Abstract
Treatment of intact human erythrocytes with pervanadate induces Tyr (Y)-phosphorylation of the transmembrane protein band 3; in parallel, the activity of the immunoprecipitated tyrosine kinases Syk and Lyn is increased. When erythrocytes are incubated with pervanadate together with PP1, a specific inhibitor of Src kinases, including Lyn, the Y-phosphorylation of band 3 is only partially reduced. Indeed, the PP1-resistant phosphorylation of band 3 precedes and is a prerequisite for its coimmunoprecipitation with Lyn, which interacts with the phosphoprotein via the SH2 domain of the enzyme, as proven by binding competition experiments. Upon recruitment to primarily phosphorylated band 3, Lyn catalyzes the secondary phosphorylation of the transmembrane protein. These data are consistent with the view that band 3 is phosphorylated in intact erythrocytes by both PP1-resistant (most likely Syk) and PP1-inhibited (most likely Lyn) tyrosine kinases according to a sequential phosphorylation process. Similar radiolabeled peptide maps are obtained by tryptic digestion of32P-band 3 isolated from either pervanadate-treated erythrocytes or red cell membranes incubated with exogenous Syk and Lyn. It has also been demonstrated by means of mass spectrometry that the primary phosphorylation of band 3 occurs at Y8 and Y21, while the secondary phosphorylation affects Y359 and Y904.
Introduction
Phosphorylation/dephosphorylation of protein tyrosine residues has been implicated in the regulation of several erythrocyte functions, including metabolism,1-4 membrane transport,5,6 cell volume, and cell shape.6-8Protein tyrosine kinases identified to date in red cells include insulin receptor tyrosine kinase9,10 and the Syk11,12 and Src-like nonreceptor tyrosine kinases.5,11,12 Among the Src-like tyrosine kinases detected in human erythrocytes, Lyn and Fgr are expressed predominantly, with Hck being found at a lower levels.5The attempt at purifying the native protein tyrosine kinases from erythrocytes led to the isolation of substantial amounts of only the 2 tyrosine kinases, Syk and Lyn.12
The tyrosine kinases belonging to the Src family share a high degree of structural similarity, with a common domain architecture and regulatory mechanisms. The Src sequence consists of a poorly conserved N-terminal segment, 2 conserved domains termed SH3 and SH2, followed by the catalytic domain, SH1.13,14 The SH3 and SH2 domains interact with protein sequences containing polyproline II helices and phosphotyrosine residues, respectively, and play a dual role in Src regulation, because they are both required for keeping Src kinase in an inactive state15,16 and targeting the enzyme to specific substrates by directing protein-protein interactions.17
The activity of Src-related kinases is modulated by phosphorylation of 2 tyrosine residues: Autophosphorylation of Tyr (Y)416 (c-Src numbering), located inside the catalytic domain, correlates with enzyme activation,14,18-20 while Csk-mediated phosphorylation of the C-terminal Y527 gives rise to inactive forms of Src kinases. Indeed, phosphorylated Y527 interacts with the SH2 domain of the Src molecule, thus triggering a reorganization of the enzyme structure that forces the kinase to adopt a locked and down-regulated conformation.15 16
p72syk contains 2 SH2 domains, a linker region that is postulated to bind cellular effectors, a catalytic domain that includes autophosphorylation sites, and a short carboxyl-terminal extension of yet undetermined function.21 A proteolytic form of p72syk displaying a Mr of 36 kd (p36syk) has been purified from erythrocytes.12 In analogy with the 36- to 40-kd forms of Syk found in other types of cells,12,22,23p36syk corresponds to the C-terminal part of p72syk and exhibits higher activity than that displayed by the full-length enzyme.24
The multifunctional transmembrane protein termed band 3 represents the major Y-phosphorylated protein in human erythrocytes (Figure 1C).8,11,25 Y8, located in the highly acidic cytoplasmic domain of band 3, has been identified as the major Y-phosphorylation site in vitro1,26,27; Y21, Y359, and Y904 have also been reported to be phosphorylated in vitro.28 Treatment of intact red cells with pervanadate has been found to increase both the Y-phosphorylation of band 3 and p72syk activity, suggesting that this tyrosine kinase is likely responsible for the Y-phosphorylation of band 3 in intact cells.11 We have provided evidence for a synergistic involvement of both Syk and Lyn in band 3 phosphorylation in isolated erythrocyte membranes.12 It has been demonstrated that the primary phosphorylation of band 3 catalyzed by p36syk (or p72syk) in erythrocyte membranes is a prerequisite for the association of added Lyn with the membranes and for the subsequent Lyn-catalyzed phosphorylation of different band 3 tyrosine residues.12 The present study shows that, in intact human red cells stimulated with pervanadate, band 3 undergoes sequential phosphorylation catalyzed by the concerted action of the Src-unrelated tyrosine kinase p72syk and the Src-related tyrosine kinase Lyn. Our findings suggest that, upon phosphorylation by p72syk, Y8 and Y21 act as docking sites for the SH2 domain of Lyn, which subsequently phosphorylates band 3 at additional secondary sites.
Y-phosphorylation of band 3 in isolated ghosts and in intact human erythrocytes.
(A) Radioactive phosphorylation pattern of human erythrocyte membranes (ghosts) incubated with γ[32P]ATP either alone (lane 1) or in the presence of 35-nmol/L Lyn (lane 2) or 15-nmol/L p36syk (lanes 3-6). Increasing concentrations of PP1 were present in the incubation medium of assays shown in lanes 4 to 6. (B) Radioactive phosphorylation pattern of erythrocyte ghosts, first phosphorylated by p36syk in the presence of unlabeled ATP as described in “Materials and methods” and further incubated for 10 minutes in the presence of γ[32P]ATP without (lane 1) or with 35-nmol/L Lyn (lanes 2-5). Assays in lanes 3 to 5 were performed in the presence of increasing concentrations of PP1 added immediately before Lyn. 32P-radiolabeled proteins were subjected to SDS-PAGE on 10% gels followed by autoradiography. (C) Anti-P-Y immunostaining of erythrocyte membrane proteins phosphorylated in vivo. Intact human erythrocytes were incubated either in the absence (lane 1) or presence (lanes 2-5) of pervanadate as described in “Materials and methods.” Increasing concentrations of PP1 were present in the assays shown in lanes 3 to 5. After incubation, erythrocyte membranes were rapidly isolated, solubilized, and submitted to SDS-PAGE followed by transfer to a nitrocellulose filter. The filter was then immunostained with anti-P-Y antibody. Other experimental details are described in “Materials and methods.” Panels are representative of at least 6 different experiments.
Y-phosphorylation of band 3 in isolated ghosts and in intact human erythrocytes.
(A) Radioactive phosphorylation pattern of human erythrocyte membranes (ghosts) incubated with γ[32P]ATP either alone (lane 1) or in the presence of 35-nmol/L Lyn (lane 2) or 15-nmol/L p36syk (lanes 3-6). Increasing concentrations of PP1 were present in the incubation medium of assays shown in lanes 4 to 6. (B) Radioactive phosphorylation pattern of erythrocyte ghosts, first phosphorylated by p36syk in the presence of unlabeled ATP as described in “Materials and methods” and further incubated for 10 minutes in the presence of γ[32P]ATP without (lane 1) or with 35-nmol/L Lyn (lanes 2-5). Assays in lanes 3 to 5 were performed in the presence of increasing concentrations of PP1 added immediately before Lyn. 32P-radiolabeled proteins were subjected to SDS-PAGE on 10% gels followed by autoradiography. (C) Anti-P-Y immunostaining of erythrocyte membrane proteins phosphorylated in vivo. Intact human erythrocytes were incubated either in the absence (lane 1) or presence (lanes 2-5) of pervanadate as described in “Materials and methods.” Increasing concentrations of PP1 were present in the assays shown in lanes 3 to 5. After incubation, erythrocyte membranes were rapidly isolated, solubilized, and submitted to SDS-PAGE followed by transfer to a nitrocellulose filter. The filter was then immunostained with anti-P-Y antibody. Other experimental details are described in “Materials and methods.” Panels are representative of at least 6 different experiments.
