shown here are representative of several experiments performed under the same conditions but with different batches of primary fibroblasts. Bars represent SEMs of 3 samples per condition in the same batch of cells.
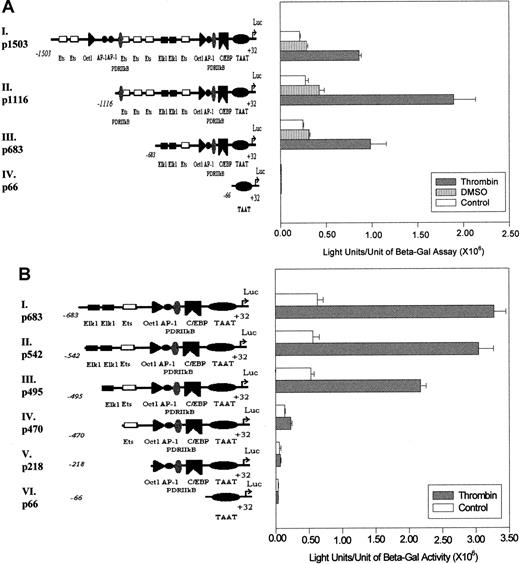
Transcription driven by different 9E3/cCAF promoter constructs after thrombin stimulation.
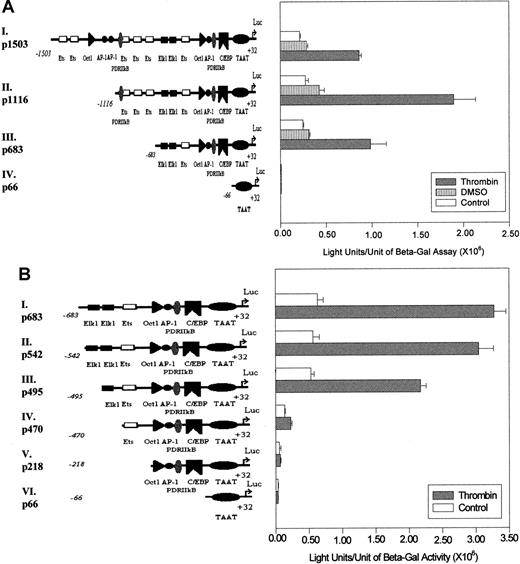
(A) The long promoter of the 9E3/cCAF gene, −1503 to +32 bp (p1503; I), was subjected to 5′ deletions to obtain 3 additional promoter fragments as shown (II-IV). The transient transfections were performed as described in Figure 1C. p683 contains the elements necessary for basal stimulation as well as thrombin-stimulated transcription. The region between −1503 and −1116 bp may contain regulatory elements that repress gene activation by p1116, after thrombin stimulation. (B) The 5′ deletions of the p683 (II-V) were obtained by restriction enzyme digests and by PCR-based methods as described in “Materials and methods.” The transient transfections were performed as described in Figure 1C. After deletion of both Elk1 binding sites, a dramatic decrease in the response to thrombin occurred (p470, IV). The experiments shown in this figure are representative of several performed under the same conditions but with different batches of primary cells. Bars represent SEMs of 3 samples per condition in the same batch of cells.
Transcription driven by different 9E3/cCAF promoter constructs after thrombin stimulation.
(A) The long promoter of the 9E3/cCAF gene, −1503 to +32 bp (p1503; I), was subjected to 5′ deletions to obtain 3 additional promoter fragments as shown (II-IV). The transient transfections were performed as described in Figure 1C. p683 contains the elements necessary for basal stimulation as well as thrombin-stimulated transcription. The region between −1503 and −1116 bp may contain regulatory elements that repress gene activation by p1116, after thrombin stimulation. (B) The 5′ deletions of the p683 (II-V) were obtained by restriction enzyme digests and by PCR-based methods as described in “Materials and methods.” The transient transfections were performed as described in Figure 1C. After deletion of both Elk1 binding sites, a dramatic decrease in the response to thrombin occurred (p470, IV). The experiments shown in this figure are representative of several performed under the same conditions but with different batches of primary cells. Bars represent SEMs of 3 samples per condition in the same batch of cells.
Analysis of the 9E3/cCAF promoter responses to activation by thrombin
To further analyze the contribution of the various consensus transcription factor binding elements to the activation of 9E3/cCAF expression, we produced 5′ deletions in p1503, linked them to the luciferase gene, and analyzed the constructs by transient tranfections (Figure 2A). The −683-bp to +32-bp promoter construct (p683) contains several elements that can potentially be involved in the stimulation of 9E3/cCAF by thrombin. In addition to the TATA box, it contains the consensus binding sequences for the transcription activators Elk1,35 AP-1,36 PDRIIκB (the chicken equivalent of nuclear factor κB37-39); and the CAAT box for C/EBP. This construct also contains the binding element for the chemokine gene repressor Oct-1,32 which is found a short distance upstream from the AP-1 binding site. The −1116-bp to +32-bp construct (p1116) contains in addition to these elements 3 Ets binding sites and another PDRIIκB element. The full-length p1503 promoter contains an additional 2 AP-1, 1 Oct-1, and 2 Ets binding sites. These 3 constructs and an additional one containing only the9E3/cCAF gene TATA box (p66) were transiently transfected into primary fibroblasts and the cells were assayed for luciferase activity in the presence or absence of thrombin stimulation (Figure2A). Because primary cells were used, variations in transfection efficiencies between batches of cells can occur. Therefore, for each experiment, internal controls were always included. Furthermore, the levels of activation are not of the magnitude of those generally seen when molecules are overexpressed or cell lines are used. Although all constructs yielded some basal level of activation, treatment with thrombin resulted in significantly higher levels of activation. The response of the full-length construct, p1503, to thrombin resulted in a 3-fold stimulation over the control (Figure 2A,I), whereas the promoter truncated at −1116 (p1116) yielded a 6-fold stimulation by thrombin (Figure 2A,II), and the one truncated at −683 (p683) exhibited activity comparable to that of p1503. These results indicate that the region encompassing −683 to +32 bp contains most, if not all, of the elements necessary for basal transcription as well as responsiveness to thrombin in intact cells. They also suggest that there might be negative regulatory elements between −1503 and −1116 bp.
Because most of the activation induced by thrombin was achieved with the p683 construct, we concentrated on determining which element(s) in this region of the promoter are the thrombin response elements. For these studies, further 5′ deletions of p683 followed by transient transfections into primary fibroblasts were performed. The results show that eliminating the sequence from −683 to −542 bp (p542) had no significant effect on the level of stimulation by thrombin (Figure2B,II), whereas deletion of the putative Elk1 binding site between −534 to −524 bp (p495) slightly reduced the level of transcription activation (Figure 2B,III). However, when the second putative Elk1 element between −493 and −483 bp was also eliminated (p470), there was a 3-fold reduction in activation of transcription in the absence of thrombin treatment and a 7-fold reduction in the presence of thrombin treatment (Figure 2B,IV). In addition, the reporter construct containing only the PDRIIkB, AP-1, and CAAT binding element (p218) showed no significant activation either in the presence or absence of thrombin (Figure 2B,V). These results taken together strongly suggest that the major functional elements for thrombin stimulation of this chemokine gene in primary normal fibroblasts are the 2 putative Elk1 binding sites between −534 and −483 bp.
