Abstract
Chronic myeloid leukemia (CML) is a malignant stem cell disease characterized by an expansion of myeloid progenitor cells expressing the constitutively activated Bcr-Abl kinase. This oncogenic event causes a deregulation of apoptosis and cell cycle progression. Although the molecular mechanisms protecting from apoptosis in CML cells are well characterized, the cell cycle regulatory event is poorly understood. An inhibitor of the cyclin-dependent kinases, p27, plays a central role in the regulation of growth factor dependent proliferation of hematopoietic cells. Therefore, we have analyzed the influence of Bcr-Abl in the regulation of p27 expression in various hematopoietic cell systems. An active Bcr-Abl kinase causes down-regulation of p27 expression in murine Ba/F3 cells and human M07 cells. Bcr-Abl blocks up-regulation of p27 after growth factor withdrawal and serum reduction. In addition, p27 induction by transforming growth factor-beta (TGF-β) is completely blocked in Bcr-Abl positive M07/p210 cells. This deregulation is directly mediated by the activity of the Bcr-Abl kinase. A Bcr-Abl kinase inhibitor completely abolishes p27 down-regulation by Bcr-Abl in both Ba/F3 cells transfected either with a constitutively active Bcr-Abl or with a temperature sensitive mutant. The down-regulation of p27 by Bcr-Abl depends on proteasomal degradation and can be blocked by lactacystin. Overexpression of wild-type p27 partially antagonizes Bcr-Abl–induced proliferation in Ba/F3 cells. We conclude that Bcr-Abl promotes cell cycle progression and activation of cyclin-dependent kinases by interfering with the regulation of the cell cycle inhibitory protein p27.
Introduction
The proliferation of hematopoietic cells is controlled by cytokines. Binding of cytokines to their specific receptors activates several signaling pathways controlling the activity of cyclin dependent kinases (CDKs). The decision for proliferation depends on the activity of CDKs. Deregulated cell growth leads to malignant cell growth and is a critical feature of most neoplastic cells. Accordingly, a variety of oncogenes like c-myc and ras target these pathways causing inappropriate activation of CDKs.1 2
An expansion of myeloid precursor cells is one of the hallmarks of chronic myeloid leukemia (CML). The oncogene bcr-abl is expressed in the leukemic clone and is known to constitutively activate several signaling pathways important for the regulation of proliferation, survival, and adhesion. These include RAS,3-6 phosphatidylinositol-3-kinase (PI3K),7 MYC,8 JAK/STAT,9 focal adhesion kinase (FAK),10 Src kinases (Hck and Lyn),11 and JUN.12 The constitutively active kinase Bcr-Abl transforms both cells of fibroblast and hematopoietic origin in vitro.5,13,15 Expression of the Bcr-Abl kinase is sufficient to cause CML-like disease in rodent models. De novo expression of Bcr-Abl protects growth factor-dependent cells from apoptotic cell death after cytokine withdrawal and renders these cells growth factor independent.15-17 Up-regulation of the antiapoptotic proteins Bcl-2 or Bcl-XL as the primary consequence of Bcr-Abl expression has been described.18According to this hypothesis, Bcr-Abl renders cells resistant to apoptotic cell death in the absence of cytokines, allowing them to accumulate secondary genetic abnormalities, leading to the expansion of the malignant cells and clonal evolution of the disease.
However, the question whether Bcr-Abl is capable of directly promoting cell cycle entry is less well established. Recently, we and others found that Bcr-Abl directly promotes cell cycle entry in the absence of growth factors both in primary leukemic cells and Bcr-Abl transfected cell lines.19,20 Primary CML cells continue to divide subsequent to growth factor withdrawal. The inhibition of the Bcr-Abl kinase restored growth factor dependency and prevented abnormal cell cycle entry of primary leukemic cells. Cortez et al20observed activation of RAS, Erk, and JNK pathways as a primary consequence of Bcr-Abl expression in murine myeloid 32D cells. They reported a Bcr-Abl driven activation of cyclin-dependent kinase 2 (CDK2) in growth factor and serum-deprived cells. CDK activity is positively regulated by its association with cyclins, the D-type cyclins for regulating CDK4 and CDK6 and cyclin E and A for regulating CDK2.21,22 Additionally, G1 kinases can be regulated by a set of small proteins that inhibit CDK activity by stoichiometric binding.21,22 Two families of CDK inhibitors have been identified. One is called the Ink4 inhibitors represented by its members p16Ink4a, p15Ink4b, p18Ink4c, and p19Ink4d, which are specific for CDK4 and CDK6.23-27 The other includes p21CIP1,28p27KIP1,29,30 and p57KIP2.31,32 In vitro, p21, p27, and p57 inhibit the activity of cyclin D-CDK4, cyclin D-CDK6, cyclin E-CDK2, as well as cyclin A-CDK2.29-31,33,34 The phenotype of p27-deficient mice indicates a central role of p27 in the control of hematopoiesis. p27 seems to have a selective effect on the self-renewing, mitogen-driven cell cycle characteristic of stem and some progenitor cells and to have little impact on terminal differentiation.35 Thus, p27-deficient mice exhibit an expansion of hematopoietic progenitor cells without any defect in the differentiation capacity of these cells. These observations are quite similar to clinical observations in CML, as immature myeloid cells are increased in chronic phase while their capacity to differentiate is retained.