Materials and methods
Materials
We purchased γ[32P]ATP and [32P]Pi from Amersham (Little Chalfont, UK). PP1 inhibitor and protease inhibitor cocktail were obtained from Calbiochem (Darmstadt, Germany) and Boehringer (Mannheim, Germany), respectively. Antiphosphotyrosine and antiband 3 monoclonal antibodies were purchased from ICN Biotechnology (Irvine, CA) and Sigma (Dorset, UK), respectively. Anti-Lyn and anti-Syk polyclonal antibodies, raised against protein residues 44 to 63 and 257 to 352, respectively, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-GST antibody was purchased from Amersham Pharmacia Biotech. Recombinant glutathione-S-transferase (GST) and the Lyn SH2 domain fused with GST were expressed as previously described for c-Fgr SH2 domain.29 The phosphopeptides band 3 (1-24) and Src (523-533) and their unphosphorylated analogues were synthesized as detailed elsewhere.30 The 40- to 45-kd fragment of the cytoplasmic domain of band 3 was prepared as described elsewhere.12 Other reagents were purchased from Sigma.
Enzymes
Isolation of human erythrocytes
Human erythrocytes were prepared from fresh blood collected from healthy donors as previously described.12
Isolation and phosphorylation of erythrocyte membranes (ghosts)
Erythrocyte membranes were isolated from hemolyzed red cells as described elsewhere.12 Erythrocyte membranes (3 μg) were phosphorylated for 10 minutes at 30°C in 30 μL of an incubation mixture containing 50-mmol/L Tris-HCl, pH 7.5; 10-mmol/L MnCl2; 30-μmol/L γ[32P]ATP (specific activity, 1000 cpm/pmol); 200-μmol/L sodium orthovanadate (basal medium); and the indicated amount of either p36syk or Lyn tyrosine kinase.
Preparation of Syk-phosphorylated ghosts
Ghosts were phosphorylated by 15-nmol/L p36syk for 10 minutes in basal medium containing unlabeled adenosine triphosphate (ATP) instead of radiolabeled ATP. Syk-phosphorylated ghosts were separated from p36syk, ATP, and other reagents by centrifugation and washed twice as described elsewhere.12Syk-phosphorylated ghosts were then secondarily32P-phosphorylated by Lyn in the basal medium.
The reactions were stopped by addition of 2% sodium dodecyl sulfate (SDS) and 1% 2-mercaptoethanol followed by 5 minutes of treatment at 100°C. The solubilized membranes were analyzed by 10% SDS-polyacrylamide gel electrophoresis (PAGE).
Treatment of intact red cells and antiphosphotyrosine immunoblotting
Packed cells, prepared as above described, were resuspended (at 16% hematocrit) in buffer A (20-mmol/L Tris-HCl, pH 7.5; 150-mmol/L NaCl; 10-mmol/L KCl; 1-mmol/L MgCl2; 100-μg/mL streptomycin; 25-μg/mL chloramphenicol; 24-mmol/L glucose; and 1-mmol/L adenosine). Resuspended cells (300 μL for each sample) were incubated for 30 minutes at 35°C in the absence or presence of pervanadate, prepared by mixing hydrogen peroxide (3 mmol/L) and sodium orthovanadate (2 mmol/L). When indicated, PP1 inhibitor was added to the incubation mixture immediately after pervanadate addition. After incubation, each sample was centrifuged, and the packed cells were hemolyzed in 1.8 mL of a hypotonic buffer containing 5-mmol/L sodium phosphate, pH 8; 0.02% NaN3; 30-μmol/L phenylmethylsulphonylfluoride; 1-mmol/L sodium orthovanadate; and protease inhibitor cocktail. Membranes were separated from hemolysates by centrifugation (20 000g for 20 minutes), and an aliquot (3 μg) was solubilized in SDS-PAGE sample buffer (50-mmol/L Tris-HCl buffer, pH 8.9, containing 5-mmol/L ethylenediaminetetraacetic acid (EDTA), 380-mmol/L glycine, 2% SDS, and 1% β-mercaptoethanol). After 5 minutes of treatment at 100°C, the solubilized membrane proteins were subjected to SDS-PAGE (10% gels), transferred to nitrocellulose membranes, and immunostained with anti-P-Y antibody.
Anti-Lyn, anti-Syk, and anti-GST immunoprecipitations
Packed membranes prepared from intact erythrocytes treated as described above were extracted for 1 hour at 4°C with 20-mmol/L Tris-HCl, pH 7.5; 10% glycerol; 1% Nonidet P-40; 1-mmol/L EDTA; 50-mmol/L NaCl; 1-mmol/L sodium orthovanadate; and protease inhibitor cocktail. After centrifugation, the supernatants were incubated for 5 hours at 4°C with the appropriate antibody bound to protein A-Sepharose. The immune complexes were washed 3 times by centrifugation and resuspension in 50-mmol/L Tris-HCl, pH 7.5, containing protease inhibitor cocktail and 1-mmol/L sodium orthovanadate.
Immune complex kinase assays
Tyrosine kinase assays of immune complexes formed with anti-Lyn and anti-Syk antibodies as described above were performed in basal medium containing either 300 ng of the cytoplasmic domain of band 312 or 200-μmol/L cdc2 (6-20) peptide,20 which served as exogenous substrates for Syk and Lyn, respectively. Following incubation for 10 minutes at 30°C, phosphorylation of the cytoplasmic domain of band 3 was analyzed by SDS-PAGE followed by autoradiography. In the experiments containing cdc2 (6-20) peptide, the reactions were stopped by spotting 25 μL of the incubation mixture onto P81 phosphocellulose paper, which was then processed as described elsewhere.20
32P-peptide mapping of band 3 phosphorylated in vivo or in vitro
In vivo phosphorylation of band 3 was performed as follows: Packed erythrocytes (400 μL), prepared as described above, were preincubated in 3.6 mL of buffer B (buffer A without glucose and adenosine) for 4 hours at 35°C to deplete endogenous ATP stores. The cells were then centrifuged at 750g for 3 minutes, resuspended in 2.1 mL of buffer A (16% hematocrit) containing carrier-free [32P]Pi (11.1 MBq), and radiolabeled for 14 hours at 35°C, followed by incubation for 30 minutes at 35°C with pervanadate. Membranes were then isolated from the red cells and solubilized in SDS-PAGE sample buffer.
Band 3 was phosphorylated in vitro by incubating erythrocyte ghosts (15 μg) for 10 minutes at 30°C with p36syk and Lyn in the presence of γ[32P]ATP as described above and then solubilized in SDS-PAGE sample buffer. Solubilized in vivo or in vitro phosphorylated proteins were separated by SDS-PAGE and electrophoretically transferred to polyvinylidine difluoride; the membrane was then treated with 2-mol/L NaOH at 55°C for 1 hour to eliminate phosphoserines and phosphothreonines, and32P-Y-labeled band 3 was localized by autoradiography, excised, and digested with trypsin.12 The resulting peptides were separated in 2 dimensions on thin-layer cellulose plates by electrophoresis in 1% ammonium bicarbonate (pH 8.9) (1 hour, 1000 V) followed by ascending chromatography in a buffer containing 25% pyridine, 7.5% acetic acid, and 37.5% butanol.12
Identification of the band 3 residues either primarily phosphorylated by Syk or secondarily phosphorylated by Lyn
Isolated membranes (60 μg) were phosphorylated, in a final volume of 500 μL, first by p36syk and unlabeled ATP and then by Lyn and γ[32P]ATP as described above. Band 3 was then isolated by SDS-PAGE and digested first with cyanogen bromide (1 mg/mL in 70% formic acid). The digest was dried in a Speed-Vac and then resuspended in 50 μL of 100-mmol/L ammonium acetate buffer, pH 8.0, containing 20 μg of sequencing-grade trypsin (Promega, Madison, WI) and incubated at 37°C for 6 hours. The digest was stopped by the addition of acetic acid to give a final pH of 3.0. The digest was then loaded onto an immobilized metal ion chromatography minicolumn. This was made by packing a 100-μL plastic pipette tip (with a plug of glass fiber at the end) with iminodiacetic acid–derivatized Sepharose (Amersham Pharmacia Biotech). The column was presaturated with FeCl3, and the sample was loaded in 0.1-mol/L acetic acid. The column was washed with 20-bed volumes of 0.1-mol/L acetic acid. Elution was performed with 0.1% ammonium acetate, pH 8.0, containing 500 -mmol/L Na2HPO4. The eluate was acidified by the addition of 10 μL of trifluoroacetic acid (TFA), and the digest was injected onto a 30-nm, 5-μm, C-18, 0.18 mm × 20 cm, reverse-phase microcolumn. A gradient was run from 100% A (0.1% TFA in water) to 70% B (0.07% TFA, 80% acetonitrile in water) over 1 hour. An ABI 120A syringe pump generated the gradient at 50-μL/min, which was split, giving a flow of 0.4 μL/min into the column. Individual peaks were collected manually (according to the UV trace) into 500-μL Eppendorf tubes containing a few beads of C-18 reverse-phase material to prevent sample loss. The radioactivity in each fraction was determined, and 5 phosphopeptide-containing fractions were obtained, 2 radioactive and 3 nonradioactive. The reverse-phase beads containing the peptides were packed individually into nanotips. The peptides were eluted with 80% acetonitrile and 5% acetic acid and were subjected to collision-activated dissociation in a Finnigan TSQ700 tandem quadrupole mass spectrometer using a collision energy of 10 to 30 eV and 1.8×10−3 mmHg argon with a parent ion window of 2 to 3 mass units (50% half height) and a daughter ion resolution of 1 to 2 mass units.
