Several randomized trials of graft-versus-host-disease (GVHD) prophylaxis have included methylprednisolone (MP),1-4 but in those studies MP was administered in both study arms in combination with other agents. A trial reported from this institution compared the effect of a combination of methotrexate (MTX) plus cyclosporine (CSP) with the 3-drug combination MTX plus CSP plus MP and failed to show an overall advantage of the addition of MP.5 The effects of the addition of MP to a standard regimen of CSP had never been studied in a randomized trial. In the early 1990s we conducted such a trial comparing CSP as a single agent to CSP plus MP as GVHD prophylaxis. The hypothesis was that the incidence of GVHD would be lower with the drug combination, but there was also concern that the addition of MP might increase the risk of infections6 and other posttransplantation complications. Because of continued interest in the role of MP for GVHD prophylaxis, we thought it would be important to provide long-term follow-up on our study.
The study involved 122 patients with advanced lymphohemopoietic malignancies who were considered to be at high risk for posttransplantation complications and relapse.7 All patients received marrow from HLA-identical sibling donors. Patient and transplantation characteristics are summarized in Table 1.
Daily CSP was started on day −1 (5 mg/kg/d IV) and given at gradually reduced doses until day 180. MP was started on day +7 at 0.5 mg/kg/d, increased to 1 mg/kg/d on day 15, started on a taper schedule on day 29, and discontinued on day 72. In the initial publication, we reported incidence rates of acute GVHD grades II-IV of 73% and 60% for patients given CSP and CSP plus MP, respectively (P = .01). Chronic GVHD, however, was more frequent among patients who received CSP plus MP (44% vs 21%; P = .02). The conditional probabilities of developing chronic GVHD were 94% and 51% for patients receiving CSP plus MP or CSP, respectively (P = .03). There was a suggestion that the risk of relapse was lower in patients who received CSP plus MP (P = .10). While early overall survival did not differ between the 2 groups (P = .44), there was a trend toward better relapse-free survival in patients on CSP plus MP (P = .07). There was no significant difference in the incidence of early posttransplantation infections between both groups.
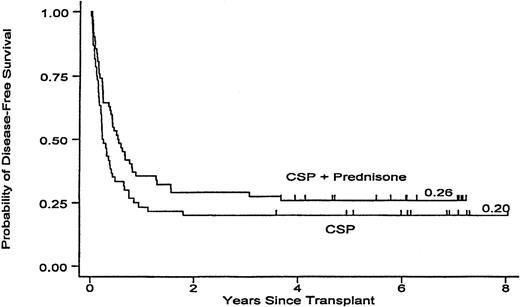
The minimum follow-up for surviving patients is now more than 3.6 years (median 6.1; maximum 8.0). The probability for overall survival is 23% among patients randomized to CSP prophylaxis and 26% among patients who received CSP plus MP. Among patients who received CSP alone, 17 relapsed 42-657 (median 81) days after transplantation, compared with 15 patients on CSP plus MP, 57-1343 (median 217) days after transplantation, for cumulative incidences of relapse of 28% and 24% for the 2 respective groups (P = .19). Currently, 12 patients in the CSP arm are surviving in remission (20%), compared with 16 patients (26%) in the CSP plus MP arm (P = .11) (Figure 1).
Leukemia-free survival in patients receiving GVHD prophylaxis with CSP alone or with CSP plus MP. P = .11.
Leukemia-free survival in patients receiving GVHD prophylaxis with CSP alone or with CSP plus MP. P = .11.
At the time of last contact, the Karnofsky performance scores were 80-100 (median 100) and 60-100 (median 100) for the CSP-treated and the CSP-plus-MP–treated patients, respectively. One patient in the CSP arm still requires immunosuppressive therapy, compared with 4 patients who had received CSP-plus-MP prophylaxis. One patient in the CSP arm and 4 in the CSP-plus-MP arm developed chronic pulmonary disease. Three patients in the CSP arm developed aseptic necrosis (involving 1-4 joints), requiring joint replacement surgery in 2, compared with 5 patients (involving 1-3 joints) in the CSP-plus-MP arm, requiring replacement surgery in 2.
Thus long-term observations in this randomized trial fail to show any significant advantage to the addition of MP to CSP as GVHD prophylaxis. Although there was a somewhat lower incidence of acute GVHD (accounted for entirely by skin involvement7) in patients who received the drug combination, the cumulative incidence of chronic GVHD was significantly increased. Earlier trends toward a reduced incidence of relapse and better relapse-free survival with the drug combination have diminished since our initial report, and neither difference is significant. At the same time, however, the lowest current Karnofsky scores were seen among patients who had received CSP plus MP, and there is a suggestion that more patients in this cohort have developed complications such as aseptic necrosis of the bone and chronic pulmonary disease and have required immunosuppressive therapy for a longer duration.
Very few patients enrolled in this trial were able to avoid the use of MP completely since MP was generally given as initial therapy when GVHD developed. But considering the overall course of these patients, the probability of developing delayed complications, and their current performance status, there is no basis on which to recommend MP plus CSP as a GVHD prophylactic regimen. Although these reuslts do not exclude the possibility that MP may be beneficial when combined with other drugs, data to support such a claim are lacking.