Abstract
Retroviral vectors based on the Moloney murine leukemia virus (MuLV) have become the primary tool for gene delivery into hematopoietic cells, but clinical trials have been hampered by low transduction efficiencies. Recently, we and others have shown that gene transfer of MuLV-based vectors into T cells can be significantly augmented using a fibronectin-facilitated protocol. Nevertheless, the relative abilities of naive (CD45RA+) and memory (CD45RO+) lymphocyte subsets to be transduced has not been assessed. Although naive T cells demonstrate a restricted cytokine profile following antigen stimulation and a decreased susceptibility to infection with human immunodeficiency virus, it was not clear whether they could be efficiently infected with a MuLV vector. This study describes conditions that permitted gene transfer of an enhanced green fluorescent protein-expressing retroviral vector in more than 50% of naive umbilical cord (UC) blood and peripheral blood (PB) T cells following CD3/CD28 ligation. Moreover, treatment of naive T cells with interleukin-7 resulted in the maintenance of a CD45RA phenotype and gene transfer levels approached 20%. Finally, it was determined that parameters for optimal transduction of CD45RA+ T cells isolated from PB and UC blood differed: transduction of the UC cells was significantly increased by the presence of autologous mononuclear cells (24.5% versus 56.5%). Because naive T cells harbor a receptor repertoire that allows them to respond to novel antigens, the development of protocols targeting their transduction is crucial for gene therapy applications. This approach will also allow the functions of exogenous genes to be evaluated in primary nontransformed naive T cells.
Clinical trials targeting hematopoietic stem cells have almost exclusively used retroviral vectors derived from the Moloney murine leukemia virus (MuLV).1,2 These MuLV-based vectors are characterized by the integration of their genetic material into target cell genomes. Although the success of clinical protocols has been impeded by low gene transfer, new techniques have significantly improved transduction efficiencies. Specifically, colocalization of retrovirus and target cells on specific adhesion domains of a recombinant fibronectin molecule has resulted in augmented transduction of both CD34+ progenitor cells and T lymphocytes.3-7 Furthermore, the use of markers that allow transduced cells to be easily and quickly identified, such as CD2, the truncated nerve growth factor receptor, and the enhanced green fluorescent protein (EGFP), have markedly improved our ability to monitor the gene transfer efficiency of various protocols.8-10
In patients with various genetic or acquired hematologic disorders, transduction of the T-cell population is highly desirable. Specifically, in patients for whom lymphocytes are reinfused to obtain a graft-versus-leukemia effect, introduction of a suicide gene that allows the T cells to be obliterated under conditions of excessive graft-versus-host disease (GVHD) would significantly minimize toxicity. Retroviral-mediated transfer of immunomodulatory cytokines and antiviral genes into T cells will enable the development of innovative therapies for patients with cancer and those with human immunodeficiency virus type 1 (HIV-1), respectively.11-14Moreover, transfer of wild-type genes into T cells with defects in enzymatic genes such as adenosine deaminase may allow the correction of these types of disorders.15-17 Although previous studies targeting T lymphocytes as a vehicle for gene therapy have focused on optimizing protocols for transduction, the advances in gene transfer technology discussed above now allow us to dissect the relative transduction levels in distinct T-cell subsets. T cells can be divided into naive and memory populations and gene transfer into the former cells is of utmost importance because it is this subgroup that theoretically maintains the capacity to respond to novel antigens. Furthermore, gene transfer into naive T cells will be required for all protocols targeting the lymphocyte population present in umbilical cord (UC) blood because the majority of UC T cells are naive.18
Expression of distinct splice variants of the cell surface CD45 tyrosine phosphatase has been used to distinguish naive and memory T cells. Naive T cells have been phenotypically identified as those lymphocytes expressing the high molecular weight CD45RA isoform, whereas the T-lymphocyte population harboring memory function exhibits a reciprocal expression of the low molecular weight isoform, CD45RO.19 After exposure of naive lymphocytes to their cognate antigen, there is a loss of CD45RA, induction of CD45RO expression, and an acquisition of effector functions. Naive CD45RA+ and memory CD45RO+ T subsets differ in their activation requirements, chemokine/cytokine secretion patterns, and adhesion molecule expression.19
The restricted lymphokine profile of naive T lymphocytes and their distinct activation requirements have been invoked to explain the observed decreased susceptibility of this T-cell subset to productive HIV-1 infection.20-23 However, the relative susceptibility of naive and memory T cells to infection with a replication-competent MuLV or with an MuLV-derived vector has never been determined. Because MuLV-derived vectors have been most extensively used for hematopoietic cell transfer in clinical trials, we assessed the capacity of naive UC and peripheral blood (PB) T cells to be transduced with an EGFP-expressing MuLV retroviral vector pseudotyped with the gibbon ape leukemia virus envelope. We find that although parameters resulting in optimal gene transfer in naive UC T cells and adult PB T cells differ, both populations can be transduced with more than 50% efficiency following CD3/CD28 stimulation. Moreover, we demonstrate that interleukin (IL)-7 cytokine treatment maintains the naive phenotype of CD45RA+ T cells and is sufficient to permit efficient gene transfer in these cells. Thus, it is feasible to exploit an MuLV-based system to introduce genes of interest in the naive T-cell subset.
Materials and methods
Vector and virus production
The PG13 packaging cell expressing the gibbon ape leukemia virus envelope and harboring the LZRS-EGFP retroviral vector was generated as previously described.7 Briefly, the EGFP complementary DNA (cDNA) (Clontech, Palo Alto, CA) was cloned downstream of the internal ribosome entry site (IRES) in the LZRS retroviral vector and expressed from the retroviral long terminal repeat. PG13 cells transduced with LZRS-EGFP were identified and sorted on a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA). PG13/LZRS-EGFP cells from this sorted pool were cloned by limiting dilution and selected based on their ability to transduce the Jurkat T cell line with high efficiency. The PG13/LZRS-EGFP cell line was grown in Dulbecco's modified Eagle's medium DMEM (GibcoBRL, Grand Island, NY) supplemented with 10% fetal calf serum (FCS), penicillin, and streptomycin. Retroviral supernatants were collected from 80% to 90% confluent plates after a 24- or 48-hour incubation period in a humidified incubator at 32°C. The collected culture medium was filtered through a 0.45-μm pore size filter (Sartorius, Göttingen, Germany) and stored at −80°C for further use. The retroviral particles produced from the PG13/LZRS-EGFP clone used here were replication incompetent as monitored by a reverse transcriptase assay.24
Monoclonal antibodies and cell cycle analysis
Fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated anti-CD5, anti-CD45RA, anti-CD45RO, and anti-CD3 antibodies were obtained from Immunotech (Marseille, France). At the indicated time points, cells were incubated with antibodies for 20 minutes and then washed in PBS before FACS analysis.
The percentage of cells in the S-G2/M phases of the cell cycle was determined by propidium iodide (PI) staining. At the indicated time points, cells were resuspended in PI (50 μg/mL, Sigma-Aldrich, St Louis, MO) diluted in PBS with 5% glycerol and 0.1% Triton-X100 and incubated for at least 15 minutes prior to analysis. Cell cycle was analyzed on a FACScan flow cytometer on the FL2-A wavelength after gating out signals due to cell debris.