Immunostaining
Proteins transferred to nitrocellulose membranes were incubated with the indicated antibodies followed by the appropriate biotinylated second antibody and developed using an enhanced chemiluminescence detection system (ECL, Amersham).
Results
In vivo and in vitro phosphorylation of human erythrocyte band 3 is synergistically catalyzed by Syk and Src-related tyrosine kinases
Consistent with previous data,12 addition of p36syk, purified from human erythrocytes, to isolated red cell membranes (ghosts) induced a marked Y-phosphorylation of band 3 (Figure 1A, lane 3). In contrast, purified Lyn behaved as a very poor phosphorylating agent (Figure 1A, lane 2). However, preincubation of membranes with p36syk and unlabeled ATP converted band 3 into an excellent substrate for Lyn (Figure 1B, lane 2). Prolonged direct incubation of the isolated membranes with Lyn alone did not obviate the requirement for p36syk as a priming agent (not shown). Similar results were obtained using p72syk purified from rat spleen instead of erythrocyte p36syk (not shown).
In an attempt to confirm sequential phosphorylation of band 3 catalyzed by Syk and Lyn tyrosine kinases in intact cells, we stimulated the Y-phosphorylation of human erythrocytes by treating them with a combination of sodium orthovanadate and hydrogen peroxide (ie, pervanadate). Control and treated cells were then hemolyzed, and erythrocyte membranes were immediately isolated as described in “Materials and methods.” Antiphosphotyrosine immunoblotting revealed band 3 as the major Y-phosphorylated protein in membranes isolated from stimulated erythrocytes (Figure 1C, lane 2). The involvement of p72syk in the Y-phosphorylation of band 3 in erythrocytes activated by pervanadate has already been suggested.11 To verify the contribution of Lyn to band 3 phosphorylation in vivo, we carried out experiments with PP1, which is a highly potent inhibitor selective for Src family tyrosine kinases,31 including Lyn.32 Accordingly, PP1 proved unable to inhibit the in vitro phosphorylation of band 3 catalyzed by p36syk (Figure 1A, lanes 4-6). In contrast, secondary 32P-phosphorylation of band 3 catalyzed by Lyn was almost completely abolished in the presence of PP1 (Figure1B, lanes 3-5). PP1 was also tested for its effect on pervanadate-stimulated Y-phosphorylation in intact erythrocytes. As shown in Figure 1C (lanes 3-5), preincubation of red cells in the presence of both pervanadate and PP1 decreased the amount of phosphotyrosine detected in band 3. This result is consistent with the involvement of Lyn or another PP1-inhibited kinase in the phosphorylation of band 3 in intact erythrocytes. The residual Y-phosphorylation of the protein observed even in the presence of 25-μmol/L PP1 (Figure 1C, lanes 3-5) discloses the contribution of Src-unrelated protein kinases, as expected assuming that the sequential model of phosphorylation also takes place in intact cells.
We also tested the effect of PP1 on band 3 Y-phosphorylation triggered by different stimuli.2 8 For this purpose, we treated intact erythrocytes for 30 minutes with diamide (1-3 mmol/L), N-ethylmaleimide (2-4 mmol/L), or with a hypertonic buffer (900 mOsm) and found that PP1 always inhibited partially the band 3 Y-phosphorylation (not shown).
Activation of p72syk and Lyn by pervanadate treatment of intact erythrocytes
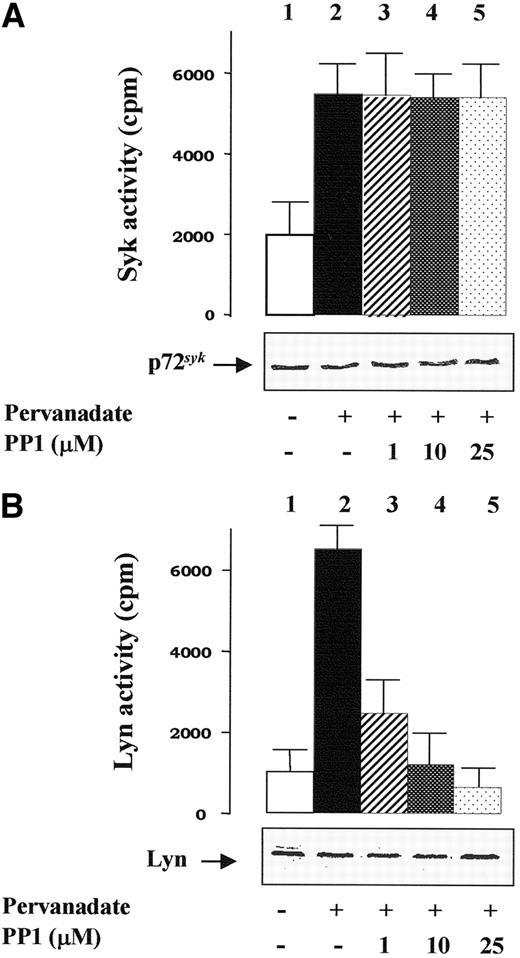
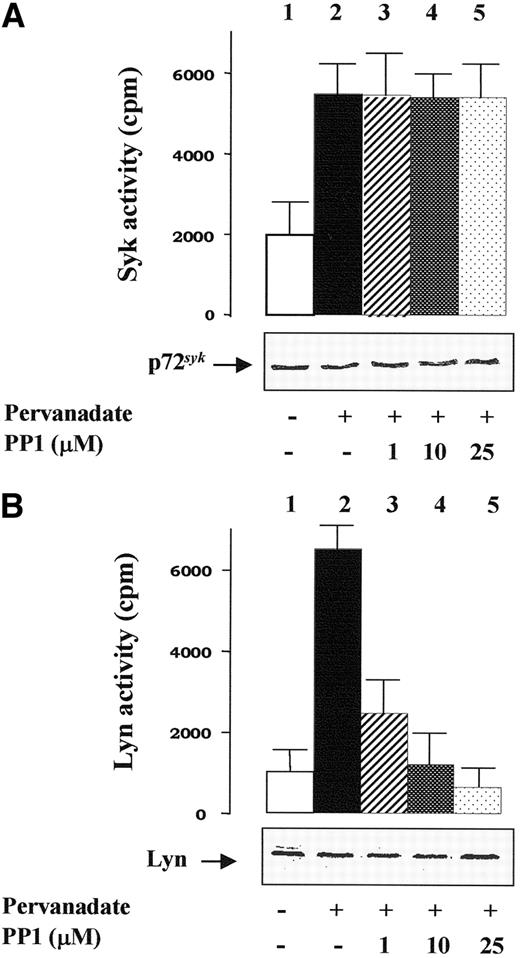
Pervanadate treatment of intact red cells correlates with an activation of p72syk.11 This is also demonstrated by the immunoprecipitation experiments shown in Figure 2A, carried out using extracts of membranes isolated from control and treated cells prepared with Nonidet P-40 as described in “Materials and methods.” After centrifugation, the extracted proteins were immunoprecipitated with anti-p72syk antibodies, and p72syk activity was tested in vitro toward the cytoplasmic domain of band 3. p72syk activity present in the anti-p72syk immunoprecipitates (IP) from stimulated cells was more than 3-fold higher relative to the basal activity found in untreated cells (compare histograms 1 and 2 in Figure 2A). As expected, PP1 treatment of erythrocytes did not affect p72syk activity (Figure 2A, histograms 3-5).