We investigated the role of Bcr-Abl on cell cycle control and p27 expression after serum reduction, transforming growth factor-beta (TGF-β) treatment and cytokine withdrawal. In both human and murine cell lines, we found Bcr-Abl–dependent deregulation of p27 expression. These data demonstrate for the first time that Bcr-Abl directly promotes cell cycle entry by decreasing the level of the CDK inhibitory protein, p27.
Material and methods
Reagents
The Abl-kinase inhibitor CGP 57148B (now renamed: STI 571) was kindly provided by Novartis (Basel, Switzerland). A stock solution (10 mg/mL) was prepared by dissolving the compound in HDMSO/H2O and kept at −20°C. CGP 57148B was used at a concentration of 1 μmol/L. Transforming growth factor-beta (TGF-β) was obtained from PBH (PBH, Hannover, Germany). The cell permeable proteasome inhibitor, Lactacystin (Calbiochem, San Diego, CA) was dissolved in DMSO and used at a concentration of 10 μmol/L. The PI3 kinase inhibitor LY294002 was obtained from Calbiochem (San Diego, CA) and used at a concentration of 5 μmol/L.
Cell culture techniques
The human cell line M07 was derived from a patient with megakaryocytic leukemia and requires granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), or steel factor for proliferation. Cells were cultured in RPMI 1640 (Seromed, Berlin, Germany) supplemented with 10% fetal calf serum (FCS) and 100 ng/mL of GM-CSF (Leukomax, Sandoz, Switzerland). The cell line M07/p210 was obtained from Dr M. Hallek, Munich, Germany.36M07/p210 cells were generated by electroporation of M07 cells with the pGD210 plasmid and subsequent selection for G418 (400 μg/mL; Gibco) resistance. The resulting M07/p210 cells, stable express p210Bcr-Abl and remain factor independent. M07/p210 were grown in RPMI 1640 supplemented with 10% FCS.
The murine pro-B lymphocyte cell line Ba/F3, which expresses p185 Bcr-Abl was a kind gift from Dr J. Duyster (Munich, Germany). The Bcr-Abl temperature sensitive mutant (tsBcr-Abl) was generated by subcloning a temperature sensitive v-Abl mutant (DP)37into pSLXBcr-Abl. Ba/F3, transfected with this vector grew only in medium containing IL-3, when cultivated at nonpermissive temperature (39°C) and were growth factor independent at the permissive temperature (33°C) as previously reported.38 At the nonpermissive temperature Ba/F3 cells were grown in suspension culture in RPMI 1640 medium containing 10% FCS and 1 ng/mL recombinant murine IL-3 (PBH, Hannover, Germany). Factor-independent Ba/F3 cultivated at the permissive temperature 33°C were grown without IL-3 supplementation. The growth factor-independent Ba/F3 clone expressing wild-type Bcr-Abl was transformed with pSLXBcr-Abl and cultivated at 37°C.
p27 overexpression
The p27 wild-type complementary DNA (cDNA) (kindly supplied by S. Dowdy, St Louis, MO) was cloned as a blunt/EcoRI fragment into the HpaI and EcoRI site of the MSCV-derived bicistronic expression vector Mig1 containing an IRES-EGFP-cassette (kindly supplied by W. Pear, Philadelphia, PA). 1.5 × 106 Ba/F3 cells stably expressing Bcr-Abl were electroporated with the p27 wild-type Mig or the empty-EGFP-vector as control with a GenePulser electroporator (Bio-Rad, München, Germany). Twenty-four to 36 hours after electroporation, DNA content was analyzed by adding 5μg/mL Hoechst 33342 dye (Sigma, Deisenhofen, Germany), subsequent incubation at 37°C for 15 minutes and subsequent measurement on a modified FACSSTAR cell sorter (Becton Dickinson, Heidelberg, Germany) equipped with 2 argon lasers running at 488 and 365 nm. EGFP-fluorescence was measured in the FL1 channel at 510 nm, Hoechst 33342 fluorescence was measured in FL4 at 450 nm. After gating vital cells in the FSC/SSC dot plot, cell doublets were excluded by setting a further gate in the FL4 pulse/FL4 area dot plot. DNA histograms were analyzed with the WinCycle software (Phoenix Flow Systems, San Diego, CA).
Survival assay
Cells were plated into 96-well plates at 1 × 105per well in their respective media supplemented with 10% FCS. After the indicated period, the viable cells in each well were assayed to their ability to transform 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide into a purple formazan. The absorbance of the samples were messured in an enzyme-linked immunosorbent assay (ELISA) reader at 570 nm.
Cell proliferation enzyme-linked immunosorbent assay
Cells were plated into 96-well plates at 0.5 × 105 per well in their respective media. After the indicated period, BrdU was added to the samples for 4 hours. The detection of incorporated BrdU during DNA synthesis was performed using the “Cell Proliferation ELISA” kit according to the instructions of the manufacturer (Boehringer Mannheim, Mannheim, Germany).
