Abstract
Lentiviral vectors have the potential to play an important role in hemophilia gene therapy. The present study used human immunodeficiency virus (HIV)-based lentiviral vectors containing an EF1 enhancer/promoter driving human factors VIII (hFVIII) or IX (hFIX) complementary DNA expression for portal vein injection into C57Bl/6 mice. Increasing doses of hFIX-expressing lentivirus resulted in a dose-dependent, sustained increase in serum hFIX levels up to approximately 50-60 ng/mL. Partial hepatectomy resulted in a 4- to 6-fold increase (P < 0.005) in serum hFIX of up to 350 ng/mL compared with the nonhepatectomized counterparts. The expression of plasma hFVIII reached 30 ng/mL (15% of normal) but was transient as the plasma levels fell concomitant with the formation of anti-hFVIII antibodies. However, hFVIII levels were persistent in immunodeficient C57Bl/6 scid mice, suggesting humoral immunity-limited gene expression in immunocompetent mice. This study demonstrates that lentiviral vectors can produce therapeutic levels of coagulation factors in vivo, which can be enhanced with hepatocellular proliferation.
Gene therapy for hemophilia has been targeted to the liver because it is the normal site of factor VIII and factor IX synthesis, although biologically active factor IX can be synthesized elsewhere in the body. To obtain expression of factors VIII and IX in vivo, retroviral vectors based on the Moloney murine leukemia virus have been used because they are capable of integrating into the host chromosome. Retroviral delivery into the livers of dogs1 or mice,2 or into the murine musculature3,4 have produced long-term but not always therapeutic levels of coagulation factors. Moreover, retroviral transduction was performed in animals that were partially hepatectomized1 or were very young2 (2-3 days old) at a time when the hepatocytes were proliferating.
To overcome these limitations, some investigators have used human immunodeficiency virus (HIV)-1–based lentiviral vectors for stable gene transfer in the liver because they found these vectors could efficiently transduce a variety of nondividing cells in vivo.5-8 Recently, we have found that lentiviral transduction in the liver is highly dependent on the progression of hepatocytes through the cell cycle.9 In this study, we tested whether lentiviral vectors could produce therapeutic concentrations of circulating human factors VIII (hFVIII) and IX (hFIX) in mice.
Study design
Helper lentiviral packaging constructs (pCMVΔR8, pCMVΔR8.2, and pCMVΔR8.74) as well as the VSV-G envelope plasmid (pMD.G) were previously described.5,6 10
The pHR2PGKhFIX transfer plasmid was provided by L. Naldini (University of Torino, Torino, Italy). The vector plasmids containing the EF1α enhancer/promoter with the hFVIII (B-domain deleted) and hFIX complementary DNAs (cDNAs) were derived from pHR2PGKhFIX plasmid by standard cloning.
The replication-defective lentivirus was produced and monitored for the presence of replication-competent virus as previously described9 except the conditioned media (15 mL/plate) was concentrated as described by Sutton et al.11 In general, lentiviral vectors that expressed lacZ in HeLa cells resulted in 2-7 × 106 transducing units/μg p24 Gag antigen.
Female C57Bl/6 and C57Bl/6 scid mice (8 weeks of age) were purchased from Jackson Laboratories. All animal procedures9and assays for hFIX12 and ALT13 were previously described. hFVIII levels were measured by enzyme-linked immunosorbent assay (ELISA) as described by the manufacturer (Affinity Biologicals, Hamilton, ON). hFVIII antibodies were measured by ELISA, using a slight modification of the method by Le et al.14
Results and discussion
Liver injury associated with lentiviral administration in vivo
Similar to our previous study,9 we found that the intraportal administration of lentiviral vectors was associated with dose-dependent, self-limited hepatic injury. Serum alanine aminotransferase (ALT) levels were increased during the first 24 hours after vector infusion in all mouse groups treated with lentivirus (but not vehicle controls) but returned to normal by day 3 (Table1). The causative factor(s) responsible for the transient liver injury is presently unknown, but either virion proteins like viral protein R (vpr) and VSV-G, which are known to have potential toxic effects in vitro,15,16 or concentrated contaminants in the viral preparations may be the responsible factors. Nevertheless, the liver injury associated with vector administration has been shown to induce hepatocellular cycling and may be involved in enhancing the efficiency of lentiviral vector transduction.9
Human factor IX expression in vivo
We injected PGK-hFIX and EF1α-hFIX lentiviral vectors into the portal vein of C57Bl/6 mice at 2 different concentrations (10 and 35 μg p24 Gag antigen). Mice administered PGK-hFIX lentivirus (n = 4) had undetectable levels of serum hFIX (< 1.6 ng/mL), whereas stable low-level serum hFIX was detected in EF1α-hFIX injected mice (n = 5). For this reason, all subsequent studies used the EF1α-hFIX lentiviral vector.