Activation of both p72syk and Lyn by pervanadate treatment of intact erythrocytes.
Human erythrocytes were incubated without (histogram 1) or with pervanadate (histograms 2-5) either in the absence (histograms 1 and 2) or presence of PP1 (histograms 3-5). Erythrocyte membranes were then rapidly isolated and treated with extraction buffer as described in “Materials and methods.” The extracted proteins were immunoprecipitated with either anti-Syk (A) or anti-Lyn (B) antibodies, and tyrosine kinase activities of the immune complexes were tested in vitro as described in “Materials and methods.” Aliquots of packed membranes were also subjected to SDS-PAGE, transferred to nitrocellulose membranes, and incubated with either anti-Syk or anti-Lyn antibody (insets in both panels). Reported values represent means of 4 separate experiments, with SE indicated by vertical bars.
Activation of both p72syk and Lyn by pervanadate treatment of intact erythrocytes.
Human erythrocytes were incubated without (histogram 1) or with pervanadate (histograms 2-5) either in the absence (histograms 1 and 2) or presence of PP1 (histograms 3-5). Erythrocyte membranes were then rapidly isolated and treated with extraction buffer as described in “Materials and methods.” The extracted proteins were immunoprecipitated with either anti-Syk (A) or anti-Lyn (B) antibodies, and tyrosine kinase activities of the immune complexes were tested in vitro as described in “Materials and methods.” Aliquots of packed membranes were also subjected to SDS-PAGE, transferred to nitrocellulose membranes, and incubated with either anti-Syk or anti-Lyn antibody (insets in both panels). Reported values represent means of 4 separate experiments, with SE indicated by vertical bars.
Using assays similar to those described above, we also performed anti-Lyn IP and Lyn activity was tested in vitro toward the synthetic peptide cdc2 (6-20), a substrate specific for Src kinases. While Lyn activity in control erythrocytes was low, it was greatly enhanced (by about 7-fold) in IP from pervanadate-treated cells (compare histograms 1 and 2 in Figure 2B). As expected, PP1 treatment of erythrocytes almost completely prevented phosphorylation of the peptide by the immunoprecipitated Lyn (Figure 2B, histograms 3-5). The high extent of Lyn activity found in pervanadate-treated erythrocytes (Figure 2B, histogram 2) is consistent with the presence of the doubly autophosphorylated (at Y396 and Y507), hyperactive Lyn conformation20 due to the blockage of phosphotyrosine phosphatase activities.
These results show that both p72syk and Lyn are activated by pervanadate treatment, ie, under conditions that trigger band 3 Y-phosphorylation. This finding, in conjunction with the effect of the Src specific inhibitor PP1, supports the view that p72syk is probably the tyrosine kinase responsible for the band 3 Y-phosphorylation that is resistant to PP1 inhibition in vivo. Moreover, comparison of data presented in Figures1C and 2B suggests that Lyn might be the Src kinase inhibited by PP1 and involved in band 3 Y-phosphorylation.
Recruitment of Lyn to Y-phosphorylated band 3 in pervanadate-treated cells
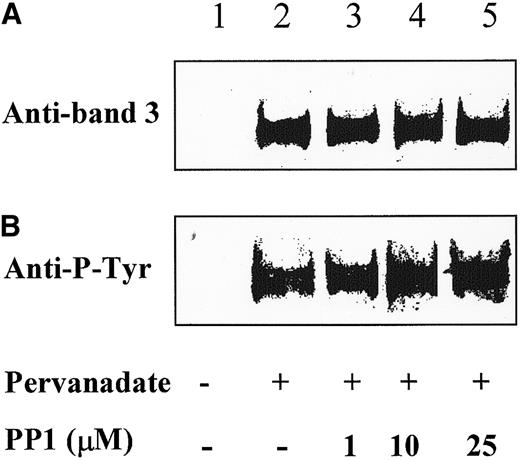
According to the sequential model of band 3 phosphorylation, p72syk generates the docking sites for the binding of Lyn to the membranes and subsequent phosphorylation of additional band 3 tyrosine residues (Figure 1B).12 To assess the actual occurrence of an interaction between Lyn and band 3 in vivo, we assayed for the presence of band 3 in the anti-Lyn IP obtained from intact red cells as described in Figure 2B. Figure3A shows that Lyn coimmunoprecipitated with band 3 when Y-phosphorylation was stimulated by pervanadate treatment (compare lanes 1 and 2 in Figure 3A), irrespective of the presence of the Lyn-specific inhibitor PP1 (Figure 3A, lanes 3-5).
Detection of Y-phosphorylated band 3 in anti-Lyn immunoprecipitates.
Anti-Lyn immune complexes, obtained as described in Figure 2B, were submitted to SDS-PAGE, transferred to nitrocellulose membranes, and immunostained with either anti-band 3 antibody (A) or anti-P-Y antibody (B). The figure is representative of 3 separate experiments.
Detection of Y-phosphorylated band 3 in anti-Lyn immunoprecipitates.
Anti-Lyn immune complexes, obtained as described in Figure 2B, were submitted to SDS-PAGE, transferred to nitrocellulose membranes, and immunostained with either anti-band 3 antibody (A) or anti-P-Y antibody (B). The figure is representative of 3 separate experiments.
Immunostaining with anti-P-Y antibody of anti-Lyn IP obtained from stimulated cells shows that the band 3 species coimmunoprecipitated with Lyn was Y-phosphorylated (compare lanes 1 and 2 in Figure 3B). Phosphotyrosine was also detectable in band 3 coimmunoprecipitated from PP1-treated erythrocytes, in accordance with the view that the primary phosphorylating agent is not Lyn but rather a Src-unrelated kinase that is also activated by pervanadate treatment. Syk is therefore the first-choice candidate to perform this task.
Peptide maps of band 3 phosphorylated in vitro or in vivo: identification of the protein residues phosphorylated by p36syk or Lyn in isolated erythrocyte membranes
We compared the 32P-peptide maps of band 3 obtained either from pervanadate-treated erythrocytes incubated in the presence of γ[32P]Pi or from ghosts32P-phosphorylated in vitro by addition of both p36syk and Lyn. Figure4 shows that the32P-Y-peptide maps obtained from trypsin-digested band 3 radiolabeled in vivo (Figure 4A) or in vitro (Figure 4B) are similar. This finding corroborates the view that the same sequential phosphorylation process outlined in vitro also operates in vivo. The peptide maps display 4 major radioactive fragments (Figure 4), suggesting that at least 4 tyrosine residues are phosphorylated in stimulated cells. Y8 has been suggested to be the main tyrosine residue of band 3 phosphorylated in isolated erythrocyte membranes incubated with γ[32P]ATP1,26,27; Y21, Y359, and Y904 have also been reported to be phosphorylated under the same in vitro conditions.28
Electrophoretic pattern of 32P-peptides obtained from tryptic digestion of band 3 phosphorylated either in vivo or in vitro by both p36syk and Lyn.
(A) Intact erythrocytes were depleted of endogenous ATP and incubated with [32P]-Pi as described in “Materials and methods.” Cell membranes were then rapidly isolated and submitted to SDS-PAGE. (B) Isolated erythrocyte membranes were phosphorylated with γ[32P]ATP in basal medium containing 15-nmol/L p36syk and 35-nmol/L Lyn and submitted to SDS-PAGE. In vivo and in vitro radiolabeled membrane proteins were transferred to polyvinylidine difluoride filters and treated with alkali to remove phosphoserine and phosphothreonine residues. 32P-Y-band 3 was excised from the filters and digested with trypsin; resulting32P-peptides were separated in 2 dimensions on thin-layer cellulose as detailed in “Materials and methods.” A similar amount of radioactivity was loaded on the cellulose plates. Shown are autoradiographs of the cellulose plates, with the origin indicated by an arrow. The figure is representative of 3 separate experiments.
Electrophoretic pattern of 32P-peptides obtained from tryptic digestion of band 3 phosphorylated either in vivo or in vitro by both p36syk and Lyn.
