Abstract
Three French families with triose phosphate isomerase (TPI) deficiency were studied, and 2 new mutations giving rise to null alleles were observed: a frameshift mutation with deletion of the 86-87 TG dinucleotide in codon 29 (TPI Alfortville) and a T→A transversion in nucleotide 2 of the initiation codon (TPI Paris). The first mutation occurred in compound heterozygosity with the frequent E105D mutation. The second mutation occurred in association with the 2-nucleotide promoter variant (−43G,−46A). In a third family, the propositus was an E105D homozygote. In the TPI Paris family, the coinheritance of the −43,−46 promoter variant appeared to exert little, if any, effect on TPI enzyme activity, a finding consistent with 2 previous reports that questioned the putative role of the promoter polymorphism as a true deficiency variant. Similarly, the further coinheritance of glucose-6-phosphate dehydrogenase (G6PD) A− (202 G→A/376 A→G) appeared to have little effect on the observed phenotype. Compound heterozygosity for the E105D mutation with the null allele TPI Alfortville appeared to lead to a more severe clinical syndrome than did E105D homozygosity, suggesting that compound heterozygosity with null alleles may lead to more profound clinical abnormalities than homozygosity with missense alleles. A simple, rapid polymerase chain reaction and restriction enzyme procedure for the E105D mutation was developed for prenatal diagnosis in one family and subsequently used for screening in the other families.
Human triose phosphate isomerase (TPI; EC 5.3.1.1) is a homodimeric enzyme1,2 that catalyzes the interconversion of glyceraldehyde-3-phosphate and dihydroxyacetone phosphate and is involved in glycolysis as well as in gluconeogenesis and triglyceride synthesis.3 The enzyme, which is expressed in all cell types, is considered ubiquitous, and it is encoded by a single gene on chromosome 12 at locus 12p13.4,5 Three processed intronless pseudogenes on different chromosomes have been described.6
TPI deficiency, an autosomal recessive disorder, has been known since 1965.7 Heterozygotes are clinically normal. Homozygotes and compound heterozygotes exhibit a somewhat variable syndrome that always includes nonspherocytic hemolytic anemia. Except for 2 instances,8,9 progressive, severe, but somewhat variable neuromuscular dysfunction has been a major clinical feature, sometimes including mental retardation or other evidence of cerebral impairment. Thirteen different mutations in the human TPI locus have been identified.10 Among these, the mutation affecting amino acid 105 (1592 G→C; E105D) is the most frequent.11
The severity of the syndrome, often manifested by death in the fetal period or in early childhood or by devastating neuromuscular dysfunction, has prompted many families to seek prenatal diagnosis.
The variable severity of the syndrome may well be associated with differing degrees of enzyme deficiency, which may in turn vary according to the specific mutation. It seems reasonable to speculate that compound heterozygotes with 1 null allele and 1 missense allele might well exhibit syndromes of greater severity than might occur in homozygotes or compound heterozygotes with missense alleles only. The paucity of known null alleles10 and the failure to find null allele homozygotes in more than 33 years of experience with TPI deficiency are supportive of this conjecture. Also of considerable note is the observation of embryo lethality in TPI null allele homozygous mice.12
In this paper we present 2 French families with previously undescribed null alleles as well as a third family with a homozygous E105D mutation. For the E105D mutation, a diagnostic technique is described that allows easy prenatal diagnosis and rapid screening for the mutation.
Materials and methods
Enzymatic analysis
Erythrocyte TPI and glucose-6-phosphate dehydrogenase (G6PD) activities were assayed as recommended by the International Committee for Standardization in Haematology.13
DNA samples
A total of 5 to 10 ml of peripheral blood was drawn in ethylenediaminetetraacetic acid from the subjects, their family members, and normal control subjects. DNA was prepared using standard techniques.14 Chorionic villus samples (CVS) for prenatal diagnosis were processed as previously described.15 In all instances, informed consent was obtained.
