Abstract
Histone deacetylase inhibitors (HDACIs) have been used to focus on the effects of inducing gene expression through the acetylation of histones which results in chromatin remodeling. The study explored whether HDACIs could induce the expression of costimulatory/adhesion molecules on acute myeloid leukemia (AML) cells, thereby effectively inducing tumor immunity. The expression of CD80, CD86, human leukocyte antigen (HLA)-DR, HLA-ABC, and intracellular adhesion molecule–1 (ICAM-1) was tested in human AML cell lines after the addition of HDACI, sodium butyrate (SB). Generally, increased expression of CD86 was observed by SB treatment in a majority of cell lines, and ICAM-1 was expressed in fewer cell lines. Essentially the same results were obtained using other HDACIs such as FR901228, trichostatin A, and trapoxin A. Quantitation of transcripts of CD86 accompanied with RNA synthesis inhibition assay and nuclear run-on assay revealed that SB up-regulates the CD86 expression transcriptionally. Furthermore, chromatin immunoprecipitation experiments showed that HDACI treatment caused remarkable acetylation on histone H3 and H4 at CD86 promoter chromatin in vivo. In 30 clinical AML samples, CD86 expression was significantly increased (P < .001) by SB treatment, and the expression of HLA-DR and ICAM-1 was moderately increased (P < .05) by SB treatment. Finally, the allogeneic mixed leukocyte reaction (allo-MLR) against HL60 cells pretreated with SB was enhanced 4-fold compared with allo-MLR obtained with non-treated HL60 cells. These results suggest that the immunotherapeutic use of HDACIs may become a novel tool for treatment of AML.
Introduction
Although a very high complete remission rate can be obtained in acute myeloid leukemia (AML) by a combination therapy of multiple drugs, chemotherapy leads to less than a 50% long-term disease-free survival.1,2 Results of allogeneic bone marrow transplantation (allo-BMT) suggest the existence of an antileukemic immune response, the so-called graft-versus-leukemia (GVL) effect. It has been indicated that donor-derived T cells play an important role in eliminating the residual leukemic cells after BMT.3 4 The current model of T-cell activation has been presumed to require 2 signals. The first signal is specific, requiring T-cell receptor recognition and binding to major histocompatability complex (MHC) complex/antigen presented by an antigen-presenting cell (APC). The second signal is nonspecific, resulting from the binding of ligands for the T-cell costimulatory receptors CD28 and CTLA-4, ie, CD80/B7-1 and CD86/B7-2, respectively.
Results of an experimentally induced animal tumor model suggest that rejection of immunogenic tumors is primarily mediated by T cells and that the engagement of the TCR by MHC complex/peptide is generally not sufficient to trigger a productive immunoresponse.5Adequate expression of costimulatory/adhesion molecules is critical to stimulate a potent T-cell response.6 Failure to receive these signals renders potential effector T cells anergic or tolerant, and such second signals provided by the APC through numerous surface molecules are required to promote complete T-cell activation. Of these molecules, the B7 family members B7-1 (CD80) and B7-2 (CD86) have been shown to be important.7-10 In addition, intracellular adhesion molecule–1 (ICAM-1), by binding to lymphocyte function–associated antigen 1 (LFA-1) on the surface of lymphocytes, can also be crucial for activating T lymphocytes.11,12Among the major mechanisms of escape from immune surveillance, the absence of a specific tumor antigen or down-regulation of MHC molecule expression induces a lack of T-cell recognition of cancer cells. Another major mechanism of escape from immune surveillance involves defective expression of ligands for the T-cell costimulatory receptors CD28 and CTLA-4, ie, CD80 and CD86, respectively, on the target cell surface. AML cells generally express MHC class I and class II molecules, but CD80, CD86, and ICAM-1 are not always expressed on AML cells; thus these molecules can be the target of immunotherapy in AML.13,14 Furthermore, it has been reported that the T-cell stimulatory properties of leukemic cells can be up-regulated. Through activation via CD40, B-cell lymphoma and leukemia cells express high levels of human leukocyte antigen (HLA) and B7 molecules and become efficient APCs for alloreactive T cells.15,16Moreover, CD40-stimulated pre-B leukemia cells have been used to generate leukemia-specific autologous cytolytic T cells.17
Chromatin modification by histone acetyltransferase (HAT) or histone deacetylase (HDAC) represents a fundamental mechanism of transcriptional regulation.18,19 It has been proposed that histone acetylation promotes transcription, probably by relaxing specific segments of DNA and facilitating the binding of transcription factor.20 HDAC inhibitors (HDACIs) are thought to inhibit the enzymatic activity of histone deacetylase and to enhance the transcriptional activity of several genes. Currently known HDACIs, including sodium butyrate (SB),21 trichostatin A (TSA),22,23 trapoxin A (TPX),24 FR901228 (FR),25 and MS-27-275,26 have been known to cause a variety of phenotypic changes, such as G1 and G2/M arrest of cell cycle, morphological reversion of transformed cells, differentiation,27,28 and cell growth inhibition.29-31 It has been shown that butyrate stimulates production of fetal hemoglobin, and some beneficial effects have been obtained in the treatment of thalassemia32 and sickle cell disease.33 In cancer therapy, clinical trails of phenylbutyrate, which is used for the treatment of several cancers, is now in phase I/II study and the drug may be a promising treatment option for acute promyelocytic leukemia (APL) that is refractory to all-trans-retinoic acid (ATRA) and arsenic trioxide.34 Furthermore, we have recently reported that a majority of AML cells other than APL cells could also be differentiated by combination treatment with HDACIs and other differentiation-inducible reagents such as ATRA and vitamin D3.28 Modulation of immune responses through acetylation and deacetylation is poorly understood. We hypothesized that acetylation and deacetylation are among the major mechanisms in the regulation of costimulatory/adhesion molecules.
In this study we examined the effects of HDACIs on the expression of these molecules in AML cell lines and freshly isolated clinical samples and showed immunomodulatory effects of HDACIs, which can be a new therapeutic tool for immunotherapy in leukemia.
Materials and methods
Cells and cell culture
We used 9 human leukemia cell lines including 7 myelomonocytic leukemia cell lines (HL60, U937, KG-1, Kasumi-1, NB4, NKM-1, and ML-1), a megakaryoblastic cell line (MEG-O1), and an erythroleukemia cell line (K562). All cell lines were cultured in Roswell Park Memorial Institute medium (RPMI 1640) containing 10% fetal calf serum (FCS) and 2 mmol/Ll-glutamine at the concentration of 5 × 105cells per mL. Clinical samples were obtained from 30 AML patients with informed consent. Diagnosis was established by cytological criteria based on French-American-British (FAB) classification, and cell surface markers were detected by flow cytometry. Karyotypes were defined according to criteria of the International System for Human Cytogenetic Nomenclature. Both freshly isolated and thawed samples were suspended in complete medium containing 20% FCS at a concentration of 5 × 105 cells per mL. We added 1.0 mmol/L SB to the cells 48 hours before assay.
HDACIs and other agents
Four HDACIs were used in the study: SB and TSA (both from Wako Pure Chemical Industries, Osaka, Japan), FR (gift from Fujisawa Pharmaceutical, Osaka, Japan), and TPX (gift from Shionogi Pharmaceutical, Osaka, Japan). The following were freshly prepared to various concentrations just before use: ATRA, cyclohexamide (CHX), and Actinomycin D (ActD) (all from Sigma Chemical, St Louis, MO) and interferon (IFN)-γ (gift from Shionogi).
