Sung et al recently reported the identification of TM5b as one of the tropomyosin isoforms present in the human erythrocyte membrane skeleton.1 Therein, they also “propose a molecular model of a short actin protofilament in erythrocytes … in which tropomodulin is associated near the N-terminal end of 1 TM molecule, which comprises either TM5 or TM5b, … and is at the pointed end of the short actin filament” (see “Results,” p 1478).1 Clearly, the identification of TM5b as one of the erythrocyte tropomyosin isoforms is important: it specifies one of the unknown components of the short erythrocyte actin filaments and will no doubt contribute to deciphering how they assemble in vivo.1 It is unfortunate, however, that the presentation of the Sung et al model leaves the impression that this model for the organization of the short erythrocyte actin filaments has not been proposed before. To the contrary, a series of research articles on tropomodulin,2-5 as well as several review articles on the erythrocyte membrane skeleton6-9 and a textbook,10 have discussed extensively the idea that tropomodulin is located at the pointed ends of the short erythrocyte actin filaments and functions with tropomyosin to restrict their length. The misrepresentation by Sung et al is disappointing because science advances by virtue of new ideas as well as facts. It is a disservice to the scientific community not to place new findings in their proper historical context.
A view on the molecular basis of erythrocyte membrane mechanics
University of California, San Diego
La Jolla, CA
We recently reported the identification of tropomyosin isoform 5b (TM5b) in human erythrocytes and the implications of tropomodulin-TM5 or tropomodulin-TM5b complexes in the protofilament and hexagonal organization of membrane skeletons.1-1 In this report, schematic drawings/models of a tropomodulin-TM complex, a short actin protofilament, and hexagonal lattices of the erythrocyte membrane skeleton were presented to illustrate the proposed structure and function of these newly characterized tropomodulin-TM complexes (shown here in the middle 3 panels of the Figure, labeled “Molecular ruler,” “Actin protofilament,” and “Hexagonal lattices,” with minor modifications). We extensively cited articles to support our statements and/or proposals, with 9 articles authored or coauthored by Dr Fowler, including the 1996 review article.1-2
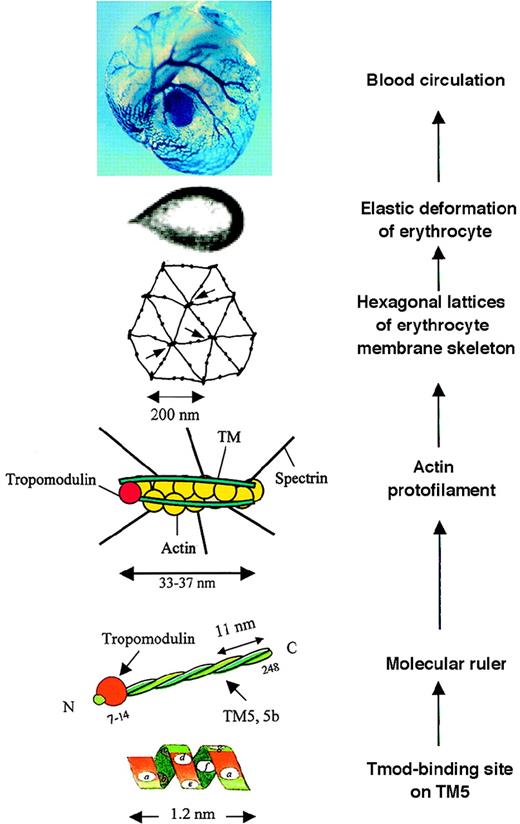
A composite illustrating a view of the possible molecular basis of erythrocyte membrane mechanics in vitro and in vivo.
Read from bottom up. At “Tmod-binding site on TM5,” residues ata, d, f, and a in the N-terminal heptad repeats of TM5,1-6 functioning as the tropomodulin-binding site. At “Molecular ruler,” a complex of tropomodulin and TM5 or TM5b, in the form of homodimer or heterodimer, functioning to protect actin filaments of an uniform length.1-1,1-6 At “Actin protofilament,” a short actin protofilament of about 33-37 nm consisting of 6 G-actin per strand protected by the molecular ruler, specifying the joining of 6 spectrin tetramers. At “Hexagonal lattices of erythrocyte membrane skeleton,” geometry of the membrane skeleton defined mainly by spectrin teramers and actin protofilaments; arrows point to junctional complexes. At “Elastic deformation of erythrocyte,” elastic deformation of an erythrocyte in a flow channel,1-7 responding to a shear stress of 4.0 dyn/cm2. At “Blood circulation,” “blue” erythrocytes circulating in blood vessels of a mouse yolk sac. (X-gal staining detected the expression of tropomodulin in erythrocytes reported by a “knocked in” lacZ reporter gene under the control of the endogenous Tmod promoter.) TheTmod−/− mutation is lethal, suffering from arrests in heart development, vasculogenesis, and definitive lineage hematopoiesis.1-8 A Tmod+/− embryo at 9.5 days of gestation is shown.
