Abstract
Glucocorticoids are able to release Epstein-Barr virus–immortalized (EBV-immortalized) lymphoblastoid B cell lines (LCLs) from the persistent growth arrest induced in these cells by retinoic acid (RA). Moreover, physiologic concentrations of glucocorticoids efficiently antagonized LCL growth inhibition induced by 13-cis-RA; 9-cis-RA; all-trans-RA; and Ro 40-6055, an RA receptor (RAR) selective agonist. RAR expression levels, however, were not affected by glucocorticoids. Glucocorticoids, but not other steroid hormones, directly promote LCL proliferation, a phenomenon that was mainly mediated by down-regulation of the cyclin-dependent kinase (CDK) inhibitor p27Kip-1. Moreover, glucocorticoids contrasted the up-regulation of p27Kip-1, which was underlying the RA-induced LCL growth arrest, thereby indicating that glucocorticoids and RA signalings probably converge on p27Kip-1. Both antagonism of RA-mediated growth inhibition and promotion of LCL proliferation were efficiently reversed by the glucocorticoid receptor (GR) antagonist RU486, indicating that all of these effects were mediated by GR. Of note, RU486 also proved to be effective in vivo and, in mice, was able to significantly inhibit the growth of untreated LCLs as well as LCLs growth-arrested by RA in vitro. These findings provide a rational background to further evaluate the possible role of glucocorticoids in the pathogenesis of EBV-related lymphoproliferations of immunosuppressed patients. Moreover, GR antagonists deserve further consideration for their possible efficacy in the management of these disorders, and the use of schedules, including both RA and a GR antagonist, may allow a more thorough evaluation of the therapeutic potential of RA in this setting.
Retinoids, including retinol (vitamin A) and its natural and synthetic derivatives, are a class of compounds of crucial importance in the regulation of numerous physiologic processes such as embryonal morphogenesis, visual response, reproduction, growth, cell differentiation, and immune function. The pleiotropic effects induced by retinoids are mediated by the binding to and activation of 2 different families of nuclear receptors, the retinoic acid (RA) receptors (RARs) and the retinoid X receptors (RXRs), which belong to the steroid–thyroid hormone receptor superfamily.1,2 Extensive data have provided evidence of a retinoid role in the prevention or reversal of premalignant lesions of the upper aerodigestive tract, skin, and cervix.3Retinoids are also effective in inhibiting the proliferation of neoplastic cell lines of various origins in vitro3,4 and, in clinical settings, all-Trans RA (ATRA) was shown to induce complete remission in most patients with acute promyelocytic leukemia (APL).5 Moreover, retinoids, alone or in combination with other drugs, have shown some activity in other hematologic malignancies including juvenile chronic myeloid leukemia, myelodysplastic syndrome, and cutaneous T-cell lymphoma.4 6
Despite these promising findings, however, the clinical usefulness of retinoids is limited. This is mainly due to the wide heterogeneity of cellular responses to these drugs. In fact, either among different types of cancers or within a single tumor histotype, not all transformed cells are sensitive to the antiproliferative effects of these compounds, and the growth of some malignancies may even be enhanced by retinoid treatment.4 6 The mechanisms underlying these phenomena are still unclear. In particular, it is presently unknown whether these contrasting effects are, at least in part, due to host factors that are able to modulate and/or interfere with retinoid-mediated signaling. Elucidation of this issue is, however, of relevance not only to gain insight into the physiopathology of retinoids but also to improve the efficacy of these drugs in the clinical setting.
Epstein-Barr virus–immortalized (EBV-immortalized) lymphoblastoid B cell lines (LCLs) are a suitable in vitro model for the study of EBV-related lymphoproliferative disorders of immunosuppressed patients. We have previously shown that 9-cis-RA, 13-cis-RA, and ATRA powerfully inhibit LCL proliferation at concentrations corresponding to therapeutically achievable plasma levels (10−6 mol/L).7 The antiproliferative effects of RA were not dependent on the induction of a terminal differentiation, and they were not mediated by a direct modulation of EBV-encoded latent antigen expression.7 We have also shown that RA treatment of EBV-immortalized B lymphocytes is associated with multiple effects on the G1 regulatory proteins including p27Kip-1 up-regulation; decreased levels of cyclins D2, D3, and A; and inhibition of CDK2, CDK4, and CDK6 activity, which ultimately results in reduced pRb phosphorylation and G0/G1 protein growth arrest.8
Interestingly, the strong growth inhibitory effect exerted by 13-cis-RA, 9-cis-RA, and ATRA on LCLs persisted in vitro for more than 10 days following drug withdrawal.7However, LCLs persistently growth-arrested by RA treatment in vitro were able to recover their proliferative activity following inoculation into severe combined immunodeficiency (SCID) mice. On these grounds, our experimental model appears particularly useful to identify factors able to antagonize RA-mediated antiproliferative effects. In this study, we show that glucocorticoids are able to release LCLs from the RA-mediated proliferative block both in vitro and in vivo and to efficiently antagonize RA-induced growth inhibition. These effects were also clearly evident at physiologic concentrations of glucocorticoids and were inhibited by the glucocorticoid receptor (GR) antagonist RU486. Moreover, we also demonstrated that glucocorticoids directly convey growth-promoting signals to LCLs that may contribute to sustain the proliferation of these cells both in vitro and in vivo. These findings provide a rational background for the design of new strategies that are potentially useful to improve the management of EBV-related lymphoproliferations of immunosuppressed patients.