In vitro amplification
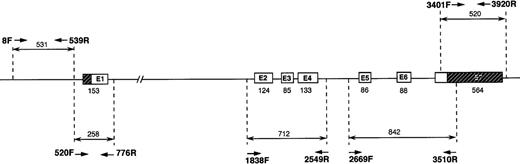
Polymerase chain reaction (PCR) amplifications were performed as previously described,15 with minor modifications. Intraintronic oligonucleotide primers were designed to amplify fragments 250 to 800 base pairs (bp) in length to include the exons, the intron-exon boundaries, and the promoter region. The primers are listed in Table 1, and the analytic strategy followed is shown in Figure 1.
Strategy followed for amplification and sequencing of the TPI gene.
Primers used and PCR conditions are described in Table 1. The strategy followed used PCR amplification of the TPI gene in 5 parts, 2 for the promoter region and 3 for the exons and intron-exon boundaries. Fragment amplified with primers 520F and 776R overlaps both the promoter and exon 1 regions.
Strategy followed for amplification and sequencing of the TPI gene.
Primers used and PCR conditions are described in Table 1. The strategy followed used PCR amplification of the TPI gene in 5 parts, 2 for the promoter region and 3 for the exons and intron-exon boundaries. Fragment amplified with primers 520F and 776R overlaps both the promoter and exon 1 regions.
Prenatal diagnosis in family A
Detection of E105D mutation.
The mutation creates a DdeI recognition site (CTNAG):GTCAG→CTCAG. Exon 3 was amplified together with exons 2 and 4 with primers 1838F and 2549R, generating a 712-bp fragment (Table 1 and Figure 1). Digestion with DdeI gives rise to 5 fragments (289, 206, 87, 75, and 55 bp) with the normal control gene and to 6 fragments (289, 178, 87, 75, 55 and 28 bp) with the E105D gene, the 206-bp fragment being cut into 2 new fragments of 178 and 28 bp.
Del 86-87 TG detection.
The mutation creates an MwoI restriction site (GCN7GC): GCN9GC→GCN7GC. Exon 1 was amplified with primers 520F and 776R, which generates a 258-bp fragment encompassing the first exon (Table 1 and Figure 1). Digestion of the PCR fragment with MwoI produces 2 fragments (190 and 68 bp) for the normal gene and 3 fragments (133, 68, and 55) for the mutant, the sum of the 2 fragments generated by the new restriction site being 188, and not 190 bp, because of the 2-bp deletion.
All resultant fragments were resolved on 1 × TBE, 4% NuSieve/Seakem agarose gel (FMC Bioproducts, Rockland, ME) and stained with ethidium bromide.
G6PD genotyping
Sequence determination
Double-stranded PCR products were sequenced using the same set of oligonucleotides used for initial amplification and internal primers. The reference nucleotide sequence for the human TPI gene was GenBank locus HSTPI1G, accession X69723. The numbering system used in this paper varies from that of GenBank and conforms to current recommendations for monogenic disorders18 in which the number 1 designates the A of the initiation ATG codon in both genomic DNA and complementary DNA as well as the corresponding coded methionine of the expressed peptide.
Results
Case reports
Family A.
A 970-g premature male infant was born at 30 weeks of gestation, 41 days after rupture of the membranes, resulting in oligohydramnios and infection with streptococcus B. At birth, the infant required artificial ventilation because of pulmonary immaturity. Physical examination showed pallor, hepatomegaly and splenomegaly, diffuse edema, and jaundice of the umbilical cord. Laboratory studies indicated hemolytic anemia (hemoglobin 77 g/L, reticulocytes 418 × 109/L) requiring blood transfusion. Despite treatment, refractory hypoxemia and clinical signs of bilirubin encephalopathy developed. The infant died at age 4 days. Postmortem examination revealed signs of hemolytic anemia (marked extramedullary hematopoiesis), signs of severe pulmonary immaturity, and pulmonary infection.
Both parents were healthy and unrelated. The father, age 60, had 5 healthy children from a previous marriage. The mother was 37 years old. In both parents, hematologic data were in the normal range except for a moderate reticulocytosis (181 × 109/L and 113 × 109/L for the father and the mother, respectively). TPI activity was approximately 50% of control values (Table 2). Unfortunately, TPI was not assayed in the infant, because a blood sample prior to exchange transfusion had not been retained.