Cell extract preparation
Extracts were prepared as follows: Cells were collected by centrifugation and dissolved in lysis buffer (50 mmol/L Tris-HCl, pH 7.6; 250 mmol/L NaCl; 0.1% Triton X-100; 5 mmol/L EDTA; 1 μg/mL leupeptin; 1 mmol/L phenylmethylsulfonyl fluoride [PMSF]; 1 mmol/L dithiothreitol [DTT]; 1 μg/mL aprotinin). The extracts were quantified using Bradford protein assay with bovine serum albumin as a protein standard.39
Western blots
Proteins (50 μg each lane) were run on sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) with the Tris glycine buffer system.40 Proteins were transferred to PVDF membranes (Boehringer Mannheim) using a semidry transfer system as recommended by the manufacturer (Bio-Rad). Loading of proteins was controlled by Ponceau staining before antigen detection. The membranes were blocked by incubating in 1% blocking solution (BS; Boehringer Mannheim) for 1 hour. The blots were then incubated with either anticyclin D3 (C-16), anticyclin D2 (C-17), anticyclin E (C-19), anti-CDK2 (M2), anti-PCNA (PC10) antibody (1:250; Santa Cruz), or with p27KIP1(G173-524) antibody (1:250; Pharmingen, San Diego, CA) in TBS with 0.5% blocking solution overnight. Blots were washed 2 times in Tris-buffered saline with Tween-20 (0.1%) (TBST) for 10 minutes and afterward blocked 2 times with 0.5% BS in TBS for 10 minutes. Then blots were incubated with 2° antibody conjugated to horseradish peroxidase (diluted 1:5000; Boehringer Mannheim) for 30 minutes, and proteins were detected by chemiluminescence (Boehringer Mannheim). Equal protein loading was controlled by reprobing with anti-GAP-DH antibody (1:10 000; Biodesign, Kennebunk, ME).
Cell cycle analysis
Cell cycle analyses were performed by determination of DNA content with propidiumiodide. Cells were washed in phosphate-buffered saline (PBS) and fixed in 70% ethanol for at least 1 hour at 4°C. Shortly before flow cytometry analysis cells were rehydrated in cold PBS, treated with 50 μg/mL RNase A (Boehringer Mannheim) for at least 15 minutes at room temperature and stained with 50 μg/mL propidiumiodide. The stained cells were analyzed by FACScan (Becton Dickinson, Heidelberg, Germany). The fraction of cells with a DNA content of less than] 2N, 2N, 2 to 4N, and 4N was determined by means of the Modfit 2.0 software (Becton Dickinson). Number of viable cells was determined by counting the number of cells, excluding trypan blue.
Results
Growth factor deprivation induces p27 protein expression in M07 cells
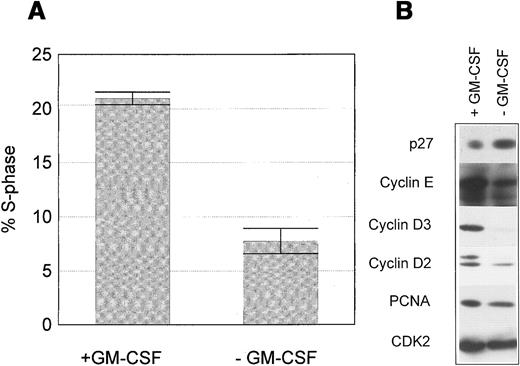
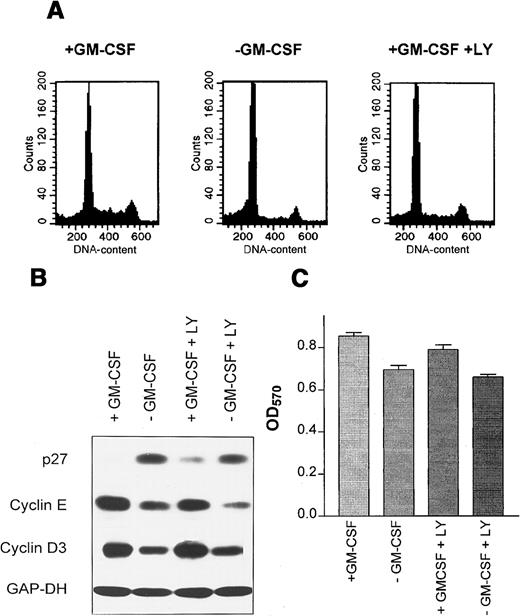
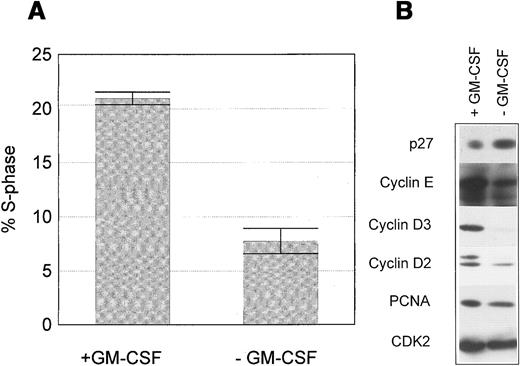
Proliferation of wild-type M07 cells is strictly dependent on the presence of exogenous growth factors GM-CSF or IL-3. Removal of growth factors reduces S-phase fraction by 21.0% ± 1.1% to 7.6% ± 1.8% after 18 hours (Figure 1A). Critical targets for the growth factor signaling pathways are cell cycle regulatory proteins regulating the activity of cell cycle-dependent kinases. Both cyclins and cyclin inhibitors are regulated by GM-CSF in M07 cells. Removal of GM-CSF for 18 hours led to a decline of cyclin E, cyclin D2, cyclin D3, and PCNA levels and to an up-regulation of the CDK-inhibitor p27 (Figure 1B). The growth factor-dependent regulation of p27 in other cell systems has been shown to be mediated by activation of the PI3K.41 We therefore studied, whether the same mechanism is operative in M07 cells. Accordingly, these experiments showed that inhibition of PI3K by its specific inhibitor LY294002 led to a modest reduction of S-phase fraction to 56.6% of controls after 18 hours. In contrast, removal of growth factor was more effective in reducing the number of DNA synthesizing and decreased the S-phase fraction to 28% of controls. (Figure 2A). Similarly, LY294002 induced up-regulation of p27 to a lesser extent in comparison to growth factor withdrawal (Figure 2B). Furthermore, inhibition of PI3K by LY294002 decreased the level of cyclin E, the cyclin D3 level remains constant (Figure 2B). The observed protein and cell cycle regulation is concordant to the cell survival measured in the MTT assay (Figure 2C). These data show that p27 expression in M07 cells is regulated by other pathways in addition to the PI3 kinase pathway.