Materials and methods
Reagents
Reagents used in the study included ATRA and 9-cis-RA (Roche, Basel, Switzerland); 13-cis-RA, mifepristone (RU486), dexamethasone (Dex), hydrocortisone (HC), estradiol, and testosterone (Sigma Chemical Co, Milan, Italy); and the RARα agonist Ro 40-6055 (also known as Am580)9 (gift from Dr W. Bollag, Hoffman-LaRoche, Basel, Switzerland). Retinoids and RU486 were dissolved in dimethylsulfoxide (DMSO) at 10−1 mol/L and diluted in culture medium to a final concentration of less than 0.01% (vol/vol). RA was handled under subdued light, and the stock solutions were stored at −20°C and protected from light and oxygen. The following human recombinant cytokines were used: interleukin-1α (IL-1α), specific activity 107 U/mg, and IL-6, specific activity 2 × 108 U/mg (Boehringer Mannheim GmbH, Mannheim, Germany), and IL-4, specific activity 107 U/mg (Genzyme, Cambridge, MA).
Cell lines and culture conditions
Establishment and characterization of DAA-3 and HDE-14 LCLs have been described elsewhere.7 The cell lines were cultured in Roswell Park Memorial Institute medium (RPMI 1640) supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 20 mmol/L L-glutamine. They were maintained in a humidified 5% carbon dioxide (CO2) incubator at 37°C. All experiments with steroid hormones were performed with cells cultured in a medium containing 15% charcoal-stripped FCS (HyClone, Logan, UT).
Cell surface immunofluorescence analysis
Cell surface immunofluorescence was performed as previously described.7 Briefly, after preincubation at 4°C for 30 minutes in binding buffer (10% rabbit serum in phosphate-buffered saline [PBS]), 5 × 105 cells were incubated with saturating concentrations of the primary monoclonal antibody (mAb) at 4°C for 30 minutes. After 3 washes in PBS, the samples were incubated at 4°C for an additional 30 minutes, with optimal dilutions of fluorescence isothiocyanate–conjugated (FITC-conjugated) second-step antibody. The samples were then washed 3 times with PBS and fixed in 1% buffered paraformaldehyde. Isotype-matched controls were used to determine nonspecific binding. All flow cytometric analyses were performed on a fluorescence activated cell sorter (FACS) (FACScan using Lysis II software; Becton Dickinson, Milan, Italy). The expression of the EBV-encoded latent membrane protein-1 (LMP-1) and EBV nuclear antigen-2 (EBNA-2) antigens was investigated by immunofluorescence on cells permeabilized and fixed using ORTHO PermeaFix (Ortho Biotech, Milan, Italy). Briefly, 5 × 105 cells were incubated with 2 mL ORTHO PermeaFix (1:2 dilution) at room temperature for 40 minutes.
After centrifugation at 400g for 10 minutes, the supernate was aspirated, and the pellets were resuspended and kept at room temperature for 10 minutes in 2 mL 10% PBS/BSA. Cells were then centrifuged, the supernate was discarded, and staining was performed as described above. Optimal dilutions of anti–LMP-1 and anti–EBNA-2 antibodies were determined by using the EBV−Burkitt's lymphoma-derived cell line DG75 as a negative control. We used the following mAbs for immunophenotypic studies: CD21, CD23, and CD71 (Becton Dickinson); phycoerythrin-conjugated (PE-conjugated) CD19 (Biosource, Camarillo, CA); PE-conjugated CD38 (PharMingen, San Diego, CA); CD39 (Serotec, Oxford, England); anti–surface immunoglobulin (sIg) (Ortho Biotech); and FITC-conjugated CD30, anti–LMP-1 (CS1.4), and anti–EBNA-2 (PE2) (brand names in parentheses; Dako, Milan, Italy). We also used isotypic controls (mouse IgG1, IgG1-PE, and IgG2a) and FITC-conjugated goat antimouse Ig (Becton Dickinson).
Cell proliferation assay
Proliferation assays were performed in 96-well plates in quadruplicate cultures. Cells were seeded at an initial density of 104 cells per well in 200 μL of medium. Appropriate dimethyl sulfoxide (DMSO) dilutions were used as controls. DMSO did not affect proliferation of any cell line. Proliferative responses to B-cell growth-promoting cytokines were evaluated in serum-free medium. At the time-points indicated, cultures were pulsed with 0.037 MBq (1 μCi) 3H-methyl thymidine (specific activity, 92.5 × 1010 Bq/mmol/L [25 Ci/mmol/L]) (Amersham International, Bucks, England) for 6 hours and subsequently harvested (Unifilter-96, GF/C filter plates; Packard, Meriden, CT). Radioactivity was measured in a liquid scintillation counter (Top Count NXT, Packard), and the results were expressed as mean counts per minute (cpm) plus or minus SD of quadruplicate wells. In some experiments, proliferation was also evaluated by counting the number of viable cells (9 aliquots per time-point) in a Bürker chamber in the presence of trypan blue dye exclusion.
Western blot analysis
Whole cell extracts were prepared by lysing 107 cells in a buffer containing 50 mmol/L Tris-HCl (tris[hydroxymethyl] aminomethane hydrogen chloride) (pH 7.5), 150 mmol/L sodium chloride (NaCl), 2 mmol/L ethylenediamine tetraacetic acid (EDTA), 2 mmol/L ethyleneglycotetraacetic acid (EGTA), 25 mmol/L sodium fluorine (NaF), 25 mmol/L β-glycerolphosphate, 0.1 mmol/L sodium orthovanadate, 0.1 mmol/L phenylmethylsulfonyl fluoride, 5 μg/mL leupeptin, 1 μg/mL aprotinin, 0.2% Tryton-X-100, and 0.3% Nonidet P-40 (lysis buffer). After 20 minutes of incubation at 0°C, the extracts were centrifuged at 12 000 rpm for 30 minutes at 4°C. The protein concentration in the lysate was determined by the Bio-Rad protein assay kit (Bio-Rad Laboratories, Richmond, CA). Aliquots of the supernatant were mixed with 2 times sodium dodecyl sulfate (SDS) sample buffer (150 mmol/L Tris, 30% glycerol, 3% SDS, 1.5 mg/100 mL bromophenol blue dye, and 100 mmol/L dithiothreitol) and denatured at 100°C for 5 minutes. Equivalent amounts (40 μg) of protein were separated on 12.5% SDS-PAGE (polyacrylamide gel electrophoresis) and transferred onto a nitrocellulose membrane (Schleicher and Schuell, Keene, NH). Ponceau S staining was performed to confirm that equal amounts of total protein were present in all the lanes.