Monoclonal antibodies and flow cytometric analysis
The following fluorescein isothiocyanate (FITC)-conjugated antihuman monoclonal antibodies (mAbs) were used: CD80 (Immunotech International, Marseilles, France), CD86 (PharMingen, San Diego, CA), HLA-DR (Nichirei, Tokyo, Japan), HLA-ABC (Immunotech), and CD54 (Dako Japan, Kyoto, Japan). The cells were treated with HDACIs 48 hours before flow cytometric assay. For combination treatment, IFN-γ and SB were added simultaneously 48 hours before assay. ATRA (10−7 mol/L) was added to the cells to induce differentiation in HL60, and the cells were incubated for 48 hours. Flow cytometric analysis was performed as described. Briefly, 1 × 105 cells were incubated with isotype-matched control antibody or anti-CD80, anti-CD86, anti-CD54, anti–HLA-DR, and anti–HLA-ABC antibodies at 4°C for 30 minutes. After washing twice with phosphate-buffered saline (PBS) supplemented with 2% FCS, the cells were subjected to flow cytometric analysis (FACSCalibur; Becton Dickinson, Mountain View, CA) with CELL Quest v3.1 software (Becton Dickinson).
Propidium iodide staining for viability
The cells were cultured in complete medium at 5 × 105 cells per mL in a 24-well plate. After treatment with HDACIs for 48 hours, the cells were collected and suspended in 0.5 mL PBS. We added propidium iodide (PI) (Sigma) at a final concentration of 1 μg/mL in PBS to each tube, and the mixture was incubated for 5 minutes at room temperature. Approximately 10 000 data events per sample were analyzed on FACSCalibur. Viable cells were defined with a forward scatter (FS)/PI gate.
Detection of histone hyperacetylation by HDACI treatment
Acid urea Triton X-100 (AUT) gel electrophoresis was performed as previously described.22 HL60 cells (2 × 106) were cultured for 6 hours with or without 1.0 mmol/L SB. Cellular histones were extracted and subjected to AUT gel electrophoresis. Histone hyperacetylation by HDACI treatment was detected in situ using antiacetylated histone H4 antibody (Upstate Biotechnology, Lake Placid, NY). HL60 cells were incubated for 3 hours with or without HDACIs and subjected to immunostaining. The antibody was used at 1:4000 dilution, and acetylated histones were detected by using labeling streptoavidine-biotin (LSAB) method.
Chromatin immunoprecipitation
HL60 cells were treated with 1.0 mmol/L SB for indicated periods. The cells (5 × 107) were fixed with formaldehyde (final concentration, 1%) at room temperature for 15 minutes. Soluble chromatin was made per manufacture's specifications (Upstate Biotechnology) with minor modification. Briefly, the cells were washed 2 times with cold PBS; suspended in 1.0 mL Pipes buffer with 5 mmol/L Pipes (pH 8.0), 85 mmol/L potassium chloride (KCl), and 0.5% NP40 containing protease inhibitors; and placed on ice for 10 minutes. After pelletizing the cells, they were resuspended in 250 μL sodium dodecyl sulfate (SDS) lysis buffer comprising 1% SDS, 10 mmol/L ethylenediamine tetraacetic acid (EDTA), and 50 mmol/L Tris-HCl (tris[hydroxymethyl] aminomethane [pH 8.1]); placed on ice for 10 minutes; and subjected to sonication of the lysate by using Bioruptor UCD-200TM (Cosmo Bio, Tokyo, Japan). After centrifugation, the supernatant fraction was diluted in 2.0 mL dilution buffer comprising 0.01% SDS, 1.1% Triton X-100, 1.2 mmol/L EDTA, 16.7 mmol/L Tris-HCl (pH 8.1), and 167 mmol/L sodium chloride (NaCl), and we used 20 μL diluted supernatant as input control. The chromatin solution was precleared by 80 μL Salmon Sperm DNA/Protein A Agarose Slurry (Upstate Biotechnology).
The supernatant fraction was divided into 2 equal parts of 950 μL each and subjected to immunoprecipitation with 5 μL antiacetylated histone H3 or H4 antibody (Upstate Biotechnology). The immunocomplex was recovered by adding 30 μL Salmon Sperm DNA/Protein A Agarose Slurry. The beads were washed 5 times per manufacturer's specifications (Upstate Biotechnology). We added 250 μL elution buffer with 1% SDS and 0.1 mmol/L NaHCO3, and the beads were rotated at room temperature for 30 minutes. Then 5 mol/L NaCl was added to reverse the formaldehyde cross-linking, and the pellets were resuspended and treated with proteinase K, extracted with phenol/chloroform, and precipitated with ethanol. The pellets were precipitated with 20 μg glycogen as carrier and resuspended in 50 μL of 10 mmol/L Tris-HCl and EDTA. We used 10 μL of 50 μL DNA solution for quantitative PCR. Quantitative PCR was performed using SYBR Green PCR Master Mix (Perkin Elmer). For making the relative standard curve, control DNA was prepared from HL60 cells, and 10-fold dilution value of the DNA was used for standard curve. Primers used were 5′-GCCCTCCTTGGTTATGCAGTC-3′ and 5′-CATGACGTTAAGATGAGCCTGAA-3′, which can amplify the 301–base pair (bp) length fragment in the CD86 promoter (−16 to −316).
The PCR mixture contained 200 nmol/L forward and reverse primers each, 25 μL SYBR Green PCR Master Mix, and 10 μL DNA solution. The total amount of reaction volume was 50 μL. Analysis was performed by the ABI-PRISM 7700 Sequence Detector System using Sequence Detector v1.6.3 software (both from Perkin Elmer). Using the same primer sets, conventional PCR was also performed with 10 μL DNA sample and 27 cycles. PCR products stained by ethidium bromide were visualized using a Gel Doc 1000 Video Gel Documentation System and quantitated by Molecular Analyst Software (Bio-Rad Laboratories, Hercules, CA).
Relative quantitation of messenger RNA using real-time PCR method
SB was added to each cell line to give a final concentration of 0.7 mmol/L in HL60 and 0.5 mmol/L in U937. CHX for protein inhibition experiments was applied 1 hour prior to SB treatment at a final concentration of 10 μg/mL. The cells were harvested at various times (0, 1, 3, 6, and 12 hours after SB treatment), and RNA was extracted from 107 cells using RNA STAT-60 (Tel-Test “B,” Friendswood, TX) according to the manufacturer's specifications. The following reverse transcription for complementary DNA (cDNA) synthesis reaction was performed by the standard methodology. Using Primer Express v1.0 software (Perkin Elmer), we designed PCR primers for the amplification of cDNA derived from the messenger RNA (mRNA) of CD86, ICAM-1, and β-actin. The primers used were as follows: CD86 forward and reverse primers 5′-GGGCCGCACAAGTTTTGA-3′ and 5′-GCCCTTGTCCTTGATCTGAA-3′, respectively; ICAM-1 forward and reverse primers 5′-TGCAGACAGTGACCATCTACAGC-3′ and 5′-TCTGAGACCTCTGGCTTCGTC-3′, respectively; β-actin forward and reverse primers 5′-ACAATGAGCTGCCTGTGGCT-3′ and 5′-ATCACGATGCCAGTGGTACG-3′, respectively. The TaqMan probe contains a reporter dye (FAM) at the 5′-end of the probe and a quencher dye (TAMRA) at the 3′-end of the probe. The following sequences were used as probe: CD86 probe: 5′-(FAM)-TCGGACAGTTGGACCCTGAGACTTCAC-(TAMRA)-3′; ICAM-1: 5′-(FAM)-TTCCGGCGCCCAACGTGATT-(TAMRA)-3′; and β-actin: 5′-(FAM)-CAGCCATGTACGTTGCTATCCAGGCTGT-(TAMRA)-3′. Real-time PCR analysis was performed by the ABI-PRISM 7700 Sequence Detector System according to the manufacturer's specifications. For relative quantitation of each mRNA, we used the separate tube method and calculated the relative quantity of each sample using relative standard curves. The normalized amount of target mRNA was calculated by dividing the amount of each target mRNA by the amount of β-actin corresponding to each sample.
mRNA synthesis inhibition
ActD was dissolved in ethanol at 1 mg/mL and used at a final concentration of 3.5 ng/μL. HL60 cells were incubated with 0.7 mmol/L SB for 3 hours to raise the basal level of CD86 mRNA. After washing twice with PBS, the cells were preincubated with ActD for 30 minutes. Aliquots of cells pretreated with ActD were further incubated for the indicated time in the presence or absence of 0.7 mmol/L SB. RNA was extracted and subjected to real-time PCR using the same set of primers and probe described above.