Elk1 is important for the activation of the9E3/cCAF gene by thrombin
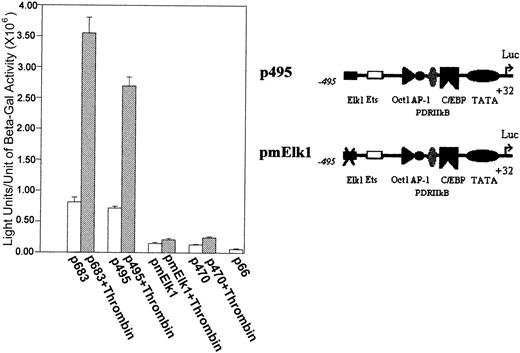
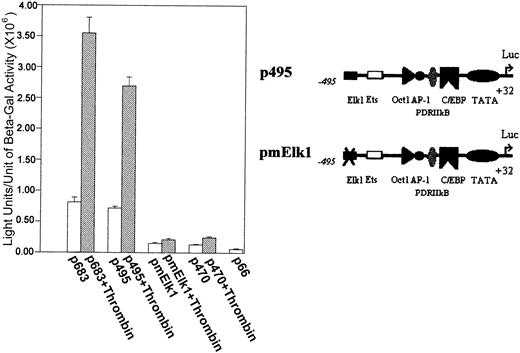
To further address the possibility that the Elk1 consensus binding sites are critical for the transcription activation of the9E3/cCAF gene, we deleted the Elk1 site starting at −534 bp, mutated the Elk1 site starting at −493 bp to obtain a construct containing −495 bp to +32 bp (pmElk1), and performed transient transfection studies similar to those described above. In contrast to the normal p495 (that contains the unaltered putative Elk1 binding site), the p495 with the mutated Elk1 yielded a much lower level of transcription and was not responsive to thrombin (Figure3).
Elk1 binding sites are critical for 9E3/cCAF activation by thrombin.
The transient transfections were performed as described in Figure 1C with a reporter construct lacking Elk1 binding sites; the −534 Elk1 site was deleted and the −493 site was mutated (CAGGAT to acGcgT, pmElk1). With the mutation, both the basal and stimulated transcription levels of p495 were decreased to the p470 level of activation.
Elk1 binding sites are critical for 9E3/cCAF activation by thrombin.
The transient transfections were performed as described in Figure 1C with a reporter construct lacking Elk1 binding sites; the −534 Elk1 site was deleted and the −493 site was mutated (CAGGAT to acGcgT, pmElk1). With the mutation, both the basal and stimulated transcription levels of p495 were decreased to the p470 level of activation.
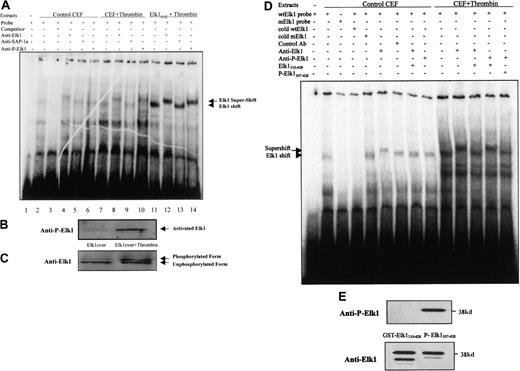
To determine whether a transcription factor in primary normal fibroblasts binds to the sites present in the 9E3/cCAF promoter, we performed EMSAs using a radiolabeled probe containing the sequence of the putative Elk1 binding element (−493 to −483 bp) present in the 9E3/cCAF promoter (Figure 4A). This probe was incubated with nuclear extracts from primary fibroblasts treated in the presence or absence of thrombin or from fibroblasts overexpressing Elk1 and treated with thrombin. Shift complexes were seen with the nuclear extracts from the primary fibroblasts, both in the absence (lane 2) and presence (lane 7) of thrombin treatment, reflecting the binding of a nuclear factor(s) from normal cells to the probe. This binding is specific in that it was competed out with 25-fold excess unlabeled oligonucleotide (lane 3). Cells overexpressing Elk1 showed the same size shift complex but in greater abundance (data not shown) and when these overexpressing cells were treated with thrombin the shift was even more obvious (lane 11). To determine whether the transcription factor in the shift complex was Elk1 or SAP1a (a closely related factor that also binds the Elk1 consensus binding element), we performed the EMSA in the presence of an antibody to the C-terminus of the Elk1 protein (aa 379-392) or an antibody to the C-terminus of SAP1a, neither of which interferes with protein-DNA binding. The antibody to Elk1 retarded the migration of protein-oligonucleotide complex resulting in the appearance of a supershifted band (lanes 4, 8, and 12), whereas the antibody to SAP1a caused no supershift (lanes 5, 9, and 13).
The Elk1 transcription factor binds to the putative Elk1 binding elements of the 9E3/cCAF promoter and is activated by thrombin.
(A) EMSAs with extracts prepared from control chick embryo fibroblasts (CEFs), CEFs treated with thrombin, and CEFs overexpressing Elk1 and treated with thrombin. The conditions for the reactions are described in detail in “Material and methods.” All lanes have radiolabeled probe containing the Elk1 binding sequence (−493 to −483 bp) present in the promoter region of 9E3/cCAF. Extracts from untreated control cells contained factors that caused shift complexes to occur (lane 2, arrowheads). These complexes did not form when the incubation with the radiolabeled probe was done in the presence of 25-fold excess of unlabeled probe (lane 3). Incubation of the extracts from cells treated with thrombin, with the radiolabeled probe, caused a shift similar to that of untreated cells but much more pronounced (lane 7). In the presence of an antibody specific for the Elk1 protein (anti-Elk1) a supershift was detected both in the absence and presence of thrombin (lanes 4 and 8; arrow). However, this complex cannot be supershifted by an antibody specific to SAP-1 (lanes 5, 9, and 13), another member of the TCF transcription factor family, which has the ability to bind to the same element. When the extracts were treated with the antibody to activated Elk1 (anti-Ser383 phosphor-Elk1), the supershift was observed for the cells treated with thrombin only, indicating that this enzyme activates the Elk1 transcription factor. These same results were verified with cells overexpressing Elk1 where the shifts and supershifts are more obvious because the levels of the Elk1 transcription factor are much higher. (B) Immunoblot analysis of the activated Elk1 after treatment with thrombin. Anti-Ser383 phosphor-Elk1, the antibody that detects activated Elk1, showed that thrombin treatment increases the phosphorylation of this transcription factor. (C) Immunoblot analysis using the anti-Elk1 antibody that detects all forms of the Elk 1 transcription factor. The higher molecular weight band represents phosphorylated Elk1. (D) EMSA prepared with extracts of cells treated in the presence and absence of thrombin to verify the specificity of Elk1 shift and supershift complexes. To test the specificity of the shift we used a mutated Elk-1 binding oligonucleotide instead of wild type. To test the specificity of the supershift, we used excess unphosphorylated and phosphorylated C-terminal peptides of Elk-1 (GST-Elk1310-428 and GST-P-Elk1307-428, respectively) as competitors for the binding of the antibodies to Elk1 and a control antibody from the same company (Ser136 phosphor-Bad). (E) Immunoblot analysis of the phosphorylated and unphosphorylated peptides using both antibodies to the Elk1 C-terminal domain. The presence of 2 bands in the blot probed with anti-Elk1 is not surprising because it is common that during the purification of GST-fusion proteins partial cleavage of the protein occurs.
The Elk1 transcription factor binds to the putative Elk1 binding elements of the 9E3/cCAF promoter and is activated by thrombin.