The membrane was blocked with 0.5% casein in PBS for 1 hour at room temperature and incubated with the appropriate antibody overnight at 4°C. After 3 washes with 0.5% casein for 5 minutes, the membranes were incubated at room temperature for 1 hour with an appropriate horseradish peroxidase–linked secondary antibody to a final concentration of 1:1000. Final washes were performed in 0.5% casein for 15 minutes, PBS/Tryton- X-100 for 5 minutes (3 times), and distilled water for 5 minutes. Immunolabeled bands were detected with the ECL Western blot detection system (Amersham). The following antibodies were used (brand names and concentrations noted in parentheses): p27Kip-1 (1:2500) and CDK2 (1:2500) (Transduction Laboratories, Lexington, KY); GR (E-20, 1:1000), RARα (C-20, 1:1000), cyclin E (C-19, 1:1000), cyclin A (H-432, 1:2000), cyclin H (C-18, 1:1000), CDK4 (H-22, 1:1000), and CDK6 (C-21, 1:1000) (Santa Cruz Technologies, Santa Cruz, CA); and CDK7 (Ab-1, 1:100) (Calbiochem, Oncogene Research, Cambridge, MA).
In vivo experiments
Female CB.17 SCID/SCID mice, 4 weeks old (Harlan-Nossan, Milan, Italy), were kept under conventional conditions during the experiments. Groups of 12 mice were used, and a suspension of 10 × 106 cells in 200 μL saline buffer was given as subcutaneous (s.c) inoculations in the right flank. RU486 (0.1 mol/L in DMSO) was dissolved in 2.5% Cremophor EL (Fluka Chemie AG, Buchs, Switzerland) in water. Twenty-four hours after s.c. LCL transplantation, RU486 was administered at a dose of 0.5 mg per day per animal (in a volume of 200 μL) for 35 days. An equal volume of the vehicle alone was administered to control mice with the same schedule. Mice were inspected weekly for the appearance and progressive growth of tumor masses. The size of s.c. tumors was measured with calipers, and tumor volumes were calculated by using the following formula: (length × width2) / 2. Statistical significance was calculated by using the 2-tailed Fisher exact test. Aliquots of tumor tissue were either formalin-fixed and paraffin-embedded or snap-frozen and stored at −80°C. For further in vitro analyses, single-cell suspensions from s.c. masses of mice with advanced tumors were purified using Ficoll-Hypaque density gradient (Pharmacia, Uppsala, Sweden).
Results
LCLs, persistently growth-arrested by RA in vitro, recover their proliferative activity following transplantation into SCID mice
As a first step, we assessed whether the proliferative block induced by RA on LCLs in vitro also persisted in vivo. To this end, groups of 12 SCID mice received s.c. injections with DAA-3 LCLs (10 × 106 cells per mouse). The LCLs were previously treated for 7 days with the solvent alone (0.001% DMSO) or with 13-cis-RA at a concentration capable of inducing a persistent (10−5 mol/L) growth arrest in vitro.7 In all groups of mice, transplanted cells gave rise to tumor masses that grew noninvasively at the site of inoculation. Although s.c. tumors induced by RA-treated DAA-3 cells appeared slightly later compared with controls, 35 days after inoculation, both groups of mice showed masses larger than 4 cm3 (not shown). DAA-3 cells purified from s.c. tumors were recultured in vitro in the presence of various concentrations of 13-cis-RA. These cells showed a responsiveness to RA-mediated growth inhibition similar to that of the parental ones (not shown), ruling out the fact that the in vivo growth of DAA-3 cells could be due to the appearance of RA-resistant variants. These findings demonstrate that the growth arrest induced by RA on LCLs, although persistent in vitro upon drug withdrawal, is reversible in vivo. These findings also indicate that host factors may allow the recovery of LCL proliferation.
Glucocorticoids, but not B-cell growth-promoting cytokines, induce a proliferative recovery of LCLs persistently growth-arrested by RA
As a next step, we determined whether cytokines known to promote B-cell proliferation were able to interfere with the antiproliferative activity of RA and, particularly, to release LCLs from RA-induced proliferative block. Preliminary experiments indicated that 100 U/mL IL-1α, 0.5 μg/mL IL-4, or 100 U/mL IL-6 enhanced the proliferation of DAA-3 cells in vitro. Nevertheless, these cytokines, either singularly or in combination, failed to recover the proliferation of DAA-3 cells persistently growth-arrested by RA (not shown). Conversely, administration of the glucocorticoid hormones Dex and HC at physiologic concentrations (10−6 to 10−7 mol/L) induced a prompt recovery of DAA-3 cells previously growth-inhibited by RA (Figure 1 and data not shown). These findings indicate that glucocorticoids are able to relieve the proliferative block induced by RA on LCLs.