(A) Intact erythrocytes were depleted of endogenous ATP and incubated with [32P]-Pi as described in “Materials and methods.” Cell membranes were then rapidly isolated and submitted to SDS-PAGE. (B) Isolated erythrocyte membranes were phosphorylated with γ[32P]ATP in basal medium containing 15-nmol/L p36syk and 35-nmol/L Lyn and submitted to SDS-PAGE. In vivo and in vitro radiolabeled membrane proteins were transferred to polyvinylidine difluoride filters and treated with alkali to remove phosphoserine and phosphothreonine residues. 32P-Y-band 3 was excised from the filters and digested with trypsin; resulting32P-peptides were separated in 2 dimensions on thin-layer cellulose as detailed in “Materials and methods.” A similar amount of radioactivity was loaded on the cellulose plates. Shown are autoradiographs of the cellulose plates, with the origin indicated by an arrow. The figure is representative of 3 separate experiments.
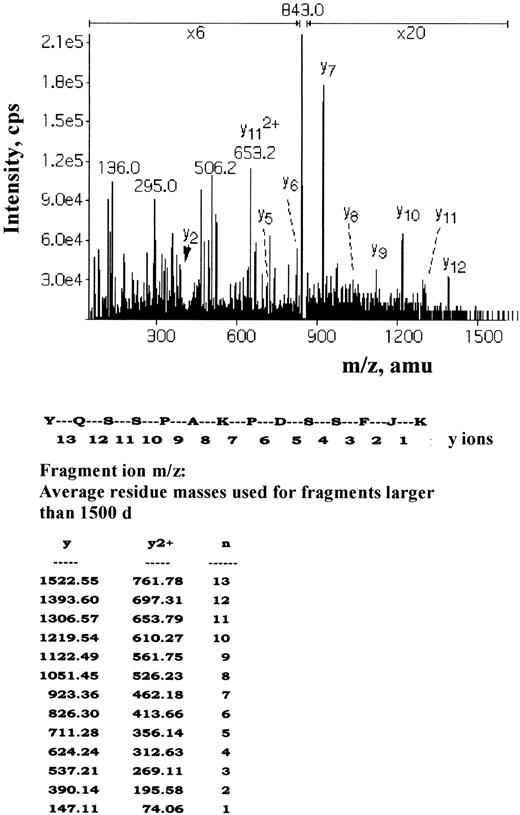
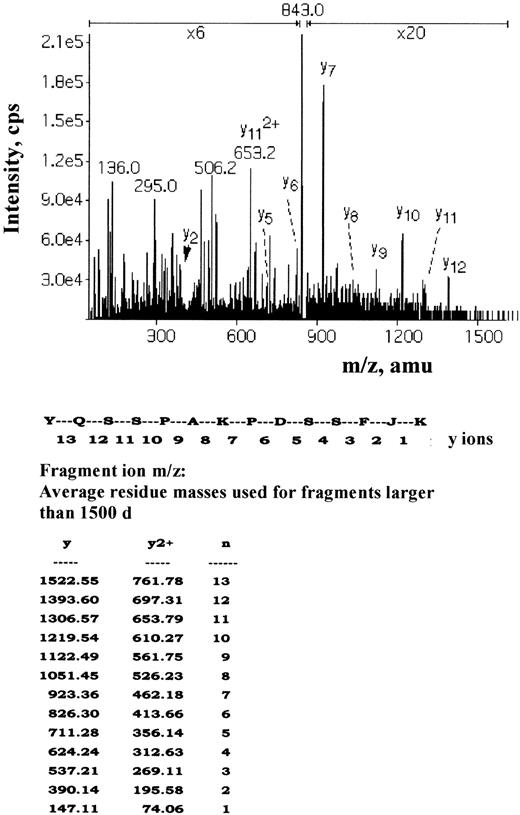
To identify the band 3 residues directly affected by Syk and Lyn, isolated erythrocyte membranes were phosphorylated with unlabeled ATP by p36syk and then secondarily radiolabeled by Lyn as described in “Materials and methods.” Five phosphopeptide-containing fractions were isolated and subjected to sequencing by mass spectrometric sequencing (MS/MS). Two main nonradioactive peaks were found corresponding to 2 band 3 peptides matching the amino acids 2 to 11, EELQDDJEDm-lac (where m-lac indicates homoserine lactone formed by the cyanogen bromide modification of methionine and J indicates phosphotyrosine), and amino acids 13 to 31, EENLEQEEJEDPDIPESQm-lac. This indicates that Y8 and Y21 are the main sites of phosphorylation by Syk. A very small amount of an unlabeled peptide corresponding to 347-to-360 YQSSPAKPDSSFJK was found. The high-performance liquid chromatography peak was eluted just before a larger radioactive peak, which contained the same peptide because both gave identical MS/MS spectra (Figure 5). This small peak is probably due to the separation of isomers arising from the cis-trans isomerization of the proline in the peptide and appears to be nonradioactive simply because of the low amount of material causing the signal to drop below background. The other main radioactive peak contained the peptide ATFDEEEGRDEJDEVAMPV corresponding to amino acids 893 to 911. Thus, Lyn phosphorylates Y904 and Y359 of band 3.
Mass spectrometric sequencing (MS/MS) of the phosphopeptide-containing Y359.
The radioactive fraction containing the 2+ ion m/z 843 isolated by immobilized metal affinity chromatography and reverse-phase chromatography was subjected to sequence analysis by collisionally activated dissociation using a triple quadrupole mass spectrometer. The resultant MS/MS spectrum is shown with the sequence ions labeled as indicated.
Mass spectrometric sequencing (MS/MS) of the phosphopeptide-containing Y359.
The radioactive fraction containing the 2+ ion m/z 843 isolated by immobilized metal affinity chromatography and reverse-phase chromatography was subjected to sequence analysis by collisionally activated dissociation using a triple quadrupole mass spectrometer. The resultant MS/MS spectrum is shown with the sequence ions labeled as indicated.
The interaction between Lyn and band 3 is mediated by the SH2 domain of the kinase
The importance of primary Y-phosphorylation of band 3 in the recruitment of Lyn to the transmembrane protein (Figure 3B) suggests the involvement of the SH2 domain of Lyn in the interaction between the enzyme and its substrate. This concept is also supported by the sequences containing the Syk-phosphorylated residues identified in vitro, MEELQDDY8EDM and EENLEQEEY21EDP, which exhibit a Src SH2-recognition motif.33 Our hypothesis was validated by competition experiments performed with band 3 phosphorylated both in vitro and in vivo.
Assays performed using synthetic peptides and isolated erythrocyte membranes revealed that peptide band 3 (1-26), phosphorylated at Y8 and Y21, but not its unphosphorylated derivative, prevented Lyn-mediated secondary phosphorylation of the transmembrane protein in a dose-dependent manner (Figure6A). The stringent specificity of this inhibition is highlighted by the observation that the Src (523-533) phosphopeptide TEPQpYQPGENL, which is highly homologous to the Lyn tail that interacts with the enzyme SH2 domain, inducing the locked Lyn conformation, was a less efficient competitor than the band 3 (1-26) phosphopeptide (Figure 6A). These data are consistent with the view that the occupancy of Lyn's SH2 domain by the specific phosphopeptide hinders both the binding of the enzyme to band 3 and the secondary phosphorylation of the protein. Similar results were obtained using recombinant Lyn SH2 domain to antagonize the secondary phosphorylation of band 3 in isolated erythrocyte membranes (Figure 6B). By specifically interacting with the primarily phosphorylated tyrosines of band 3, recombinant GST-SH2 fusion protein prevented the secondary phosphorylation of the transmembrane protein mediated by Lyn.
Specific inhibition of band 3 secondary phosphorylation by either band 3 (1-26) phosphopeptide or recombinant Lyn SH2 domain.
The ability of Lyn to phosphorylate band 3 following phosphorylation by p36syk with unlabeled ATP was measured in the presence of γ[32P]ATP and increasing concentrations of either the indicated synthetic phosphopeptides and their unphosphorylated homologues (A) or recombinant GST and GST-SH2 domain (B). Incubations were carried out for 5 minutes under conditions described in “Materials and methods.” The samples were submitted to SDS-PAGE, and the radioactivity incorporated into band 3 was evaluated either by analysis on a Packard Instant Imager or by autoradiography and scintillation counting of the identified radiolabeled bands. Lyn activity is expressed as the percentage of the control values obtained in the absence of effectors. Reported values represent means of 4 separate experiments, with SE indicated by vertical bars.