(A) EMSAs with extracts prepared from control chick embryo fibroblasts (CEFs), CEFs treated with thrombin, and CEFs overexpressing Elk1 and treated with thrombin. The conditions for the reactions are described in detail in “Material and methods.” All lanes have radiolabeled probe containing the Elk1 binding sequence (−493 to −483 bp) present in the promoter region of 9E3/cCAF. Extracts from untreated control cells contained factors that caused shift complexes to occur (lane 2, arrowheads). These complexes did not form when the incubation with the radiolabeled probe was done in the presence of 25-fold excess of unlabeled probe (lane 3). Incubation of the extracts from cells treated with thrombin, with the radiolabeled probe, caused a shift similar to that of untreated cells but much more pronounced (lane 7). In the presence of an antibody specific for the Elk1 protein (anti-Elk1) a supershift was detected both in the absence and presence of thrombin (lanes 4 and 8; arrow). However, this complex cannot be supershifted by an antibody specific to SAP-1 (lanes 5, 9, and 13), another member of the TCF transcription factor family, which has the ability to bind to the same element. When the extracts were treated with the antibody to activated Elk1 (anti-Ser383 phosphor-Elk1), the supershift was observed for the cells treated with thrombin only, indicating that this enzyme activates the Elk1 transcription factor. These same results were verified with cells overexpressing Elk1 where the shifts and supershifts are more obvious because the levels of the Elk1 transcription factor are much higher. (B) Immunoblot analysis of the activated Elk1 after treatment with thrombin. Anti-Ser383 phosphor-Elk1, the antibody that detects activated Elk1, showed that thrombin treatment increases the phosphorylation of this transcription factor. (C) Immunoblot analysis using the anti-Elk1 antibody that detects all forms of the Elk 1 transcription factor. The higher molecular weight band represents phosphorylated Elk1. (D) EMSA prepared with extracts of cells treated in the presence and absence of thrombin to verify the specificity of Elk1 shift and supershift complexes. To test the specificity of the shift we used a mutated Elk-1 binding oligonucleotide instead of wild type. To test the specificity of the supershift, we used excess unphosphorylated and phosphorylated C-terminal peptides of Elk-1 (GST-Elk1310-428 and GST-P-Elk1307-428, respectively) as competitors for the binding of the antibodies to Elk1 and a control antibody from the same company (Ser136 phosphor-Bad). (E) Immunoblot analysis of the phosphorylated and unphosphorylated peptides using both antibodies to the Elk1 C-terminal domain. The presence of 2 bands in the blot probed with anti-Elk1 is not surprising because it is common that during the purification of GST-fusion proteins partial cleavage of the protein occurs.
Activation of the Elk1 transcription factor involves the phosphorylation of this factor on Ser383 and Ser389.40 41To determine if thrombin stimulates the phosphorylation of this transcription factor, we performed the supershift assays with an antibody specific for the Ser383 phosphorylated form of Elk1 (anti-Ser383 phosphor-Elk1). This antibody caused a supershift to occur with extracts from cells stimulated with thrombin (lanes 10 and 14), but not with extracts from cells that were not stimulated (lane 6). These results show that Elk1 binds the −493- to −483-bp site and suggest that binding is independent of activation of Elk1 by thrombin because the supershift was observed with anti-Elk1 in both nonstimulated (lane 4) and thrombin-stimulated (lanes 8 and 12) assays, whereas the anti-Ser383 phosphor-Elk1 only supershifted the cells stimulated by thrombin (lanes 10 and 14).
To confirm that the Elk1 transcription factor is phosphorylated on Ser383 in response to thrombin, the nuclear extracts used in Figure 4A were examined by immunoblot analysis with the anti-Ser383 phosphor-Elk1 antibody. The results show that thrombin stimulation resulted in a significant increase of Elk1 phosphorylation on Ser383 (Figure 4B). Similar expression levels and even loading of the Elk1 protein were demonstrated by reprobing the blot with the antibody to the C-terminus of Elk1 (Figure 4C). Because this antibody detects all of the Elk1 protein in the cells (phosphorylated and unphosphorylated), 2 bands were observed; the lower band represents the unphosphorylated form of the Elk1 protein, whereas the higher band corresponds to phosphorylated Elk1.
To further verify the specificity of the shift complex, we show an EMSA using the mutated Elk-1 binding element (Figure 4D). The shift complex formed with the wild-type oligonucleotide (lane 2) but did not form with the mutated oligonucleotide (lane 3). Moreover, the complex was competed out by excess (50-fold) unlabeled wild-type (lane 4) but not mutated oligonucleotide (lane 5). These findings indicate that this complex is specific for the conserved Elk1 binding element. To verify the specificity of the supershift complex, we used a control antibody and the C-terminal peptides of Elk-1 (phosphorylated and unphosphorylated) as competitors for the transcription factor binding to its antibodies. The supershift complex obtained with the anti-Elk1 antibody (lane 6) did not form with the control antibody (lane 7) and was competed out when the antiElk1 antibody was preincubated with unphosphorylated peptide competitor (lane 8; the latter was obtained by expressing a GST-Elk1310-428 fusion protein that does not contain the DNA binding domain but contains the unphosphorylated Elk1 C-terminus). Furthermore, in control cells, the supershift complex also did not form when the anti–P-Elk1 antibody was used (lane 9) but, when cells were stimulated with thrombin, the supershift occurred with both Elk1 antibodies (compare lanes 10, 11, and 13). Unlabeled GST-Elk1310-428 was able to compete out the supershift obtained with anti-Elk1 (lane 12) but not the supershift obtained with anti-P-Elk1 (lane 13). The latter was competed out (lane 14) by a GST-P-Elk1307-428 C-terminus peptide phosphorylated on Ser383. Figure 4E shows that the GST-Elk1310-428 is recognized by the anti-Elk1 antibody but not by anti–P-Elk1, whereas the phosphorylated GST-P-Elk1307-428 protein was recognized by both antibodies. These results show that the supershift is specific for Elk1.
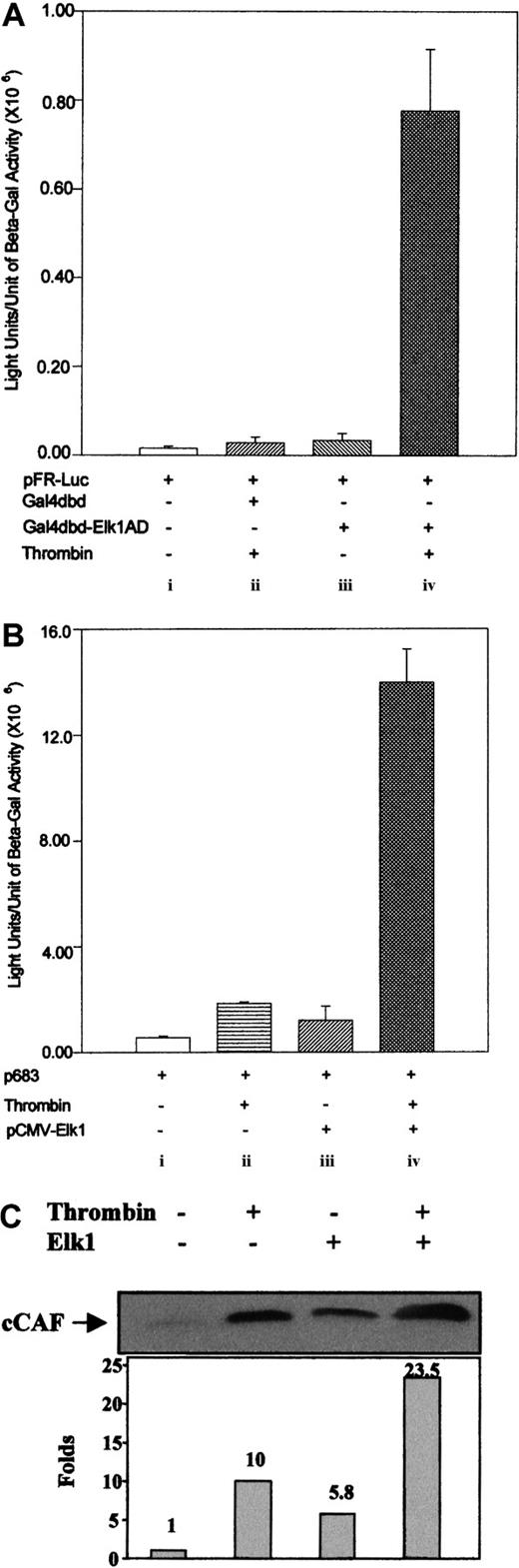
To determine whether the thrombin-induced phosphorylation of Elk1 enhances the activation of this factor, we used a heterologous expression system with a Gal4-Elk1 fusion protein and a Gal4 luciferase reporter (Stratagene). Cells were cotransfected with the pFR-Luc construct, which contains 6 Gal4 DNA binding elements in tandem upstream of the luciferase gene, and with the pFA-Gal4dbd-Elk1AD construct, which is a cytomegalovirus-driven expression vector for a fusion protein containing the Gal4 DNA binding domain (Gal4dbd) and the Elk1 activation domain (Elk1AD). The Gal4dbd part of this fusion protein binds to the Gal4 response elements in pFR-Luc and the Elk1AD portion of the fusion protein activates the luciferase gene after phosphorylation. As a negative control we used an expression vector for the Gal4 DNA binding domain alone (pFC-Gal4dbd). It was observed that, when pFC-Gal4dbd was cotransfected with the reporter construct (pFR-Luc) followed by treatment with thrombin, the activation of the luciferase gene was not significantly different from that of the control (Figure 5Ai-ii); the same was observed when pFA-Gal4dbd-Elk1AD was cotransfected with the reporter construct in the absence of thrombin treatment (Figure 5Aiii). However, when thrombin was used to stimulate the cells cotransfected with the pFA-Gal4dbd-Elk1AD and the reporter construct, we observed a 16-fold increase in activation (Figure 5Aiv). These results show that the signals generated after stimulation of cells by thrombin activate the Elk1 transcription factor.