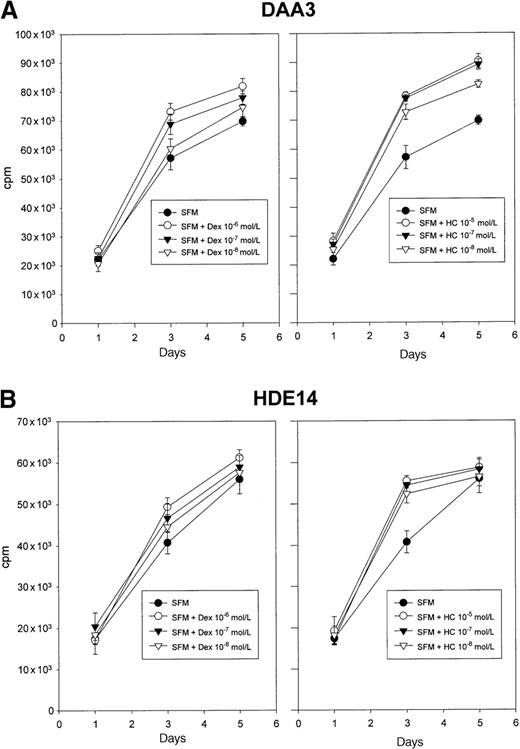
Glucocorticoids are able to release LCLs from DAA-3 cells growth-arrested by RA.
DAA-3 cells were treated with 10−5 mol/L 13-cis-RA for 7 days and then recultured without RA in medium alone or supplemented with 2 different concentrations of Dex (10−6 and 10−7 mol/L). Proliferation was evaluated at different time-points by3H-thymidine uptake. The results of 1 representative experiment out of 3 are shown. Each point represents the mean plus or minus SD of values obtained from triplicate wells. Similar findings were obtained with HC.
Glucocorticoids are able to release LCLs from DAA-3 cells growth-arrested by RA.
DAA-3 cells were treated with 10−5 mol/L 13-cis-RA for 7 days and then recultured without RA in medium alone or supplemented with 2 different concentrations of Dex (10−6 and 10−7 mol/L). Proliferation was evaluated at different time-points by3H-thymidine uptake. The results of 1 representative experiment out of 3 are shown. Each point represents the mean plus or minus SD of values obtained from triplicate wells. Similar findings were obtained with HC.
To verify whether glucocorticoids preferentially induced the outgrowth of phenotypically distinct cell clones, we investigated the expression of several differentiation and activation markers on the DAA-3 LCLs released by Dex or HC from RA-induced growth arrest. The analysis revealed that consistent with the recovery of LCL proliferation, Dex and HC reversed RA-induced CD71 down-regulation (Figure2 and data not shown). Moreover, while the expression of CD23, CD30, CD39, and sIg tended to return to basal levels after the decrease induced by RA treatment, RA-mediated CD19 and CD21 down-regulation and CD38 up-regulation persisted in cells recovered by Dex and HC (Figure 2 and data not shown). Nevertheless, separate experiments showed that glucocorticoids administered to untreated LCLs induced a marked decreased of CD19 and CD21 expression, which occurred concomitantly with up-regulation of CD38 (not shown). There was an apparent lack of reversibility of RA-induced changes relative to CD19, CD21, and CD38 antibodies, and this irreversibility was probably due to the direct effect exerted on the expression of these markers by glucocorticoids. These findings, together with the observation that all these effects were reproducibly induced by glucocorticoids in a large panel of LCLs, including those monoclonal for Ig gene rearrangements (not shown), argue against the possibility that Dex and HC may stimulate the preferential growth of distinct cell clones.
Immunophenotypic profile of DAA-3 cells growth-arrested by RA and reversed by HC.
Cells were treated with 10−6 mol/L 13-cis-RA for 6 days and then replated either in medium alone or with 10−6 mol/L HC (indicated as RA + HC). Immunophenotype was evaluated after 7 days of culture, when cells exposed to HC fully recovered their proliferation. Data relative to the percentage of positive cells (A) and mean fluorescence intensity (B) are shown. The results of 1 representative experiment out of 3 are reported. Similar findings were observed with Dex.
Immunophenotypic profile of DAA-3 cells growth-arrested by RA and reversed by HC.
Cells were treated with 10−6 mol/L 13-cis-RA for 6 days and then replated either in medium alone or with 10−6 mol/L HC (indicated as RA + HC). Immunophenotype was evaluated after 7 days of culture, when cells exposed to HC fully recovered their proliferation. Data relative to the percentage of positive cells (A) and mean fluorescence intensity (B) are shown. The results of 1 representative experiment out of 3 are reported. Similar findings were observed with Dex.
Glucocorticoids, but not other steroid hormones, enhance LCL proliferation in vitro
To gain further insight into the effects exerted on LCLs by glucocorticoids, we investigated whether various steroid hormones, particularly Dex and HC, were able to affect LCL proliferation in vitro. Because normal FCS contains variable concentrations of steroid hormones, including glucocorticoids, we first investigated whether deprivation of steroid hormones in the culture medium had any effect on LCL proliferation. These experiments showed that DAA-3 and HDE-14 cells grown in steroid-free medium proliferated less efficiently than those cultured with normal FCS, with a 35%-40% decrease in3H-thymidine incorporation on day 3 of culture (not shown). This indicates that LCL proliferation is enhanced by FCS-derived steroid hormones. Glucocorticoids probably accounted for most of the growth-promoting activity exerted by FCS-derived steroids because only Dex and HC (Figure 3), but not progesterone, estradiol, or testosterone, enhanced LCL proliferation in steroid-free medium (not shown). As shown in Figure 3, the enhancement of LCL proliferation induced by various concentrations (from 10−6 to 10−8 mol/L) of both Dex and HC was largely dose-dependent, with slightly more pronounced effects induced by HC. Compared with the 3H-thymidine uptake induced by 10−7 mol/L HC, administration of supraphysiologic doses (10−5 mol/L) of this steroid did not result in any further increase in LCL proliferation rates (Figure 3). These findings were confirmed on a larger panel of LCLs (not shown). We also verified that the growth-promoting effect exerted on LCLs by glucocorticoids was associated with changes in the expression of EBV-encoded latent antigens. While the levels of LMP-1 were substantially unaffected by 10−6 mol/L Dex and HC, HDE-14 and DAA-3 cells exposed to these steroids showed a slight EBNA-2 up-regulation and an increase in mean fluorescence intensity of usually less than 20%-30% compared with controls (not shown).