Specific inhibition of band 3 secondary phosphorylation by either band 3 (1-26) phosphopeptide or recombinant Lyn SH2 domain.
The ability of Lyn to phosphorylate band 3 following phosphorylation by p36syk with unlabeled ATP was measured in the presence of γ[32P]ATP and increasing concentrations of either the indicated synthetic phosphopeptides and their unphosphorylated homologues (A) or recombinant GST and GST-SH2 domain (B). Incubations were carried out for 5 minutes under conditions described in “Materials and methods.” The samples were submitted to SDS-PAGE, and the radioactivity incorporated into band 3 was evaluated either by analysis on a Packard Instant Imager or by autoradiography and scintillation counting of the identified radiolabeled bands. Lyn activity is expressed as the percentage of the control values obtained in the absence of effectors. Reported values represent means of 4 separate experiments, with SE indicated by vertical bars.
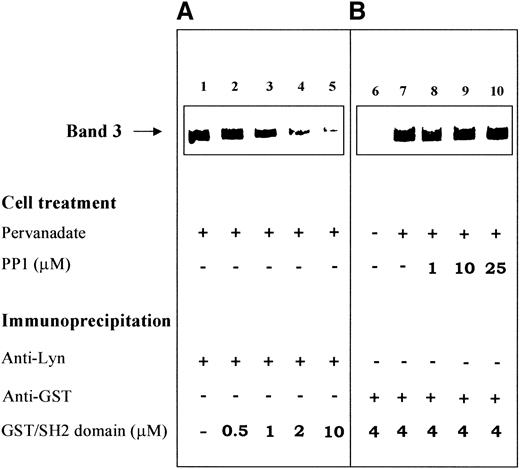
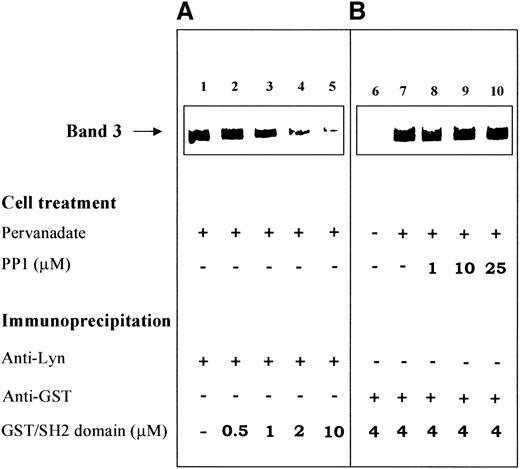
The specific involvement of the SH2 domain in the recruitment of Lyn to band 3 is also validated by competition experiments performed with band 3 phosphorylated in intact cells. Figure7A shows the coimmunoprecipitation of phospho-band 3 with Lyn in anti-Lyn IP obtained from pervanadate-stimulated erythrocytes. The presence of increasing concentrations of recombinant Lyn SH2 domain, added during the immunoprecipitation, progressively hindered the interaction between the primarily phosphorylated protein and Lyn (Figure 7A, lanes 2-5). The same conclusion is supported by the experiments shown in Figure 7B. Human red cells treated as described in Figure 2B were immunoprecipitated with anti-GST antibody in the presence of GST-SH2 domain. Upon Y-phosphorylation by pervanadate treatment of intact cells, band 3 coimmunoprecipitated with recombinant Lyn (GST)/SH2 domain (compare lanes 6 and 7 in Figure 7B). The same interaction was detected in IP obtained from cells stimulated in the presence of the Src inhibitor PP1 (Figure 7B, lanes 8-10), thereby confirming the hypothesis that band 3 phosphorylation catalyzed by a Src-unrelated tyrosine kinase is a prerequisite for the binding of Lyn to the protein.
Recombinant GST-SH2 domain counteracts the interaction between Lyn and phosphorylated band 3.
(A) Immunostaining of band 3 coimmunoprecipitated in anti-Lyn IP. Erythrocytes were treated with pervanadate, and the proteins extracted from the cell membranes were immunoprecipitated with anti-Lyn antibody as described in “Materials and methods.” The immunoprecipitations shown in lanes 2 to 5 were performed in the presence of increasing concentrations of recombinant GST/SH2 domain. (B) Immunostaining of band 3 coimmunoprecipitated in anti-GST IP. Human erythrocytes were treated as in Figure 2B, and the proteins extracted from the cell membranes were immunoprecipitated with anti-GST antibody in the presence of 4-μmol/L recombinant GST-SH2 domain. Immune complexes were submitted to SDS-PAGE, transferred to nitrocellulose membranes, and immunostained with anti-band 3 antibody. Experimental conditions are detailed in “Materials and methods.” The figure is representative of 5 separate experiments.
Recombinant GST-SH2 domain counteracts the interaction between Lyn and phosphorylated band 3.
(A) Immunostaining of band 3 coimmunoprecipitated in anti-Lyn IP. Erythrocytes were treated with pervanadate, and the proteins extracted from the cell membranes were immunoprecipitated with anti-Lyn antibody as described in “Materials and methods.” The immunoprecipitations shown in lanes 2 to 5 were performed in the presence of increasing concentrations of recombinant GST/SH2 domain. (B) Immunostaining of band 3 coimmunoprecipitated in anti-GST IP. Human erythrocytes were treated as in Figure 2B, and the proteins extracted from the cell membranes were immunoprecipitated with anti-GST antibody in the presence of 4-μmol/L recombinant GST-SH2 domain. Immune complexes were submitted to SDS-PAGE, transferred to nitrocellulose membranes, and immunostained with anti-band 3 antibody. Experimental conditions are detailed in “Materials and methods.” The figure is representative of 5 separate experiments.
Discussion
It has previously been reported that p72sykmay associate with band 3 and has been suggested that this tyrosine kinase is potentially involved in the pervanadate-induced Y-phosphorylation of band 3.11 The present experiments validate the hypothesis that activation of p72syk induces the phosphorylation of specific tyrosine residues of band 3 (Figures 1, 3, and 4). Moreover, we present the first direct evidence that, in pervanadate-stimulated red cells, a Src-related kinase is activated and involved in the phosphorylation of band 3, as judged by its reduced Y-phosphorylation brought about in vivo by PP1, an inhibitor specific for Src kinases (Figures 1C and 2B). The following evidence suggests that Lyn is the most likely candidate, among Src kinases, to phosphorylate the band 3: (1) Lyn is the only Src kinase we could purify in substantial amount from erythrocytes,12 (2) Lyn activity is highly increased in parallel with pervanadate-induced band 3 Y-phosphorylation (Figure 2B), (3) 32P-radiolabeled peptide maps of band 3 phosphorylated either in intact erythrocytes or in cell membranes by exogenous p36syk and Lyn are similar (Figure 4), and (4) other authors found that Lyn activity, at variance with that of Src and Fyn, is enhanced under conditions that also induce an increase of band 3 Y- phosphorylation.6
The present results also demonstrate that band 3 is phosphorylated according to a sequential model of phosphorylation. Upon stimulation, a primary tyrosine kinase, Src-unrelated and PP1-resistant, initiates the phosphorylation by acting on the primary phosphorylation sites of band 3. The experimental evidence points toward p72syk as the most likely candidate to perform this task and indicates Y8 and Y21 as specific targets of this enzyme. These residues are located at the N-terminal end of the flexible cytosolic domain of band 3, which contains the binding sites for cytoskeletal proteins and glycolytic enzymes.34 The primary phosphorylation most likely takes place by simple recognition of the sequences containing Y8 and Y21, MEELQDDY8EDM and EENLEQEEY21EDP, which display the consensus sequence specific for p72syk.35-38 In fact, in contrast with Src kinases, p72syk has been shown to be endowed with remarkable site specificity, exhibiting a stringent requirement for acidic residues located at the N-terminal side of the target residue. Moreover, the requirement for acidic/acidic/hydrophobic residues at positions +1, +2, and +3 with respect to the phosphorylatable tyrosine enables p72syk to generate binding sites for the SH2 domain of Src tyrosine kinases.36 37
The primary phosphorylation of band 3 catalyzed by p72syk generates the SH2 binding motifs that are a prerequisite for the following recruitment of Lyn. The interaction between the band 3 docking sites generated by the primary phosphorylation and the Lyn SH2 domain is proven unambiguously by the following evidence: (1) The specific occupancy of Lyn's SH2 domain by the synthetic peptide band 3 (1-26) phosphorylated at Y8 and Y21 counteracts the secondary phosphorylation of band 3 catalyzed in vitro by Lyn (Figure 6A); (2) recombinant Lyn (GST)-SH2 domain, which sequesters the protein docking sites, acts as a specific antagonist of the in vitro Lyn-mediated phosphorylation of band 3 (Figure 6B); (3) recombinant Lyn (GST)-SH2 domain prevents the coimmunoprecipitation of phospho-band 3 with Lyn in anti-Lyn IP from stimulated erythrocytes by competing with endogenous Lyn for binding to band 3 docking sites (Figure 7A); and (4) recombinant Lyn (GST)-SH2 domain coimmunoprecipitates with primary phosphorylated band 3 as judged by anti-GST IP obtained from intact red cells stimulated either in the absence or presence of the Lyn inhibitor PP1.