Direct activation of Elk1 by thrombin.
(A) Heterologous expression of the Elk1 transcription factor in primary fibroblasts and activation by thrombin. All transfections were performed as in Figure 1C except that the cells were cotransfected with 2 μg of a reporter construct containing 6 Gal4 binding elements in series immediately upstream of the luciferase gene (pFR-Luc) and with 2 μg of the expression vector for the fusion protein pFA-Gal4dbd-Elk1AD, which contains the Gal4 protein DNA binding domain (Gal4dbd) and the Elk1 protein activation domain (Elk1AD). Stimulation of the cotransfected cells with thrombin resulted in 16-fold increase over the control (iv). As controls, cells were transfected with 2 μg of pFR-Luc and 2 μg of pcDNA3.0 vector (i), or cotransfected with pFR-Luc and 2 μg of the expression vector for the Gal4dbd and then stimulated by thrombin (ii), or cotransfected with pFR-Luc and 2 μg of the pFA-Gal4dbd-Elk1AD in the absence of stimulation by thrombin (iii). (B) Overexpression of the Elk1 transcription factor and thrombin treatment on 9E3/cCAF expression. The transient transfections were performed as described in Figure 1C. Cotransfection of 2 μg of pCMV-Elk1 with 2 μg of p683 in primary fibroblasts resulted in a 2-fold increase in activation of the reporter gene (compare i and iii), whereas without Elk1 overexpression, thrombin stimulation enhanced transcription 3-fold (compare i and ii and with Figures 2 and 3, but in the latter note the scale differences). When the cotransfected cells were treated with thrombin, a synergistic effect was observed (iv). The transfections for conditions (i) and (ii) also included 2 μg of pcDNA3.0 vector. (C) Immunoblot analysis using an antibody specific for cCAF. Supernatants of fibroblasts treated as in panel B were resolved in 20% polyacrylamide-glycerol gel. The synergistic effect on transcription activation of 9E3/cCAF is reflected in an increase in the amount of cCAF protein produced by the fibroblasts. This figure shows representative experiments in all cases. Each experiment was performed at least twice, using different batches of cells. Bars in panels A and B represent SEMs of 3 samples/condition in the same batch of cells.
Direct activation of Elk1 by thrombin.
(A) Heterologous expression of the Elk1 transcription factor in primary fibroblasts and activation by thrombin. All transfections were performed as in Figure 1C except that the cells were cotransfected with 2 μg of a reporter construct containing 6 Gal4 binding elements in series immediately upstream of the luciferase gene (pFR-Luc) and with 2 μg of the expression vector for the fusion protein pFA-Gal4dbd-Elk1AD, which contains the Gal4 protein DNA binding domain (Gal4dbd) and the Elk1 protein activation domain (Elk1AD). Stimulation of the cotransfected cells with thrombin resulted in 16-fold increase over the control (iv). As controls, cells were transfected with 2 μg of pFR-Luc and 2 μg of pcDNA3.0 vector (i), or cotransfected with pFR-Luc and 2 μg of the expression vector for the Gal4dbd and then stimulated by thrombin (ii), or cotransfected with pFR-Luc and 2 μg of the pFA-Gal4dbd-Elk1AD in the absence of stimulation by thrombin (iii). (B) Overexpression of the Elk1 transcription factor and thrombin treatment on 9E3/cCAF expression. The transient transfections were performed as described in Figure 1C. Cotransfection of 2 μg of pCMV-Elk1 with 2 μg of p683 in primary fibroblasts resulted in a 2-fold increase in activation of the reporter gene (compare i and iii), whereas without Elk1 overexpression, thrombin stimulation enhanced transcription 3-fold (compare i and ii and with Figures 2 and 3, but in the latter note the scale differences). When the cotransfected cells were treated with thrombin, a synergistic effect was observed (iv). The transfections for conditions (i) and (ii) also included 2 μg of pcDNA3.0 vector. (C) Immunoblot analysis using an antibody specific for cCAF. Supernatants of fibroblasts treated as in panel B were resolved in 20% polyacrylamide-glycerol gel. The synergistic effect on transcription activation of 9E3/cCAF is reflected in an increase in the amount of cCAF protein produced by the fibroblasts. This figure shows representative experiments in all cases. Each experiment was performed at least twice, using different batches of cells. Bars in panels A and B represent SEMs of 3 samples/condition in the same batch of cells.
To extend the studies with the heterologous expression system to the full-length Elk1 protein, we determined whether the overexpression of Elk1 in the presence of thrombin stimulation would cause a higher level of activation than with the endogenous factor alone. Thrombin induced about a 3-fold increase in activation by the endogenous Elk1 with p683 alone (Figure 5Bii; see also Figure 2A-B, but note the difference in the units of the Y-axis), whereas overexpression of Elk1 in the absence of thrombin caused an approximate 2-fold increase above the p683 basal level (Figure 5Biii) (this could be due to the trace amounts of phosphorylated Elk1 seen when Elk1 is overexpressed in the absence of thrombin, Figure 4B). However, simultaneous Elk1 overexpression and thrombin treatment of cells transfected with p683 produced a strong synergistic effect (about 28-fold over control; Figure 5Biv). To correlate these activation levels with those of the cCAF protein, cells were treated in a similar manner and the supernatants were analyzed by immunoblotting using an antibody to cCAF. We found that the levels of endogenous cCAF protein parallel the levels of the luciferase activity driven by the cCAF promoter (Figure 5C).
