Abstract
The authors have identified a 12-residue carboxyl-terminal extension of Lys-Ser-Pro-Met-Arg-Arg-Phe-Leu-Leu-Phe-Cys-Met in a dysfibrinogen derived from a woman heterozygotic for this abnormality and associated with severe bleeding. This extension is due to a T-to-A mutation that creates AAG encoding Lys at the stop (TAG) codon, thus translating 36 base pairs in the noncoding region of the Bβ gene. The extra Cys residues appear to be involved in 1 or 2 disulfide bonds between 2 adjacent abnormal fibrinogen molecules, forming a fibrinogen homodimer as indicated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Indeed, about half of the fibrinogen molecules exist as end-linked dimers oriented in parallel or with an angle, as observed by transmission electron microscopy. These end-linked dimers may well alter the conformations of D and DD regions on fibrin assembly, leading to increased fiber branching at their sites in the growing protofibrils. By scanning electron microscopy, the Osaka VI fibrin network appears to have a lacelike structure composed of highly branched, thinner fibers than the normal fibrin architecture. Such fibrin networks may be easily damaged to form large pores when fluids are allowed to pass through the gels. The fragility of Osaka VI fibrin clots, further confirmed by permeation and compaction studies, may account for the massive bleeding observed in this patient.
Introduction
Fibrinogen is a 340-kd plasma protein that participates in the final step of blood coagulation. Fibrinogen is composed of 2 identical molecular halves, each molecular half being composed of 3 nonidentical polypeptides, Aα, Bβ, and γ chains, held together by multiple disulfide bonds.1,2 Electron microscopy revealed a trinodular structure3,4 connected by 2 coiled-coil regions. Part of the fibrinogen molecule has recently been clarified by x-ray crystallographic analysis at high resolution,5,6 and very recently the crystal structure of modified bovine fibrinogen has been determined at lower resolution.7 These data show that the outer nodules are made up of the 2 independently folded β and γ chains, confirming the closely positioned globular domains previously observed by electron microscopy.3 8
The 3 chains are encoded by 3 independent genes clustered on chromosome 4. During the synthesis of fibrinogen, individual chains are translated, processed, and assembled, and the mature fibrinogen molecule is eventually secreted into blood circulation. Although different assembly pathways have been proposed,9 10 a molecular half, AαBβγ, is formed as an intermediate, and then 2 molecular halves are held together with 5 disulfide bonds to form the dimeric structure of mature fibrinogen (AαBβγ)2.
The fibrinogen-to-fibrin conversion resulting in a fibrin network consists of highly ordered molecular interactions, including binding with thrombin, thrombin-catalyzed cleavage of fibrinopeptides A and B, formation of staggered double-stranded fibrin protofibrils, and their lateral associations. In these molecular interactions, several binding modes have been proposed, and some of them have been confirmed by electron microscope and x-ray crystallographic analyses.1,2,5-7 11
A hereditary dysfibrinogen is a fibrinogen molecule that is unable to exert its physiologic functions because of a structural alteration(s) determined at the gene level.12 Among 60 or more structural alterations so far identified at molecular level, those with a Cys substitution are rather frequent, although the status of the Cys in those dysfibrinogens has been elucidated only in some cases.13 In this paper, we describe the presence of 2 types of end-linked fibrinogen homodimers formed by a single or pair of disulfide bridges between the Cys residues in the carboxyl-terminal extension of a mutant Bβ chain of fibrinogen. Thus, very fragile fibrin networks composed of highly branched, thin fibers, probably consisting of only a few protofibrils, are formed.
Materials and methods
Fibrinogen was purified from normal and patient-citrated plasma. Human α-thrombin, recombinant 2-chain tissue-type plasminogen activator (t-PA), and factor XIII and its thrombin-activated transglutaminase, factor XIIIa, were prepared as described previously.14-16 Lysylendopeptidase was purchased from Wako Chemical Co, Osaka, Japan, and ancrod, the purified fraction of a thrombin-like snake venom derived from Agkistrodon rhodostoma, was a gift from Mochida Pharmaceutical Co, Tokyo, Japan. The following high-performance liquid chromatography (HPLC) columns were used: phenyl-5PW-RP and TSK-GEL ODS-80 Ts from Tosoh, Tokyo, Japan.