Our data demonstrate that the secondary phosphorylation of band 3 both in isolated membranes and in intact cells depends on the ability of Lyn's SH2 domain to recognize and interact with the protein after it has been primed by the first phosphorylation. Accordingly, only a slight phosphorylation of band 3 is detected when Lyn alone is added to isolated erythrocyte membranes (Figure 1A). Upon recruitment to Syk-phosphorylated band 3, Lyn catalyzes the secondary phosphorylation of the protein. Y359 and Y904 have been identified as the band 3 residues specifically phosphorylated by Lyn. While Y359 is located at the junction between the N-terminal cytosolic domain and the transmembrane domain of band 3, Y904 is situated at the C-terminal end of the protein. Most likely, the phosphorylation of these residues by Lyn is not directly dictated by local specificity determinants but by conformational features. The observation that the addition of either band 3 (1-26) phosphopeptide or the recombinant SH2 domain of Lyn prevents the band 3 secondary phosphorylation catalyzed by Lyn indicates that these conformational requirements rely primarily on the interaction between phospho-band 3 and the SH2 domain of Lyn. This hypothesis is validated by the finding that specific sequences are not stringently required for site recognition by Src kinases, apart from the frequent presence of a hydrophobic residue at position n-1.36,37 39
A sequential process of phosphorylation synergistically mediated by Syk and Src tyrosine kinases has already been demonstrated in vitro for the hematopoietic lineage-specific protein HS1.40 41Here we present the first in vivo evidence of a protein sequentially phosphorylated and propose that Syk or related kinases may play a general role in generating docking sites for Src SH2 domains in proteins, thereby converting them into good substrates for Src tyrosine kinases.
We also suggest that the involvement of a Src kinase in band 3 Y-phosphorylation might be a general theme in human erythrocytes. In fact, we found that PP1 inhibits also the band 3 Y-phosphorylation induced by cell treatment with the oxidizing agents diamide and N-ethylmaleimide or cell exposure to hypertonic conditions.
Band 3 is a multifunctional transmembrance protein, which serves as anion transporter, anchor for cytoskelton, hemoglobin, and glycolitic enzymes, as well as a senescence antigen.34 The 43 kd N-terminal domain of band 3 protrudes into the cytosol as a flexible finger and contains the binding sites for cytoskeletal proteins and glycolitic enzymes, whereas the intramembrane domain mediates anion transport.34 Because Syk and Lyn phosphorylate band 3 at both cytosolic and membrane domains, Y-phosphorylation/dephosphorylation is likely involved in the regulation of several erythrocyte functions (ie, glycolysis, cell shape, cytoskeleton movements, and anion transport.
Acknowledgments
We are grateful to Dr Oriano Marin for generously providing synthetic phosphopeptides. The technical assistance of Miss Carla Munari is gratefully acknowledged.
Supported by the Armenise-Harvard Foundation, AIRC, the Italian Ministry of Health (Project AIDS), Italian MURST (PRIN, 1997), and CNR (Target Project on Biotechnology).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Arianna Donella-Deana, Dipartimento di Chimica Biologica, Viale G. Colombo, 3, 35121 Padova, Italy; e-mail: arianna@ck2.bio.unipd.it.

![Fig. 1. Y-phosphorylation of band 3 in isolated ghosts and in intact human erythrocytes. / (A) Radioactive phosphorylation pattern of human erythrocyte membranes (ghosts) incubated with γ[32P]ATP either alone (lane 1) or in the presence of 35-nmol/L Lyn (lane 2) or 15-nmol/L p36syk (lanes 3-6). Increasing concentrations of PP1 were present in the incubation medium of assays shown in lanes 4 to 6. (B) Radioactive phosphorylation pattern of erythrocyte ghosts, first phosphorylated by p36syk in the presence of unlabeled ATP as described in “Materials and methods” and further incubated for 10 minutes in the presence of γ[32P]ATP without (lane 1) or with 35-nmol/L Lyn (lanes 2-5). Assays in lanes 3 to 5 were performed in the presence of increasing concentrations of PP1 added immediately before Lyn. 32P-radiolabeled proteins were subjected to SDS-PAGE on 10% gels followed by autoradiography. (C) Anti-P-Y immunostaining of erythrocyte membrane proteins phosphorylated in vivo. Intact human erythrocytes were incubated either in the absence (lane 1) or presence (lanes 2-5) of pervanadate as described in “Materials and methods.” Increasing concentrations of PP1 were present in the assays shown in lanes 3 to 5. After incubation, erythrocyte membranes were rapidly isolated, solubilized, and submitted to SDS-PAGE followed by transfer to a nitrocellulose filter. The filter was then immunostained with anti-P-Y antibody. Other experimental details are described in “Materials and methods.” Panels are representative of at least 6 different experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/4/10.1182_blood.v96.4.1550/4/m_h81600024001.jpeg?Expires=1766253941&Signature=NS1f1kLhh5iJTmWaDGCas5PiWBzMWgOoSclNtyvhWqW7gwsROdfGgFRAhCrrITP7V86dMb~5XmIkNuoCMJxDe95PV2RLwQ5sJolYsUlzRDJ7P4nqEyMebFxAnvg2b0psao52PneH2s6L-0j2S7-s3SE4QTN63j-x3GWEG1mMJeC-FyERXk7EQEFojDF7XW~LHtNaNJCGx8ibJ-YGnjMsapp4~Ovap9GeFNz9j5cpkRbAoEJzxE0cOcTHyjrWy6PcfiGXnbrSAPs9mDZZVLjwc8w2L9dgPhxKZ9OqMD2m3wvk5sBdAm3KG1d~0bO0ic7ZrVXemMkTrZ6r0cNji5EAJQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Electrophoretic pattern of 32P-peptides obtained from tryptic digestion of band 3 phosphorylated either in vivo or in vitro by both p36syk and Lyn. / (A) Intact erythrocytes were depleted of endogenous ATP and incubated with [32P]-Pi as described in “Materials and methods.” Cell membranes were then rapidly isolated and submitted to SDS-PAGE. (B) Isolated erythrocyte membranes were phosphorylated with γ[32P]ATP in basal medium containing 15-nmol/L p36syk and 35-nmol/L Lyn and submitted to SDS-PAGE. In vivo and in vitro radiolabeled membrane proteins were transferred to polyvinylidine difluoride filters and treated with alkali to remove phosphoserine and phosphothreonine residues. 