Nuclear run-on assay
HL60 cells (5 × 107) were incubated for 3 hours with or without 1.0 mmol/L SB. The cells were washed twice with PBS and further incubated with 5 mL lysis buffer, which was made of 10 mmol/L Tris-HCl (pH 7.4), 10 mmol/L NaCl, 3 mmol/L magnesium dichloride (MgCl2), and 0.5% NP40, for 3 minutes on ice. Similar conditions were used for 3 washing steps, and the final nuclear pellet was suspended in 100 μL nuclear freezing buffer with 50 mmol/L Tris-HCl (pH 8.3), 40% glycerol, 5 mmol/L MgCl2, and 0.1 mmol/L EDTA. The nuclei were then mixed with 30 μL run-on buffer comprising 25 mmol/L Tris-HCl (pH 8.0); 12.5 mmol/L MgCl2; 750 mmol/L KCl; 1.25 mmol/L each of adenosine, guanosine, and cytidine 5′-triphosphate (ATP, GTP, and CTP, respectively) (Roche Diagnostics, Mannheim, Germany); and 3.7 MBq α–32P-UTP (phosphorous 32–uridine 5′-triphosphate) (Amersham Pharmacia Biotech, Tokyo, Japan). After incubation at 30°C for 30 minutes, 15 μL deoxyribonuclease I (DNase I) was added, and the assay was incubated for another 15 minutes. Labeled transcripts were isolated using RNA STAT-60 (Tel-Test “B”) and purified according to the manufacturer's specifications. Full-length human CD86 DNA (gift from Dr Lewis Lanier) and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) DNA were applied to the membrane (Hybond-N, Amersham) using Dott Blotter DB-200 (Sanplatec, Osaka, Japan) and fixed to the membrane using ultraviolet (UV) cross-linker. An equal amount of radioactivity (approximately 3 × 106 counts per minute of labeled RNA) was used for the membrane hybridization assay. Data were normalized for the content of GAPDH in each sample using the NIH Image version 1.6 software (National Institutes of Health, Bethesda, MD).
Allogeneic mixed leukocyte reaction
To evaluate the immunomodulatory effects of SB against allogeneic leukocytes, HL60 and K562 cells treated with SB and peripheral blood mononuclear cells (PBMNCs) from healthy volunteers were prepared as the stimulator and responder, respectively. The cells were treated with 0.7 mmol/L SB for 48 hours, washed, and resuspended in RPMI 1640 supplemented with 10% human serum obtained from a volunteer donor. After irradiation with 75 Gy, 5 × 102to 1 × 105 stimulator cells were added to 1 × 105 PBMNCs in each well of 96-well flat-bottomed culture plates (Microtest 96, Becton Dickinson). Lymphocyte proliferation after 5 days of culture was measured with the addition of hydrogen 3 (3H)-thymidine (ICN Biomedicals, Costa Mesa, CA) (37kBq [1 μCi] per well) for 18 hours prior to harvesting and determining incorporated radioactivity.
Statistical methods
Differences in the expression of each molecule (CD80, CD86, HLA-DR, HLA-ABC, and ICAM-1) between SB- and medium-treated clinical AML samples were analyzed using the Wilcoxon rank sum test.
Results
SB enhances expression of costimulatory/adhesion molecules in AML cell lines in a cell-type–specific manner
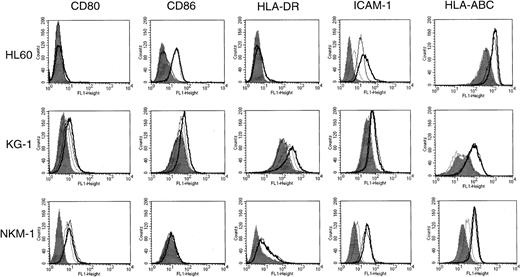
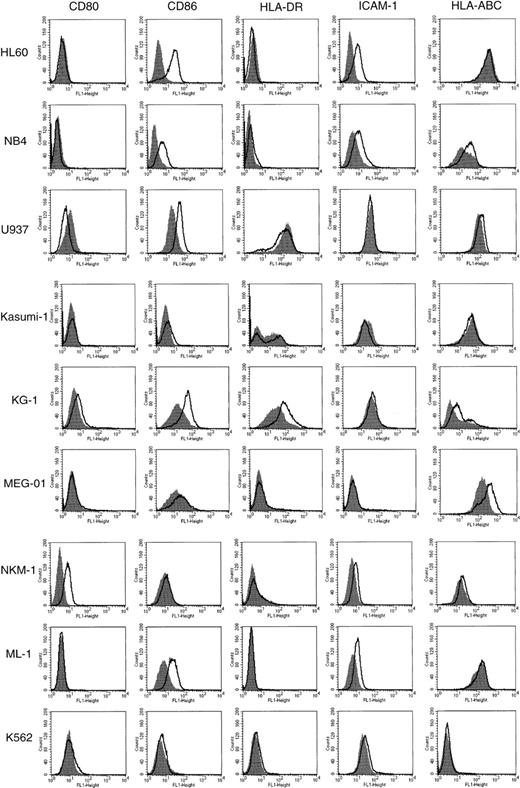
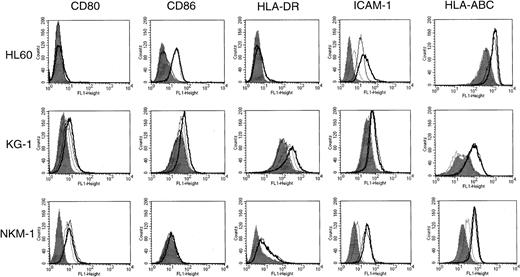
To determine the effect of HDACIs on the expression of CD80, CD86, HLA-DR, HLA-ABC, and ICAM-1 on the cell surface of AML, we tested 9 human AML cell lines for the action of SB by flow cytometric analysis (Figure 1). The cells were incubated for 48 hours in the presence or absence of SB at various concentrations. Cell viability was examined by both trypan blue dye exclusion and PI staining methods. The maximal concentration of SB for each cell line was decided by the fact that 70% of cells were kept viable during the analyses. The expression of CD80 was exclusively increased in NKM-1 cells and down-regulated in U937 cells by the addition of SB. In contrast, the expression of CD86 was up-regulated in 5 of 9 AML cell lines examined. CD86, but not CD80, was induced by SB in HL60, NB4, KG-1, ML-1, and U937 cells. In terms of ICAM-1, a significant increase in its expression was observed in HL60, NB4, and ML-1. The expression of HLA-DR, an MHC class II family member, was not increased by SB except in the case of KG-1 cells, which expressed HLA-DR at a high level before treatment. The expression of HLA-ABC, MHC class I antigen, was up-regulated in megakaryoblastic cell line MEG-O1. None of the molecules examined were affected in erythroleukemia cell line K562.
Expression of CD80, CD86, HLA-DR, ICAM-1, and HLA-ABC in AML cell lines incubated in the presence or absence of SB.
The 9 AML cell lines indicated were incubated with or without SB for 48 hours. HL60 cells were treated with 0.7 mmol/L SB, and all other cells were treated with 0.5 mmol/L SB. The cells were then stained with FITC-conjugated antibodies and analyzed by flow cytometry. Histograms represent the log of fluorescence (horizontal axis) versus the relative cell number (vertical axis). Closed histograms (in black) represent medium-treated cells. Open histograms represent SB-treated cells.
Expression of CD80, CD86, HLA-DR, ICAM-1, and HLA-ABC in AML cell lines incubated in the presence or absence of SB.