Studies on purified fibrinogen
Coagulation studies were performed according to standard procedures. Aggregation studies of preformed and acid-solubilized fibrin monomer, factor XIIIa–catalyzed cross-linking of fibrin and fibrinogen, and the enhancement of t-PA–catalyzed activation of plasminogen by the polymerizing fibrin monomer were performed essentially as described previously.14,15 Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was carried out with 7.5%-to-15% gradient gels (Figure1A). For the identification of the doublet bands denoted as Fbg′ and Fbg, SDS-PAGE was carried out with 4.5% acrylamide gels in a first run and, after staining the proteins, the doublet bands were cut from the gels and individually reduced by dithiothreitol and then put on the top of the lanes of the second 7.5%-to-15% gradient gels and electrophoresed.
Subunit polypeptides of the patient's fibrinogen examined by SDS-PAGE.
(A,B) Lane 1, normal fibrinogen; lane 2, the patient's fibrinogen. The bands representing 2 molecular weight species of the patient's fibrinogen indicated by Fbg and Fbg′ (lane 2 in panel A) in 4.5% gel were cut out, and SDS-PAGE was rerun on a 7.5% to 15% gradient gel under nonreducing (C) and reducing conditions (D). (C,D) Lane 1, normal fibrinogen; lane 2, the higher molecular weight species; lane 3, the normal fibrinogen species. Note that only an aberrant Bβ chain (Bβ′) is present in the Fbg′ species.
Subunit polypeptides of the patient's fibrinogen examined by SDS-PAGE.
(A,B) Lane 1, normal fibrinogen; lane 2, the patient's fibrinogen. The bands representing 2 molecular weight species of the patient's fibrinogen indicated by Fbg and Fbg′ (lane 2 in panel A) in 4.5% gel were cut out, and SDS-PAGE was rerun on a 7.5% to 15% gradient gel under nonreducing (C) and reducing conditions (D). (C,D) Lane 1, normal fibrinogen; lane 2, the higher molecular weight species; lane 3, the normal fibrinogen species. Note that only an aberrant Bβ chain (Bβ′) is present in the Fbg′ species.
Lysylendopeptidase mapping of the fibrinogen Bβ chain
The 3 subunits of fibrinogen were separated after reduction and S-pyridylethylation (Pe) by HPLC using a TSK gel phenyl-5PW-RP column (4.6 × 75 mm). They were eluted in the order of Aα, Bβ, and γ chains with a linear gradient (30 minutes) from 30% to 50% acetonitrile. The Pe-Osaka VI Bβ chain (0.1 mg/mL, 130 μg) was digested with lysylendopeptidase (E/S = 1/50, wt/wt) at 37 °C for 18 hours in 50 mmol/L Tris-HCl, pH 9.0, containing 3 mol/L urea. The lysylendopeptidase digests of Pe-Bβ chains derived from normal and Osaka VI fibrinogens were injected onto a TSK-GEL ODS-80 TS column (4.6 × 150 mm), and the peptides were eluted with a linear gradient from 0% to 40% acetonitrile in 100 minutes. Three peptides from Osaka VI, K16′, K17′, and K34′, and 2 peptides from normal K16 and K17 were subjected to the amino-terminal amino acid sequence analysis.
Amino acid composition and sequence analysis
The amino acid sequence analysis of intact fibrinogen Osaka VI was performed with a Protein Sequencer, model 476A (PE Biosystems, Foster City, CA), by using 100 μg of protein up to 6 cycles. Peptide analyses of K16′ and K17′ and normal K16 and K17 were conducted by using a 20% to 25% volume of each fraction, and nearly a half of K34′ was used for sequence analysis.
Permeation and compaction studies
Permeation study was performed according to the methods of Nair and Dhall17 with a slight modification as described previously.16 Compaction experiments were performed by a minor modification of a previously described method.16Percent compaction was expressed as volume percent of the original volume (0.75 mL) of the clot.
Scanning electron microscopy of Osaka VI clots
Specimens for scanning electron microscopy (SEM) were prepared in 30-μL Plexiglas microdialysis cells perforated for solvent perfusion.18 Fibrinogen (1 mg/mL) was incubated with α-thrombin (0.16 NIH U/mL) in a microdialysis cell in 50 mmol/L Tris-HCl, pH 7.4, containing 0.1 mol/L NaCl at 25 °C for 45 minutes or overnight. Specimens were washed 3 times with the rinse buffer (50 mmol/L cacodylate buffer, pH. 7.4) to remove excess salt, fixed for 2 hours in 2% glutaraldehyde in the rinse buffer, and then rinsed 3 times. The samples were dehydrated in a gradient series of ethanol concentrations through 100% over a period of 1.5 hours. The clots were critical point–dried with CO2 in an HCP-2 (Hitachi, Tokyo, Japan), mounted, and finally coated with about 12.5 nm of gold-palladium with an Hitachi Ion Sputter, E-1030. Specimens were observed and photographed using an Hitachi SEM-S4100 scanning electron microscope.