Two years later, the mother was again pregnant. At 11 weeks of gestation, CVS molecular diagnosis demonstrated that the fetus was a heterozygote who had inherited the paternal mutant allele and the maternal normal allele. The infant was born after a normal full-term pregnancy, weighing 3060 g. Physical examination at birth was normal, and at age 2, at the time this article was submitted, the child remained perfectly healthy.
Family B.
The propositus was the second living child of clinically normal unrelated parents. The father was of French origin and the mother was from Madagascar. She had had 4 pregnancies with only 2 living children. The first pregnancy resulted in death in utero of a female fetus at 7 months, and the third pregnancy ended spontaneously at 6 weeks. The birth of the propositus (fourth pregnancy) was apparently normal, with spontaneous delivery at 41 weeks.
Psychomotor retardation was noted at 4 months, and a seizure crisis occurred at 7 months. Further clinical evolution was marked by severe convulsive microcephalic encephalopathy and growth retardation. At 8 years, the child was unable to walk alone and had not developed language skills.
The results of investigations, including karyotyping, neuroradiologic imaging, electrophysiologic testing, and muscle biopsies, were normal. There were no laboratory data indicative of hemolytic anemia. Erythrocyte TPI activity was reduced to heterozygous levels in the father and was in the low normal range in the mother and healthy brother. TPI activity in the propositus was in the heterozygous range, essentially the same as in the father. Erythrocyte G6PD activity was markedly reduced in the patient and moderately reduced in the mother (enzyme data presented in Table 2).
Family C.
The parents were unrelated and had a normal older daughter. The propositus was born after a normal full-term pregnancy. Shortly after birth, severe hemolytic anemia (hemoglobin 40 g/L) was noted. Transfusions were performed on day 2 and again at 3 and 6 weeks. Chronic hemolytic anemia persisted, with hemoglobin values ranging 90 to 100 g/L, marked by a hemolytic crisis with a drop of hemoglobin to 70 g/L. Although neuromuscular development was initially normal, slowing of development was noted at the end of the first year. At 27 months, mental development was apparently normal, but the patient was unable to walk without assistance. Distal weakness, hypotonia, and amyotrophy were noted. Electromyographic studies and motor and sensory nerve conduction velocities were compatible with spinal motor neuron involvement. Muscle biopsy confirmed fiber-type grouping and target fibers, both of which are characteristic of denervation. A nerve biopsy was normal.
At age 30 months, complete hematologic evaluation revealed TPI deficiency in the propositus and lowered TPI activity in the heterozygous range in both clinically unaffected parents (Table 2).
Slowly progressive motor deterioration has continued. Language is normal, but school-type skills appear to be delayed. Hemolytic anemia is well tolerated, with hemoglobin values about 100 g/L, at the age of 6.
Molecular examination of TPI genes
PCR fragments that encompassed the promoter, exons, and intron-exon boundaries in the TPI gene (Figure 1) were generated, purified, and subsequently sequenced. In the case of the promoter, primers selected initially had to be changed during the course of this study because we detected errors (subsequently corrected) in the published sequence (GenBank X69723).
A technique for rapid diagnosis, using PCR amplification followed by digestion with the restriction enzyme DdeI, was developed for the most frequent mutation (E105D) (Figure2). This technique, initially devised for prenatal diagnosis in family A, was subsequently employed for samples from subjects in the other families and was of special importance in the evaluation of family C.
PCR digestion analysis in family A.