The 9 AML cell lines indicated were incubated with or without SB for 48 hours. HL60 cells were treated with 0.7 mmol/L SB, and all other cells were treated with 0.5 mmol/L SB. The cells were then stained with FITC-conjugated antibodies and analyzed by flow cytometry. Histograms represent the log of fluorescence (horizontal axis) versus the relative cell number (vertical axis). Closed histograms (in black) represent medium-treated cells. Open histograms represent SB-treated cells.
The potential of various HDACIs to induce expression of CD86
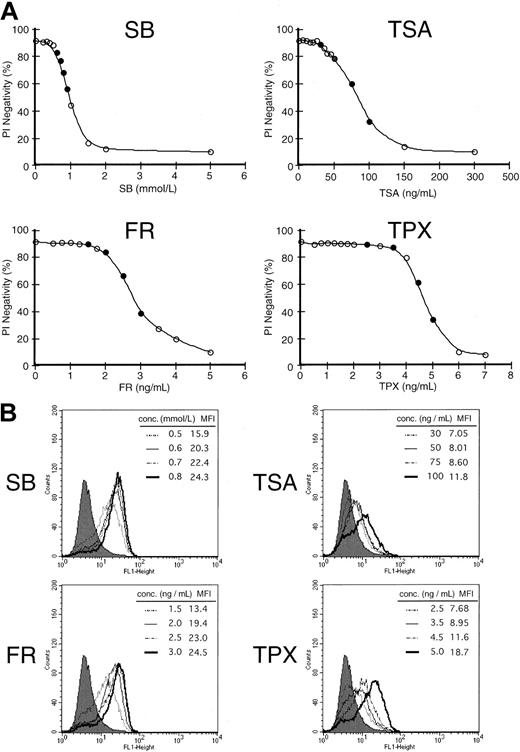
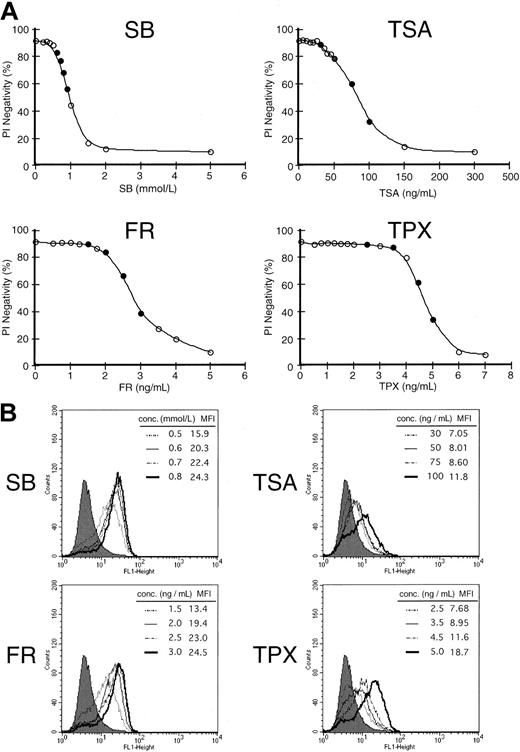
We then examined the difference in the ability to enhance the expression of CD86 among several HDACIs in HL60 cells. To define the ideal dose for the respective HDACI, various concentrations of each HDACI were added to the cells, and viability was examined by the PI exclusion method 48 hours after treatment (Figure2A). We thereafter used each agent at the concentrations indicated as a closed circle (●) to determine the expression of CD86. As shown in Figure 2B, the induction of CD86 increased in a dose-dependent manner for all 4 HDACIs examined. However, the ability to enhance the expression seemed to be different among HDACIs compared with their cytotoxic effects. The treatment with SB and FR seemed to enhance the expression more drastically than the expression induced by TSA and TPX. Namely, treatment with 0.5 mmol/L SB (the dose at which more than 80% of the cells were kept viable 48 hours after treatment) increased the mean fluorescence intensity (MFI) by 3-fold relative to the control level. However, 50 ng/mL TSA (the concentration at which equivalent viability was obtained compared to 0.5 mmol/L SB) could only exhibit a 1.4-fold increase of MFI. These results suggest that the potential to induce the expression of CD86 may vary among HDACIs.
Differential capacity to induce costimulatory molecules among 4 histone HDACIs.
(A) Cell viability curve of HL60 cells incubated by various concentrations of HDACIs for 48 hours. Cell viability was examined by PI exclusion method. Each agent was subsequently used at the concentrations indicated by a closed circle (●) for the induction of CD86. (B) Expression of CD86 was examined in the presence of each HDACI (SB, TSA, FR, and TPX) for 48 hours at the concentrations indicated. Closed histograms (in black) represent medium-treated cells (MFI = 5.5), whereas open histograms with solid or broken lines represent HDACI-treated cells at the various concentrations indicated in each box.
Differential capacity to induce costimulatory molecules among 4 histone HDACIs.
(A) Cell viability curve of HL60 cells incubated by various concentrations of HDACIs for 48 hours. Cell viability was examined by PI exclusion method. Each agent was subsequently used at the concentrations indicated by a closed circle (●) for the induction of CD86. (B) Expression of CD86 was examined in the presence of each HDACI (SB, TSA, FR, and TPX) for 48 hours at the concentrations indicated. Closed histograms (in black) represent medium-treated cells (MFI = 5.5), whereas open histograms with solid or broken lines represent HDACI-treated cells at the various concentrations indicated in each box.
Acetylation of histones was observed by HDACI treatment
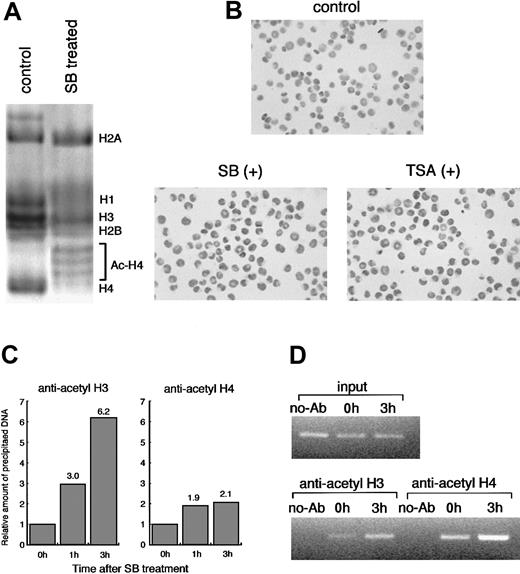
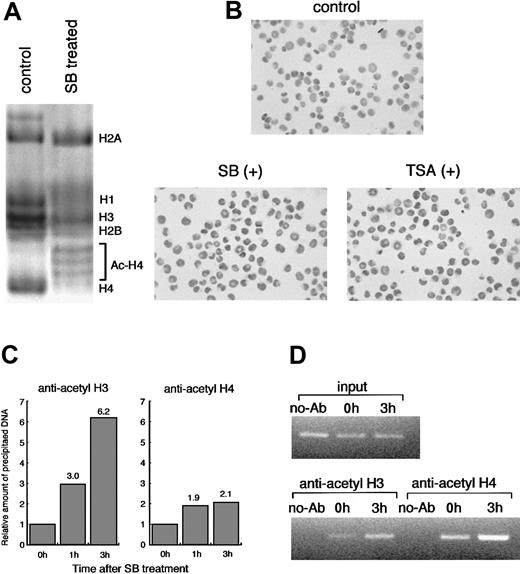
To confirm whether HDACI treatment leads to histone acetylation, we performed AUT gel electrophoresis assay and immunostaining in situ using antiacetylated histone H4 antibody. AUT gel electrophoresis allows separation of each cellular histone (H1, H2A, H2B, H3, and H4), and acetylated histones can be separated as slower migrating proteins than unacetylated ones. The highly acetylated histone H4 was exclusively detected by the SB treatment for 6 hours (Figure3A). We further analyzed histone acetylation by in situ immunostaining. The cells treated with TSA and SB were positively immunostained in situ by antiacetylated histone H4 antibody within 1 hour, and the number of positive cells increased gradually up to 3 hours after treatment (Figure 3B).