Electron microscopy of individual molecules
Fibrinogen samples were prepared by spraying a dilute solution (25 μg/mL) of molecules in a volatile buffer (25 mmol/L ammonium formate, pH 7.4) containing 35% (vol/vol) glycerol onto freshly cleaved mica.8 Shadowing was performed with platinum at an angle of 8 degrees and carbon at 90 degrees on a rotary stage in a vacuum evaporator, VE-2000 (Vacuum Device, Ibaraki, Japan). The specimens were examined in a JEM-1010 transmission electron microscope (JEOL, Tokyo, Japan) at 80 kV and a magnification of 50 000.
Results
Description of the patient
The patient was a 36-year-old Japanese woman with a history of massive postpartum hemorrhage on each occasion of her previous 2 childbirths, for which 1300 mL and 2500 mL, respectively, of fresh blood had been transfused to control the bleeding. For her third childbirth, she delivered a baby uneventfully with the aid of 2.0 g of human fibrinogen fraction infused just before the delivery. Among the blood coagulation and hemostatic tests, the prothrombin time and the activated partial thromboplastin time were both moderately prolonged: 17.7 seconds (control 13.3 seconds) and 44.0 seconds (control 36.0 seconds), respectively. The thrombin time and the ancrod time were markedly prolonged: 50.8 seconds and 78.8 seconds (control 13.3 seconds and 16.8 seconds), respectively. The plasma fibrinogen concentration evaluated by the thrombin time method was less than 40 mg/100 mL, whereas that by the turbidimetric methods was in the normal range. Although the presence of a mild hepatic dysfunction seemed to account at least partly for the prolonged prothrombin time and activated partial thromboplastin time, decreased levels of plasma fibrinogen determined by the 2 different methods suggested the presence of a congenital dysfibrinogenemia. These data prompted us to conduct further investigations, including determination of structural defects and their relevance to the postpartum hemorrhages. The plasma of 2 of 3 daughters also gave prolonged thrombin and ancrod times.
Abnormality of purified fibrinogen
The purified patient-derived fibrinogen has 2 Bβ-chain species, denoted as Bβ′ and Bβ, as evidenced by SDS-PAGE (Figure 1A). The Bβ-chain species corresponded to the normal Bβ chain, whereas the Bβ′-chain species was found to have a molecular weight higher by about 2000 than the normal Bβ chain. Under nonreducing conditions, the patient-derived fibrinogen also migrated as a doublet, one at the position for normal fibrinogen denoted as Fbg and the other at a position equivalent to a 2-fold higher molecular weight protein, Fbg′. No trimer or higher molecular weight proteins were seen. This was the first dysfibrinogen that could be seen as a doublet band with no other high molecular weight species. In the second SDS-PAGE run under reducing conditions, the Fbg′ species was found to have Bβ′ chains alone, suggesting that Fbg′ exists as a homodimer consisting of 2 fibrinogen molecules with the Bβ′ chains only. On the other hand, Fbg was found to consist of normal Bβ-chain species only (Figure 1B). The Aα and γ chains appeared to be normal.
The thrombin time was moderately prolonged (27 seconds, control 14.4 seconds), and Ca++ partially corrected the delay. Profiles of α-thrombin–released fibrinopeptides A and B (FPA and FPB) were indistinguishable from those of normal fibrinogen, as evidenced by SDS-PAGE and HPLC elution profiles (data not shown). The aggregation profile of the patient-derived fibrin monomer disclosed a vast increase in turbidity at the initial time of mixing the solution, followed by a slow increase, resulting in loss of the typical sigmoidal curve with a lag phase (Figure 2). However, the patient's polymerizing fibrin monomer was able to enhance t-PA–mediated plasminogen activation, and the factor XIIIa-catalyzed cross-linking of the γ chain took place in a normal fashion (data not shown). These data suggest that double-stranded protofibrils may have been constructed normally via a set of ′A′-′a′ polymerization sites.
Aggregation profiles of acid-solubilized fibrin monomers derived from normal and Osaka VI fibrinogen.
Aggregation of acid-solubilized fibrin monomer was studied by monitoring absorbance at 350 nm.
Aggregation profiles of acid-solubilized fibrin monomers derived from normal and Osaka VI fibrinogen.
Aggregation of acid-solubilized fibrin monomer was studied by monitoring absorbance at 350 nm.