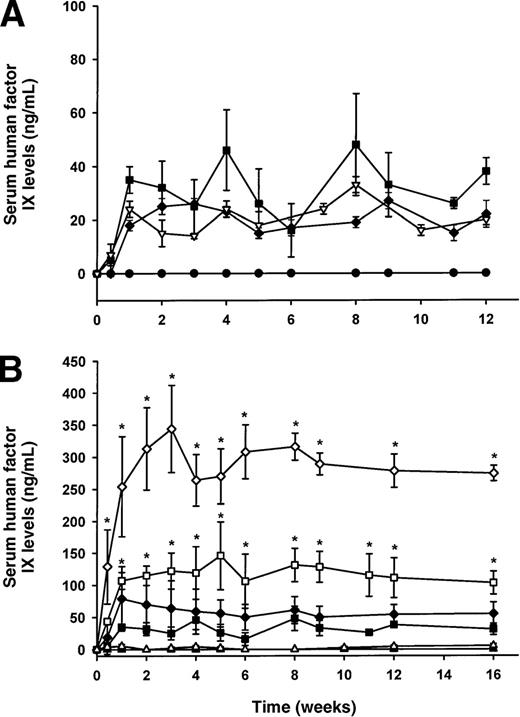
The issue of whether hepatic gene transfer is influenced by the presence of accessory proteins in the vector remains unclear.8,9 In this study, C57Bl/6 mice injected with lentiviral vectors 36 μg p24 containing (ΔR8.2) or lacking (ΔR8.74) accessory proteins produced similar levels of serum hFIX (∼20-30 ng/mL; Figure 1A) consistent with our earlier observations9 that lentiviral transduction in the liver does not depend on accessory proteins. Similar levels of serum hFIX were observed in immune-competent versus immune-deficient mice (Figure 1A), suggesting the absence of an immune response against hFIX. In addition, the absence of accessory proteins in lentiviral vectors will help to avoid the potential generation of replication-competent lentiviruses and also the potential toxicity associated with the accessory proteins like vpr.15 Thus, subsequent experiments were performed with vectors produced with the pCMVΔR8.74 packaging construct deficient in the accessory genes.
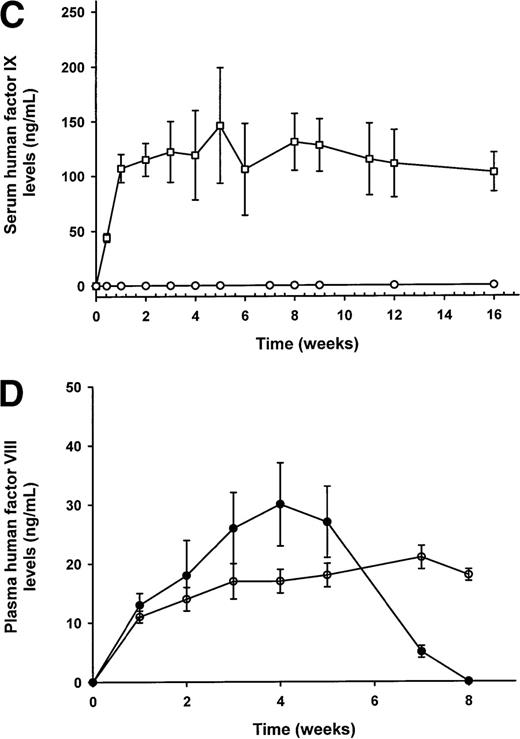
Serum human factor IX (hFIX; Figure 1A-C) and plasma human factor VIII (hFVIII; Figure 1D) levels as measured by ELISA.
(A) hFIX expression following administration of lentivirus 36 μg p24 with (□) and without (▪, ▿) viral accessory proteins in C57Bl/6 (□, ▪) and C57Bl/6 scid (▿) mice. As a negative control for the expression of hFIX, C57Bl/6 mice were given vehicle solution (•). (B) The role of hepatocellular proliferation on lentiviral transduction and expression of hFIX in C57Bl/6 mouse serum. Increasing doses of lentivirus from 8 (▵, ▴) to 36 (□, ▪) and to 108 (⋄, ♦) μg p24 Gag antigen were injected into the portal vein of mice with (▵, □, ⋄) or without (▴, ▪, ♦) prior partial hepatectomy. (C) The role of integrase on the expression of hFIX in partially hepatectomized C57Bl/6 mice. Lentiviral vectors with functional (○) or nonfunctional integrase (•) were administered into mice, and the expression of hFIX was measured by ELISA. (D) Plasma levels of hFVIII in C57Bl/6 (•) and C57Bl/6 scid (○) mice (n = 3/group), which have been partially hepatectomized 48 hours prior to lentiviral administration of 140 μg and 108 μg p24 Gag antigen, respectively. Mean values ± SEM are shown in the figures. *P < .005 comparing hepatectomized versus nonhepatectomized mice in the same dose group. The significance of differences between groups at the same dose of lentivirus (with or without a partial hepatectomy) was tested by a one-way ANOVA with the use of StatView 5.0 software. If a probability value of P < .05 was obtained, the Tukey test was then used for comparison for each individual group with the appropriate control.