Histone acetylation by HDACIs in HL60 cells.
(A) HL60 cells were incubated with or without 1.0 mmol/L SB for 6 hours. Each histone fraction was extracted and analyzed on an AUT gel. The gel was stained with Coomassie blue. (B) Histone hyperacetylation by HDACIs was detected in situ using antiacetylated histone H4 antibody. HL60 cells were incubated for 3 hours with either 1.0 mmol/L SB or 100 ng/mL TSA and subjected to immunostaining. In comparison with the control, almost all the cells were stained positively after 3 hours of treatment with both SB and TSA. (C) Soluble chromatin preparations from cell cultures treated with or without SB were immunoprecipitated with the antibody against acetylated histone H3 or H4. Precipitated DNA was quantitated by real-time PCR using SYBR Green Master Mix. On the other hand, aliquots of chromatin solution were directly subjected to DNA extraction followed by real-time PCR, and these were used as input DNA. The ratio of the DNA amount at the indicated time relative to the corresponding input DNA amount was calculated. Finally, the relative amount of each precipitated DNA normalized by input DNA was shown. (D) The PCR products with 27 cycles of conventional PCR using the same primers and DNA template were visualized with ethidium bromide staining. No-Ab indicates that antibodies were not added for ChIP.
Histone acetylation by HDACIs in HL60 cells.
(A) HL60 cells were incubated with or without 1.0 mmol/L SB for 6 hours. Each histone fraction was extracted and analyzed on an AUT gel. The gel was stained with Coomassie blue. (B) Histone hyperacetylation by HDACIs was detected in situ using antiacetylated histone H4 antibody. HL60 cells were incubated for 3 hours with either 1.0 mmol/L SB or 100 ng/mL TSA and subjected to immunostaining. In comparison with the control, almost all the cells were stained positively after 3 hours of treatment with both SB and TSA. (C) Soluble chromatin preparations from cell cultures treated with or without SB were immunoprecipitated with the antibody against acetylated histone H3 or H4. Precipitated DNA was quantitated by real-time PCR using SYBR Green Master Mix. On the other hand, aliquots of chromatin solution were directly subjected to DNA extraction followed by real-time PCR, and these were used as input DNA. The ratio of the DNA amount at the indicated time relative to the corresponding input DNA amount was calculated. Finally, the relative amount of each precipitated DNA normalized by input DNA was shown. (D) The PCR products with 27 cycles of conventional PCR using the same primers and DNA template were visualized with ethidium bromide staining. No-Ab indicates that antibodies were not added for ChIP.
SB treatment causes hyperacetylation of histone H3 and H4 at CD86 promoter chromatin in vivo
To directly assess whether HDACI-induced CD86 expression correlates with changes in the acetylation of histones, we took advantage of chromatin immunoprecipitation (ChIP) assay.35 36 Antibodies specific to acetylated histone H3 (lysines 9 and 14) and H4 (lysines 5, 8, 12, and 16) were used to immunoprecipitate soluble chromatin from cells treated with or without SB. The resulting genomic DNA was then analyzed by quantitative PCR using primers spanning approximately a 300-bp fragment of theCD86 promoter gene. We used SYBR Green PCR Master Mix for quantitative PCR. ChIP analysis with antibodies to acetylated H3 and H4 revealed that SB treatment actually caused a remarkable increase in acetylation of histone H3 and H4 at CD86 promoter chromatin in vivo within 3 hours. The acetylation level of histone H3 and H4 increased by 6.2- and 2.1-fold, respectively, after 3 hours of treatment with SB (Figure 3C). Another 2 experiments independently performed showed almost the same results (data not shown). For visualization, semiquantitative PCR was also performed for 27 to 30 cycles using the same primer sets and template DNA. The amplified products with 27 cycles are shown in Figure 3D. Similar to the data from real-time PCR, the increased acetylation level of histone H3 and H4 on the CD86 promoter was observed.
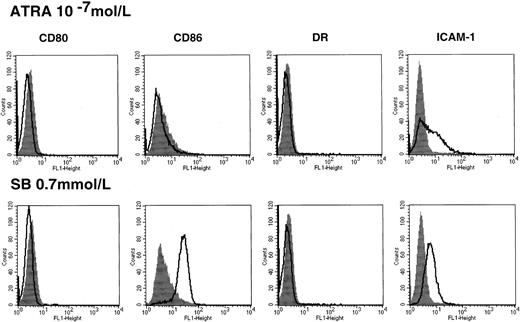
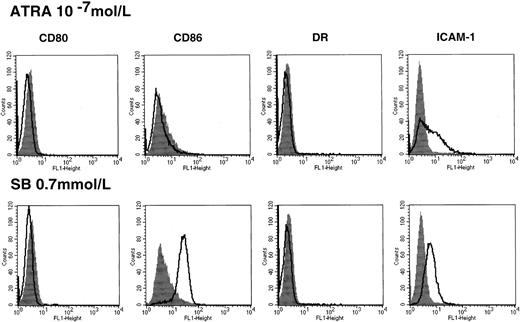
Up-regulation of costimulatory molecules by HDACIs is not correlated with ATRA-induced differentiation in HL60
In the case of HL60, HDACIs alone used in the present study increased the expression of myeloid-lineage cell surface markers, but they were not sufficient to induce terminal differentiation revealed by morphological change and functional markers such as nitroblue tetrazolium (NBT) reduction activity.28 In contrast, treatment with ATRA significantly promotes cell differentiation of HL60, as indicated by morphology and functional assay.37We thus examined the effect of ATRA on the induction of costimulatory/adhesion molecules. Treatment with ATRA alone for 48 hours could not enhance the CD86 expression (Figure4), and this was consistently observed during the treatment with ATRA for 5 days (data not shown). This suggests that up-regulation of CD86 is not associated with cell differentiation in the case of HL60.
Costimulatory/adhesion molecules in HL60 cells treated with ATRA.
Cells were incubated in the presence or absence of 10−7mol/L ATRA for 48 hours followed by flow cytometry (upper panel). The results of treatment with 0.7 mmol/L SB were shown for comparison (lower panel). Closed histograms (in black) represent medium-treated cells, whereas open histograms represent ATRA- or SB-treated cells.
Costimulatory/adhesion molecules in HL60 cells treated with ATRA.
Cells were incubated in the presence or absence of 10−7mol/L ATRA for 48 hours followed by flow cytometry (upper panel). The results of treatment with 0.7 mmol/L SB were shown for comparison (lower panel). Closed histograms (in black) represent medium-treated cells, whereas open histograms represent ATRA- or SB-treated cells.
The potential to modulate the expression of costimulatory molecules is different between SB and IFN-γ
IFN-γ has been revealed to up-regulate several costimulatory/adhesion molecules in monocytes and AML blasts.13 38 We used 6 AML cell lines (HL60, NKM-1, KG-1, Kasumi-1, U937, and ML-1) to compare the effects between SB and IFN-γ and to evaluate whether SB enhances IFN-γ–dependent action. Each chemical was added either independently or simultaneously, incubated for 48 hours, and then analyzed by FCM. The data from 3 representative cell lines (HL60, NKM-1, and KG-1) are shown in Figure5. In KG-1 cells, IFN-γ alone induced CD80 expression but not CD86, whereas SB up-regulated CD86 but not CD80. The minimal additive effect was observed by the combination treatment on the expression of both CD80 and CD86. In NKM-1 cells, single treatment with either IFN-γ or SB increased CD80 expression to a similar degree, but no additive effect was observed. By a single treatment with IFN-γ, CD80 expression was up-regulated in 3 of 6 cell lines (KG-1, NKM-1, and U937), whereas the induction of CD86 expression was observed in none of the 6 cell lines. HLA-DR expression was weakly up-regulated in Kasumi-1 and NKM-1, whereas the expression of HLA-ABC was highly up-regulated in all 6 cell lines. However, again there was no observed additive effect of combination treatment on the expression of HLA-DR and HLA-ABC in any of the 6 cell lines.