Identification of an aberrant peptide in lysylendopeptidase digests of Pe-Bβ chain
In the mapping profiles of the lysylendopeptidase digests of Pe-Bβ chain derived from the patient's fibrinogen, there was an aberrant peptide peak, K34′, and a peak with a wide shoulder, K16′ (Figure 3). They were not present in the mapping profiles of digests of normal fibrinogen. By amino acid sequence analyses, K16′ was found to comprise the Bβ (454-461) segment with an additional Lys residue linked to its carboxyl terminus, whereas no amino acids were detectable at each cycle of the normal counterpart, K16 (Table 1). Because the K16 peak was absent in the mapping profile of fibrin Pe-β chain digests, normal K16 peptide is the amino-terminal Bβ (1-21) segment whose amino-terminal amino acid is pyroglutamic acid, which is not detectable by sequence analysis. These data suggested that the patient-derived K16′ contains 2 peptides, the Bβ (1-21) peptide and an aberrant peptide corresponding to the 454-to-461 residues that is linked with an additional Lys residue at its carboxyl-terminal Gln461. By sequence analysis of the neighboring K17′ peptide, the normal carboxyl-terminal Bβ (454-461) segment was identified as expected.
HPLC profile of lysylendopeptidase digests of Bβ chain.
The lysylendopeptidase digests of Pe-Bβ chains derived from normal fibrinogen and fibrinogen Osaka VI were injected onto a TKS GEL ODS-80 column (4.6 × 150 mm), and the peptides were eluted with a linear gradient from 0% to 40% acetonitrile in 100 minutes. Three Osaka VI peaks, K16, K17, and K34, and normal K16 and K17 were collected and subjected to N-terminal amino acid sequence analysis.
HPLC profile of lysylendopeptidase digests of Bβ chain.
The lysylendopeptidase digests of Pe-Bβ chains derived from normal fibrinogen and fibrinogen Osaka VI were injected onto a TKS GEL ODS-80 column (4.6 × 150 mm), and the peptides were eluted with a linear gradient from 0% to 40% acetonitrile in 100 minutes. Three Osaka VI peaks, K16, K17, and K34, and normal K16 and K17 were collected and subjected to N-terminal amino acid sequence analysis.
On the other hand, K34′ was found to have a sequence of Ser-Pro-Met-Arg-Arg-Phe-Leu-Leu-Phe-Cys(PeCys)-Met, which seemed to be equivalent to those translated by a 36-base noncoding region following the stop codon (TGA). Because K16′ was found to have Lys at its carboxyl terminus, one base exchange that creates a codon for Lys was expected. Indeed, a single T-to-A mutation at nucleotide 1464 of the Bβ chain, creating a codon AAG for K instead of TAG for the stop, was confirmed (data not shown). With this exchange, an additional 12 residues were translated until the appearance of a new stop codon (TGA). However, the content of K34′ was only 15% of K17′, whereas the K16′ was about 60% that of K17′, which is in good agreement with the ratio of Bβ chain over Bβ′ chain in staining density (Figure 1A). The reason for the low recovery of K34 was unclear.
Although there was an extra Cys residue in this extended peptide segment, no free sulfhydryl groups were detected by a sulfhydryl titration experiment of the patient's fibrinogen using a fluorogenic titrant, N-pyrenyl-maleimide (data not shown). Amino acid sequence analysis of a whole fibrinogen molecule also showed that no albumin was linked to the extra Cys residue of the aberrant Bβ chain (data not shown). The abnormal molecules would instead exist as disulfide-bridged dimers, as deduced from the presence of a much higher, apparently double-molecular weight species of fibrinogen in nonreduced SDS-PAGE (Figure 1B). To confirm this assumption, we conducted electron microscope analysis of individual molecules.
Ultrastructure of individual fibrinogen molecules
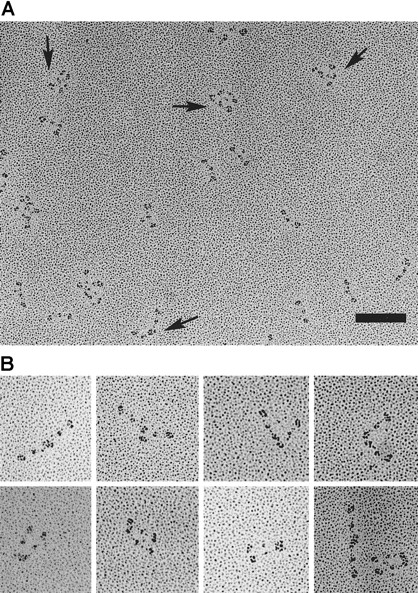
The patient's fibrinogen molecules were rotary shadowed with platinum and examined by transmission electron microscopy (TEM), and the results from the several preparations are summarized in Figure4. In our TEM images, the carboxyl-terminal β and γ chains were separately observed in the D region at higher magnifications, but additional small nodules corresponding to the αC domain19 were not observed. Of 1860 images of the patient's fibrinogen, about 52% had a simple trinodular structure, 35% had a dimeric form connected at the D regions, and 13% appeared to be multimers or irregular aggregates (Figure 4B). No trimers in any forms were observed, but all the aggregates were composed of even numbers of fibrinogen molecules.