Growth factor withdrawal induces p27 in M07 cells.
M07 cells were cultured for 18 hours with or without GM-CSF. (A) Proportion of S-phase cells in the presence or absence of GM-CSF. Data represent the mean ± SE of 3 independent examinations. (B) Relative changes in expression levels of p27, cyclin D2, cyclin D3, cyclin E, CDK2, and PCNA after growth factor withdrawal for 18 hours. Lysates were prepared and subjected to Western blot analysis using anti-p27, anticyclin D3, anticyclin E, anticyclin D2, anti-PCNA, and anti-CDK2 antibodies. Data shown are representative of at least 5 independent examinations.
Growth factor withdrawal induces p27 in M07 cells.
M07 cells were cultured for 18 hours with or without GM-CSF. (A) Proportion of S-phase cells in the presence or absence of GM-CSF. Data represent the mean ± SE of 3 independent examinations. (B) Relative changes in expression levels of p27, cyclin D2, cyclin D3, cyclin E, CDK2, and PCNA after growth factor withdrawal for 18 hours. Lysates were prepared and subjected to Western blot analysis using anti-p27, anticyclin D3, anticyclin E, anticyclin D2, anti-PCNA, and anti-CDK2 antibodies. Data shown are representative of at least 5 independent examinations.
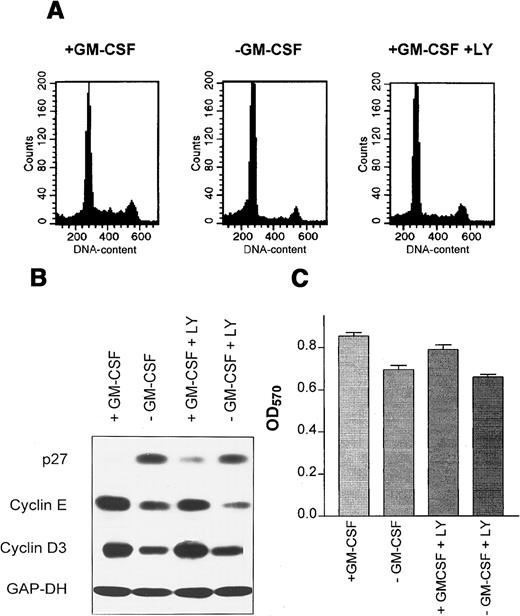
PI3K activity down-regulates p27 in M07 cells.
M07 cells were cultured for 18 hours in the presence or absence of GM-CSF with or without 5 μmol/L LY294002. (A) A sample of cells was assayed for cellular DNA content by propidium iodide staining and FACS. (B) Levels of p27, cyclin D3, and cyclin E were analyzed by immunoblotting of 50 μg total cell extracts. Equal protein loading was controled by reprobing with anti-GAP-DH antibody (1:10 000). (C) Cells were plated into 96-well plates at 1 × 105 per well. The viable cells in each well were assayed to their ability to transform 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide into a purple formazan. The absorbance of the samples was measured in an ELISA reader at 570 nm. Data represent the mean ± SE of 5 examinations.
PI3K activity down-regulates p27 in M07 cells.
M07 cells were cultured for 18 hours in the presence or absence of GM-CSF with or without 5 μmol/L LY294002. (A) A sample of cells was assayed for cellular DNA content by propidium iodide staining and FACS. (B) Levels of p27, cyclin D3, and cyclin E were analyzed by immunoblotting of 50 μg total cell extracts. Equal protein loading was controled by reprobing with anti-GAP-DH antibody (1:10 000). (C) Cells were plated into 96-well plates at 1 × 105 per well. The viable cells in each well were assayed to their ability to transform 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide into a purple formazan. The absorbance of the samples was measured in an ELISA reader at 570 nm. Data represent the mean ± SE of 5 examinations.
Bcr-Abl inhibits the up-regulation of p27 after serum reduction in M07 cells
Several authors reported that Bcr-Abl expression renders M07 cells growth factor independent.42 Therefore, Bcr-Abl constitutively activates signaling pathways that are normally used by the growth factors IL-3 and GM-CSF. As proliferation of M07/p210 cells is independent of GM-CSF, response to growth factor deprivation cannot be studied in bcr-abl–transfected M07 cells. Therefore, we have chosen to study response to serum deprivation. Reduction of serum content from 10% to 1% for 42 hours reduced the expression of the proliferation marker PCNA in both wild-type or in Bcr-Abl–transfected cells (data not shown). Levels of cyclin D3 and cyclin E were not affected by this reduction of serum (Figure3). However, after 42 hours, a significant increase of the p27 level was observed in M07 cells both after GM-CSF withdrawal and serum reduction. The magnitude of up-regulation was significantly higher after deprivation of GM-CSF in comparison to serum reduction. In contrast, neither in the presence nor in the absence of GM-CSF p27 levels were increased after serum reduction in M07/p210 cells (Figure 3). Therefore, we conclude that the regulation of the CDK inhibitor p27 is impaired in M07/p210 cells.