Comparison of the effect of SB with IFN-γ for the induction of costimulatory molecules.
The cells were treated with 500 IU/mL IFN-γ in the presence or absence of SB for 48 hours then analyzed by flow cytometry. Closed histograms (in black) represent medium-treated cells. Open histograms with the broken line and thin line represent SB-treated and IFN-γ–treated cells, respectively. Bold line histograms represent cells treated with SB and IFN-γ simultaneously 48 hours before the assay.
Comparison of the effect of SB with IFN-γ for the induction of costimulatory molecules.
The cells were treated with 500 IU/mL IFN-γ in the presence or absence of SB for 48 hours then analyzed by flow cytometry. Closed histograms (in black) represent medium-treated cells. Open histograms with the broken line and thin line represent SB-treated and IFN-γ–treated cells, respectively. Bold line histograms represent cells treated with SB and IFN-γ simultaneously 48 hours before the assay.
In terms of the expression of ICAM-1, a single treatment with IFN-γ up-regulated its expression in all 6 cell lines examined irrespective of the various patterns of induction by SB. In U937, KG-1, and NKM-1 cells, IFN-γ alone, but not SB alone, could enhance the expression of ICAM-1 drastically. However, only a small additive effect by dual treatment was observed in HL60 cells, and no additive or synergistic effect was obtained in other cells. These results suggest that the mechanisms by which costimulatory/adhesion molecules are induced are somewhat different between HDACIs and IFN-γ.
Up-regulation of CD86 and ICAM-1 by SB is observed at the mRNA level and does not require new protein synthesis
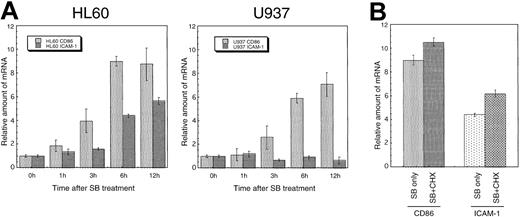
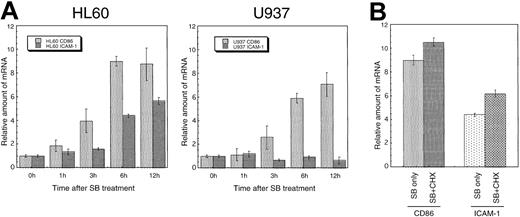
To confirm whether the induced expression of costimulatory/adhesion molecules by SB was regulated transcriptionally or posttranscriptionally, transcripts of these genes were assayed quantitatively by a real-time PCR method. Total RNA was prepared at various times (0-12 hours) after treatment. The cDNA synthesis reaction was performed using an equal amount (1 μg) of RNA. Analyses of real-time PCR and subsequent calculations were performed by the ABI-PRISM 7700 with Sequence Detector v1.6.3 software. The CD86 mRNA increased up to 7- to 10-fold at 12 hours after treatment in a time-dependent manner in both HL60 and U937. Similarly, the ICAM-1 mRNA increased approximately 6-fold relative to the control in HL60 cells. However, transcripts of ICAM-1 remained at the basal level in U937 cells. The alteration of mRNA in both CD86 and ICAM-1 is well correlated with the protein expression on the cell surface detected by flow cytometry (Figure6A).
Induced expression of CD86 and ICAM-1 mRNA in a cell-type–specific and time-dependent manner.
(A) HL60 and U937 cells were incubated with SB for the indicated time, and transcripts of CD86 and ICAM-1 were quantitated by real-time PCR method. Each real-time PCR was performed in quadruplicate, and the average fold induction relative to time 0 was shown with SD. (B) De novo protein synthesis is not required for the SB-induced expression of CD86 and ICAM-1 mRNA. HL60 cells were incubated for 6 hours with 0.7 mmol/L SB in the presence or absence of 10 μg/mL CHX.
Induced expression of CD86 and ICAM-1 mRNA in a cell-type–specific and time-dependent manner.
(A) HL60 and U937 cells were incubated with SB for the indicated time, and transcripts of CD86 and ICAM-1 were quantitated by real-time PCR method. Each real-time PCR was performed in quadruplicate, and the average fold induction relative to time 0 was shown with SD. (B) De novo protein synthesis is not required for the SB-induced expression of CD86 and ICAM-1 mRNA. HL60 cells were incubated for 6 hours with 0.7 mmol/L SB in the presence or absence of 10 μg/mL CHX.
To further elucidate the mechanism of the up-regulation of transcripts, we examined whether active protein synthesis is necessary for the SB-induced expression of CD86 and ICAM-1. HL60 cells were preincubated in the presence of the protein-synthesis inhibitor CHX followed by further incubation for the indicated time in the presence or absence of 0.7 mmol/L SB. The representative data obtained 6 hours after SB treatment are shown in Figure 6B. The addition of CHX to SB-treated HL60 cells did not decrease the enhanced CD86 or ICAM-1 mRNA expression. In addition, the administration of CHX to untreated HL60 cells caused a superinduction of the basal CD86 and ICAM-1 mRNA, which is consistent with the previous report.39 This suggests that protein synthesis is not required for the SB-induced up-regulation of CD86 and ICAM-1 mRNA.
The enhanced expression of CD86 by SB is regulated at the transcriptional level
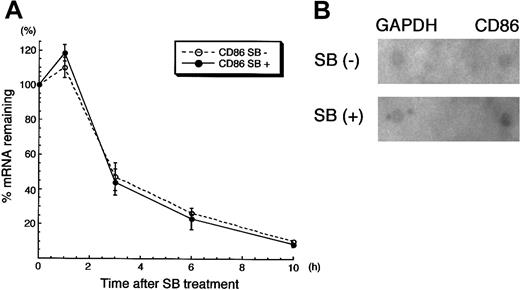
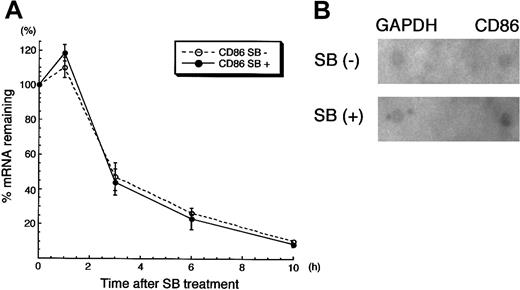
We next sought to unveil whether the up-regulation of CD86 mRNA by SB treatment was induced transcriptionally or via posttranscriptional modification. HL60 cells were pretreated with SB for 3 hours, subsequently incubated with ActD for 30 minutes, and then treated again with SB for the indicated time. Quantitative reverse transcriptase (RT)-PCR was performed to see how CD86 mRNA was down-regulated. As shown in Figure 7A, SB did not affect the disappearance of CD86 mRNA in cells treated with ActD. This suggests that the increase in expression of CD86 mRNA is not due to the altered stability of the RNA. We further performed a nuclear run-on assay to see whether de novo synthesis of CD86 mRNA is modified by SB. Nuclei from HL60 cells treated with or without SB for 3 hours were used for the assay of in vitro synthesis of mRNA. The production of CD86 mRNA was preferentially increased in nuclei from SB-treated cells compared with the untreated cells (1.68-fold increase), whereas no alteration of newly synthesized GAPDH mRNA was observed (1.07-fold) (Figure 7B). Namely, the newly synthesized CD86 mRNA normalized with GAPDH was increased by 1.5-fold by SB treatment. Overall, we conclude that SB-treated up-regulation of CD86 expression is regulated transcriptionally.
CD86 mRNA was up-regulated transcriptionally by SB treatment.