Electron microscopy of individual rotary-shadowed molecules of fibrinogen Osaka VI.
(A) Field of the patient's fibrinogen showing 3 species of fibrinogen molecules, ie, simple trinodular molecules, end-linked dimers, and end-linked bilayer dimers (arrows), and aggregates. Bar, 100 nm. (B) Gallery of typical end-linked dimers.
Electron microscopy of individual rotary-shadowed molecules of fibrinogen Osaka VI.
(A) Field of the patient's fibrinogen showing 3 species of fibrinogen molecules, ie, simple trinodular molecules, end-linked dimers, and end-linked bilayer dimers (arrows), and aggregates. Bar, 100 nm. (B) Gallery of typical end-linked dimers.
Interestingly, many of the dimers appeared to be end-linked, in which the 2 molecules were arranged with the ends of 2 molecules near each other, ie, with the molecular backbones at an angle, straight, or in parallel. In some dimers, the 2 D regions appeared to be fused into one elongated nodule. The straight form of dimer had some resemblance to the end-to-end joined fibrinogen dimers, which were covalently cross-linked by factor XIIIa.20 21 About 25% of the dimers were in the end-linked bilayer form in which the 2 molecules were aligned next to each other in parallel and appeared to be linked at D regions at both ends. In contrast to the Osaka VI fibrinogen molecule, most of the control fibrinogen molecules manifested simple trinodular structures with only a few aggregates. The presence of dimeric fibrinogen molecules may well disturb the fibrin monomer assembly and thus produce the abnormal fibrin network structure to be described next.
Scanning electron micrographs of Osaka VI–fibrin clots
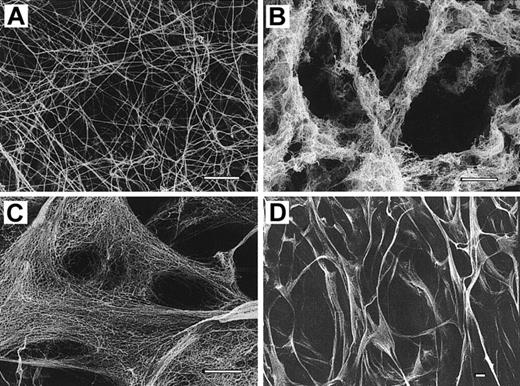
When examined by SEM, the patient's fibrin clots were very different in appearance from control clots (Figure5). The patient's clots obtained after 45 minutes of incubation with thrombin revealed spongelike gels composed of thinner fibers and large pores, as compared with the normal fibrin architecture (Figure 5A,B). Free fiber ends were often observed at the boundary between the networks and the pores so that the clots were very porous. When the gels were fully incubated with thrombin for 17 hours, however, a significantly different nature of appearance was commonly observed. Completely gelled Osaka VI clots were found to be composed of highly branched thin fibers creating a lacelike, uniform mesh structure (Figure 5C). There were very few free fiber ends or thick bundles, but large pores or open areas bounded by lacelike networks were frequently observed (Figure 5D). Many fibers were estimated to be about 15 to 25 nm in diameter and 210 to 300 nm in length between the branch points when calculated from the images at higher magnifications. On the contrary, the fibers and fiber bundles of normal fibrin clots were of a uniform thicker size with very few fiber ends. Branching structures seemed to be regularly spaced.
SEM-images of fibrin clots.
(A) Normal fibrin clots as control. (B) Clot formed from the patient's fibrinogen after 45 minutes of incubation with thrombin showing thin fibers forming a secondary network of spongelike structure. (C,D) Clots formed after 17 hours of incubation with thrombin, showing large pores bounded by smaller secondary, highly branched lacelike networks. Bar, 2 μm.
SEM-images of fibrin clots.