Preparation of primary T cells and lymphocyte purification
The UC blood samples were obtained from full-term uncomplicated deliveries at the Clinique Saint Roch (Montpellier, France) and PB samples were obtained from healthy adult donors after informed consent was obtained. PB mononuclear cells (PBMC) and umbilical cord mononuclear cells (UCMC) were separated by Ficoll-Hypaque (Sigma) density gradient centrifugation. To obtain purified CD3+ T cells, PBMC and UCMC were incubated with an anti-CD3 antibody conjugated with Dynabeads (CD3 PanT M-450 Dynal, Oslo, Norway). After a 45-minute incubation at 4°C with continuous shaking, the bead-bound cells were recovered using a magnet (Dynal). The bead-bound cells were washed at least 5 times to remove contaminating cells. This separation process resulted in an average 92% purity of positively selected cells. In some experiments, CD3+ cells were purified by negative selection. UCMC were incubated with a cocktail of tetrameric antibodies complexes containing anti-CD14, anti-CD16, anti-CD19, anti-CD56, and anti-glyA (StemSep, Stem Cell Technologies Inc, Vancouver, Canada). After 30 minutes of incubation at 4°C, magnetic colloid was added for another 30 minutes. Samples were then loaded in a magnetic column where antibody-bound cells were retained. The purity of the released population was approximately 90%.
To purify the CD45RA+ and CD45RO+ PB T-cell subsets, PBMC were first costained with FITC-conjugated anti-CD5 and PE-conjugated anti-CD45 RA or RO antibodies. CD5+ PBMC were sorted on a FACS Vantage Cell Sorter into reciprocal subsets of negative-expressing cells for either the RO or RA isoform of the CD45 membrane phosphatase. After sorting, the isolated negative-staining subset were purified to at least 95%.
Lymphocyte activation and retroviral transductions
Purified as well as nonpurified T-cell populations were grown in RPMI medium (GibcoBRL) with 10% FCS supplemented with IL-2 (75 U/mL) (Chiron Corporation, Emeryville, CA). Before transduction, lymphocytes (1 × 106/well) were stimulated with 2 immobilized anti-CD3 antibodies (1μg/mL)-UCHT1 (the generous gift of G. Boonen, Utrecht, Netherlands) and OKT3 (ATCC, Bethesda, MD) together with an anti-CD28 antibody (9.3, 1μg/mL; kindly provided by C. June, Bethesda, MD) on non-tissue culture-treated plates. In some experiments, cells were stimulated with IL-7 alone (10 ng/mL) (Peprotech, London, England) and transduced in the presence of IL-7 as described below.
At the indicated time points, cells were transduced on fibronectin-coated plates essentially as described by Moritz et al.3 The recombinant fibronectin fragment (CH-296), which contains the connecting segment, cell-binding domain, and heparin-binding domain,25 was kindly provided by Takara Shuzo Company (Otsu Shiga, Japan). Non-tissue culture-treated 24-well plates were coated with fibronectin (8 μg/cm2) for 2 hours at room temperature. The unbound fibronectin was then removed, plates were blocked with 1% bovine serum albumin (BSA) for 20 minutes at 37°C, and subsequently washed once with PBS prior to use. Lymphocytes (1 × 106) in 0.5 mL of RPMI medium were then incubated with 0.5 mL of retroviral supernatant on these fibronectin-coated wells. After a 6-hour exposure to retrovirus at 37°C, cells were centrifuged and resuspended in fresh medium with IL-2. When multiple transductions were performed, the retroviral supernatant was removed after 6 hours and cells were left on fibronectin with fresh medium before the transduction procedure was repeated the following day. Transduction efficiencies were assessed 48 hours later. Transduced cells were identified by their FL-1 autofluorescence on a FACScan.
Southern blot analysis
Transduced UC and PBL samples were FACS sorted on the basis of EGFP expression and genomic DNA was isolated from both EGFP− and EGFP+ populations with a commercially available kit (Gentra Systems, Minneapolis, MN). Ten micrograms of genomic DNA was incubated with XbaI for 9 hours at 37°C, electrophoresed overnight in 0.8% agarose gels, and transferred onto Hybond N+ membranes (Amersham, Uppsala, Sweden) by capillary action. Membranes were hybridized at 42°C overnight with an α-[32P]dCTP random-primed labeledNotI-NotI EGFP probe (1328 bp). Following washes in 2X standard saline citrate/2% sodium dodecyl sulfate at 68°C, filters were exposed to x-ray film at −80°C.
T-cell repertoire analysis
Total RNA was prepared using the TRIzol reagent (GibcoBRL). RNA (2 μg) was reverse transcribed with random hexanucleotides (Pharmacia Biotech, Uppsala, Sweden) using M-MuLV reverse transcriptase (GibcoBRL). cDNAs were amplified (40 cycles) in a 25-μL reaction mixture with 1 of the 24 TCRBV subfamily-specific primers and a Cβ primer recognizing the 2 constant regions Cβ1 and Cβ2 of the beta chain of the T-cell receptor (TCR), as previously described.26,27 Two microliters of the 24 TCRBV/Cβ-first run polymerase chain reaction (PCR) products were subjected to 2 cycles of elongation (run-off) using a Cβ dye labeled (6-Fam) primer allowing PCR products to be detected on a 337 automated DNA sequencer (Applied Biosystems, Foster City, CA). One microliter of the run-off PCR products was loaded on a 24-cm 6% acrylamide sequencing gel and analyzed for size and fluorescence intensity using the Immunoscope software. The TCRBV nomenclature proposed by Arden and colleagues was used in this study.28
Results
Phenotype of naive T cells after TCR stimulation
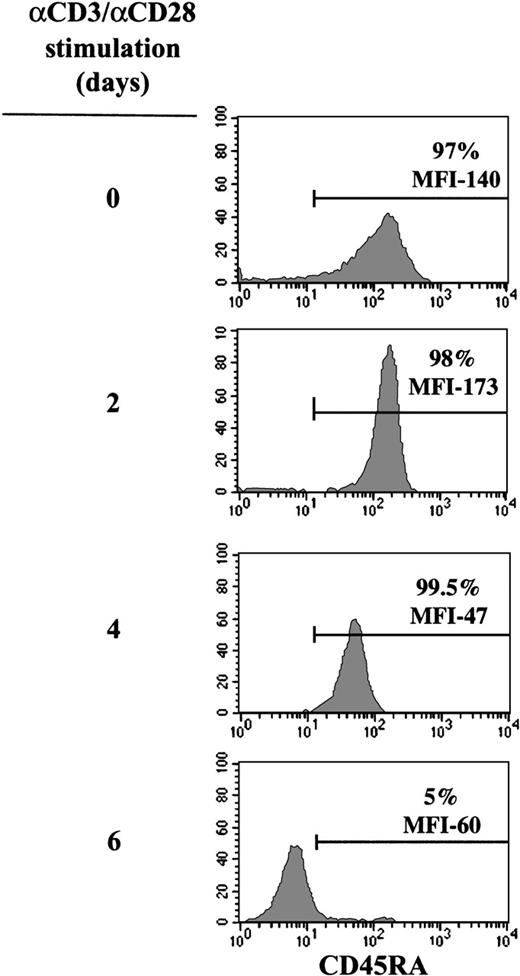
Expression of the RA isoform of the CD45 cell surface glycoprotein is one of the best described phenotypic criteria for distinguishing naive T cells from memory T cells. Because the majority of UC T cells are immature and express CD45RA on the cell surface,18homogenous populations of naive T lymphocytes can be isolated from this source. Furthermore, the use of UC cells ensures that the CD45RA+ T-cell population is truly naive and does not represent a “back-conversion” of memory CD45RO T cells.18 29-32 Because transduction of T cells with an MuLV-based vector requires that the cells are prestimulated, we first monitored expression of CD45RA on the cell surface following engagement of the CD3 and CD28 receptors. More than 95% of CD3+cells, positively selected from UC samples using anti-CD3-coated magnetic beads, routinely expressed the CD45RA antigen (Figure1). Stimulation of these naive T cells with immobilized anti-CD3 and anti-CD28 monoclonal antibodies resulted in an initial increase in CD45RA expression (between 24 and 48 hours after activation), demonstrated by an augmented mean fluorescence intensity (MFI) of CD45RA on the cell surface (Figure 1). Following this early response, there was a concomitant down-regulation of CD45RA and an up-regulation of the memory marker CD45RO, such that by day 6 of mitogen treatment, essentially all cells converted to a CD45RA−/RO+ phenotype (Figure 1 and data not shown).