Serum human factor IX (hFIX; Figure 1A-C) and plasma human factor VIII (hFVIII; Figure 1D) levels as measured by ELISA.
(A) hFIX expression following administration of lentivirus 36 μg p24 with (□) and without (▪, ▿) viral accessory proteins in C57Bl/6 (□, ▪) and C57Bl/6 scid (▿) mice. As a negative control for the expression of hFIX, C57Bl/6 mice were given vehicle solution (•). (B) The role of hepatocellular proliferation on lentiviral transduction and expression of hFIX in C57Bl/6 mouse serum. Increasing doses of lentivirus from 8 (▵, ▴) to 36 (□, ▪) and to 108 (⋄, ♦) μg p24 Gag antigen were injected into the portal vein of mice with (▵, □, ⋄) or without (▴, ▪, ♦) prior partial hepatectomy. (C) The role of integrase on the expression of hFIX in partially hepatectomized C57Bl/6 mice. Lentiviral vectors with functional (○) or nonfunctional integrase (•) were administered into mice, and the expression of hFIX was measured by ELISA. (D) Plasma levels of hFVIII in C57Bl/6 (•) and C57Bl/6 scid (○) mice (n = 3/group), which have been partially hepatectomized 48 hours prior to lentiviral administration of 140 μg and 108 μg p24 Gag antigen, respectively. Mean values ± SEM are shown in the figures. *P < .005 comparing hepatectomized versus nonhepatectomized mice in the same dose group. The significance of differences between groups at the same dose of lentivirus (with or without a partial hepatectomy) was tested by a one-way ANOVA with the use of StatView 5.0 software. If a probability value of P < .05 was obtained, the Tukey test was then used for comparison for each individual group with the appropriate control.
Dose response and the role of hepatocellular proliferation on hFIX expression
C57Bl/6 mice were injected with EF1α-hFIX into the portal vein with increasing doses of lentivirus (8, 36, and 108 μg p24). Although serum hFIX levels were undetectable at the lowest dose of lentivirus used (n = 5), when the dose of lentivirus was increased to 36 μg (n = 4) or 108 μg p24 (n = 4), serum hFIX levels became persistently detectable (Figure 1B). At the highest dose of lentivirus (108 μg p24), therapeutic levels of hFIX were observed (∼50-60 ng/mL, ∼1% of normal human levels).
A surgical partial hepatectomy was performed 48 hours prior to lentiviral administration to maximize liver regeneration and to enhance lentiviral transduction. As the dose of lentivirus was increased from 8 to 108 μg p24 in mice, there was a dose-dependent increase in serum hFIX. The data in Figure 1B revealed that at the lowest dose, 8 μg p24, very low levels of serum hFIX were detected (∼2-3 ng/mL; n = 5). Partially hepatectomized animals receiving lentivirus at a dose of 36 μg p24 (Figure 1B) had significantly more (∼4-fold;P < .005) circulating hFIX (∼105 ng/mL; n = 6) than their nonhepatectomized counterparts. At the highest dose of lentivirus (108 μg p24), mice given a partial hepatectomy had an approximate 6-fold increase in serum hFIX concentration, reaching from 270 to 350 ng/mL (∼6% of normal) compared with nonhepatectomized mice (P < .005; Figure 1B). This finding demonstrates that hepatocellular proliferation significantly enhanced lentiviral expression of hFIX from mouse liver. Note that the 4- to 6-fold increase in serum factor IX observed with partial hepatectomy was less than the 11- to 30-fold increase in hepatocellular transduction observed with a β-galactosidase vector. The most likely explanation for the difference is due to lentiviral transduction and hFIX production from nonparenchymal liver and splenic cells that are also transduced in the absence of a partial hepatectomy during intraportal infusion of the virus.9
Previous studies using Moloney-based retroviruses showed maximal serum hFIX concentrations in the range of 2-10 ng/mL, even with a partial hepatectomy.1 This serum level of factor IX was substantially less than the approximate 300 ng/mL achieved in the current study with lentiviral vectors. The reason lentiviral vectors appear to have greater efficacy than Moloney vectors despite the fact that both require cell cycle progression may be due to several parameters. First, in contrast to lentiviruses, Moloney viruses require nuclear membrane breakdown for transduction.17 In the liver, DNA synthesis can occur in the absence of cellular or nuclear division. Second, lentiviral vectors may be able to persist in a pre-integration state longer than Moloney vectors so that lentiviral transduction may occur over time as cells cycle.9 Although the use of lentiviral vectors for the expression of hFIX is promising, the production of hFIX at a therapeutic level occurs only at a dose that results in liver injury. In comparison, adeno-associated virus vectors using either an EF1α-hFIX, retroviral LTR, or liver-specific enhancer/promoter can produce higher levels of hFIX, ranging from 200 to 20 000 ng/mL18-20 at safe, nontoxic doses. Further improvements in lentiviral vectors will likely be required to achieve therapeutic levels of hFIX at a dose that is considered safe.