Glucocorticoids enhance LCL proliferation.
Treatment with Dex (10−6 to 10−8mol/L) or HC (10−5 to 10−8 mol/L) induced a dose-dependent increase of 3H-thymidine uptake in DAA-3 and HDE-14 cells cultured in steroid-free medium (SFM). The results of 1 representative experiment out of 3 are shown. Each point represents the mean plus or minus SD of values obtained from triplicate wells.
Glucocorticoids enhance LCL proliferation.
Treatment with Dex (10−6 to 10−8mol/L) or HC (10−5 to 10−8 mol/L) induced a dose-dependent increase of 3H-thymidine uptake in DAA-3 and HDE-14 cells cultured in steroid-free medium (SFM). The results of 1 representative experiment out of 3 are shown. Each point represents the mean plus or minus SD of values obtained from triplicate wells.
Glucocorticoids antagonize the antiproliferative activity exerted on LCLs by RA
To assess whether glucocorticoids could interfere with RA-mediated LCL growth inhibition, we investigated the effects of different concentrations of various steroid hormones (Dex, HC, progesterone, estradiol, and testosterone) on the antiproliferative activity exerted on DAA-3 LCLs by 10−5 mol/L 13-cis-RA. Evaluation of 3H-thymidine uptake over a 7-day period of time showed that only Dex and HC were able to significantly counteract RA-induced LCL growth inhibition. Antagonistic activity of both Dex and HC was clearly evident at all concentrations investigated, with more pronounced effects at 10−6 to 10−7 mol/L (not shown). Similar findings were obtained with ATRA and 9-cis-RA, the 2 other RA isomers active on LCLs (not shown). The antagonistic effect exerted by glucocorticoids on the antiproliferative activity of RA was also confirmed by further cotreatment experiments in which proliferation was evaluated by counting viable cells by trypan blue dye exclusion (not shown). Moreover, the analysis of an additional group of 9 LCLs yielded similar results (not shown), indicating that the effects exerted by glucocorticoids on RA-induced growth inhibition are of general relevance in the LCL system.
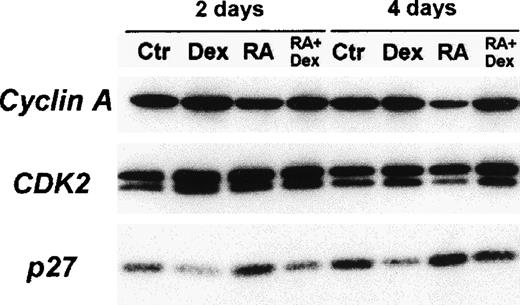
Immunoblot analysis of cyclin A, CDK2, and p27Kip-1 proteins in DAA-3 cells.
The cells were cultured for 2 and 4 days in SFM (center) alone or supplemented with either 10−6 mol/L 13-cis-RA (RA), 10−6 mol/L Dex, or a combination of these 2 drugs. For CDK2, faster migrating bands represent the phosphorylated active forms of this kinase. Dex induced a decrease in the amount of p27Kip-1 protein and a markedly contrasted p27Kip-1 up-regulation induced by RA. Dex also increased the levels of the phosphorylated active forms of CDK2 and antagonized an RA-induced decrease of CDK2 phosphorylation. Similar findings were obtained with the HDE-14 LCLs (not shown). We subjected 50 μg extract proteins from each lysate to immunoblot analysis. The cellular proteins visualized in each panel are indicated to the left.
Immunoblot analysis of cyclin A, CDK2, and p27Kip-1 proteins in DAA-3 cells.
The cells were cultured for 2 and 4 days in SFM (center) alone or supplemented with either 10−6 mol/L 13-cis-RA (RA), 10−6 mol/L Dex, or a combination of these 2 drugs. For CDK2, faster migrating bands represent the phosphorylated active forms of this kinase. Dex induced a decrease in the amount of p27Kip-1 protein and a markedly contrasted p27Kip-1 up-regulation induced by RA. Dex also increased the levels of the phosphorylated active forms of CDK2 and antagonized an RA-induced decrease of CDK2 phosphorylation. Similar findings were obtained with the HDE-14 LCLs (not shown). We subjected 50 μg extract proteins from each lysate to immunoblot analysis. The cellular proteins visualized in each panel are indicated to the left.
Effects of glucocorticoids on cell cycle regulatory proteins
To better understand the mechanisms of action of glucocorticoids involved in the promotion of LCL growth, we investigated the expression of several cell cycle regulatory proteins in DAA-3 and HDE-14 cells cultured for 2, 4, and 7 days in steroid-free medium with or without 10−6 mol/L Dex. At all time-points, cells treated with Dex showed significantly increased amounts of the phosphorylated form of CDK2, whereas there was no observed change in the levels of CDK4, CDK6, and CDK7 (Figure 4 and data not shown). Also, the expression of cyclin E, A, and H was not modulated by Dex (Figure 4 and data not shown). Of note, Dex induced a marked down-regulation of p27Kip-1 that was evident since day 2 of treatment (Figure4). These findings indicate that the decreased availability of this CDK inhibitor in Dex-treated LCLs probably accounted for the enhanced phosphorylation of CDK2 in these cells and resulted in enhanced CDK2 kinase activity and accelerated G1-to-S transition. Thus, Dex-induced p27Kip-1 down-regulation probably constitutes the key factor responsible for the growth-promoting activity exerted on LCLs by glucorticoids.