DNA was prepared from peripheral blood for patients I 1 and I 2. Patient II 1 died in the perinatal period and was not available for the study. For the fetus (II 2), DNA was obtained from CVS. “nal” indicates normal control sample, MWS (molecular weight standard) = ΦX174 RF/HaeIII fragments. (A) Pedigree of family A. (B) PCR digestion of exon 3. Amplification of exons 2, 3, and 4 with the 1838 F and 2549 R set of primers was followed by digestion with DdeI (normal fragment 206 bp, mutants fragments 178 and 28 bp). Patients I 1 and II 2 are heterozygous for the mutation; patient I 2 is normal. (B) PCR digestion of exon 1. Amplification with the 520 F and 776 R set of primers followed by digestion with MwoI (normal fragment 190 bp, mutant fragments 133 and 56 bp). Patients I 1 and II 2 are normal, and patient I 2 is heterozygous for the mutation del 86-87.
PCR digestion analysis in family A.
DNA was prepared from peripheral blood for patients I 1 and I 2. Patient II 1 died in the perinatal period and was not available for the study. For the fetus (II 2), DNA was obtained from CVS. “nal” indicates normal control sample, MWS (molecular weight standard) = ΦX174 RF/HaeIII fragments. (A) Pedigree of family A. (B) PCR digestion of exon 3. Amplification of exons 2, 3, and 4 with the 1838 F and 2549 R set of primers was followed by digestion with DdeI (normal fragment 206 bp, mutants fragments 178 and 28 bp). Patients I 1 and II 2 are heterozygous for the mutation; patient I 2 is normal. (B) PCR digestion of exon 1. Amplification with the 520 F and 776 R set of primers followed by digestion with MwoI (normal fragment 190 bp, mutant fragments 133 and 56 bp). Patients I 1 and II 2 are normal, and patient I 2 is heterozygous for the mutation del 86-87.
The father in family A was a TPI E105D heterozygote. The mother was a heterozygote for a novel deletion TG of the 2 bases at positions 86 and 87 in codon 29 within exon 1, a mutation that we designated TPI Alfortville. Prenatal diagnosis in this family at week 11 of a second pregnancy revealed that the fetus was a TPI E105D heterozygote, and we predicted correctly that the child would be unaffected (Figure 2).
In family B, the propositus had 2 TPI gene variations. The first one involved a point mutation in the initiation codon with nucleotide 2 in the ATG codon replaced by an A, giving rise to an AAG coding for a hypothetical lysine. We designated this mutant as TPI Paris. The second variation was the well-known tightly linked pair of transitions in positions −43 and −46 in the promoter region, A→G and G→A, respectively.19-21 The father was heterozygous for the initiation codon mutation, while the mother was heterozygous for the promoter transition pair. The brother with normal TPI enzyme activity, like the mother, had only the double transition. The propositus and mother additionally had G6PD deficiency, with the G6PD mutation characterized by sequencing and restriction analysis as A− (202 G→A/376 A→G).
In family C, the propositus, with severe hemolytic anemia, was homozygous for the E105D mutation. Using the rapid technique described above, it was shown that both parents were heterozygous for TPI E105D. The sister of the propositus had a normal genotype.
Hematologic, enzymatic, and genotype results for all of the patients and their families are summarized in Table 2.
Discussion
Hemolysis and progressive neurologic disease (neuromuscular impairment with spasticity or mental retardation) are closely associated in almost all reported instances of TPI deficiency.10 However, 2 subjects are known who had no evident neurologic impairment even though hemolysis was present.9,22 The relative preponderance, one to the other, of hemolysis and neurologic dysfunction appears to vary from family to family. This is somewhat surprising because TPI is a ubiquitous housekeeping enzyme. However, it has been suggested that protein-protein interactions may result in compartmentalization, with differential enzyme expression in one tissue versus another.9,22 23
TPI E105D, the most frequently occurring TPI mutation,10,11affects subjects of diverse geographic and ethnic origins.24 In a worldwide study of DNA samples from key subjects in almost all known TPI E105D kindreds, including the families reported here, complete linkage disequilibrium with 2 markers was demonstrated for the E105D mutation. Haplotype analysis demonstrated that all the E105D chromosomes carried the same otherwise uncommon haplotype,25 consistent with the hypothesis that all E105D subjects are descendants of a common ancestor, a finding supported by a subsequent study by others.26 The predominance of the E105D mutation has practical significance because questions concerning prenatal diagnosis are often paramount among the concerns of affected families.