Bcr-Abl prevents the up-regulation of p27 after serum reduction.
M07 and M07/p210 cells were cultured with either 10% or 1% serum content in the presence or absence of GM-CSF. After 42 hours, cell lysates were prepared and analyzed by Western blot analysis.
Bcr-Abl prevents the up-regulation of p27 after serum reduction.
M07 and M07/p210 cells were cultured with either 10% or 1% serum content in the presence or absence of GM-CSF. After 42 hours, cell lysates were prepared and analyzed by Western blot analysis.
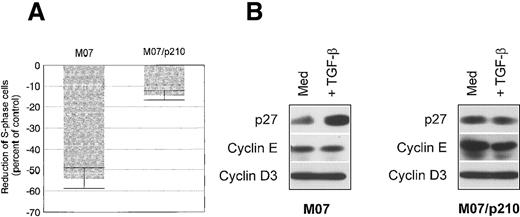
TGF-β up-regulates p27 in wild-type M07 cells, whereas Bcr-Abl expression renders M07/p210 cells resistant toward TGF-β
TGF-β efficiently inhibits the proliferation of wild-type M07 cells.43 Treatment of M07 cells with 10 ng/mL TGF-β over 18 hours resulted in a 53.2% ± 3.5% reduction of the S-phase fraction (Figure 4A). Several mechanisms by which TGF-β can induce a G1-arrest have been described. Depending on the cell type, TGF-β down-regulates cyclin levels, up-regulates CDK-inhibitors like p27, or down-regulates CDK4 levels.44-46 In M07 cells, levels of cyclin D3 and cyclin E were not affected by TGF-β (Figure 4B). In addition, treatment with TGF-β did not affect levels of cyclin D2, CDK4, CDK2, and p21 (data not shown). However, TGF-β up-regulated the CDK inhibitor p27 in M07 cells (Figure 4B). Treatment of M07/p210 cells with 10 ng/mL of TGF-β only slightly reduced the S-phase fraction, compared with wild-type M07 cells (Figure 4A). Furthermore, p27 was not induced in TGF-β treated M07/p210 cells (Figure 4B). Also, neither levels of cyclin E nor of cyclin D3 were affected by TGF-β treatment. These data further indicate a defective p27 regulation in Bcr-Abl expressing M07/p210 cells.
TGF-β inhibits proliferation in M07 cells through an up-regulation of the CDK inhibitor p27.
(A) Reduction of the proportion of S phase cells in comparison to control after 48 hours of cultivation in either the presence of 10 ng/mL TGF-β and GM-CSF (100 U/mL) for M07 cells or with 10 ng/mL TGF-β for M07/p210 cells. Values reflect the mean ± SE of at least 3 independent determinations. (B) Levels of p27, cyclin E, and cyclin D3 in control (GM-CSF, 100 U/mL) and TGF-β (5 ng/mL)-treated M07 and M07/p210 cells after 18 hours. Protein expression was determined by means of Western blot analysis.
TGF-β inhibits proliferation in M07 cells through an up-regulation of the CDK inhibitor p27.
(A) Reduction of the proportion of S phase cells in comparison to control after 48 hours of cultivation in either the presence of 10 ng/mL TGF-β and GM-CSF (100 U/mL) for M07 cells or with 10 ng/mL TGF-β for M07/p210 cells. Values reflect the mean ± SE of at least 3 independent determinations. (B) Levels of p27, cyclin E, and cyclin D3 in control (GM-CSF, 100 U/mL) and TGF-β (5 ng/mL)-treated M07 and M07/p210 cells after 18 hours. Protein expression was determined by means of Western blot analysis.
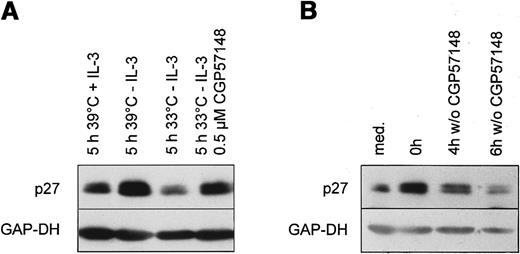
The Bcr-Abl-kinase prevents up-regulation of p27 after growth factor withdrawal
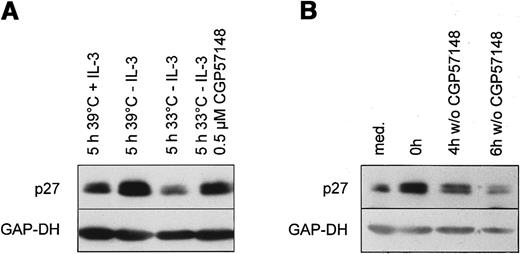
Ba/F3 cells expressing temperature sensitive Bcr-Abl protein were cultured at the nonpermissive temperature 39°C. At this temperature Ba/F3 cells are strictly dependent on the presence of exogenous IL-3. Similar to the results in the human cell line, growth factor withdrawal over 14 hours led to an up-regulation of p27 (Figure5A). Readdition of IL-3 to growth factor starved cells resulted in a rapid decline of p27 at 39°C within 5 hours (Figure 5A). Prolonged withdrawal of IL-3 over 5 hours led to a further elevation of p27 levels. Activating the Bcr-Abl kinase by temperature shift resulted in a decline of p27, although exogenous IL-3 was not present (Figure 5A). Therefore, the activation of the Bcr-Abl kinase directly decreases p27 levels in the absence of growth factors.