To gain insights into the mechanisms underlying the antagonism of glucocorticoids on RA-mediated growth inhibition, we also investigated the effects of 10−6 mol/L Dex on the expression of the same cell cycle regulatory proteins (as given above) in HDE-14 cells exposed to 10−6 mol/L 13-cis-RA. The analysis showed that treatment with Dex contrasted with, although not completely, RA-induced p27Kip-1 up-regulation (Figure 4). Consistently, Dex-treated cells also showed higher levels of the phosphorylated active form of CDK2 (Figure 4). These effects were evident since day 2 of treatment (Figure 5). Moreover, RA-induced cyclin A down-regulation observed on day 4 was almost entirely abrogated by Dex, which is consistent with the full recovery of the proliferative activity of these cells (Figure 4). Similar findings were also observed in the DAA-3 LCLs (not shown).
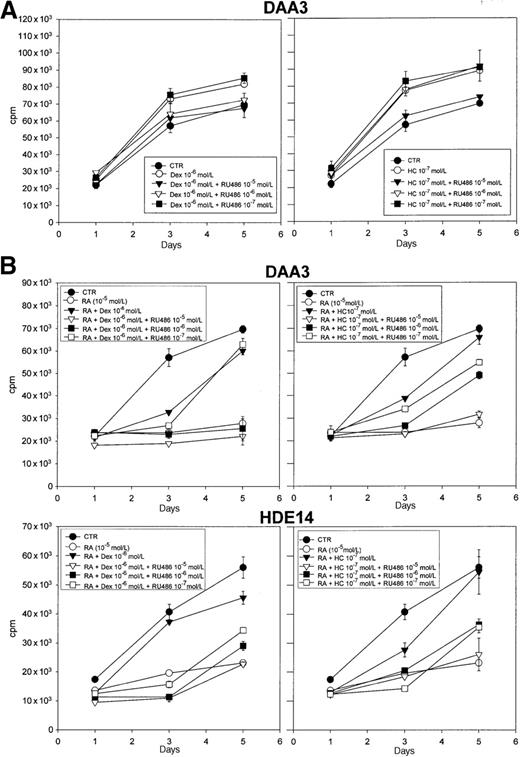
The GR antagonist RU486 fully reversed the LCL growth-promoting effects and RA antagonism mediated by glucocorticoids.
(A) DAA-3 cells were incubated in SFM (CTR, control) for the indicated times with or without RU486 (from 10−5 to 10−7 mol/L) in the presence or absence of 10−6 mol/L Dex (left panel) or 10−7mol/L HC (right panel). (B) The panel depicts the effects of different concentrations of RU486 (from 10−5 to 10−7 mol/L) on the antagonistic activity exerted by 10−6 mol/L Dex (left panels) or 10−7 mol/L HC (right panels) against DAA-3 and HDE-14 cell growth inhibition induced by 10−5 mol/L 13-cis-RA. Proliferation was evaluated at different time-points by 3H-thymidine uptake. The results of 1 representative experiment out of 3 are shown. Each point represents the mean plus or minus SD of values obtained from triplicate wells.
The GR antagonist RU486 fully reversed the LCL growth-promoting effects and RA antagonism mediated by glucocorticoids.
(A) DAA-3 cells were incubated in SFM (CTR, control) for the indicated times with or without RU486 (from 10−5 to 10−7 mol/L) in the presence or absence of 10−6 mol/L Dex (left panel) or 10−7mol/L HC (right panel). (B) The panel depicts the effects of different concentrations of RU486 (from 10−5 to 10−7 mol/L) on the antagonistic activity exerted by 10−6 mol/L Dex (left panels) or 10−7 mol/L HC (right panels) against DAA-3 and HDE-14 cell growth inhibition induced by 10−5 mol/L 13-cis-RA. Proliferation was evaluated at different time-points by 3H-thymidine uptake. The results of 1 representative experiment out of 3 are shown. Each point represents the mean plus or minus SD of values obtained from triplicate wells.
Glucocorticoid-mediated effects are abrogated by the GR antagonist RU486
To determine whether the effects exerted by Dex and HC on LCLs are mediated by GRs, we investigated the ability of the GR antagonist RU486 to suppress the activity of these steroids. Preliminary experiments showed that 10−5 to 10−7 mol/L RU486 alone had no significant effect on the proliferation of LCLs grown in steroid-free medium (not shown). Also, the expression of LMP-1 and EBNA-2 was not affected by RU486 (not shown). As shown in Figure5A, RU486 was able to abrogate the growth-promoting stimulus exerted by Dex and HC on DAA-3 LCLs. In particular, 10−5 to 10−6 mol/L RU486 was active against 10−6 mol/L Dex, and 10−5 mol/L RU486 was active against 10−7 mol/L HC, confirming the stronger effects exerted by this latter steroid hormone. Similar results were obtained with the HDE-14 LCL (not shown). Consistently, RU486 also suppressed the antagonism exerted by glucocorticoids on RA-mediated LCL growth inhibition. In fact, RU486 concentrations as low as 10−7 mol/L efficiently counteracted the activity of 10−6 mol/L Dex and 10−7 mol/L HC in HDE-14 cells (Figure 5B). In DAA-3 cells, RU486 concentrations higher than 10−6 mol/L completely inhibited the effects of 10−6 mol/L Dex. However, the activity of 10−7 mol/L HC was suppressed by RU486 in a dose-dependent fashion, with maximal inhibition observed at 10−5 mol/L (Figure 5B). These findings indicate that the effects of glucocorticoids reported here were mediated by GRs. Western blot analysis, which was carried out with a polyclonal antibody specific for both GRα and GRβ isoforms, showed that HDE-14 and DAA-3 LCLs constitutively expressed detectable amounts of GRα (not shown). The GRβ isoform, a physiologic antagonist of GRα,10 was not expressed in these cells. While 13-cis-RA did not affect GRα expression levels, exposure to 10−6 mol/L Dex induced a marked GRα protein down-regulation that was evident since day 2 of treatment (not shown); 13-cis-RA had no effect on Dex-induced down-regulation of GRα (not shown).