MEK1/ERK2 involvement in the stimulation of 9E3/cCAF by thrombin
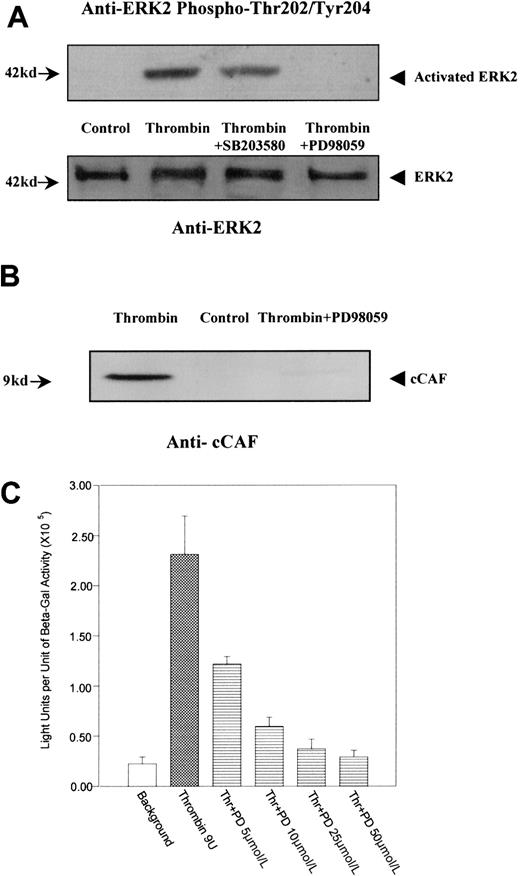
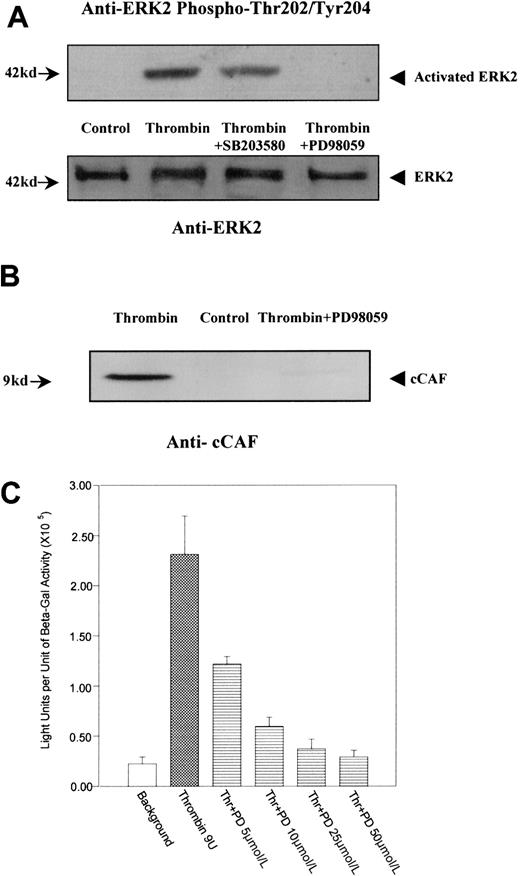
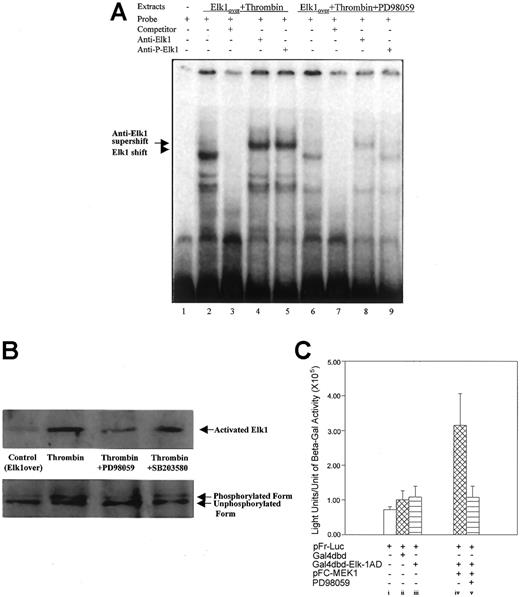
We have previously shown that ERK2 is differentially phosphorylated on tyrosines after primary fibroblasts are stimulated by thrombin.12 These findings raise the possibility that ERK2 is involved in the stimulation of 9E3/cCAF by thrombin. Western blot analysis (Figure 6A) using an antibody against activated ERK2 (phosphorylation on Thr202 and Tyr204) showed that this kinase was highly phosphorylated and activated after stimulation by thrombin. This effect was inhibited by PD98059, the highly selective inhibitor of MEK1, which is the kinase that is directly upstream from ERK2 in the MAP kinase cascade and phosphorylates and directly activates ERK2.42 The inhibitor to MAP kinase p38, SB203580, used under the same conditions, showed minimal effects on thrombin-induced ERK2 phosphorylation. Similarly, thrombin stimulation of these cells caused an increase in production of cCAF, whereas treatment by thrombin in the presence of PD98059 resulted in inhibition of cCAF production (Figure 6B). The specificity of the PD98059 effects were further confirmed by transient transfection analysis. Activation of the p683 promoter by thrombin was down-regulated by PD98059 in a dose-dependent manner (Figure 6C). Similarly, EMSA experiments (Figure 7A) performed with the nuclear extracts from cells treated with thrombin in the presence of PD98059 showed that this inhibitor decreases the binding of Elk1 to the probe (compare lanes 2 and 6). Furthermore, this complex (lane 6) was supershifted by anti-Elk1 (lane 8) but not by anti-Ser383 phosphor-Elk1 (lane 9) indicating that MEK1/ERK2 is involved in the phosphorylation of Elk1 by thrombin in primary fibroblasts. To confirm that the decreased Elk1 binding with PD98059 treatment is not due to the down-regulation of nuclear Elk1 protein levels, but through the Ser383 phosphorylation, Western blot analysis was performed with the same nuclear extracts used in the EMSA. The results indicate that a similar amount of Elk1 is overexpressed and present in the nuclei even with the inhibitors. PD98059 dramatically decreases Elk1 Ser383 phosphorylation, whereas the p38 MAP kinase inhibitor SB203580 had only a very small effect (Figure 7B). To show that MEK1/ERK2 acts via Elk1 in our cells, the Gal4-Elk1 fusion construct described above (pFA-Gal4dbd-Elk1AD) was cotransfected with a MEK1 expression vector (pFC-MEK1) and the reporter construct (pFR-Luc). Even in the absence of thrombin stimulation, the reporter gene was activated by MEK1 overexpression (Figure 7Civ), indicating that overexpression of this kinase activated its substrate ERK2 followed by activation of the Elk1 transcription factor. Furthermore, addition of PD98059 eliminated the activation of the reporter gene by MEK1/ERK2 (Figure 7Cv). Taken together, these results support the conclusion that the Elk1 transcription factor is activated by thrombin through the MEK1/ERK2 kinase cascade.
MEK1/ERK2 involvement in activation of 9E3/cCAF by thrombin.
(A) Immunoblot analysis of ERK2 phosphorylation. Extracts prepared from cells treated in the presence or absence of thrombin and in the presence of thrombin plus the MEK1 inhibitor PD98059 or the p38 inhibitor SB203580 were analyzed by immunoblot using antibodies to activated ERK2. Thrombin caused a high level of phosphorylation of ERK2; PD98059 dramatically decreased phosphorylation of ERK2 but SB203590 had virtually no effect. The same blot was stripped and reprobed with anti-ERK2 antibodies to show the levels of the ERK2 protein in each lane. (B) Immunoblot analysis with an antibody to the cCAF chemokine. Thrombin stimulated high expression of cCAF, whereas blocking phosphorylation of ERK2 via MEK1 inhibition by PD98059 inhibited the production of cCAF in cells treated with thrombin. (C) Dose-dependent effect of PD98059 on p683-induced activation of gene expression after stimulation by thrombin. Primary fibroblasts were transiently cotransfected as discussed in the Figure 2 legend. PD98059 lowered the stimulation of p683 by thrombin to levels comparable to those of the control in a dose-dependent manner.
MEK1/ERK2 involvement in activation of 9E3/cCAF by thrombin.
(A) Immunoblot analysis of ERK2 phosphorylation. Extracts prepared from cells treated in the presence or absence of thrombin and in the presence of thrombin plus the MEK1 inhibitor PD98059 or the p38 inhibitor SB203580 were analyzed by immunoblot using antibodies to activated ERK2. Thrombin caused a high level of phosphorylation of ERK2; PD98059 dramatically decreased phosphorylation of ERK2 but SB203590 had virtually no effect. The same blot was stripped and reprobed with anti-ERK2 antibodies to show the levels of the ERK2 protein in each lane. (B) Immunoblot analysis with an antibody to the cCAF chemokine. Thrombin stimulated high expression of cCAF, whereas blocking phosphorylation of ERK2 via MEK1 inhibition by PD98059 inhibited the production of cCAF in cells treated with thrombin. (C) Dose-dependent effect of PD98059 on p683-induced activation of gene expression after stimulation by thrombin. Primary fibroblasts were transiently cotransfected as discussed in the Figure 2 legend. PD98059 lowered the stimulation of p683 by thrombin to levels comparable to those of the control in a dose-dependent manner.
Elk1 activation by thrombin occurs via MEK1/ERK2.