Evolution of CD45RA expression in CD3/CD28-stimulated UC T cells.
Cell surface expression of CD45RA on purified UC T cells was monitored using a PE-conjugated anti-CD45RA monoclonal antibody following 0, 2, 4, and 6 days of mitogen stimulation with immobilized anti-CD3 and anti-CD28 monoclonal antibodies. The percentage of CD45RA+cells and the mean fluorescence intensity (MFI), indicative of the level of expression, are indicated. Results are representative of data obtained with 10 different donors.
Evolution of CD45RA expression in CD3/CD28-stimulated UC T cells.
Cell surface expression of CD45RA on purified UC T cells was monitored using a PE-conjugated anti-CD45RA monoclonal antibody following 0, 2, 4, and 6 days of mitogen stimulation with immobilized anti-CD3 and anti-CD28 monoclonal antibodies. The percentage of CD45RA+cells and the mean fluorescence intensity (MFI), indicative of the level of expression, are indicated. Results are representative of data obtained with 10 different donors.
Comparison of gene transfer efficiency in naive UC T cells and PB T cells
We and others have demonstrated a high level of retroviral-mediated gene transfer into primary adult T cells using a fibronectin-mediated protocol.5-7 Nevertheless, adult T cells comprise both CD45RA+ and CD45RO+ subsets and the relative abilities of these 2 populations to be transduced with an MuLV retroviral expression vector has not been assessed. It remained to be determined whether naive T cells, with their restricted lymphokine profile and impaired ability to sustain productive HIV-1 infection, could be efficiently transduced with an MuLV-based vector. Because we found that gene transfer in adult T cells was optimal at 2 days after mitogen stimulation,7 we first assessed whether UC T cells, which express high levels of CD45RA at this time point (Figure 1), could be efficiently transduced. Purified CD3+ UC T cells were stimulated for 48 hours with immobilized anti-CD3/CD28 monoclonal antibodies and cells were then exposed to PG13/LZRS-EGFP retroviral supernatant on fibronectin-coated plates for 6 hours on 2 consecutive days. The PG13/LZRS-EGFP clone used here produces GALV-pseudotyped virions harboring the replication-incompetent MuLV-based LZRS vector expressing EGFP.7 Following transduction, cells were expanded with IL-2 and gene transfer was monitored 48 hours later by flow cytometry.
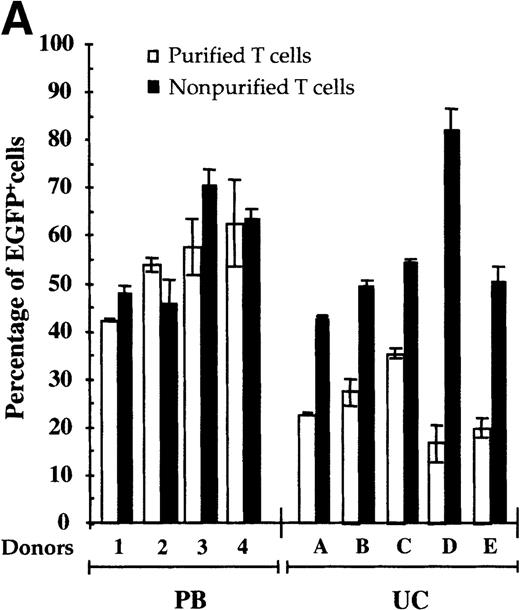
Although the level of gene transfer in purified UC T cells was relatively high, it was significantly lower than that observed in identically treated adult PB T cells (24.6 ± 7.3% versus 53.9 ± 8.6%) (Figure 2A). Specifically, gene transfer in purified UC T cells ranged from 15% to 35%, whereas transduction of PB T cells purified from individual donors varied from 40% to 75% (Figure 2A). Additionally, the level of EGFP expression in PB T cells, monitored by the MFI, was higher than that observed in purified UC T cells (MFI > 1000 versus MFI ∼ 500) (data not shown).
Autologous accessory cells enhance retroviral-mediated gene transfer into naive UC T cells.