Bcr-Abl kinase activity directly down-regulates the CDK inhibitor p27 in murine Ba/F3 cells.
(A) Ba/F3 cells expressing the temperature sensitive Bcr-Abl were cultivated for 14 hours at the nonpermissive temperature (39°C) in the absence of IL-3 (t = 0). Then cells were either restimulated for 5 hours with fresh medium with or without 1 ng/mL IL-3 at 39°C or shifted to the permissive temperature (33°C) in the absence of IL-3 with or without 1 μmol/L CGP 57148B. Lysates were prepared and subjected to Western blot analysis using anti-p27, and anti-GAP-DH antibody (1:10 000). (B) Ba/F3 cells expressing p185 Bcr-Abl were cultivated at 37 °C in the presence (t = 0) or absence (med) of 1 μmol/L CGP 57148B for 14 hours. Subsequently, cells were washed with PBS and further cultivated without CGP 57148B for the indicated periods. p27 and GAP-DH protein expression were determined by means of Western blot analysis.
Bcr-Abl kinase activity directly down-regulates the CDK inhibitor p27 in murine Ba/F3 cells.
(A) Ba/F3 cells expressing the temperature sensitive Bcr-Abl were cultivated for 14 hours at the nonpermissive temperature (39°C) in the absence of IL-3 (t = 0). Then cells were either restimulated for 5 hours with fresh medium with or without 1 ng/mL IL-3 at 39°C or shifted to the permissive temperature (33°C) in the absence of IL-3 with or without 1 μmol/L CGP 57148B. Lysates were prepared and subjected to Western blot analysis using anti-p27, and anti-GAP-DH antibody (1:10 000). (B) Ba/F3 cells expressing p185 Bcr-Abl were cultivated at 37 °C in the presence (t = 0) or absence (med) of 1 μmol/L CGP 57148B for 14 hours. Subsequently, cells were washed with PBS and further cultivated without CGP 57148B for the indicated periods. p27 and GAP-DH protein expression were determined by means of Western blot analysis.
To further confirm this result, we tested whether the inhibition of the Bcr-Abl kinase by a specific Abl-kinase inhibitor CGP 57148B can prevent the observed decline of p27.47 Ba/F3 cells expressing temperature sensitive Bcr-Abl were shifted to 33°C in the presence of 1 μmol/L of CGP 57148B. Inhibition of the Bcr-Abl kinase with 1 μmol/L of CGP 57148B reduced intracellular phosphotyrosine level and abolished autophosphorylation of Bcr-Abl (data not shown). As shown, inhibition of the Bcr-Abl kinase by CGP 57148B partially prevented down-regulation of p27 (Figure 5A).
CGP 57148B renders Ba/F3 cells stably expressing constitutively active Bcr-Abl to a factor dependency (data not shown). Incubation of Bcr-Abl–expressing Ba/F3 with 1 μmol/L CGP 57148B for 14 hours resulted in an up-regulation of p27 (Figure 5B). p27 declined again after removing CGP 57148. So Bcr-Abl kinase activity down-regulates p27 in the absence of growth factors.
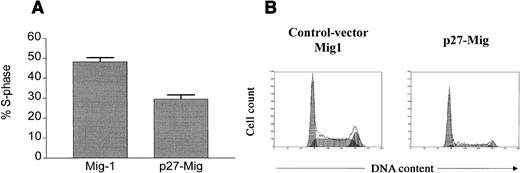
Proliferation of Bcr-Abl transformed cells is antagonized by overexpression of p27
To examine whether Bcr-Abl transformation is dependent on p27-regulation, we transiently transfected wild-type p27 into a Ba/F3 cell clone stably transfected with p185-bcr-abl. We used an expression vector with an IRES-EGFP cassette, enabling us to compare differences in cell cycle activity between high and low p27-expressing cells by EGFP/Hoechst33342 double staining and subsequent FACS analysis. The same vector without the p27 cDNA was used as a control. Transfection efficiency ranged between 15% to 25% when cells were analyzed for EGFP fluorescence. Compared to transfection with the EGFP control vector, overexpression of p27 significantly inhibited the proliferation of Bcr-Abl transformed cells (Figure6). In 4 independent experiments, high expression of p27 (cells with high EGFP expression) led to an approximately 40% reduction of cells in the S-phase compared with control cells. The mean value for cells in S-phase was 29.5%‖± 2.06% for p27 transfected cells versus 48.25% ± 2.28% for control cells (Figure 6A). Figure 6B shows a representative cell cycle analysis of highly EGFP-expressing cells.
Cell-cycle analysis of Ba/F3-p185 cells transfected with Mig1-control or p27-Mig vector.