(A) RNA synthesis inhibition assay. The detailed condition of pretreatment with SB and ActD was described in “Materials and methods.” After the addition of ActD for 30 minutes, RNA was extracted from cells after additional incubation in the presence or absence of SB for 1, 3, 6, and 10 hours. The amount of CD86 mRNA was normalized by the amount of GAPDH mRNA; both were obtained from real-time PCR. The relative amounts of normalized CD86 mRNA are presented. (B) Nuclear run-on assay. HL60 cells treated in the presence or absence of 1 mmol/L SB for 3 hours were subjected to the nuclear run-on assay. The membranes, on which full-length cDNAs of CD86 and GAPDH were dot-blotted, were hybridized with in vitro synthesized RNA. The intensity of the CD86 dot-hybridized with SB-treated, radio-labeled de novo RNA was 1.68-fold higher than that of the CD86 dot-hybridized with untreated, radio-labeled de novo RNA. The intensity of GAPDH dots was not altered by SB treatment (1.07-fold).
CD86 mRNA was up-regulated transcriptionally by SB treatment.
(A) RNA synthesis inhibition assay. The detailed condition of pretreatment with SB and ActD was described in “Materials and methods.” After the addition of ActD for 30 minutes, RNA was extracted from cells after additional incubation in the presence or absence of SB for 1, 3, 6, and 10 hours. The amount of CD86 mRNA was normalized by the amount of GAPDH mRNA; both were obtained from real-time PCR. The relative amounts of normalized CD86 mRNA are presented. (B) Nuclear run-on assay. HL60 cells treated in the presence or absence of 1 mmol/L SB for 3 hours were subjected to the nuclear run-on assay. The membranes, on which full-length cDNAs of CD86 and GAPDH were dot-blotted, were hybridized with in vitro synthesized RNA. The intensity of the CD86 dot-hybridized with SB-treated, radio-labeled de novo RNA was 1.68-fold higher than that of the CD86 dot-hybridized with untreated, radio-labeled de novo RNA. The intensity of GAPDH dots was not altered by SB treatment (1.07-fold).
Induction of costimulatory/adhesion molecules by SB in AML samples
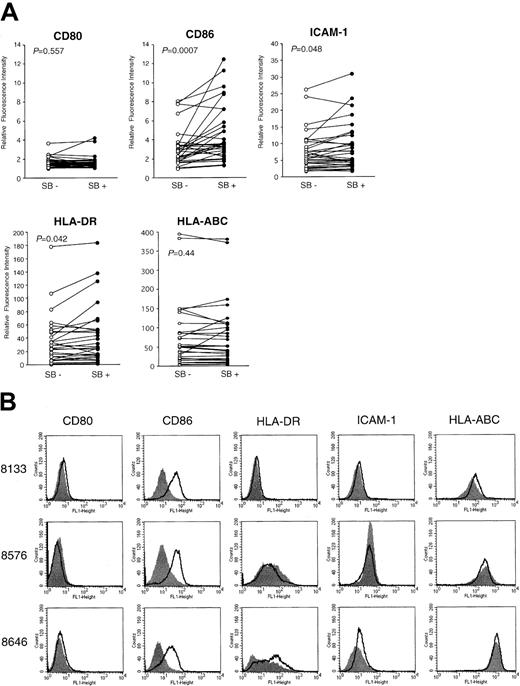
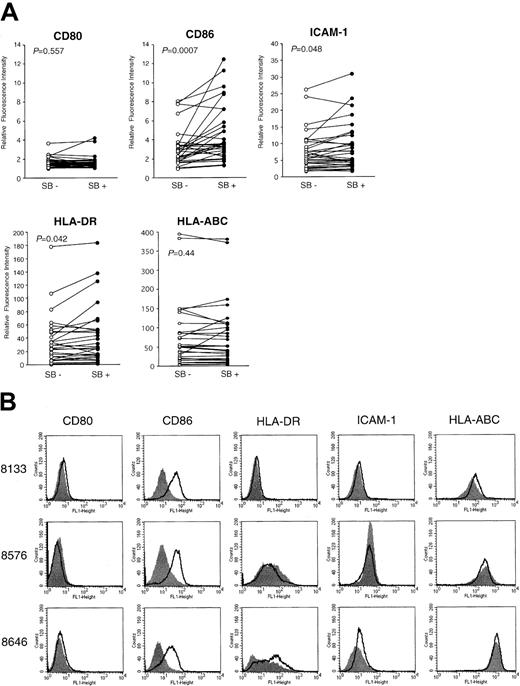
To see how efficiently SB induces the costimulatory/adhesion molecules in clinical samples, we examined the effect of SB on 30 freshly isolated leukemic blasts. Viability was assessed by the trypan blue dye exclusion test. All samples contained more than 80% blast cells with viability greater than 80%. The fold induction of the intensity of expression derived from SB-treated AML cells relative to that from untreated cells is summarized in Table1. The responsiveness of CD86 to SB treatment was not significantly different among FAB subtypes. When 30 samples were classified into 3 groups according to the karyotypes (normal karyotypes, karyotypes including t(8;21), and other abnormalities), only the marginal significance of the increase in CD86 expression was observed between normal karyotypes and t(8;21) (P = .04), as revealed by the Student t test. We further show the relative fluorescence intensity (RFI) of SB-pretreated and medium-treated cells according to the markers (Figure8A). The expression of CD86 was significantly increased (P = .0007), as revealed by the Wilcoxon rank sum test. The alteration in the expression of HLA-DR and ICAM-1 showed borderline significance (P = .042 andP = .048, respectively). In contrast, the expression of CD80 and HLA-ABC was not significantly changed (P = .58 and P = .44, respectively). Four of 30 samples exhibited more than a 3-fold increase in expression of CD86, and the data from 3 of these 4 samples (UPN 8133, 8576, and 8646) are shown as the highest responders (Figure 8B). The fact that a significant increase in CD86 and a moderate increase in HLA-DR and ICAM-1 were observed in clinical AML samples by SB treatment is highly consistent with the results from AML cell lines.
Up-regulation of costimulatory molecules in clinical AML samples.
(A) Thirty clinically isolated AML cells were incubated with SB for 48 hours to detect the altered expression of CD80, CD86, HLA-DR, HLA-ABC, and ICAM-1 by flow cytometry. Relative fluorescence intensity of medium-treated (SB−) and SB-treated (SB+) cells are shown as open and closed circles, respectively. Statistical significance of each expression between medium-treated and SB-treated samples was analyzed by the Wilcoxon rank sum test, and P is shown. (B) Four of 30 samples exhibited more than a 3-fold increase in the expression (MFI) of CD86. The data of 3 of these 4 samples (UPN 8133, 8576, and 8646) are shown as the highest responders.
Up-regulation of costimulatory molecules in clinical AML samples.
(A) Thirty clinically isolated AML cells were incubated with SB for 48 hours to detect the altered expression of CD80, CD86, HLA-DR, HLA-ABC, and ICAM-1 by flow cytometry. Relative fluorescence intensity of medium-treated (SB−) and SB-treated (SB+) cells are shown as open and closed circles, respectively. Statistical significance of each expression between medium-treated and SB-treated samples was analyzed by the Wilcoxon rank sum test, and P is shown. (B) Four of 30 samples exhibited more than a 3-fold increase in the expression (MFI) of CD86. The data of 3 of these 4 samples (UPN 8133, 8576, and 8646) are shown as the highest responders.