(A) EMSA with extracts prepared from cells overexpressing Elk1 and treated with thrombin in the absence and presence of the MEK1 inhibitor PD98059. The conditions for the reactions are described in “Material and methods.” All lanes have radiolabeled probe containing the Elk1 binding sequence present in the promoter region of 9E3/cCAF (−493 to −483 bp). Extracts from cells treated with thrombin contained factors that caused shift complexes to occur (lane 2, arrowheads). These complexes did not form when the incubation with the radiolabeled probe was done in the presence of 25-fold excess of unlabeled probe (lane 3). Extracts from cells treated with thrombin in the presence of PD98059 contained reduced amounts of factors but caused similar, albeit less pronounced, shift complexes (lane 6, arrowhead). Incubation of the extracts from lanes 2 and 6 with the radiolabeled probe in the presence of an antibody specific for the Elk1 protein (anti-Elk1) resulted in a supershift (lanes 4 and 8, arrow). However, when the extracts from lanes 2 and 6 were incubated with an antibody to the activated Elk1 (anti-Ser383 phosphor-Elk1) only the extracts from cells treated with thrombin in the absence of PD98059 resulted in a supershift (compare lanes 5 and 9). (B) Immunoblot analysis of activated Elk1 detected with anti-Ser383 phosphor-Elk1 in the presence of PD98059 or SB203580. The latter inhibitor slightly decreases thrombin-induced phosphorylation of Elk1, whereas the former prevented phosphorylation. (C) Effect of MEK1 on activation of the Elk1 transcription factor. The same heterologous expression system used in Figure 5 was used for these experiments, and the conditions for the transfections were described in the Figure 2legend. When appropriate, the pcDNA 3.1 plasmid was used to maintain the total amount of DNA transfected at 6 μg. When cells were cotransfected with 2 μg of pFR-Luc and 1 μg of the fusion protein plasmid pFA-Gal4dbd-Elk1AD, no significant activation of the reporter was observed (iii), but cotransfection of the same amounts of pFR-Luc and pFA-Gal4dbd-Elk1AD in conjunction with 1 μg of the plasmid for MEK1 overexpression, resulted in a significant activation of the reporter gene (iv). This activation was inhibited by PD98059 (v). As a negative control, transfection with pFR-Luc alone (2 μg) or cotransfections of the Gal4 DNA binding domain (Gal4dbd; 1 μg) and pFR-Luc (2 μg) were performed. These experiments were performed several times with different batches of primary cells. Here we show representative experiments. Bars represent SEMs of 3 samples/condition in the same batch of cells.
Elk1 activation by thrombin occurs via MEK1/ERK2.
(A) EMSA with extracts prepared from cells overexpressing Elk1 and treated with thrombin in the absence and presence of the MEK1 inhibitor PD98059. The conditions for the reactions are described in “Material and methods.” All lanes have radiolabeled probe containing the Elk1 binding sequence present in the promoter region of 9E3/cCAF (−493 to −483 bp). Extracts from cells treated with thrombin contained factors that caused shift complexes to occur (lane 2, arrowheads). These complexes did not form when the incubation with the radiolabeled probe was done in the presence of 25-fold excess of unlabeled probe (lane 3). Extracts from cells treated with thrombin in the presence of PD98059 contained reduced amounts of factors but caused similar, albeit less pronounced, shift complexes (lane 6, arrowhead). Incubation of the extracts from lanes 2 and 6 with the radiolabeled probe in the presence of an antibody specific for the Elk1 protein (anti-Elk1) resulted in a supershift (lanes 4 and 8, arrow). However, when the extracts from lanes 2 and 6 were incubated with an antibody to the activated Elk1 (anti-Ser383 phosphor-Elk1) only the extracts from cells treated with thrombin in the absence of PD98059 resulted in a supershift (compare lanes 5 and 9). (B) Immunoblot analysis of activated Elk1 detected with anti-Ser383 phosphor-Elk1 in the presence of PD98059 or SB203580. The latter inhibitor slightly decreases thrombin-induced phosphorylation of Elk1, whereas the former prevented phosphorylation. (C) Effect of MEK1 on activation of the Elk1 transcription factor. The same heterologous expression system used in Figure 5 was used for these experiments, and the conditions for the transfections were described in the Figure 2legend. When appropriate, the pcDNA 3.1 plasmid was used to maintain the total amount of DNA transfected at 6 μg. When cells were cotransfected with 2 μg of pFR-Luc and 1 μg of the fusion protein plasmid pFA-Gal4dbd-Elk1AD, no significant activation of the reporter was observed (iii), but cotransfection of the same amounts of pFR-Luc and pFA-Gal4dbd-Elk1AD in conjunction with 1 μg of the plasmid for MEK1 overexpression, resulted in a significant activation of the reporter gene (iv). This activation was inhibited by PD98059 (v). As a negative control, transfection with pFR-Luc alone (2 μg) or cotransfections of the Gal4 DNA binding domain (Gal4dbd; 1 μg) and pFR-Luc (2 μg) were performed. These experiments were performed several times with different batches of primary cells. Here we show representative experiments. Bars represent SEMs of 3 samples/condition in the same batch of cells.
AP-1 is not critical for the activation of 9E3/cCAF in normal fibroblasts
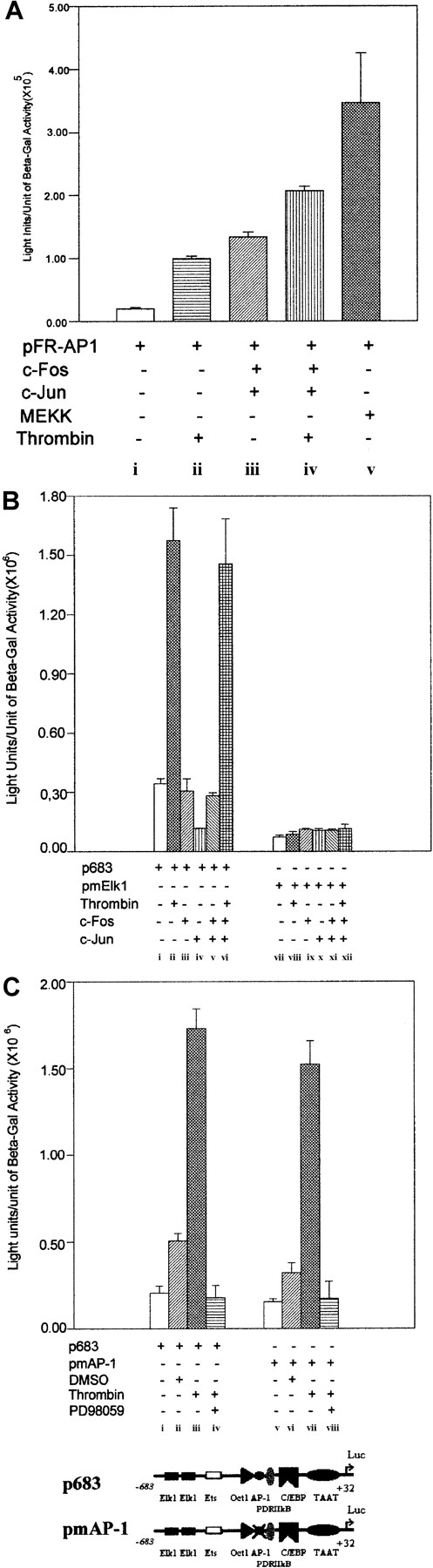
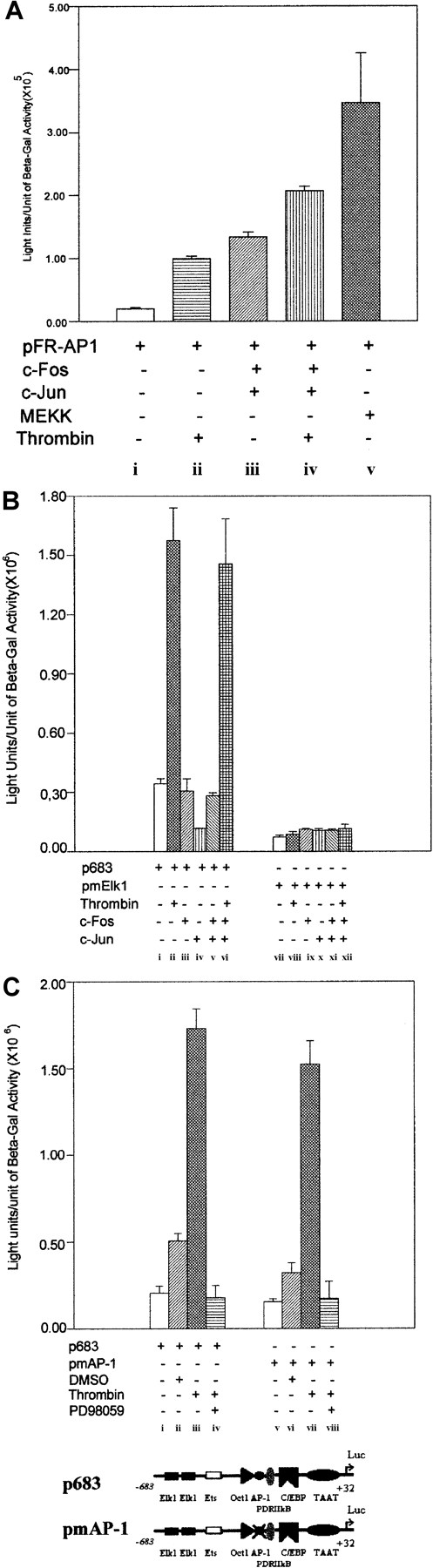