Role of lentiviral integration on hFIX expression
A point mutation (D64V) in the integrase gene of the lentivirus is known to severely diminish proviral integration in vitro and in vivo, but it does not affect preceding steps in the lentivirus life cycle.5,6,21 Previous in vitro and in vivo studies have demonstrated that an integrase-defective lentivirus injected into the brain of mice failed to express β-galactoside, demonstrating that episomal forms of lentiviral particles are incapable of expressing transgene products.5 Moreover, our previous in vivo liver studies9 have shown that the provirus integrates into the host chromosome in a cell cycle-dependent manner. For this reason, we determined the relationship between proviral integration and the expression of hFIX. To maximize expression of hFIX from an integrase-defective (ΔR8) lentiviral vector, a partial hepatectomy was performed on C57Bl/6 mice (n = 4) 48 hours prior to administration of the lentiviral vector at a dose of 36 μg p24. Results showed that, in the absence of proviral integration, no hFIX was detected in mouse serum (< 1.6 ng/mL; Figure 1C) over a 16-week period (n = 4; Figure 1B), whereas mice that received lentiviral particles with a functional integrase gene produced therapeutic levels of hFIX (∼105 ng/mL; Figure 1B and re-graphed in Figure 1C). Therefore, lentiviral integration was essential for hFIX expression in mouse serum.
Human factor VIII expression in vivo
Lentiviral vectors using the EF1α enhancer/promoter expressing B-domain deleted hFVIII cDNA were injected into the portal vein of mice, who had undergone a surgical partial hepatectomy 48 hours prior to vector administration. C57Bl/6 mice administered lentivirus at a dose of 140 μg p24 transiently expressed hFVIII concomitant with the formation of anti-hFVIII antibodies (not shown). Plasma hFVIII levels reached a peak of 30 ± 7 ng/mL (∼15% of normal) by week 4, but, by week 8, the levels were undetectable (< 1.6 ng/mL; Figure1D). It is likely the loss of hFVIII was immune related because C57Bl/6scid mice (n = 3) injected with a dose of 108 μg p24 of lentivirus had persistent expression of plasma hFVIII levels for 8 weeks. The immunogenic response against hFVIII in C57Bl/6 mice has been observed in some but not all gene transfer studies.2,22Variables that might affect antibody formation include differences in the variety of cell types transduced, liver injury due to the vector, promoter selection, and substrain variation in mice obtained from different vendors. Lentiviruses have been found to transduce splenic cells and nonparenchymal cells in the liver, which can potentially function as robust antigen-presenting cells.9 Moreover, in the context of a recombinant adenovirus vector, the comparison of a hepatocyte-specific promoter with an ubiquitous one has been shown to reduce antibody production against the transgene product.23The factor(s) involved in mediating an antigenic response against hFVIII in the studies performed here remains to be determined.
Future development of lentiviral-mediated gene therapy for hemophilia
This study demonstrates that lentiviral vectors can produce therapeutic levels of coagulation factors, but it raises several issues that need to be addressed. Better purification methods for lentiviral vectors are needed to help prevent or to reduce the liver injury associated with lentivirus administration. The present study, in conjunction with our previous work,9 demonstrates that viral injury causes normally quiescent hepatocytes to progress into the cell cycle to enhance lentiviral transduction. For this reason, to produce therapeutic levels of coagulation factors, techniques need to be developed to promote cell cycle progression in a safer, nonsurgical manner, such as the use of growth factors like hepatocyte growth factor or keratinocyte growth factor.13 24
Acknowledgment
The authors appreciate the help from Leonard Meuse for the handling of the mice.
Supported by grant HL53682 from the National Institutes of Health.
F.P. is a recipient of the Judith Graham Pool Fellowship through the National Hemophilia Foundation.
K.O. is a recipient for the Japan Society for the Promotion of Science Fellowship.
Reprints:Mark A. Kay, Department of Pediatrics and Genetics, Stanford University, Stanford, CA 94305; e-mail:markay@leland.stanford.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.