(A) Normal fibrin clots as control. (B) Clot formed from the patient's fibrinogen after 45 minutes of incubation with thrombin showing thin fibers forming a secondary network of spongelike structure. (C,D) Clots formed after 17 hours of incubation with thrombin, showing large pores bounded by smaller secondary, highly branched lacelike networks. Bar, 2 μm.
Compaction and permeation studies
As shown in Figure 6, the patient's fibrin was considerably more compressible than normal fibrin, indicating that the patient's fibrin network was made up of fragile fibers, such as thin fibers with larger pores, a finding that is consistent with the appearance of the SEM images (Figure 5D).
Compaction studies of the patient's fibrinogen.
The fibrin clot was formed in a conical microfuge tube with 0.60 mg/mL fibrinogen and 0.06 NIH U/mL thrombin in Tris-buffered saline. Following incubation at 25°C for 2 hours, the tubes were centrifuged at 4200g for 30 seconds 3 times, and each volume of the expelled buffer was withdrawn and measured with a Hamilton syringe. Percent compaction was expressed as the volume percent of the original volume (0.75 mL) of the clot.
Compaction studies of the patient's fibrinogen.
The fibrin clot was formed in a conical microfuge tube with 0.60 mg/mL fibrinogen and 0.06 NIH U/mL thrombin in Tris-buffered saline. Following incubation at 25°C for 2 hours, the tubes were centrifuged at 4200g for 30 seconds 3 times, and each volume of the expelled buffer was withdrawn and measured with a Hamilton syringe. Percent compaction was expressed as the volume percent of the original volume (0.75 mL) of the clot.
Permeation studies were also conducted on Osaka VI fibrin. However, less satisfactory data were obtained because channeling along the walls or the other irregularities of flow through the fibrin gels were often observed soon after the flow experiment was started. From these results, we considered the Osaka VI clots as particularity fragile gels in which enlargement of the pores inside the networks leading to the destruction of the network easily occurred.
Discussion
We have identified a unique 12-residue extension at the carboxy terminus of the Bβ chain of a dysfibrinogen, fibrinogen Osaka VI, derived from a heterozygous proband. This dysfibrinogen could be categorized as part of a group of Cys mutants that have an extra Cys residue in the aberrant subunit polypeptides. As clearly demonstrated by nonreducing SDS-PAGE, there are 2 fibrinogen species in the patient's fibrinogen fraction—one (Fbg) being apparently equivalent to normal fibrinogen and the other (Fbg′) being about twice as large as the other in terms of molecular size. Thus, the Fbg′ species seems to exist as a fibrinogen dimer. On reducing SDS-PAGE, the Bβ-chain band of Fbg migrated as a single band exactly at the same position as that of the normal Bβ chain, whereas the Bβ-chain band of Fbg′ (Bβ ′) migrated also as a single band but a little more slowly. Therefore, the Fbg′ species is thought to possess 2 Bβ′ chains only and to exist as a fibrinogen dimer of 2 abnormal fibrinogen molecules. If this is the case, 2 abnormal fibrinogen molecules could be bound most likely by a single- or 2-disulfide bridge(s) via a Cys residue residing in the carboxy-terminal 12-residue elongated segment of the aberrant Bβ chain.
A variety of Cys mutants have been reported, but no free sulfhydryl groups have so far been reported in any of these mutant molecules.12 In some molecules, the Cys substitution is linked with a single Cys as shown in fibrinogen Osaka II13and, in another, with serum albumin as in fibrinogens Dusart22 and Nijmegen and IJmuiden.23 There is a unique Cys residue at Aα-442 in fibrinogen Marburg that is partly linked with serum albumin because of loss of its disulfide partner AαCys-472 in the 150–amino acid truncated segment of the aberrant Aα chain.16,24 In some others, however, the Cys substitution in one molecular half is linked with its counterpart in the other within the same molecule, as shown in fibrinogens Kawaguchi and Osaka with an AαCys-16 substitution, thus contributing to the formation of intramolecular disulfide bonds.25 In this regard, Osaka VI fibrinogen molecules seem to be unique: namely, the Cys residue residing in the 12-residue–extended Bβ chain in one molecule seems to be linked with its corresponding Cys residue in another molecule to form 1 or 2 intermolecular disulfide bonds, which leads to the formation of a fibrinogen dimer, as represented by (AαBβ′γ)2-S-S-(AαBβ′γ)2.