A composite illustrating a view of the possible molecular basis of erythrocyte membrane mechanics in vitro and in vivo.
Read from bottom up. At “Tmod-binding site on TM5,” residues ata, d, f, and a in the N-terminal heptad repeats of TM5,1-6 functioning as the tropomodulin-binding site. At “Molecular ruler,” a complex of tropomodulin and TM5 or TM5b, in the form of homodimer or heterodimer, functioning to protect actin filaments of an uniform length.1-1,1-6 At “Actin protofilament,” a short actin protofilament of about 33-37 nm consisting of 6 G-actin per strand protected by the molecular ruler, specifying the joining of 6 spectrin tetramers. At “Hexagonal lattices of erythrocyte membrane skeleton,” geometry of the membrane skeleton defined mainly by spectrin teramers and actin protofilaments; arrows point to junctional complexes. At “Elastic deformation of erythrocyte,” elastic deformation of an erythrocyte in a flow channel,1-7 responding to a shear stress of 4.0 dyn/cm2. At “Blood circulation,” “blue” erythrocytes circulating in blood vessels of a mouse yolk sac. (X-gal staining detected the expression of tropomodulin in erythrocytes reported by a “knocked in” lacZ reporter gene under the control of the endogenous Tmod promoter.) TheTmod−/− mutation is lethal, suffering from arrests in heart development, vasculogenesis, and definitive lineage hematopoiesis.1-8 A Tmod+/− embryo at 9.5 days of gestation is shown.
We proposed that TM in the protofilament is composed of TM5 or TM5b in the form of either homodimer or heterodimer based on our new findings. There was no intention to impress the scientific community that this was the only model ever proposed. Gilligan and Bennett (1993),1-3 Lux and Palek (1995 and earlier),1-4Fowler (1996),1-2 and others1-5 have in fact proposed several models for the short actin filament in erythrocytes. We unfortunately did not take the opportunity to discuss the variations among these models. For example, in the 1996 Fowler model, the actin protofilament is about 60 nm long, consisting of 18 G-actin, with 2 TM molecules located at one (pointed) end associating with tropomodulin and several spectrin molecules located at the other (barbed) end associating with adducin tails. In the models of Gilligan and Bennett and of Lux and Palek, protofilaments are about 35 nm long, consisting of about 12 G-actin. The end of TM to which tropomodulin binds and how 6 spectrin tetramers per protofilament are spaced, however, are not specified. We proposed that it is the common properties shared by TM5 and TM5b that contribute to the formation of the actin protofilament (Figure) and that the 6 pairs of G-actin in the double helix define the hexagonal arrangement of spectrin in the filament. The properties shared by TM5 and TM5b include the same number of G-actin that they protect, the high tropomodulin and actin affinity they both possess, and their unique ability to form both homodimers and heterodimers with each other.
As to how tropomodulin functions with TM to restrict the actin filament length: Fowler's 1996 review article stated that the actin filaments in the native membrane skeleton are likely to be about 67 nm long and that “strict control of the relative amounts of tropomyosin, tropomodulin and adducin with respect to the amounts of actin, spectrin and other associated components could act to limit the filaments to the length of one tropomyosin rod plus the actin subunits required for spectrin binding” (p 90).1-2 In contrast, our model was based on the precise information provided by TM5 and TM5b, and the article explained, step by step, why the erythrocyte protofilament has only one TM in length (see “Discussion,” p 1478). The significance of identifying TM5b, therefore, goes beyond merely specifying one of the unknown components of the short erythrocyte actin filament.
The scientific community is invited to read the article by Sung et al, as well as the references cited, as all new findings or ideas need to be judged in the historical context by the scientific community. The Figure represents a personal view in terms of the roles of tropomodulin and TM5 or TM5b in the attempt to understand the molecular basis of erythrocyte membrane mechanics. Here I acknowledge my current and former collaborators, many outstanding investigators, including Dr Fowler, who have contributed to the advancement of this field, and those who have developed ingenious technologies that made these studies possible.