The subpopulation of highly EGFP-expressing cells was analyzed for cell cycle distribution. (A) Proportion of EGFP+ cells in S-phase transfected with p27-Mig in comparison to Mig-controls. Values reflect the mean ± SE of at least 4 independent analyses. (B) Representative result of cell cycle distribution in EGFP-expressing cells. Transfection with p27-Mig leads to a marked reduction of cells in S/G2M-phase (G1:64%, S:28%, G2/M:8%), whereas cells transfected with the Mig-control vector show a high cycling activity (G1:38%, S:46%, G2/M:16%).
Cell-cycle analysis of Ba/F3-p185 cells transfected with Mig1-control or p27-Mig vector.
The subpopulation of highly EGFP-expressing cells was analyzed for cell cycle distribution. (A) Proportion of EGFP+ cells in S-phase transfected with p27-Mig in comparison to Mig-controls. Values reflect the mean ± SE of at least 4 independent analyses. (B) Representative result of cell cycle distribution in EGFP-expressing cells. Transfection with p27-Mig leads to a marked reduction of cells in S/G2M-phase (G1:64%, S:28%, G2/M:8%), whereas cells transfected with the Mig-control vector show a high cycling activity (G1:38%, S:46%, G2/M:16%).
Down-regulation of p27 protein is based on a proteasomal driven degradation and is mediated by PI3K dependent and PI3K independent mechanisms
To examine the mechanism by which Bcr-Abl kinase down-regulates p27 protein, we studied whether PI3K is involved as a mediator of RAS function.55 As shown in Figure7B, we found LY294002 (LY) incompletely prevented the Bcr-Abl–mediated p27 down-regulation in Ba/F3 cells expressing the temperature sensitive Bcr-Abl mutant. Accordingly, LY294002 only slightly reduced the growth of Bcr-Abl positive cells by 18.4% or 6.1%, respectively, when assayed in an MTT or BrdU ELISA assay. In contrast, inhibition of the Bcr-Abl kinase by CGP57148 reduced cell growth by 89% in the MTT assay (data not shown). Thus, the extent of p27 regulation is closely related to the regulation of cell growth in Bcr-Abl positive cells. These data suggest that PI3K activity plays some role in the p27 regulation in Bcr-Abl positive cells, but other mechanisms must be involved for the deregulation of this protein by Bcr-Abl.
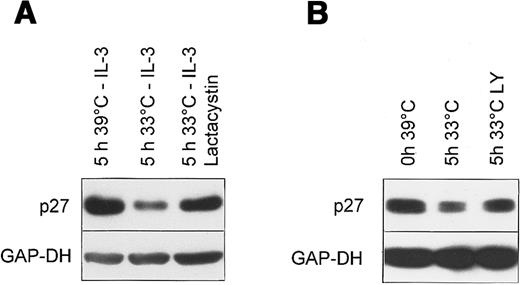
Bcr-Abl targets p27 to proteasomal degradation via both PI3K dependent and independent mechanism.
Western blot of total cell lysates from Ba/F3 cells expressing the temperature sensitive Bcr-Abl. (A) Cells were cultivated without growth factor for 5 hours either at the nonpermissive temperature (39°C) or at the permissive temperature (33°C) in the presence or absence of 10 μmol/L Lactacystin after incubation at 39°C without IL-3 for 14 hours. (B) Cells were incubated at 39°C in the absence of IL-3 for 14 hours (0 hour 39°C), followed by an incubation for 5 hours at the permissive temperature 33°C in the absence (5 hours 33°C) or presence of 5 μmol/L LY294002 (5 hours 33°C LY).
Bcr-Abl targets p27 to proteasomal degradation via both PI3K dependent and independent mechanism.
Western blot of total cell lysates from Ba/F3 cells expressing the temperature sensitive Bcr-Abl. (A) Cells were cultivated without growth factor for 5 hours either at the nonpermissive temperature (39°C) or at the permissive temperature (33°C) in the presence or absence of 10 μmol/L Lactacystin after incubation at 39°C without IL-3 for 14 hours. (B) Cells were incubated at 39°C in the absence of IL-3 for 14 hours (0 hour 39°C), followed by an incubation for 5 hours at the permissive temperature 33°C in the absence (5 hours 33°C) or presence of 5 μmol/L LY294002 (5 hours 33°C LY).
It has been shown that the regulation of p27 levels, both in normal and transformed human cells, occurs via the ubiquitin-proteasome pathway.48 To confirm that the decline was due to proteasomal degradation we examined the effect of Lactacystin, a cell-permeable proteasome inhibitor. Lactacystin inhibited the decline of p27 after temperature shift to the permissive temperature in Ba/F3 cells expressing temperature sensitive Bcr-Abl (Figure 7A).
Discussion
It is well documented that Bcr-Abl directly promotes cell cycle entry in the absence of growth factors both in primary leukemic andbcr-abl–transfected cell lines.19 20 In this study, we show that this effect is at least partially dependent on the regulation of the CDK inhibitor p27.