Among the TEM images of the patient-derived fibrinogen, we were able to see 2 types of end-linked dimers, ie, an end-to-end–linked dimer and a bilayer dimer in addition to individual trinodular fibrinogen molecules and some aggregates. Although some of the end-to-end–linked dimers resemble factor XIIIa cross-linked dimers,20 21 most of those dimers appear to be arranged with a variety of angles between the molecules, indicating that the disulfide-linked abnormal fibrinogen dimers are certainly different from the rigid, factor XIIIa cross-linked fibrinogen dimers. On the other hand, the end-linked bilayer dimer seems to have a unique structure in which the D regions of 2 fibrinogen molecules seem to be connected at both ends of both molecules (Figure 7). The 22-residue peptide backbone length, 80 Å at maximum, may be long enough to form the end-linked bilayer dimer. Although there might be high structural tension in maintaining the alignment of the 2 molecules in parallel, these dimers were clearly discernible. Because only even numbers of molecules were observed in aggregates in TEM images of the patient's fibrinogen, the aggregates are most likely to be produced by noncovalent interactions of the dimers. If we consider the aggregates as derivatives of dimers, the ratio of dimers to single fibrinogen molecules, (AαBβ′γ)2-(AαBβ′γ)2 : (AαBβγ)2, is almost 1:1, which is in good agreement with the heterozygosity for this abnormality in the patient.
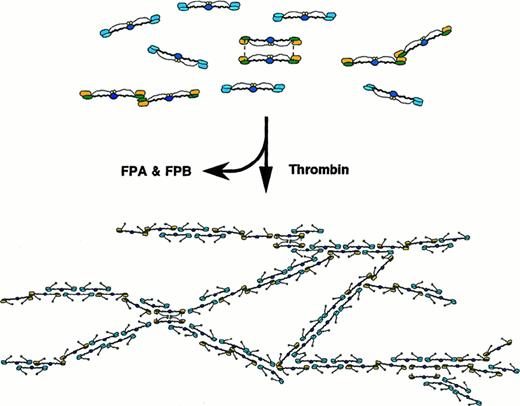
Schematic model of the patient's fibrinogen and its fibrin assembly.
Schematic model of the patient's fibrin assembly, illustrating interruption of protofibril elongation and frequent fibrin fiber branching at the loci, where the end-linked dimers are aligned. Normal molecules (blue) and end-linked dimers (yellow) are distributed based on the counts of respective molecular species on TEM images.
Schematic model of the patient's fibrinogen and its fibrin assembly.
Schematic model of the patient's fibrin assembly, illustrating interruption of protofibril elongation and frequent fibrin fiber branching at the loci, where the end-linked dimers are aligned. Normal molecules (blue) and end-linked dimers (yellow) are distributed based on the counts of respective molecular species on TEM images.
Theoretically, both of the autosomal loci of genomic DNA for fibrinogen could be operative, and thus 3 molecular species would be synthesized in the hepatocytes of the heterozygous patients. They are the fibrinogen molecules consisting of (1) two normal, (2) one normal and one abnormal, and (3) two abnormal molecular halves. In this patient's fibrinogen, however, we could see only homodimeric fibrinogens, consisting of normal-normal or abnormal-abnormal molecular halves, as deduced from SDS-PAGE profiles. Namely, the band (Fbg) was found to have normal Bβ chains only (Figure 1C-D, lane 1) and the band (Fbg′) to have Bβ′ chains only (Figure 1C-D, lane 2). Notably, no hybrid form of fibrinogen consisting of one each of normal and abnormal molecular halves (AαBβ′γ and AαBβγ) was observed in this patient. The reason for the absence of such hybrids is not clear. One can presume that such hybrid molecules may be degraded much more rapidly in hepatocytes or may not be secreted into the blood because of the structural derangement. If this is the case, the abnormal homodimeric molecules may be similarly or more highly susceptible to the intracellular enzymes and would not be secreted. Furthermore, the ratio of concentrations of Fbg′:Fbg in the purified fibrinogen fraction is close to 1 (Figure 1A, lane 2), and the patient's fibrinogen concentration always fell within the normal range. These data seem to indicate that the abnormal homodimeric fibrinogen molecules may be synthesized and transported in the hepatocytes and then secreted into the blood in a normal fashion. This may also imply that the hybrid molecule is not synthesized in this patient, although synthesis and secretion of such hybrid molecules may take place in different types of fibrinogen mutants.26
In the first step of biosynthesis of fibrinogen, the formation of Aα-γ and Bβ-γ intermediates depends primarily on hydrophobic interactions of amino acids located in the coiled-coil region of the molecule,27 and similar types of interaction may be involved in the formation of a half molecule, AαBβγ, and its dimerization. Such interactions imply that the extended portion of Bβ′ would not participate in the initial molecular assembly because the remainder of the Bβ′ chain has the complete segment required for the formation of intermediates. This may account for the fact that the 2 extra Cys residues in the extended region bonded with each other and formed a homodimeric abnormal fibrinogen. A similarity was observed in a recently identified human fibrinogen subclass, Fib420.28Fig420 is also synthesized as a homodimeric fibrinogen, (AαEBβγ)2, and its Aα chain, AαE, contains a 40-kd extended region due to an alternative splicing during the Aα chain transcription that forms a completely independent domain.28