32P-Y-band 3 was excised from the filters and digested with trypsin; resulting32P-peptides were separated in 2 dimensions on thin-layer cellulose as detailed in “Materials and methods.” A similar amount of radioactivity was loaded on the cellulose plates. Shown are autoradiographs of the cellulose plates, with the origin indicated by an arrow. The figure is representative of 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/4/10.1182_blood.v96.4.1550/4/m_h81600024004.jpeg?Expires=1766253941&Signature=Ecqb32P015JTb~Hd1syQusy08x9SC2Kq0fVnkwsbvT4o7e7u1AlnNOQ-WCnxLAgZkT5ItMyM27whBMV6Rlc7sLeHdrYQ~p93lKGzOZQYeV-F7xnebS7XmrIs~j~AoFzlRaGS~rY8MYeo8IRc0gYHdCjdiF9ExIGJyE7Dk~9nTawej-3XNS0MdhnibOZKDMGO~qZmG67w4axrzmoCXUP1j70k-Ux6LZn5i4vX4ECqHyf-~uyOBIMhthxb7t743f~-gmVpX7meZXaBet4eK6QLOznTbK9dfcMCx6RPvSK6mikKr4slkFJMKgkAr4ujff07WYXrAV3lAemOgkQCRTiRlQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Specific inhibition of band 3 secondary phosphorylation by either band 3 (1-26) phosphopeptide or recombinant Lyn SH2 domain. / The ability of Lyn to phosphorylate band 3 following phosphorylation by p36syk with unlabeled ATP was measured in the presence of γ[32P]ATP and increasing concentrations of either the indicated synthetic phosphopeptides and their unphosphorylated homologues (A) or recombinant GST and GST-SH2 domain (B). Incubations were carried out for 5 minutes under conditions described in “Materials and methods.” The samples were submitted to SDS-PAGE, and the radioactivity incorporated into band 3 was evaluated either by analysis on a Packard Instant Imager or by autoradiography and scintillation counting of the identified radiolabeled bands. Lyn activity is expressed as the percentage of the control values obtained in the absence of effectors. Reported values represent means of 4 separate experiments, with SE indicated by vertical bars.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/4/10.1182_blood.v96.4.1550/4/m_h81600024006.jpeg?Expires=1766253941&Signature=g0jXUeZiRviIDBheevA0mnPhxT-MgKtg08flCIVqxb3hjGTeI8FU1ZjBN0zz2bbDTgR~LKOxXwQI5xpD-WermpmMlHVJkYmvjPNKtAJ-sWQ-gDldSqsU5acQiAOoQQxog093MMTE3mnlId96eXUG5ZbBQ7kRYcVrXOWQQXPFQeCWwNOD7tJySoqA6DtJ45wRgaIRRVQQg2sZ7Fo4DPG8kvifz95UqMKNTpKn1hUK7kqb0hYypASLGWgjOUNWTzrfUrYibswyqI~LgIxYhi~jv-qdQMOV4Bv9-gj8OirPNX6wjuH3RPIq5uqdcdV98bVJmErypfyZiKJBeLD4lA9uiA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. Y-phosphorylation of band 3 in isolated ghosts and in intact human erythrocytes. / (A) Radioactive phosphorylation pattern of human erythrocyte membranes (ghosts) incubated with γ[32P]ATP either alone (lane 1) or in the presence of 35-nmol/L Lyn (lane 2) or 15-nmol/L p36syk (lanes 3-6). Increasing concentrations of PP1 were present in the incubation medium of assays shown in lanes 4 to 6. (B) Radioactive phosphorylation pattern of erythrocyte ghosts, first phosphorylated by p36syk in the presence of unlabeled ATP as described in “Materials and methods” and further incubated for 10 minutes in the presence of γ[32P]ATP without (lane 1) or with 35-nmol/L Lyn (lanes 2-5). Assays in lanes 3 to 5 were performed in the presence of increasing concentrations of PP1 added immediately before Lyn. 32P-radiolabeled proteins were subjected to SDS-PAGE on 10% gels followed by autoradiography. (C) Anti-P-Y immunostaining of erythrocyte membrane proteins phosphorylated in vivo. Intact human erythrocytes were incubated either in the absence (lane 1) or presence (lanes 2-5) of pervanadate as described in “Materials and methods.” Increasing concentrations of PP1 were present in the assays shown in lanes 3 to 5. After incubation, erythrocyte membranes were rapidly isolated, solubilized, and submitted to SDS-PAGE followed by transfer to a nitrocellulose filter. The filter was then immunostained with anti-P-Y antibody. Other experimental details are described in “Materials and methods.” Panels are representative of at least 6 different experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/4/10.1182_blood.v96.4.1550/4/m_h81600024001.jpeg?Expires=1766312603&Signature=5EmaHzEk6RjIuykkbHxfhLOFrGLK8Ab2Mc8j25SmafWVkUZbPmZHAf2v1kxO89fy6NPi6g6GHrUphgKW0pFRQKomHhdubkatproM0Tjc2jU9dxIB6BqJTGKSvKz5XwoDjYtdzHFxILGS1RWrBxbb2EtSLsQPztlHTSCa8rONdjXlLMIgfHZGYUfu4lrgaSGy7GrFscdYzH5OCE2yH18tTHpv4iD32qiiXXFS7LO7eFC-FKr2aQv3wRi8KllqLSld4mBR2UEx9FC27AATg2TWLA~aREXvYU9bPGr19VC7QD2mrBQB5lnD11K3Qiv~n8e6XAjGCX8BEQTiPgP2SxH40A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 4. Electrophoretic pattern of 32P-peptides obtained from tryptic digestion of band 3 phosphorylated either in vivo or in vitro by both p36syk and Lyn. / (A) Intact erythrocytes were depleted of endogenous ATP and incubated with [32P]-Pi as described in “Materials and methods.” Cell membranes were then rapidly isolated and submitted to SDS-PAGE. (B) Isolated erythrocyte membranes were phosphorylated with γ[32P]ATP in basal medium containing 15-nmol/L p36syk and 35-nmol/L Lyn and submitted to SDS-PAGE. In vivo and in vitro radiolabeled membrane proteins were transferred to polyvinylidine difluoride filters and treated with alkali to remove phosphoserine and phosphothreonine residues. 32P-Y-band 3 was excised from the filters and digested with trypsin; resulting32P-peptides were separated in 2 dimensions on thin-layer cellulose as detailed in “Materials and methods.” A similar amount of radioactivity was loaded on the cellulose plates. Shown are autoradiographs of the cellulose plates, with the origin indicated by an arrow. The figure is representative of 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/4/10.1182_blood.v96.4.1550/4/m_h81600024004.jpeg?Expires=1766312603&Signature=CfZlRFdL9y9y6e9epyeLqHM6TMH~6FHK0ROwayCzb4egsmeygen0hNNd0kD7M3xOxRNDHhiEq4r14w2Bs1zfemvTjVgeWWYXjMJMJEU-DEV2UdPVkG7a7qyMiR4kqsEqd844o5iGzoaaHvSkLchSqG7V1MZIu4ITUL-F2~QUCPu7TwllRYN2Rfiz3nmA5f1imJjv9oTN~Yu14RoR8QdRK~mD9CT94hGrmdlZOHpYvrzQ9XJUM0hluhxJjQSkdBxzoKIemz5FhmKw0j5DBI6VuWRlUMw1EOzs-dClABggTPeWQf2BlxxLGUjgXMIMNUqsyBhFfhAU5vPV0OyxiEjDSA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Specific inhibition of band 3 secondary phosphorylation by either band 3 (1-26) phosphopeptide or recombinant Lyn SH2 domain. / The ability of Lyn to phosphorylate band 3 following phosphorylation by p36syk with unlabeled ATP was measured in the presence of γ[32P]ATP and increasing concentrations of either the indicated synthetic phosphopeptides and their unphosphorylated homologues (A) or recombinant GST and GST-SH2 domain (B). Incubations were carried out for 5 minutes under conditions described in “Materials and methods.” The samples were submitted to SDS-PAGE, and the radioactivity incorporated into band 3 was evaluated either by analysis on a Packard Instant Imager or by autoradiography and scintillation counting of the identified radiolabeled bands. Lyn activity is expressed as the percentage of the control values obtained in the absence of effectors. Reported values represent means of 4 separate experiments, with SE indicated by vertical bars.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/4/10.1182_blood.v96.4.1550/4/m_h81600024006.jpeg?Expires=1766312603&Signature=rdL0Tiixjh71U5ZoDQozHlICBgvbeHRApKmgvJCOq3wy8qWavEjcfHORPONfs3t-GagcRS8Ov0oHKhWQlR3IUkNJhFO4yx6MkyTmj57q8Wn4S6QD6HU9EU5HqDUFJoveGlBpP~0PprK4zD5HQHOALV0AMzg6PW~61a95ZJUFPnPbpOdFT2LCO6aWfQ78WIR7EQgWQ2m4O8M6UiGDqHLytom5WwUiA1KjeZF3ZzeHjL5h0iGLgZ59lMYsvPzVvNQyDcwsJ9bUbphT7UlU-oBwojbY0NuE591Qnpe~wx6wnjJWLKAM8pX5KAhOKLc-qLQmRriMi-LLmpsn0Ex26~o3mA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)