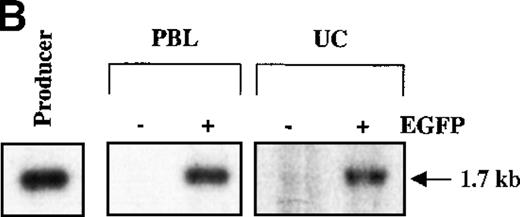
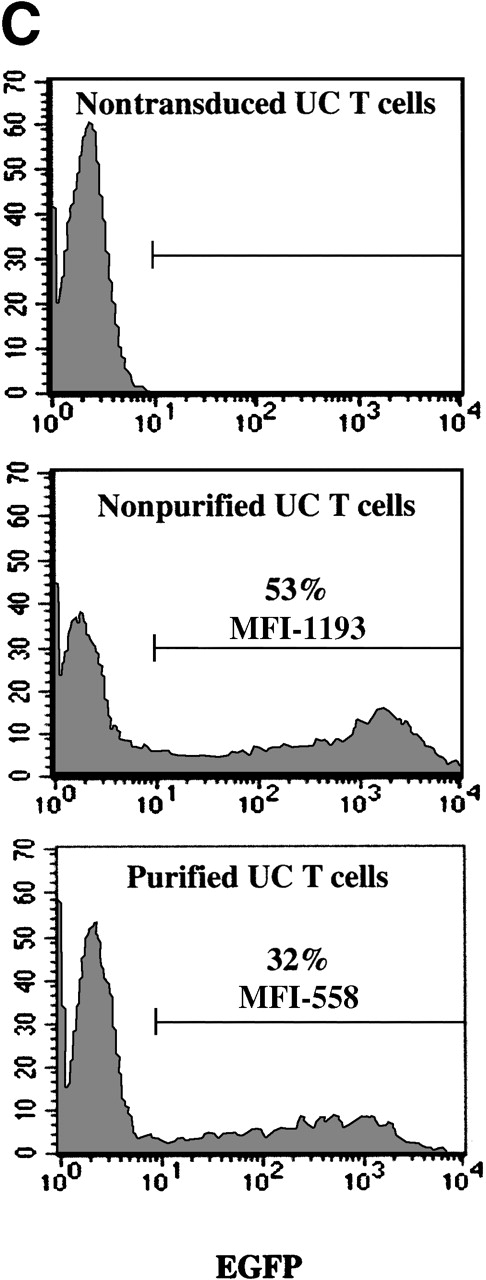
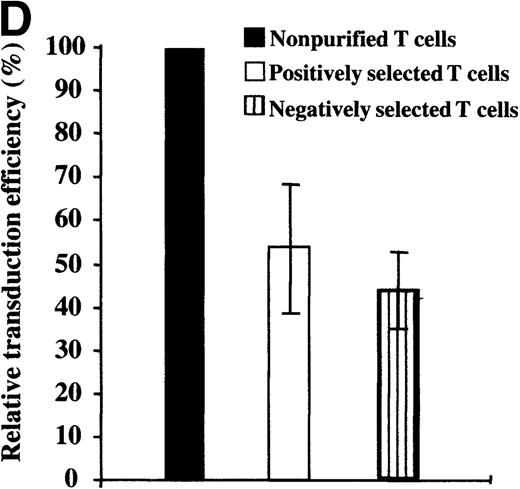
Mononuclear cells from UC blood and PB were obtained by Ficoll-Hypaque density centrifugation. To obtain a highly purified T-cell population devoid of accessory cells such as monocytes, T cells were positively selected using an anti-CD3 antibody conjugated to magnetic beads (Dynabeads). These purified T cells or nonpurified T-lymphocyte populations were then stimulated for 2 days with immobilized anti-CD3 and anti-CD28 antibodies and exposed twice to LZRS-EGFP retrovirus on fibronectin-coated plates. (A) Transduction efficiency of purified and nonpurified PB and UC T cells derived from individual donors. All transductions were performed in duplicate on fibronectin-coated plates and the mean percentage of EGFP-expressing cells was monitored 48 hours after transduction. (B) The presence of the LZRS-EGFP provirus in the EGFP− and EGFP+ UC and PB CD3+ subsets was monitored by Southern blot. CD3+ cells were purified by Dynal selection and transduced as described above. EGFP− and EGFP+populations were FACS sorted, genomic DNA was isolated, digested withXbaI, and hybridized with the 1.3-kb EGFP probe. The PG13/LZRS-EGFP producer clone was used as a positive control. The 1.7-kb fragment encompassing EGFP and the 3′LTR is indicated. (C) FACS profile of nontransduced and transduced UC T cells. The percentage of EGFP+ cells as well as the level of EGFP expression (MFI) is indicated. (D) Relative gene transfer in UC T cells following purification by either a positive (Dynal) or negative selection method (Stemsep) is compared to that obtained in a nonpurified T-cell population. The mean ± SD of EGFP+ cells in 2 to 5 independent experiments is shown.
Autologous accessory cells enhance retroviral-mediated gene transfer into naive UC T cells.
Mononuclear cells from UC blood and PB were obtained by Ficoll-Hypaque density centrifugation. To obtain a highly purified T-cell population devoid of accessory cells such as monocytes, T cells were positively selected using an anti-CD3 antibody conjugated to magnetic beads (Dynabeads). These purified T cells or nonpurified T-lymphocyte populations were then stimulated for 2 days with immobilized anti-CD3 and anti-CD28 antibodies and exposed twice to LZRS-EGFP retrovirus on fibronectin-coated plates. (A) Transduction efficiency of purified and nonpurified PB and UC T cells derived from individual donors. All transductions were performed in duplicate on fibronectin-coated plates and the mean percentage of EGFP-expressing cells was monitored 48 hours after transduction. (B) The presence of the LZRS-EGFP provirus in the EGFP− and EGFP+ UC and PB CD3+ subsets was monitored by Southern blot. CD3+ cells were purified by Dynal selection and transduced as described above. EGFP− and EGFP+populations were FACS sorted, genomic DNA was isolated, digested withXbaI, and hybridized with the 1.3-kb EGFP probe. The PG13/LZRS-EGFP producer clone was used as a positive control. The 1.7-kb fragment encompassing EGFP and the 3′LTR is indicated. (C) FACS profile of nontransduced and transduced UC T cells. The percentage of EGFP+ cells as well as the level of EGFP expression (MFI) is indicated. (D) Relative gene transfer in UC T cells following purification by either a positive (Dynal) or negative selection method (Stemsep) is compared to that obtained in a nonpurified T-cell population. The mean ± SD of EGFP+ cells in 2 to 5 independent experiments is shown.
These data suggested that purified UC T cells were transduced by an MuLV-based vector at significantly lower levels than purified PB T lymphocytes. However, another explanation was that the 2 populations were transduced at equivalent levels but retroviral transgene expression was silenced or less robust in immature UC T cells. In the latter case, we would expect to detect a significant level of provirus in the EGFP− UC T-cell subset. To test this hypothesis, transduced CD3+ UC and PB cells were FACS-sorted on the basis of EGFP expression and the presence of provirus was assessed by Southern analysis using a32P-labeled EGFP probe. Although provirus was clearly detected in both the UC and PB CD3+ populations, which were EGFP+, provirus was at the limits of detection in the EGFP− populations (Figure 2B). These data strongly suggest that EGFP expression correlates with retroviral integration in purified PB and UC T cells. Moreover, it appears that transcription from the retroviral 5′ long terminal repeat is not negatively affected in UC T cells.
We next assessed whether the presence of autologous mononuclear cells would result in an increased level of gene transfer in UC T cells. UCMC isolated from the same donors used in the above-described experiments were transduced without further purifying the T-cell population. Importantly, under these conditions, the efficiency of transduction doubled as compared to that observed in purified T cells, from a mean of 24.6 ± 7.3% to 56.5 ± 5.4 (Figure 2A). Furthermore, the MFI of EGFP expression in these cells was more than 1000, equivalent to that observed in PB T cells (Figure 2C). It is important to point out that although a mixture of mononuclear cells was initially present in the cell culture, these assays monitored the transduction of the T-lymphocyte population. Specifically, at the time point at which gene transfer was assessed, 4 days following mitogen treatment, the entire EGFP+ population expressed the CD3 antigen on the cell surface (data not shown). Finally, it is notable that the presence of autologous mononuclear cells did not alter the level of gene transfer detected in identically treated PB T cells (53.9 ± 8.6 versus 57.4 ± 11.9%). These data demonstrate that there is a significant difference in the optimal conditions for transduction of naive UC T lymphocytes and adult PB T-cell populations.