Glucocorticoids antagonize the growth inhibition mediated by an RAR–selective agonist without affecting RAR expression levels
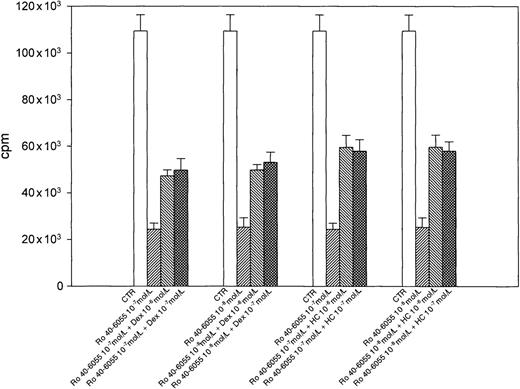
We have previously demonstrated that RA-induced LCL growth arrest is mediated by RARα.11 As shown in Figure6, both Dex and HC were able to antagonize the growth inhibition induced in DAA-3 cells by the RARα selective agonist Ro 40-6055 at all concentrations. To assess whether the antagonistic effects exerted by glucocorticoids were mediated by changes in RARα expression, RARα protein levels were investigated in HDE-14 and DAA-3 cells cultured in steroid-free medium with either 10−6 mol/L Dex, 10−5 mol/L 13-cis-RA, or both for 2, 4, and 7 days. No significant change in RARα expression levels was observed in cells exposed to Dex at all time-points considered, whereas a decrease in the amount of RARα protein was seen in 13-cis-RA–treated cells since day 2 (not shown). Cells treated with both Dex and 13-cis-RA showed RARα expression levels similar to those observed in cells exposed to 13-cis-RA alone (not shown).
Glucocorticoids antagonize LCL growth inhibition induced by the RAR selective agonist Ro 40-6055.
Concentrations of Dex and HC, ranging from 10−6 to 10−7 mol/L, efficiently antagonized the antiproliferative effects exerted by 10−7 and 10−8 mol/L Ro 40-6055 on DAA-3 LCLs. Cell proliferation was evaluated after 7 days in SFM alone (center). The results of 1 representative experiment out of 3 are shown. Each histogram represents the mean plus or minus SD of values obtained from triplicate wells.
Glucocorticoids antagonize LCL growth inhibition induced by the RAR selective agonist Ro 40-6055.
Concentrations of Dex and HC, ranging from 10−6 to 10−7 mol/L, efficiently antagonized the antiproliferative effects exerted by 10−7 and 10−8 mol/L Ro 40-6055 on DAA-3 LCLs. Cell proliferation was evaluated after 7 days in SFM alone (center). The results of 1 representative experiment out of 3 are shown. Each histogram represents the mean plus or minus SD of values obtained from triplicate wells.
RU486 decreases both the in vivo recovery of LCLs persistently growth-arrested by RA and the growth of untreated LCLs transplanted into SCID mice
The effects exerted on LCLs by glucocorticoids in vitro and particularly their ability to counteract RA-mediated growth inhibition at physiologic concentrations suggested a likely relevant role of these steroids in the prompt in vivo recovery of LCLs persistently growth-arrested by RA in vitro. To directly address this issue, we investigated the in vivo effects of RU486 on the growth of RA-treated DAA-3 cells following s.c. inoculation into SCID mice. Administration of 0.5 mg per day RU486 significantly reduced both the number and the volume of s.c. tumor masses (Table 1) without evidence of toxic effects. In particular, after 35 days of treatment, only 3 of 12 animals (25%) treated with RU486 carried s.c. masses as large as those grown in control mice (P = .0003) (Table 1). Of note, 5 of 12 treated mice (42%) showed no evidence of tumor formation whereas 4 of 12 animals (33%) showed the growth of very small s.c. masses (from 0.04-0.5 cm3). These findings indicate that endogenous glucocorticoids probably constitute the main host factors responsible for the in vivo recovery of LCLs that are persistently growth-arrested by RA.
We also investigated whether the in vivo growth of untreated LCLs was influenced by endogenous glucocorticoids. To this end, we evaluated the effects of 0.5 mg per day RU486 on the growth of untreated DAA-3 cells following transplantation into SCID mice. These experiments showed that RU486 also markedly reduced the growth of untreated DAA-3 cells. In fact, after 35 days of treatment, approximately 42% of the animals exposed to RU486 carried s.c. masses significantly smaller than those of controls (P = .019), with 2 of 12 mice (16.7%) showing no evidence of tumor formation.
Discussion
In the present study, we demonstrate that glucocorticoids exert antagonistic effects on RA-mediated LCL growth inhibition both in vitro and in vivo. In fact, Dex and HC, but not B-cell growth-promoting cytokines such as IL-1α, IL-4, and IL-6, were able to recover the in vitro proliferation of LCLs persistently arrested in G0/G1 protein by RA. Moreover, the recovery of these cells occurring in vivo was significantly reduced by treating the SCID mice with the steroid antagonist RU486, which indicates that endogenous glucocorticoids are probably the most relevant host factors responsible for the release of LCLs from RA-induced proliferative block. Consistently, physiologic concentrations of glucocorticoids directly antagonized the antiproliferative activity exerted by 13-cis-RA, 9-cis-RA, and ATRA, even when these retinoids were administered at high doses (10−5mol/L). It is worth noting that glucocorticoids efficiently antagonized RA-mediated growth inhibition in a large panel of LCLs, indicating that this is a generalized effect in the LCL system.