The structure of the fibrin network is usually influenced by the substances coexisting in the reaction mixture.29 However, some of the fibrin networks of abnormal fibrinogens, such as Dusart, Caracas II, and Marburg fibrins,30-33 were found to be composed of thinner and highly branched fibers due to the presence of covalently bound albumin or extra sugar on the molecules. These abnormal fibrinogens were noted to have defects in fibrin fiber assembly. The major defect of fibrinogen Osaka VI was observed in the step of lateral association of the double-stranded protofibrils. Formation of double-stranded protofibrils seemed to proceed at normal speed up to a certain level, as evidenced by normal release of fibrinopeptide B and normal t-PA–catalyzed plasmin generation. Nevertheless, we presumed that the presence of end-linked dimers may have affected longitudinal elongation of the protofibrils together with their lateral association. The reasons for this conclusion are as follows: (1) the normal half-staggered arrangement of fibrin monomer in an overlapping manner could hardly be formed at the sites of the end-linked dimers, especially at the sites of end-linked bilayer dimers that are aligned. This disturbance may well yield many branch points; (2) steric constraints in the end-linked dimers may result in a decrease or a loss of the conformational freedom required for the longitudinal alignment of the fibrin dimers and thus increase the possibility to have a fiber end or a bent form that would tend to make branching junctions; and (3) the disulfide bond between the extended regions of Bβ′ chains may well affect the B:b binding because of a conformational change in the D and DD regions, because the b site has also been shown to reside in the carboxyl-terminal β-chain segment of the D region5 and probably close to the extended regions of the Bβ chains. However, the B:b interactions are not well understood. For these reasons, we think that abnormal lateral association takes place in Osaka VI fibrin assembly as indicated by the thinner fibers in the network.
Schematic models of Osaka VI fibrin assembly are shown in Figure 7. The Osaka VI fibrin clots were transparent and very fragile and have some resemblance to the Caracas II clots32 or the “fine clots” formed under high ionic strength conditions, because they have similar properties of thinner and more highly branched fibers than normal clots.34 However, these 3 networks were completely different on the basis of fiber thickness and branch-point density in the network and, especially, the network strength toward physical pressure. Each Osaka VI fibrin fiber seemed to form a twisted fiber with only a few protofibrils, though our estimation of the fiber width (< 20 nm) from the images of SEM may contain many latent errors produced by critical-point drying and metal vapor coating. The uniformity of the fiber width and the lacelike structure was distinctive to the Osaka VI fibrin networks.
One of the most characteristic features of the fibrinogen Osaka VI clots is their fragility. More branching by itself might tend to make the clots very stiff, like fibrinogen Dusart clots. However, the Osaka VI clots have many points of weakness throughout because of the defective assembly of the protofibrils (Figure 7). There are many places where the bonding will be weak because the molecules cannot assemble with normal interaction.
This type of network may be more easily damaged to form many channels or large pores inside the gels when fluids pass through the gels. Because of this breakage, we could not get any significant values in the permeation experiments of the Osaka VI fibrin, whereas “fine clots” exhibited lower permeation than normal clots (data not shown), and higher permeation was reported in the case of Caracas II fibrin.32 Compaction, the collapsibility of the network under constant centrifugal forces, was also higher than that of normal clots, indicating that there are many large pores inside the gels. The weakness of the Osaka VI fibrin clot may well explain the massive postpartum bleeding in this proband.
Acknowledgments
We thank Sumiko Murakami, Shinji Adachi, Kiyomi Inose, and Chandrasekar Nagaswami for their technical assistance and Michiko Takano for her secretarial assistance.
Supported in part by Scientific Research Grants-in-Aid for Scientific Research 11470250 and for International Scientific Research Program, Joint Research Grants 09044329, 10044316, and 11694308 from the Ministry of Education, Science and Culture of the Government of Japan, and National Institutes of Health HL 30954.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michio Matsuda, Division of Cell and Molecular Medicine, Center for Molecular Medicine, Jichi Medical School, Yakushiji 3311-1, Tochigi, 329-0498, Japan; e-mail:thmichi@jichi.ac.jp.