A rapid procedure for prenatal CVS detection of the E105D mutation, developed for use in family A, later proved useful for prenatal diagnosis and screening in other families. In the past, prenatal diagnosis of TPI deficiency has been performed by direct TPI enzyme activity and substrate analysis of fetal red blood cells collected by cordocentesis,27,28 trophoblast homogenates, amniotic cells,29 or by PCR amplification from nucleated cord-blood cells.30 The availability of a simpler technique using CVS, as described by Arya et al31 or in this paper, provides a rapid, reliable method for the screening of the most frequent TPI mutation.
The occurrence of a significant number of known amino acid alterations in TPI that are associated with markedly decreased enzyme activity has been the basis of inquiries leading to at least a partial understanding of the structure-function relationships of the enzyme protein.32-34
The TPI Alfortville mutation almost certainly functions as a null allele. The 2-base deletion in codon 29 results in synthesis of an out-of-phase peptide of aberrant sequence extending for 40 residues downstream before terminating at residue 70. This gross alteration affects almost the entire peptide structure, predicting a total loss of TPI function. The structural alteration is so profound that dimer formation in TPI Alfortville/TPI E105D compound heterozygotes must almost certainly be limited to homodimers of the TPI E105D protein product alone, with total failure of heterodimer production and predicted major quantitative diminution of expressed protein. This alteration provides a ready explanation for the severe hemolysis that contributed to the early death of the propositus in family A, a highly likely but undocumented compound heterozygote for TPI Alfortville and TPI E105D.
In family B, the allele with an initiation codon mutation (TPI Paris) may well cause complete failure of production of the protein, thus functioning as a null allele. A similar situation, in which the initiation codon is replaced by a lysine codon, has been reported by Waye et al35 for the β-globin gene, resulting in a β0-thalassemia syndrome, ie, a gene functioning as a null allele.
Rather than complete failure of protein synthesis, an alternative mechanism might be reinitiation at the next available ATG codon, 14 codons downstream, as suggested earlier.32 Such a mechanism has in fact been recently demonstrated by Zhang and Maquat.36 Using constructs of the chloramphenicol acetyltransferase (CAT) reporter gene linked to modified fragments of the TPI gene, they noted that such reinitiation occurs with stop codons in positions 1, 2, or 10. They further demonstrated that the stability of the corresponding messenger RNAs was relatively unimpaired, in contrast to the messenger RNAs expressed with stop codons further downstream in the TPI gene.37,38 However, the predicted protein product of reinitiation would lack the entire β-1 sheet as well as asparagine-12 and lysine-14, both residues contributing to substrate binding and lysine-14 being additionally known to play a key role in catalysis.33 Additionally, the protein product would have 3 missing positive charges, further contributing to instability of the 3-dimensional structure.
Consequently, even though we have not had the opportunity to perform expression studies to demonstrate unequivocally the effect of the initiation codon mutation, it is almost certain that TPI Paris would function as a null allele regardless of which of the 2 aforementioned mechanisms is at play.
Families A and C are both affected by the most frequently described mutation, TPI E105D. This missense mutation results in an enzyme with decreased activity. However, some residual activity remains, in sharp contrast to the totally abolished activity predicted for the protein products of presumed null alleles such as TPI Alfortville and TPI Paris.
It is noteworthy that the E105D homozygous patient in family C exhibited a phenotype that was less severe than that of the presumed but undocumented compound heterozygote in family A. Both would be expected to express the same mutant protein (homodimers of E105D peptide), but enzyme activity in the compound heterozygote would be predicted to be one-half that of the homozygote.
It is also noteworthy that there have been no reports of TPI deficiency null alleles occurring as homozygotes or as compound heterozygotes involving 2 differing null alleles.10 It is likely that such combinations would be incompatible with life, as is apparently the case with homozygosity for TPI null alleles in mouse embryos.12
The promoter transition pair in positions −43 and −46 was originally reported by Watanabe et al in 1996 associated with TPI heterozygosity as assessed by intermediate reduction of TPI enzyme activity.19 The linked pair of transitions is located within the promoter region in the CAP proximal element (CPE) described by Boyer and Maquat close to the transcription initiation site.39 Watanabe et al reasoned that the mutation would reduce messenger RNA synthesis and resultant TPI enzyme activity, a concept reinforced by the finding of yet a third abnormality in a small number of TPI −43,−46 subjects—a transversion at position −62 within the TATA box.