Glucocorticoids are known to influence B-cell survival, activation, and proliferation by inducing variable effects depending on the functional and differentiation status of these cells.12-14Nevertheless, the biologic effects exerted by these steroids on preactivated B cells, such as EBV-immortalized B lymphoblasts, are still poorly defined. Here we show that glucocorticoids directly promote LCL proliferation by conveying stimulatory signals that dominate over the growth-inhibitory stimulus induced by RA. In particular, we found that the proliferation of a large panel of LCLs cultured in steroid-free medium was enhanced by Dex or HC but not by other steroid hormones, which indicates that glucocorticoids probably account for most of the growth-promoting activity exerted on LCLs by FCS-derived steroids. Moreover, we provide evidence indicating that endogenous glucocorticoids also have a contributory role in sustaining LCL growth in vivo. In fact, administration of RU486 markedly reduced both the number and size of s.c. tumor masses induced by transplantation of normal LCLs into SCID mice.
The LCL growth-promoting activity of glucocorticoids may be, at least in part, due to the slight increase in the levels of EBNA-2 expression induced by these steroids, although this issue awaits further elucidation. It is unlikely, however, that glucocorticoids antagonize the effects of RA by up-regulating EBNA-2. RA does not require a direct modulation of EBV-latent antigens to inhibit LCL growth and probably acts downstream to the signaling(s) activated by these viral proteins. To gain further insight into the mechanisms underlying the growth-promoting activity exerted on LCLs by glucocorticoids, we investigated the effects of these steroids on cell cycle regulatory proteins. Our findings strongly suggest that glucocorticoids enhance LCL proliferation mainly by down-regulating p27Kip-1. In fact, in LCLs treated with glucocorticoids, the reduced levels of this inhibitor were associated with and were probably responsible for the increased amount of the active phosphorylated form of CDK2, a phenomenon that is relevant for the enhancement of CDK2 kinase activity and, ultimately, for cell cycle progression.15
These results are consistent with recent findings indicating that p27Kip-1 is one of the targets modulated by glucocorticoid signaling to regulate lymphocyte proliferation.16 The observation that p27Kip-1 down-regulation underlies the growth-promoting effects exerted on LCLs by glucocorticoids is intriguing. This is particularly true in light of our previous findings indicating that up-regulation of p27Kip-1 has a central role in mediating the antiproliferative effects induced by RA on the same cells.8 Besides strengthening the relevance of p27Kip-1 in controlling LCL growth, the results presented herein also suggest that RA and glucocorticoid signaling probably converge on p27Kip-1 to differentially modulate the proliferation of these cells.
Both the promotion of LCL proliferation and antagonism of RA-mediated growth inhibition exerted by glucocorticoids were reversed by the GR antagonist RU486. These findings strongly suggest that all these effects were mediated by GR. Glucocorticoids and RA exert their biologic activities through nuclear receptors that share a similar structural organization and belong to the same family of ligand-activated transcription factors.17 Considering that both RA and glucocorticoids can cross-regulate the expression of several members of the same nuclear receptor superfamily,18-22 it appeared of interest to assess whether glucocorticoids antagonized the activity of RA by affecting the expression of relevant RARs or RXRs. We have recently shown that RA-induced LCL growth inhibition was mainly mediated by RARα,11 and here we report that glucocorticoids also efficiently antagonized the antiproliferative activity of the RARα selective agonist Ro 40-6055. While both RA and glucocorticoids down-regulated their own relevant receptors (RARα and GRα, respectively), probably by homologous regulation,2,17 23glucocorticoids had no effect on RARα protein levels. These findings indicate that the interference exerted on RA signaling by glucocorticoids probably occurs at a level beyond the modulation of RARα concentration. However, further studies are required to elucidate the mechanisms underlying the cross-talk between RARα and GRα in the LCL system.
EBV-immortalized LCLs are the in vitro counterpart of the cells that give rise to EBV-related lymphoproliferations of immunosuppressed patients.24,25 In particular, LCLs have biologic features that are highly reminiscent of posttransplantation lymphoproliferative disorders (PTLDs).24-26 PTLD, which occurs in 1%-10% of all cases, constitutes a life-threatening complication that may arise after transplantation of solid organs.24,26 Several lines of evidence indicate that the functional impairment of EBV-specific cytotoxic T lymphocytes (CTLs) due to immunosuppressive therapy is the major factor responsible for the uncontrolled proliferation of EBV-immortalized B lymphocytes occurring in the early phases of PTLD development.27 Nevertheless, the demonstration that physiologic concentrations of glucocorticoids promote LCL growth both in vitro and in vivo suggests that endogenous glucocorticoids may be involved in the pathogenesis of PTLD. Our findings are in fact consistent with the possibility that after EBV immortalization, the in vivo growth of EBV-infected B lymphoblasts may be at least in part promoted and sustained by endogenous glucocorticoids. Similar growth-promoting effects could also be induced by synthetic glucocorticoids administered to these patients in combination with other immunosuppressive drugs to prevent or control graft rejection. These relevant issues deserve to be directly addressed by further studies. Moreover, although the regression of most PTLDs occurring after reduction or withdrawal of immunosuppressive therapy is largely due to restoration of EBV-specific CTL responses,27,28 it would be of interest to verify whether a decreased glucocorticoid-mediated growth-promoting stimulus may also contribute to this phenomenon. Finally, our results also may have other implications for the management of EBV-associated lymphoproliferations of immunocompromised patients, and the use of schedules including both RA and a GR antagonist, such as RU486,29 may allow a more thorough evaluation of the therapeutic potential of RA in this setting.
Acknowledgments
The authors thank Prof Werner Bollag (Hoffmann-La Roche) for supplying Ro 40-6055 and Dr P. Tonel and Mrs P. Pistello for help with the manuscript.
Supported in part by a grant (R.D.) from the Italian Association for Cancer Research (AIRC), Milan, Italy. P.Z. is the recipient of a fellowship from the Italian Foundation for Cancer Research (FIRC), Milan, Italy.
Reprints:Mauro Boiocchi, Division of Experimental Oncology 1, Centro di Riferimento Oncologico, via Pedemontana Occidentale 12, 33081 Aviano (PN), Italy; e-mail: mboiocchi@ets.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.