Because the naive UC T cells used in the previously described experiments were isolated by positive selection, it was possible that the presence of an antibody-ligand interaction on the cell surface influenced retroviral transduction of these cells. To test this hypothesis, we monitored gene transfer in UC T cells that were purified by negative selection using the Stemsep method (StemCell Technologies Inc). This technique resulted in a purity of more than 90%. Importantly, transduction of both positively and negatively selected UC T cells was equivalent, with a 50% to 70% decrease as compared with nonpurified UC T cells from the same donors (Figure 2D). Collectively, these results indicate that the presence of autologous mononuclear cells is required for optimal gene transfer in naive UC T cells.
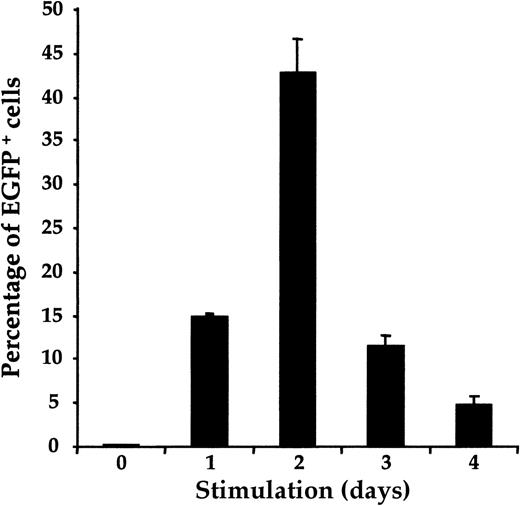
To further optimize conditions for the fibronectin-facilitated transfer of a retroviral vector into UC T cells, we assessed whether the efficiency of gene transfer varied with the duration of CD3/CD28 activation. Gene transfer in nonpurified UC T cells was monitored after a single exposure to retrovirus on days 0 to 4 of mitogen stimulation. Gene transfer was not observed in cells infected with retroviral supernatant before stimulation (day 0) but increased from 15% to approximately 42% between days 1 and 2 of mitogen treatment in a representative UC donor (Figure 3). Transduction efficiencies were strikingly diminished thereafter, decreasing to 5% by day 4 following CD3/CD28 ligation. These data are similar to those that we previously reported for PB T cells7 demonstrating that transduction of naive UC T cells and adult PB T cells with PG13/LZRS-EGFP retrovirus follow similar kinetics.
Kinetics of retroviral transduction efficiency in UC T cells.
Nonpurified UC T cells were exposed once to LZRS-EGFP retroviral supernatant on fibronectin-coated plates at various time points after anti-CD3/CD28 mitogenic stimulation. All transductions were performed in duplicate and the mean number of EGFP+ cells is depicted.
Kinetics of retroviral transduction efficiency in UC T cells.
Nonpurified UC T cells were exposed once to LZRS-EGFP retroviral supernatant on fibronectin-coated plates at various time points after anti-CD3/CD28 mitogenic stimulation. All transductions were performed in duplicate and the mean number of EGFP+ cells is depicted.
Cell cycle entry of CD3/CD28-stimulated UC T cells is not affected by the purification process
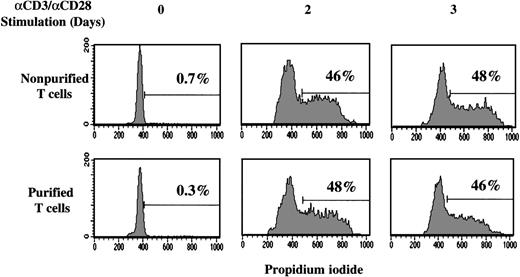
Because previous studies demonstrated defects in CD3/CD28-mediated signaling events in UC T cells33,34 and infection by Moloney-based retroviruses requires cell mitosis,35-37 we next assessed the status of cell cycle entry in UC T cells on CD3/CD28 ligation. Moreover, because the presence of autologous mononuclear cells was required for optimal retroviral transduction in UC T cells (Figure 2), we monitored the potential of these former cells to modulate the kinetics of T-cell division. Purified and nonpurified UC T cells were stained with PI, a measure of DNA content, at various time points following stimulation with immobilized anti-CD3/CD28 antibodies. More than 99% of freshly isolated UC T cells were in the G0/G1 phases of the cell cycle. However, by 2 days after mitogen treatment, approximately 50% of UC T cells were in S-G2/M and this level remained essentially constant over the next 24 hours (Figure 4). These data are essentially equivalent to the results we previously reported for adult PB T cells.7 Notably, the percentage of UC T cells in cycle was not altered by the presence of mononuclear cells or the purification process (Figure 4). Because the transductions described above were performed on fibronectin-coated plates, we also determined whether cell cycle entry was modulated by this extracellular matrix molecule. However, cell cycle entry of neither purified nor nonpurified UC T cells was affected by the presence of fibronectin (data not shown). Therefore, differences in cell cycle entry do not account for the lower transduction levels observed in purified UC T cells.
Cell cycle entry of UC T cells is not affected by the purification process.
Nonpurified or purified UC T cells (Dynal) were stimulated with immobilized anti-CD3 and anti-CD28 antibodies. Cell cycle entry was monitored at 0, 2, and 3 days by assessing DNA content of PI-stained cells on a FACScan cytometer. The percentage of cells in the S and G2/M phases of the cell cycle are indicated and results are representative of data obtained in 1 of 5 representative experiments.
Cell cycle entry of UC T cells is not affected by the purification process.
Nonpurified or purified UC T cells (Dynal) were stimulated with immobilized anti-CD3 and anti-CD28 antibodies. Cell cycle entry was monitored at 0, 2, and 3 days by assessing DNA content of PI-stained cells on a FACScan cytometer. The percentage of cells in the S and G2/M phases of the cell cycle are indicated and results are representative of data obtained in 1 of 5 representative experiments.