In family B, the mother and 1 brother were TPI −43,−46 heterozygotes. However, TPI enzyme activities of both were within the normal range, a finding differing from the report of Watanabe et al19 but consistent with 2 more recent reports20,21 that question the putative role of the paired −43,−46 substitution as a deficiency allele.19Additionally, the propositus, a compound heterozygote for both the paternal and maternal TPI variants, had enzymatic activity no less than that of the father, a TPI heterozygote. This evidence strongly suggests that the maternal −43,−46 variant did not contribute to the clinical phenotype of the propositus. Similarly, coinheritance of G6PD A− (202 G→A/376 A→G) by both the mother and the propositus added little, if anything, to the observed phenotype, a phenomenon previously reported with the combination of G6PD deficiency and TPI heterozygosity.40
Nevertheless, the family history of repeated spontaneous abortion followed by a living child with severe progressive neuromuscular dysfunction is typical of TPI deficiency, and it is tempting to speculate that the propositus in this family may in fact represent a forme fruste of TPI deficiency characterized by neurologic disease in the absence of hemolytic anemia, a combination not previously described, even though the reverse combination, hemolytic anemia without neurologic disease, is well known.8,9 Demonstration of this provocative but entirely hypothetical possibility would depend upon information not yet available, perhaps including demonstration of tissue-specific TPI deficiency in the central nervous system, along with increased dihydroxyacetone phosphate concentration as evidence of perturbed glycolysis. However, such information cannot be obtained from a living patient. Metabolic block would be an unexpected finding in red cells from a subject without hemolytic anemia and with intermediate erythrocyte TPI enzyme activity in the heterozygous range. However, it has been suggested that cultured lymphocytes may be an appropriate surrogate for neural tissue for many metabolic studies.41At least for the present, such studies have been precluded by geographic distance and reluctance of the parents to submit their child to further scientific inquiry without clear, direct clinical benefit.
In 1998, Schneider et al revisited the putative role of the −43,−46 variant in TPI deficiency and found that the −43,−46 variant occurred in approximately 20% of African American subjects.20 Their findings suggested that the reduction in TPI activity occurred in a continuum ranging from the high normal range to values suggestive of deficiency heterozygosity. They further suggested that these variant substitutions, when associated with the lower range of TPI enzyme activity, might contribute to TPI deficiency if coinherited along with a missense or nonsense deficiency allele. To our knowledge, only 2 such compound heterozygotes are known. One is a well-defined instance of clinical TPI deficiency in a compound heterozygote for the −43,−46,−62 variant along with the E105D mutation,10,20 and the other is the propositus in our family B. Observation of additional families with the TPI −43,−46 promoter variant in compound heterozygosity with TPI missense or null alleles should be useful in arriving at a clearer delineation of the role of TPI −43,−46 as a putative deficiency variant, an open question discussed by Watanabe et al,19 Schneider et al,20 and Humphries et al,21 and again posed by our findings in family B.
Acknowledgments
The authors thank Jean-Marc Costa and Michel Videau for their help during the PCR experiments, Emmanuelle Girodon for her help in prenatal diagnosis, and Lynne E. Maquat for discussions of our sequencing results. Ernest Beutler and Constantin T. Craescu are acknowledged for multiple helpful discussions. This paper is dedicated to Raymonde and Jean Rosa, who first described most of the patients and gave us constant encouragement.
Supported by grants from INSERM, the Université Paris XII, and the National Institutes of Health (grant HL25 552).
Reprints:Michel Cohen-Solal, Unité INSERM U474, Maternité de Port-Royal, 123 Boulevard de Port-Royal, 75014 Paris, France; e-mail: mcs@cochin.inserm.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.