SB induces allogeneic MLR in myelomonocytic leukemia cell line HL-60
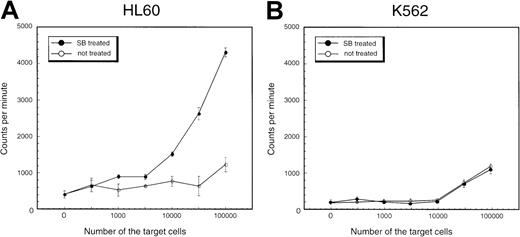
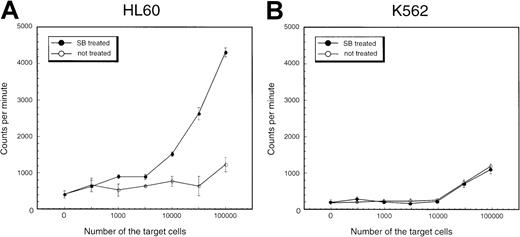
To investigate the functional properties of SB-treated AML cells, the ability to stimulate an allo-MLR of HL60 cells treated with SB was compared with that of untreated cells. HL60 cells cultured with or without 0.5 mmol/L SB were used as the stimulator in allogeneic one-way MLR, with PBMNCs from healthy donors as responders. Proliferation was assessed by the uptake of 3H-thymidine during the last 18 hours. Results of a representative experiment are shown in Figure9A. It can be seen that HL60 cells treated with SB had an enhanced capacity to stimulate lymphocyte proliferation compared with untreated HL60 cells. Essentially the same results were obtained in NB4 cells and KG-1 cells: SB-treated cells indicated higher potential to stimulate allo-MLR than medium-treated cells (data not shown). In contrast, SB treatment did not affect the allo-MLR in K562 cells, in which neither CD86 nor ICAM-1 was induced by SB (Figure 9B). These results support our notion that SB treatment stimulates allo-MLR partially through the up-regulation of CD86 and ICAM-1.
Stimulation of proliferation of normal allogeneic leukocytes by HL60 and K562 cells treated with SB.
PBMNCs of healthy donors were incubated for 5 days with (A) HL60 cells and (B) K562 cells treated or untreated with 0.7 mmol/L SB. Proliferation was measured by incorporation of 3H-thymidine during the last 18 hours of culture. The values are expressed as mean ± SD derived from quadruplicate cultures.
Stimulation of proliferation of normal allogeneic leukocytes by HL60 and K562 cells treated with SB.
PBMNCs of healthy donors were incubated for 5 days with (A) HL60 cells and (B) K562 cells treated or untreated with 0.7 mmol/L SB. Proliferation was measured by incorporation of 3H-thymidine during the last 18 hours of culture. The values are expressed as mean ± SD derived from quadruplicate cultures.
Discussion
One of the novel therapeutic approaches currently being explored against hematologic malignancies is the manipulation of costimulatory molecules expressed on APCs or on tumor cells engineered to act as APCs. HDACIs are currently considered to be promising anticancer30,40 and differentiation-inducible agents in experimental models.28,41 Moreover, promising results are being found from clinical trials investigating the therapeutic efficacy of sodium phenylbutyrate in APL.34 However, the immunomodulatory effects of HDACIs are poorly understood.42 43 To our knowledge this is the first report that shows the possibility that HDACIs can be applied as immunomodulatory therapeutic modalities. In the present study we show that HDACIs up-regulate the expression of costimulatory/adhesion molecules on the AML cell lines and freshly isolated AML clinical samples.
It is of interest that CD86 expression was up-regulated in 5 of 9 cell lines examined, and this enhancement was also seen in clinical samples with statistical significance. Additionally, ICAM-1 expression was also up-regulated in some cell lines and clinical samples. The role of CD86 expression in tumor immunity is not clear, but several tumor model systems do derive great benefit from CD86 expression.44-48Knockout mice of CD80 display normal immune responses, whereas CD86 knockout mice are severely immunocompromised.49 More recently, it was reported that ICAM-1/LFA-1 T-cell costimulatory pathways are independent of CD86/CD28 pathways and that they may synergistically expand T-cell responses in vivo.11 Taken together with our present finding that allo-MLR is actually enhanced by HDACIs, it is possible that HDACIs can enhance the immunogeneity of leukemic cells through up-regulation of costimulatory/adhesion molecules.
According to up-regulation of CD86 and ICAM-1 expression on the cell surface, the enhancement of transcripts correlated well with that of protein expression revealed by FCM. Our data indicate that CD86 mRNA was up-regulated by HDACIs through transcriptional level, as evidenced by mRNA synthesis inhibition, protein synthesis inhibition assays, and nuclear run-on assay. In addition, we have shown that the CD86 promoter was really targeted by HDACIs in vivo, as revealed by ChIP assay. Only a few papers have reported on the regulation of CD80 and CD86 promoters. Recently characterization of CD86 promoter has been reported.50 They show that CD86 transcription induced by CD40 ligation in APC depends on the nuclear factor (NF)-κB consensus site within the CD86 promoter. Similarly, NF-κB activation previously has been shown to drive the transcription of CD80.51Recently CBP/p300 acetyltransferases have been shown to associate with NF-κB to transactivate gene expression.52 53 The detailed mechanism of how HDACIs up-regulate CD86 expression is currently unknown. It is possible that HDACIs not only acetylate histones on the CD86 promoter through the suppression of HDAC activity, but they also support the NF-κB–mediated transcription, possibly through the association with CBP/p300. In the present study HDACIs preferentially up-regulate CD86 expression rather than CD80 expression in a majority of AML cells. It remains to be elucidated whether HDACIs differentially regulate the transcription of these B7 molecules in an NF-κB–dependent or NF-κB–independent manner.
It is interesting to find that the ability of TSA to enhance the CD86 expression was weaker than that of the other 3 HDACIs. To date, 8 isotypes of human HDAC genes have been identified.54-59 The selectivity and/or redundancy function of each HDAC, however, is not clear. Nonetheless, the selectivity of various HDACIs to release transcriptional repression caused by these different HDAC genes remains unveiled. Interestingly, the intensity to inhibit the enzymatic activity ofHDACs by various HDACIs, including these 4 compounds used in the present study, varies widely (Dr M. Yoshida, Tokyo University, personal communication). The differences of the potential to induce CD86 gene expression may be a reflection of these differences. Future study to identify the expression profiles, such as using the DNA microarray technology, may clarify target genes of each HDACI including these costimulatory/adhesion molecules.
The potential therapeutic implications of this work are substantial. HDACIs have been considered cell growth suppressors, apoptosis inducers, and differentiation inducers. We here offer another modality of HDACIs as antitumor drugs for use in immunotherapy. Ex vivo treatment of AML cells with HDACIs may generate functional APCs derived from AML cells and may be used in adoptive immunotherapies. Furthermore, the use of HDACIs in vivo may lead to the effective induction of antitumor CTL by the enhancement of costimulatory/adhesion molecules on AML blasts, which may eradicate leukemia blasts in association with the proapoptotic effect of HDACIs. In applying these HDACIs for clinical use, it may be necessary to recognize the heterogeneity of AML. Actually, the pattern of increase in the expression of costimulatory/adhesion molecules varies from patient to patient. Clarification of the mechanisms of the up-regulation may also explain the heterogeneity.
In conclusion we have demonstrated that a single pharmacological agent interfering with the deacetylation of histone and nonhistone proteins establishes the induced expression of costimulatory/adhesion molecules in AML cells, which can then act as accessory molecules that are important for successful antigen presentation and recognition, T-cell activation, and leukemia-restricted cytotoxicity. The immunomodulatory effects of HDACIs on tumor cells in vivo, however, have not yet been reported. We are now starting to investigate whether HDACIs effectively stimulate the expression of these costimulatory/adhesion molecules in a mouse model. The functional consequences of HDACI treatment remain to be further elucidated.
Acknowledgments
The authors are grateful to Drs N. Emi, T. Naoe, and M. Yoshida for helpful suggestions. We thank M. Isomura, Y. Nakamura, C. Wakamatsu, and S. Suzuki for technical assistance. We also thank Drs Y. Morishita, Y. Kodera, I. Sugiura, T. Takeo, T. Kataoka, and K. Kitamura for the collaboration.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Masayuki Towatari, First Department of Internal Medicine, Nagoya University School of Medicine, Nagoya 466-8550, Japan; e-mail: towatari@med.nagoya-u.ac.jp.