Relative transduction levels in naive and memory PB T cells
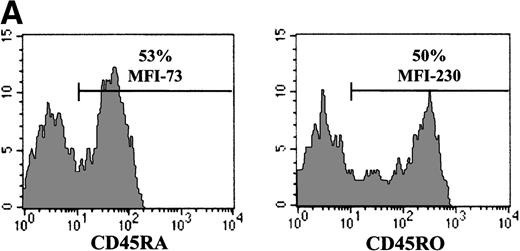
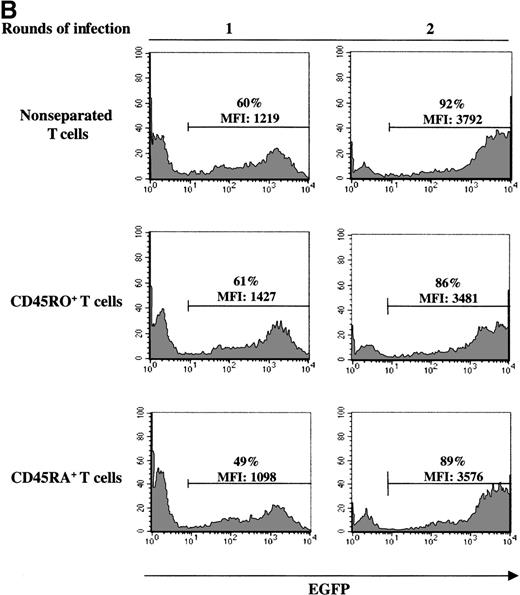
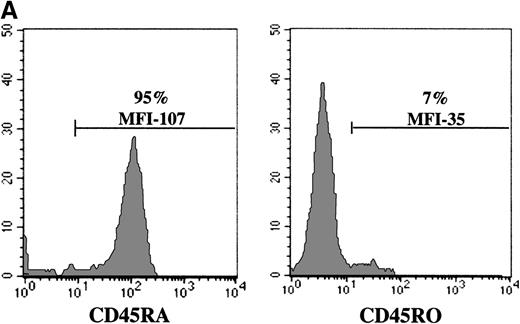
The experiments described above, assessing gene transfer in naive T cells, were all performed in cells isolated from UC blood donors. To more precisely evaluate the relative abilities of naive and memory T cells to be infected with an MuLV-based vector, we used PB lymphocytes because naive and memory T-cell subsets can be obtained from the same donor. In normal adults, CD45RA+/RO− and CD45RA−/RO+ lymphocytes are present at approximately equal levels (Figure 5A). Cells were first FACS sorted on the basis of CD5 expression, resulting in a population where more than 90% of cells expressed the T-cell specific marker CD3 (data not shown). To eliminate any bias from cells expressing both CD45RA and CD45RO, CD45RA+ naive and CD45RO+ memory cells were then purified by FACS sorting of cells that were negative for the RO and RA isoform, respectively. Although transduction of both CD45RA+ and CD45RO+ subsets was significant after a single exposure to retroviral supernatant (49% versus 61% in a representative experiment), gene transfer was higher in the memory subset (Figure 5B). Furthermore, the level of expression of the EGFP marker gene was approximately 30% higher in the memory CD45RO+ population than in the transduced naive CD45RA+ T-cell subset from the same donor (MFI of 1427 versus 1098). Interestingly, EGFP expression in the nonseparated T-cell population, containing an approximately 1:1 ratio of CD45RA:RO cells, was intermediate, with an MFI of 1219. Collectively, these results demonstrate that although the memory T-cell subset is more susceptible than naive T cells to retroviral-mediated gene transfer, both populations could be efficiently transduced. Importantly, after 2 exposures to retrovirus, the transduction efficiency of the naive and memory T-cell populations was essentially equivalent, with a gene transfer level of more than 80% in T cells from a representative donor (Figure 5B). Thus, differences in the transduction of adult CD45RA+ and CD45RO+ T cells could be eliminated by increasing the number of exposures to cell-free retrovirus. Moreover, these experiments demonstrate that the CD45RA+ T cell populations present in adult PB and UC are distinct with the former demonstrating a significantly higher capacity to be transduced with an MuLV vector.
Decreased levels of gene transfer in naive PB T cells can be overcome by increasing the number of exposures to retroviral supernatant.
(A) CD45RA and CD45RO expression on freshly purified adult PB T cells was monitored by FACS analysis. The percentage of positive cells is indicated. (B) FACS-purified CD5+, CD5+/CD45RO−(RA+), and CD5+/CD45RA−(RO+) cells, stimulated for 2 days with immobilized anti-CD3 and anti-CD28 antibodies, were transduced either once or twice with LZRS-EGFP retroviral supernatants on fibronectin-coated plates. The T cells used in the 2 experiments presented here were isolated from different donors. The percentage of EGFP+ cells and the level of EGFP expression (MFI) are indicated.
Decreased levels of gene transfer in naive PB T cells can be overcome by increasing the number of exposures to retroviral supernatant.
(A) CD45RA and CD45RO expression on freshly purified adult PB T cells was monitored by FACS analysis. The percentage of positive cells is indicated. (B) FACS-purified CD5+, CD5+/CD45RO−(RA+), and CD5+/CD45RA−(RO+) cells, stimulated for 2 days with immobilized anti-CD3 and anti-CD28 antibodies, were transduced either once or twice with LZRS-EGFP retroviral supernatants on fibronectin-coated plates. The T cells used in the 2 experiments presented here were isolated from different donors. The percentage of EGFP+ cells and the level of EGFP expression (MFI) are indicated.
Efficient retroviral transduction of naive UC T cells stimulated with the IL-7 cytokine
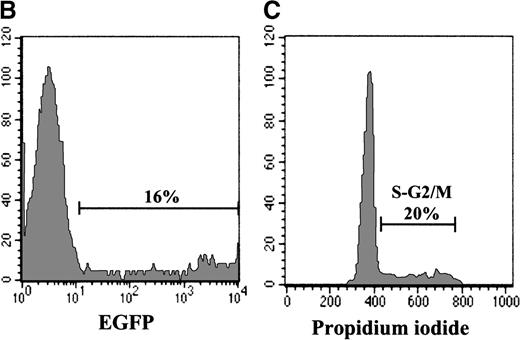
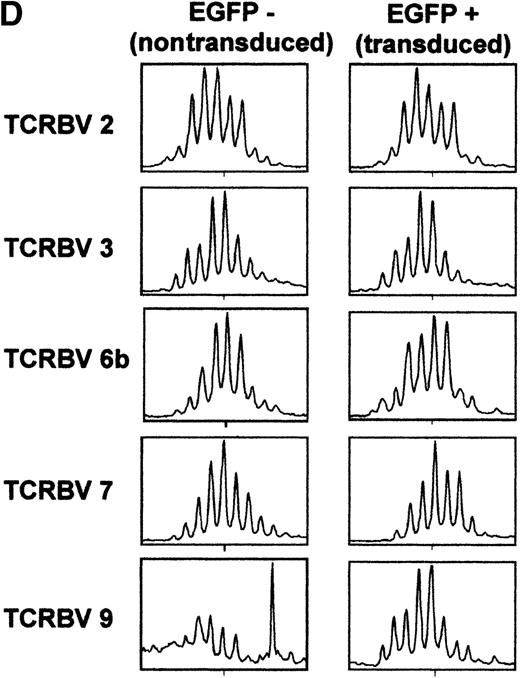
Although individual CD3/CD28-stimulated naive T cells maintain their specific TCRBV rearrangements, it is clear that their phenotype has changed; they rapidly acquire the “memory” marker of CD45RO expression.19 It is therefore questionable whether these cells, activated through the TCR, maintain the characteristics attributed to naive T cells. Thus, we monitored the ability of naive T cells that remain CD45RA+ to be transduced with an MuLV-based retroviral expression vector. Because IL-7 treatment does not induce a switch in CD45 expression from RA+ to RO+,38 39 purified UC T cells were activated with IL-7 and then exposed to retroviral supernatant. Importantly, UC T cells expanded in the presence of IL-7 alone for more than 30 days expressed high levels of CD45RA on the cell surface and did not acquire CD45RO (Figure 6A and data not shown). Transduction efficiencies in UC T cells that were activated with IL-7 (for 4 days) and then exposed to 2 rounds of PG13/LZRS-EGFP retroviral supernatant were significant, reaching levels of approximately 20% (Figure 6B). However, gene transfer after IL-7 treatment was lower than that observed in UC T cells stimulated with anti-CD3/CD28 monoclonal antibodies (Figure 2). We determined that this difference was likely due to a decreased level of cells in cycle; the maximum percentage of UC T cells in cycle following IL-7 treatment was approximately 20%, whereas 50% of cells could be induced to cycle by CD3/CD28 ligation (Figures 4 and 6C). Importantly, there was a persistence of all analyzed TCRBV subfamilies in the gene-modified cells. A representative analysis of the distribution within 5 TCRBV subfamilies is shown in Figure 6D. Notably, the T-cell repertoire displayed a gaussian-like profile for most TCRBV subfamilies, which was strikingly similar to that observed in the nontransduced population (Figure 6D). This type of profile is indicative of a polyclonal T-cell repertoire. Thus, even with a low level of gene transfer, the protocol used here allowed a diverse polyclonal population of IL-7-stimulated UC T cells to be transduced.