The active Bcr-Abl kinase interferes with the up-regulation of p27 after exposure to different p27 inducers. This was observed in 2 growth factor-dependent hematopoietic cells lines of human and mouse origin. Although under optimal growth conditions, expression levels of p27 were identical in bcr-abl–transfected and –untransfected cells, a deficient p27 up-regulation after growth factor deprivation or TGF-β treatment was observed in Bcr-Abl positive cells. Furthermore, p27 levels rapidly declined after activation of the Bcr-Abl kinase by temperature shift in murine Ba/F3 pro-B cells expressing temperature sensitive Bcr-Abl. In addition, blocking of Bcr-Abl–kinase activity by the specific Abl-kinase inhibitor CGP 57148B in constitutively Bcr-Abl–expressing Ba/F3 cells caused an increase in p27 expression, which could be rapidly reversed subsequent to removal of the inhibitor. Acute overexpression of p27 inhibited the proliferation of Bcr-Abl positive cells, indicating that Bcr-Abl induced transformation is dependent on the level of p27 protein.
One mechanism underlying Bcr-Abl–dependent p27 down-regulation can possibly be ascribed to the constitutive activation of the PI3K-signaling pathway by active Bcr-Abl kinase observed by Cortez et al.20 It has been shown that PI3K mediates p27 down-regulation in late G1-phase, both by the suppression of synthesis and the stimulation of protein degradation.41 Inhibition of PI3K abolished the mitogene-induced down-regulation of p27.41 55 Indeed, we observed that inhibition of PI3K by LY294002 in wild-type M07 cells led to an up-regulation of p27. Moreover, this inhibitor decreased the Bcr-Abl induced down-regulation of p27 to some extent. However, inhibition of PI3K in Ba/F3 pro-B cells expressing active Bcr-Abl kinase failed to completely block Bcr-Abl–induced down-regulation of p27. Thus, both PI3K-dependent and PI3K-independent mechanisms are involved in Bcr-Abl–mediated p27 down-regulation.
We found a rapid decline in p27 protein after activation of the Bcr-Abl kinase, whereas in Bcr-Abl kinase inactive cells, the half-life of p27 in the absence of IL-3 was more than 6 hours (not shown); thus in this context, p27 seems not to be regulated by a transcriptional or translational mechanism. The observation that Lactacystin, a cell permeable proteasome inhibitor completely prevented the Bcr-Abl–mediated p27 down-regulation, implies that Bcr-Abl interferes with an ubiquitin regulated proteasomal degradation pathway of the p27 protein. This is in accordance with other reports recently published. Dai et al49 reported a Bcr-Abl–driven proteasomal degradation of Abl-interactor (Abi) proteins. In Bcr-Abl–expressing cells, these Abi proteins were not detectable but inhibition of proteasomal function led to a reexpression of these proteins. A similar mechanism may be operative for Bcr-Abl–induced down-regulation of p27 after growth factor withdrawal.
However, under optimal growth conditions p27 level is not changed in Bcr-Abl kinase active cells in comparison with Bcr-Abl kinase inactive cells. Another pathway regulating p27 expression after growth factor withdrawal has recently been characterized. Pause et al50observed a deregulation of p27 expression in von Hippel-Lindau tumor suppressor gene (VHL) negative cell lines, which was only apparent after serum withdrawal. The half-life of p27 was equal in VHL positive and negative cells under optimal growth conditions. The VHL gene product associates in the cells with elongin B and C (VBC).51,52 The trimeric VBC complex associates in vivo and in vitro with Hs-CUL-2, which is a member of a multigene family, the cullins.53 The cullins have been implicated in the regulation of the cell cycle exit through the ubiquitin-mediated degradation of cyclin-dependent kinase inhibitors.54 Pause et al50 reported that growth rates and p27 expression of VHL negative and positive cells were indistinguishable under normal growth conditions. However, stabilization of p27 after serum withdrawal did not occur inVHL negative cells corresponding with their failure to arrest the cell cycle. In this context, Bcr-Abl would affect the regulation of p27 by inhibition of VHL function. Experiments regarding the regulation of VHL in Bcr-Abl–expressing cells are currently underway.
In conclusion, our data demonstrate that 2 critical pathways are targeted by Bcr-Abl. In addition to the induction of antiapoptotic proteins, the cell cycle regulatory machinery is rapidly and directly affected by the activated Bcr-Abl kinase. The activity of CDK2 is induced at the G1 checkpoint.20 We are able to demonstrate a deficient up-regulation of p27, one of the critical regulators of this activity. This parallel up-regulation of positive and down-regulation of negative cell cycle regulatory proteins exactly mimicks the biologic consequences of exogenous growth signals. The simultaneous targeting of antiapoptotic and cell cycle regulatory pathways may then lead to cell division under inappropriate conditions such as DNA damage or suboptimal growth factor concentrations. This corresponds precisely to the phenotype of primary CML cells as previously shown in experiments performed in our laboratory and by other investigators.19,56,57 Moreover, the defective checkpoint combined with increased survival might explain the genetic instability causing clonal evolution of the disease in vivo and in vitro.58 We conclude that a thorough understanding of these mechanisms is required for identification of the critical intermediates responsible for the biology of CML to develop strategies for the control of the disease.
Acknowledgments
We acknowledge S. Dowdy for the p27, W. Pear for the Mig1 cDNA, and Dr J. Ellwart, GSF Munich, for excellent technical assistance with DNA-staining and FACS-analysis.
Supported by research grants of the German research council (DFG) project Nr. Au 129/1 and the Robert Bosch foundation project Nr. 08-97.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Walter E. Aulitzky, Robert-Bosch-Krankenhaus, Auerbachstr 110, 70376 Stuttgart, Germany; e-mail:walter.aulitzky@rbk.de.