It has been shown that phorbol-12-myristate-13-acetate (PMA) activates the IL-8 chemokine gene through the AP-1 complex43 in human Jurkat T cells, and that thrombin can stimulate mitogenesis of CCL39 hamster fibroblasts through rapid and persistent activation of AP-1 via the MAP kinase cascade.44,45 Furthermore, it has also been shown that AP-1 is involved in the activation of 9E3/cCAF in Rous sarcoma virus–transformed fibroblasts.38,46 Although the results presented above (Figures 2B and 3A) show that in primary normal fibroblasts the promoter construct containing the AP-1 binding element but lacking the Elk1 sites (p470) does not respond to thrombin stimulation, it is possible that in this particular case AP-1 cooperates with Elk1. To address this possibility, we first overexpressed c-Fos and c-Junsimultaneously in primary cells and used a reporter construct containing 7 AP-1 consensus binding elements linked to the luciferase gene (pFR-AP1; Stratagene) to determine whether thrombin can activate the AP-1 complex. Expression of c-Fos and c-Junsimultaneously should result in the formation of the Fos/Jun complex that constitutes the active AP-1 transcription factor.36We found that pFR-AP1 yielded a very low level of basal activity (Figure 8Ai), whereas treatment with thrombin resulted in a 3-4 fold increase in activation (Figure 8Aii). Similar results were observed when c-Fos andc-Jun were overexpressed together in the absence of thrombin treatment (Figure 8Aiii), but when both thrombin treatment and overexpression of the AP-1 complex occurred, the transcription activity increased significantly (Figure 8A, compare iii and iv). MEK kinase was used as positive control to verify that the pFR-AP-1 was working correctly (Figure 8Av). Thus, the AP-1 transcription factor complex made from the expression vectors is functional in primary fibroblasts and can be activated by thrombin.
AP-1 is not crucial for the activation of9E3/cCAF in normal fibroblasts.
The transfection conditions were described in Figure 2B and Figure 5. (A) Overexpression of the AP-1 complex (c-Fos andc-Jun) to determine its functionality in primary normal fibroblasts. A heterologous promoter system containing 7 AP-1 binding elements in series in front of the luciferase reporter gene (Cis-Path Detect System from Stratagene) was used for these experiments. Cells transfected with pFR-AP1 showed that in the context of the tandem of AP-1 binding sites, thrombin can stimulate AP-1–dependent transcription (ii). Overexpression of c-Fosand c-Jun by cotransfection of 1 μg of each plasmid and 2 μg of pFR-AP-1 in the absence (iii) or presence (iv) of thrombin resulted in a 6- and 11-fold increase, respectively, in transcription of the reporter system. MEK kinase (MEKK) overexpression was used as a positive control for the system; cells cotransfected with 2 μg of pFR-AP-1 and 1 μg of the MEKK expression vector showed a high activation of the reporter gene (v). (B) Activation of the AP-1 binding element in the context of the 9E3/cCAF promoter. The fibroblasts were cotransfected with 2 μg of p683 (i-vi) or pmElk1 (vii-xii) and 1 μg of the expression vectors forc-Fos and c-Jun. Transfections with p683,c-Fos, and c-Jun individually or in combination did not stimulate expression of the reporter gene above that of the control (iii,iv,v); overexpression of c-Fos andc-Jun had no effect on stimulation by thrombin (compare ii and vi). With the mutated Elk1 construct (pmElk1), there was no enhancement of reporter transcription with thrombin stimulation (viii), AP-1 component(s) overexpression (ix,x,xi) or their combination (xii). (C) Mutation of the AP-1 binding element within the context of the p683 promoter. An insignificant reduction in reporter activation by pmAP-1 was observed when compared to the p683 control. DMSO, the vehicle for the PD98059 inhibitor, stimulated a small increase (2 ×) for both the mutated and nonmutated p683. PD98059, a highly selective inhibitor of MEK1, abrogated thrombin stimulation of the reporter. For each experiment, representative data are shown in which all conditions were performed simultaneously in the same batch of cells. Each type of experiment was performed at least twice, using different batches of cells. Bars represent SEMs of 3 samples per condition.
AP-1 is not crucial for the activation of9E3/cCAF in normal fibroblasts.
The transfection conditions were described in Figure 2B and Figure 5. (A) Overexpression of the AP-1 complex (c-Fos andc-Jun) to determine its functionality in primary normal fibroblasts. A heterologous promoter system containing 7 AP-1 binding elements in series in front of the luciferase reporter gene (Cis-Path Detect System from Stratagene) was used for these experiments. Cells transfected with pFR-AP1 showed that in the context of the tandem of AP-1 binding sites, thrombin can stimulate AP-1–dependent transcription (ii). Overexpression of c-Fosand c-Jun by cotransfection of 1 μg of each plasmid and 2 μg of pFR-AP-1 in the absence (iii) or presence (iv) of thrombin resulted in a 6- and 11-fold increase, respectively, in transcription of the reporter system. MEK kinase (MEKK) overexpression was used as a positive control for the system; cells cotransfected with 2 μg of pFR-AP-1 and 1 μg of the MEKK expression vector showed a high activation of the reporter gene (v). (B) Activation of the AP-1 binding element in the context of the 9E3/cCAF promoter. The fibroblasts were cotransfected with 2 μg of p683 (i-vi) or pmElk1 (vii-xii) and 1 μg of the expression vectors forc-Fos and c-Jun. Transfections with p683,c-Fos, and c-Jun individually or in combination did not stimulate expression of the reporter gene above that of the control (iii,iv,v); overexpression of c-Fos andc-Jun had no effect on stimulation by thrombin (compare ii and vi). With the mutated Elk1 construct (pmElk1), there was no enhancement of reporter transcription with thrombin stimulation (viii), AP-1 component(s) overexpression (ix,x,xi) or their combination (xii). (C) Mutation of the AP-1 binding element within the context of the p683 promoter. An insignificant reduction in reporter activation by pmAP-1 was observed when compared to the p683 control. DMSO, the vehicle for the PD98059 inhibitor, stimulated a small increase (2 ×) for both the mutated and nonmutated p683. PD98059, a highly selective inhibitor of MEK1, abrogated thrombin stimulation of the reporter. For each experiment, representative data are shown in which all conditions were performed simultaneously in the same batch of cells. Each type of experiment was performed at least twice, using different batches of cells. Bars represent SEMs of 3 samples per condition.