Efficient retroviral-mediated gene transfer in naive CD45RA+ UC T cells upon IL-7 stimulation.
(A) CD45RA and CD45RO expression on naive UC T cells stimulated with IL-7 for 10 days. (B) Naive UC T cells were cultured for 4 days in the presence of IL-7 and then transduced for 2 consecutive days with LZRS-EGFP retroviral supernatants. EGFP expression was monitored by FACS analysis 2 days following transduction. (C) Cell cycle entry of the UC T cells used for transduction was determined by PI staining at day 4 of cytokine stimulation. (D) Comparison of T-cell receptor CDR3 size distribution (Immunoscope profiles) of nontransduced (EGFP−) and transduced (EGFP+) IL-7-stimulated UC T cells. Twenty-four PCR products were generated by reverse transcriptase-PCR with 24 different TCRVB subfamily-specific primers and 1 Cβ consensus primer, followed by a run-off reaction with a fluorescent Cβ primer. The graphs represent fluorescence intensity in arbitrary units (y-axis) plotted against CDR3 size (x-axis). Representative results for TCRVB 2, TCRVB 3, TCRVB 6b, TCRVB 7, and TCRVB 9 are shown. A gaussian-like profile is observed for the first 4 TCRVB families; there is a skewed profile for TCRBV 9 in the nontransduced population.
Efficient retroviral-mediated gene transfer in naive CD45RA+ UC T cells upon IL-7 stimulation.
(A) CD45RA and CD45RO expression on naive UC T cells stimulated with IL-7 for 10 days. (B) Naive UC T cells were cultured for 4 days in the presence of IL-7 and then transduced for 2 consecutive days with LZRS-EGFP retroviral supernatants. EGFP expression was monitored by FACS analysis 2 days following transduction. (C) Cell cycle entry of the UC T cells used for transduction was determined by PI staining at day 4 of cytokine stimulation. (D) Comparison of T-cell receptor CDR3 size distribution (Immunoscope profiles) of nontransduced (EGFP−) and transduced (EGFP+) IL-7-stimulated UC T cells. Twenty-four PCR products were generated by reverse transcriptase-PCR with 24 different TCRVB subfamily-specific primers and 1 Cβ consensus primer, followed by a run-off reaction with a fluorescent Cβ primer. The graphs represent fluorescence intensity in arbitrary units (y-axis) plotted against CDR3 size (x-axis). Representative results for TCRVB 2, TCRVB 3, TCRVB 6b, TCRVB 7, and TCRVB 9 are shown. A gaussian-like profile is observed for the first 4 TCRVB families; there is a skewed profile for TCRBV 9 in the nontransduced population.
Discussion
In this report, we show that naive T cells can be efficiently transduced using an MuLV-based retroviral vector. Because previous studies reported low levels of T-cell gene transfer using retroviral vectors,40,41 it was not feasible to ascertain the relative levels of transduction in T-cell subsets. Recent advances in gene transfer technology, including the use of the extracellular matrix molecule fibronectin, which augments T cell transduction efficiency by colocalizing retrovirus particles and the target cell,5-7have now made these types of studies possible. Furthermore, development of markers such as EGFP,9,42 43 which eliminate the toxicity inherent in selection for drug resistance, permits transduced cell populations to be easily and readily detected after transduction. Use of these technologies has enabled us to establish conditions for optimal transduction of the naive T-cell subset isolated from both UC and adult donors.
Although no previous studies have assessed the ability of naive T cells to be infected with either a replication competent murine leukemia retrovirus or murine leukemia-based retroviral vectors, several groups have reported that CD3/CD28-stimulated naive CD45RA+ T cells are less susceptible to productive HIV-1 infection than memory T cells.20-22 The basis for this difference is not known, but it does not appear to be due to a diminished proliferative potential of the naive T cells.21,22,44 Indeed, we report here that the CD3/CD28-induced cell cycle entry of naive UC T cells was as high as that observed in PB T cells, with approximately 50% of cells in cycle by 2 days following activation. However, research from many groups has demonstrated that the responses of CD45RA+ naive T cells to CD3/CD28 ligation are distinct from that observed in CD45RO+ memory cells. Indeed, UC T cells exhibit impaired expression of cell surface molecules, including IL-2R, IL-12Rβ1, CD40L, and FasL.33,45-48 Furthermore, UC T cells demonstrate decreased secretion of IL-4, IL-12, and interferon-γ following activation.49-52 Finally, production of the β-chemokines RANTES and macrophage inflammatory protein (MIP)-1α and MIP-1β is severely compromised in TCR-stimulated naive T cells.53 In light of this innate signaling defect, it was interesting to observe a significant level of gene transfer in purified naive UC T cells using a GALV-pseudotyped MuLV-based retroviral vector (24.6 ± 7.3%). Nevertheless, this level of transduction was much lower than that observed in purified adult PB T cells composed of a mixed population of memory and naive subsets (53.9 ± 8.6%).
Because cytokine secretion differs significantly in stimulated cultures of UC and adult PB T cells, it is possible that the decreased gene transfer in UC T cells may be the result of a suboptimal cytokine/chemokine environment for efficient transduction or expression of the marker transgene. In this regard, the finding that several cytokines can significantly influence retroviral infection is noteworthy.54 Furthermore, the ability of autologous mononuclear cells to significantly augment gene transfer in UC T cells suggests that the former cells can modify the environment in which the UC T cells are transduced. In contrast, autologous mononuclear cells did not modify transduction in PB T cells, which are composed of both naive and memory subsets.
Interestingly, the actual levels of gene transfer in purified CD45RA+ UC T cells (24.6 ± 7.3%) were significantly lower than that observed in purified CD45RA+ PB T cells (> 50%). Thus, intrinsic differences between naive UC T cells and naive PB T cells likely influence their ability to be transduced with an MuLV-based vector. Indeed, although UC T cells represent a truly naive population, CD45RA+ PB T cells may be contaminated by cells with an intermediate naive/memory phenotype as well as by memory T cells that revert from a CD45RO+ to RA+phenotype.18,29-32 Moreover, other differences between UC CD45RA+ T cells and PB CD45RA+ T cells have been documented; the former are significantly more susceptible to spontaneous apoptosis ex vivo and maintain significantly longer telomeres after ex vivo expansion.39
The ability to transduce resting T cells stimulated solely with cytokines has significant applications for gene therapy protocols. This would be vital in situations in which TCR activation is defective, as in many genetic severe combined immunodeficiencies. Furthermore, it may be desirable to genetically manipulate T cells in the absence of TCR activation. Specifically, it is not clear whether CD3/CD28-stimulated T cells that switch from a CD45RA+ to RO+phenotype maintain their ability to respond to novel antigens in vivo. Moreover, it has recently been demonstrated that there is a significant and variable skewing of some TCRBV families after 7 days of CD3-induced ex vivo expansion.55 These data have negative implications for T-cell gene therapy protocols, which require TCR engagement and extended in vitro culture, because the reinfused T cells would have a T-cell repertoire diversity that is significantly reduced compared with that of the starting population. The use of shorter ex vivo expansion times or stimulation with the IL-7 cytokine may alleviate these problems. Soares and colleagues recently reported that treatment of CD45RA+ T cells with IL-7 during an 8-day ex vivo expansion period did not result in a selective expansion of any particular TCRBV family.39 We have verified these results by Immunoscope analysis and, importantly, demonstrate that the population of transduced IL-7-expanded UC T cells remains polyclonal with a diverse TCRBV repertoire. Furthermore, IL-7-treated UC T cells did not lose CD45RA expression and remained phenotypically naive38 39(and this report).
It is notable that the kinetics of transduction of CD3/CD28- and IL-7-stimulated naive T cells differed with optimal gene transfer at 2 and 4 days, respectively (Figure 3 and data not shown). Because MuLV-based retroviral transfer requires the entry of cells into mitosis,35-37 this difference was likely due to a longer lag time before initiation of cell division in IL-7-treated T cells than in CD3/CD28-stimulated lymphocytes. Indeed, the generation of daughter T cells in IL-7-treated cultures was not observed until 4 days after stimulation, whereas progeny were already observed after 2 days of CD3/CD28 stimulation (V.D. and N.S., unpublished observations). We achieved gene transfer efficiencies in naive IL-7-treated T cells approaching 20% and, interestingly, Unutmaz and colleagues recently reported similar transduction levels in cytokine-stimulated naive T cells using an HIV-1-derived vector (4-15%).56Importantly, the HIV vector system, like our MuLV-based retroviral system, did not support expression of ectopic genes in resting T cells. HIV-1, unlike MuLV, has the capacity to infect nondividing cells but infection of quiescent T cells is blocked before integration, likely due to incomplete reverse transcription or failure of the viral preintegration complex to be transported to the nucleus.57-60 Nonetheless, these researchers concluded that an HIV-based system was superior to an MuLV-derived vector because they observed only minimal infection of cytokine-stimulated naive T cells using a vesicular stomatitis virus–G protein (VSV-G)-pseudotyped EGFP-expressing MuLV-based vector (< 0.5%). It is possible that we observed significantly higher transduction levels because we used virus obtained from a stable PG13 producer clone, selected for its ability to infect the Jurkat T cell line at high levels.7 Furthermore, a GALV-pseudotyped retrovirus may transduce human T cells with a higher efficiency than a VSV-G-pseudotyped vector. Notably, we have also observed similar high gene transfer efficiencies in naive T cells on pseudotyping our MuLV-based EGFP-expressing vector with the amphotropic leukemia virus envelope (H.S., unpublished observations). Although a vector system based on HIV-1 has potential gene therapy applications, no clinical studies have as yet used this system. Thus, it is significant to determine that a simple and clinically applicable protocol using virus from stable PG13 clones harboring MuLV-based vectors can result in gene transfer efficiencies of more than 50% in CD3/CD28-activated naive T cells and 20% in IL-7-treated cells.
The approach described here can be used for gene therapy applications as well as for the dissection of signaling pathways in primary naive T cells. Because of the extreme difficulty in achieving high gene transfer levels in primary lymphocytes, almost all signaling studies performed following introduction of mutant genes have been limited to transformed T-cell lines. These cell lines clearly have inherent peculiarities and are phenotypically different from primary lymphocytes, which require periodic antigen stimulation for their growth and expansion. Importantly, expression of introduced genes from the LZRS retroviral vector is stable and does not appear to vary with the activation state of the cell. These properties have enabled us to use this system to characterize the role of the ZAP-70 kinase in resting primary human T cells.61
Finally, the ability to successfully transduce UC T cells is of crucial importance because patients with various malignancies and genetic disorders who do not have an HLA-identical bone marrow donor are increasingly being transplanted with UC blood.62 Like bone marrow, UC blood contains a large number of hematopoietic stem cells.61 However, in contrast with individuals in whom the hematopoietic system is reconstituted with allogeneic bone marrow, there is a reduced level of severe GVHD in patients transplanted with UC blood. GVHD is due to the activation of alloreactive T cells in the UC or bone marrow inoculum and the decreased severity of GVHD in individuals transfused with UC blood is hypothesized to be due to the innate immaturity of the naive T cells present in this latter source.63 64 Therefore, the ability to transduce the T-lymphocyte subset of UC hematopoietic cells has significant therapeutic potential. Moreover, under conditions where inducing an increased T-cell response, such as a graft- versus-leukemia response, would be desirable, it might be possible to augment the response of naive UC T cells by gene transfer of specific signaling molecules or cytokines. Taken together, the experiments presented here have major implications for gene therapy protocols involving the transduction of naive T cells.
Acknowledgments
We are indebted to Madame Niudan and her staff at Clinique Saint Roch. This work would not have been possible without their time and dedication. We are very grateful to Johann Soret for his expertise, advice, and support under stressful circumstances. Lionel Pintard and Claire Bonnerot also provided us with valuable assistance and important information. We thank Christophe Duperray for his proficiency and assistance with FACS sorting. We are beholden to Marc Sitbon and Hans Yssel for stimulating and helpful discussions throughout the course of this work. Dr Ikunoshin Kato and Setsuko Yoshimura of Takara Shuzo Company are generously acknowledged for providing the recombinant fibronectin fragment and for their continuing assistance, and C. June, as well as G. Boonen, generously provided us with reagents.
V.D. and S.J. are funded by fellowships from the French Ministère de la Recherche and Programa PRAXIS XXI, Fundação para a Ciência e Tecnologia, Portugal (Grant PRAXIS XXI BD/19929/99), respectively. N.N. was supported by fellowships from the Association Française contre les Myopathies and a grant from the March of Dimes. This work was supported by grants from the Association Française contre les Myopathies, March of Dimes (Grant #6-FY99-406), and Association pour la Recherche sur le Cancer (to N.T.).
Reprints:Naomi Taylor, Institut de Génétique Moléculaire de Montpellier, CNRS UMR 5535, 1919 Route de Mende, 34293 Montpellier, Cedex 5 France; e-mail: taylor@igm.cnrs-mop.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.