To determine whether the AP-1 element in the context of the 9E3/cCAF promoter plays a role in transcription activation stimulated by thrombin, the cells were cotransfected with the p683 reporter construct (which contains the AP-1 element and both Elk1 binding sites) and the expression vectors for c-Fos and c-Jun in the presence or absence of thrombin treatment. Expression ofc-Fos and c-Jun individually or in combination did not stimulate transcription activation above that of the control (Figure 8Biii-v), and overexpression of the complex in cells treated with thrombin resulted in a level of activation that was not significantly different from that in cells treated with thrombin alone (Figure 8B, compare ii and vi). Furthermore, transfections performed with the promoter containing the mutated Elk1 elements (pmElk1) showed that treatment with thrombin (Figure 8Bviii) or coexpression ofc-Fos and c-Jun in cells treated with thrombin, did not result in transcription activation of the reporter system (Figure 8Bxii). These results show that in primary normal fibroblasts, overexpression of a functionally competentc-Fos/c-Jun complex does not activate the 9E3/cCAF promoter. To confirm these results, we performed site-directed mutagenesis on the AP-1 binding site in the p683 construct and found that neither the basal level nor the thrombin-stimulated level of transcription was significantly altered (Figure 9C). Thus, we conclude that AP-1 is not a significant factor in the thrombin activation of the9E3/cCAF gene in primary fibroblasts.
Discussion
Thrombin, an enzyme released on wounding, is known to play key roles in wound healing and in the development of tumor stroma and to stimulate the expression of chemokines to high levels in cells importantly involved in these processes. Understanding the mechanism of activation of chemokines by agents such as thrombin could lead to ways of modulating expression of these small cytokines in conditions of injury and tumorigenesis where thrombin plays important functional roles. Here we show that in primary normal fibroblasts, cells that are critical in wound healing and in the development of tumor stroma: (1) thrombin very rapidly activates the expression of the 9E3/cCAF chemokine at the transcriptional level; (2) the 2 Elk1 binding elements in the promoter of this chemokine gene starting at −534 bp and −493 bp are the major response elements; (3) Elk1 and not SAP1a binds specifically to these promoter elements; (4) activation of the Elk1 transcription factor by thrombin is not dependent on simultaneous activation of the AP-1 transcription complex; (5) overexpression of Elk1 and thrombin treatment synergistically increases9E3/cCAF gene expression; (6) Elk1 is directly activated by the MEK1/ERK2 kinase cascade after thrombin stimulation.
Previous work using the region of the 9E3/cCAF promoter between −214 bp and +36 bp in fibroblasts transformed by Rous sarcoma virus demonstrated that activation of this gene is controlled by multiple pathways and that the most important cis-elements for the v-src response are AP-1 (beginning at −119 bp) and PDRIIκB (beginning at −95 bp).38 These investigators called this region of the promoter the v-src responsive unit (SRU). In the studies presented here, we show that the p218 construct, which contains the SRU, does not confer activation of 9E3/cCAF in primary normal fibroblasts stimulated by thrombin (Figure 2). However, with a longer promoter, 683 bp, we observed a strong activation of the reporter system. Furthermore, this activation was comparable to that observed with the 1500-bp promoter but lower than that observed with a promoter of intermediate size (1116 bp), suggesting that in primary fibroblasts there are negative regulatory elements between −1117 and −1503 bp that may play a role in preventing an excessive response even under stress conditions. Finally, within the p683 sequence we show that the Elk1 elements between −534 and −483 bp and the Elk1 transcription factor are importantly involved in activation of 9E3/cCAF by thrombin. In addition, we point out that mutation of the −483-bp Elk1 element also resulted in reduction of the basal transcription level by a factor of 3, suggesting that Elk1 may also participate in basal transcription.
The results presented here showing that Elk1 but not AP-1 is crucial for the stimulation of 9E3/cCAF gene activation by thrombin are supported by previous findings. For example, the kinetics of gene activation stimulated by thrombin and v-src are different. As shown in Figure 1A, thrombin stimulation of 9E3/cCAF mRNA occurred very rapidly after thrombin addition to the cells, reached a maximum within 3 hours, and declined to near background levels by 18 hours. Similar patterns of activation by thrombin have also been shown for MCP-1 in vascular smooth muscle cells47 and for IL-8 in human umbilical vein endothelial cells and in fibroblasts stimulated by serum, which contains thrombin.48,49 On the other hand, stimulation by v-src is first seen at 3 hours after switching transformed cells to the permissive temperature, requires 8 to 12 hours to reach its peak, and remains high for 24 hours.38,46 The differences in the 2 patterns of stimulation imply that different mechanisms are involved in the activation processes stimulated by thrombin and by the src oncogene. Indeed, stimulation of 9E3/cCAF by thrombin and v-src are additive.12
Our results also show that Elk1 can stimulate 9E3/cCAF expression in absence of the serum response element (SRE) and serum response factor (SRF). Elk1 was originally characterized as a component of a ternary complex, which contains an Elk1 transcription factor and 2 SRFs. Elk1 contains various functional domains: an N-terminal DNA binding domain, an SRF-interacting region, a C-terminal transactivation domain that contains phosphorylation sites that regulate transcription,41 and a region for binding of ERK2, which then phosphorylates and activates Elk1.26 In the ternary complex, Elk1 can bind to the SRE with the cooperation of the 2 SRFs and activate gene expression.50-53 However, the capability of Elk1 to activate transcription is not dependent on its interaction with the SRF.54 Rather, activation of transcription by Elk1 depends on phosphorylation of the (S/T)P motif in the transcription domain,40,50 which is carried out by the ERK/JNK on binding of the pertinent kinase. Therefore, it has been proposed that Elk1-dependent gene activation can occur independently of SRE/SRF if it can bind to the promoter efficiently.50 This proposition is supported by the finding that the phosphorylation of Elk1 by ERK2 increases its ability to bind to DNA without interacting with SRF54,55 and by the fact that Elk1 binding is facilitated in transfection experiments with a reporter gene containing multiple high-affinity Elk1 binding sites40,56 or phosphorylation in its C-terminus.55,57 Even though the 9E3/cCAF promoter does not contain an SRE, Elk1 binds to the Elk1 elements and activates transcription, suggesting that thrombin-induced Elk1 activation does not require cooperation with SRF. Furthermore, the 9E3/cCAF promoter contains several Elk1-Ets elements in tandem, which may be instrumental in promoting efficient binding of Elk1 to its element. Thus, the work presented here strongly supports the previous proposition that Elk1 can function independently of SRF/SRE.50 58
Our results taken together suggest that the Elk1 transcription factor may normally be bound to its response element on the promoter of the9E3/cCAF gene and that signal transduction pathways triggered by thrombin lead to the phosphorylation and activation of this transcription factor. In support of our findings are the observations showing that Elk1 can bind its DNA response element even when it is not activated.52,56,58 However, only when the cells are stimulated by 12-O-tetradecanoylphorbol-13-acetate (TPA) was Elk1 able to activate transcription.58,59 Furthermore, very recently it has been shown that MAP kinases can stabilize Elk1 binding and activation and that this is mediated by phosphorylation of the C-terminus of the protein.57
In conclusion, our studies show that thrombin can stimulate stress responses leading to chemokine gene expression through the classical MAP kinase cascade (MEK1/ERK2) normally associated with mitogenesis, but that it does so by activating the Elk1 transcription factor rather than SAP1a or AP-1. These observations lend support to the previous proposition that Elk1-driven gene activation occurs in the absence of SRE/SRF cooperation. The consistent presence of Elk1 binding elements in the promoter regions of many immediate early response genes (eg,c-Fos60; Zif268,MKP-161; egr-162;pip9263) and the rapid kinetics of stress-induced transduction signals suggest that this pathway may play an important role in the responses of normal cells in vivo to other stress-inducing agents.
Acknowledgments
We thank W. Wong for technical assistance and ACG Design for assistance with computer graphics used to prepare the figures. We are indebted to Drs R. Tjian (University of California, Berkeley, CA) for the expression vector for c-Fos and c-Jun, R. Davis (University of Michigan Medical Center) for the expression vector of Elk1, A. Sharrocks (University of Manchester, United Kingdom) for the vector of GST-Elk1310-428, and D. Strauss and S. Gill (University of California, Riverside) for use of equipment in their laboratories.
Supported in part by National Institutes of Health grant GM48436 (M.M-G.) by National Institute of General Medical Sciences.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Manuela Martins-Green, Department of Cell Biology and Neuroscience, University of California, Riverside, CA 92521; e-mail: mmgreen@ucrac1.ucr.edu.