Abstract
The role of the platelet glycoprotein (GP) Ib-V-IX receptor in thrombin activation of platelets has remained controversial although good evidence suggests that blocking this receptor affects platelet responses to this agonist. The mechanism of expression of procoagulant activity in response to platelet agonists is also still obscure. Here, the binding site for thrombin on GPIb is shown to have a key role in the exposure of negatively charged phospholipids on the platelet surface and thrombin generation, in response to thrombin, which also requires protease-activated receptor-1, GPIIb-IIIa, and platelet-platelet contact. Von Willebrand factor binding to GPIb is not essential to initiate development of platelet procoagulant activity. Inhibition of fibrinogen binding to GPIIb-IIIa also failed to block platelet procoagulant activity. Both heparin and low molecular weight heparin block thrombin-induced platelet procoagulant activity, which may account for part of their clinical efficacy. This study demonstrates a new, critical role for platelet GPIb in hemostasis, showing that platelet activation and coagulation are tightly interwoven, which may have implications for alternative therapies for thrombotic diseases.
Introduction
The platelet glycoprotein (GP) Ib complex is important in primary hemostasis as demonstrated by the bleeding problems in patients with Bernard-Soulier syndrome where this receptor is either absent or defective.1 GPIb is the receptor for von Willebrand factor (vWf) on resting platelets and slows down platelets by a transient interaction so that they adhere to damaged vessel wall subendothelium in the first stage of a complex repair process.2,3 The primary structure of the components of the complex is known4-7 and models of the tertiary structure have been proposed.8 These indicate that the binding site for vWf is held out from the platelet surface by a rodlike, highlyO-glycosylated domain. Binding of vWf to GPIb induced by ristocetin or botrocetin causes signal transduction and activation of GPIIb-IIIa.9-13
Although much evidence has accumulated for GPIb as a thrombin receptor,14-18 particularly at low doses of thrombin, this has sometimes been controversial with several authors claiming that the protease-activated receptor-1 (PAR-1) is sufficient to explain all aspects of platelet activation by thrombin.19,20 In most cases this conflict was due to inadequate attention being paid to the GPIb effect being apparent only at low doses so that amounts were used such that its role might have been masked. As well as these problems, studies on the platelet thrombin receptors have been complicated by the inability to explain the high-affinity receptor (KD∼10−9-10−10 mol/L), present in 50 to 100 copies per platelet, which several binding studies have demonstrated.21,22 There is general agreement that the PAR-1 receptor, present in 1500 to 2000 copies per platelet is probably the moderate affinity receptor (Kd10−8-10−9 mol/L). A Kdof 5 × 10−7-10−8 mol/L has been determined for thrombin to the GPIb (or glycocalicin)-binding site.18,23,24 The recently discovered protease-activated receptor-4 (PAR-4) on platelets,25,26 which is also activated by thrombin, may explain some of the human platelet responses to thrombin not elicited by thrombin receptor-activating peptide (TRAP); however, the fact that it needs higher levels of thrombin than PAR-1 for optimal activation makes it unlikely that PAR-4 is responsible for the differences in response at low doses of thrombin.27 Mazzucato and coworkers28 examined thrombin binding to platelets at various temperatures and concluded that theoretical binding conditions were only met at 4°C. The binding constant at 4°C resembles that of thrombin binding to glycocalicin (10−8 mol/L). However, although these conditions meet theoretical considerations for equilibrium measurements, it must be noted that they are not physiologic and may therefore exclude modes of interaction of thrombin with platelets via GPIb, requiring platelet-platelet interactions, or participation of other molecules, or internalization of receptors.
The lack of GPIb in Bernard-Soulier syndrome affects not only the platelet responses to vWf and thrombin, which show defects in activation similar to those when GPIb receptor sites are blocked, but in addition there is a defect in procoagulant activity. Basal levels of procoagulant activity are higher than controls,29 and yet the expression of procoagulant activity in response to agonists is reduced.30 The raised basal levels could be due to a reduced stabilization of the membrane by fewer links with the membrane-associated cytoskeleton, but the reduced response on activation has never been satisfactorily explained. Development of procoagulant activity on platelets is a complicated process including the formation of prothrombinase complex. After platelet activation, negatively charged aminophospholipids (phosphatidylserine, -ethanolamine, and -inositol) are exposed in the outer leaflet of the plasma membrane and bind factors Va and Xa in the presence of Ca++. Prothrombin can then bind to this complex and is rapidly cleaved to thrombin. Measurement of thrombin generation is a classic method of assessing this activity.31 Because exposure of negatively charged aminophospholipids at the platelet surface is essential for procoagulant activity, its measurement by binding of suitably labeled annexin V is a good method for determining the first steps of this process.32,33 We found that measurement of thrombin generation or of annexin V binding led to comparable estimates of procoagulant activity. The development of procoagulant activity and formation of microvesicles, by shedding of membrane, are linked processes.34 35
Some controversy still exists over the binding between GPIb and thrombin and the sites that are involved on each of these molecules. The anionic exosite II on thrombin36 or the anonic exosite I37 may be the binding site for GPIb involving the highly negatively charged 268 to 287 sequence on GPIbα. This may also be the heparin-binding site on thrombin and could explain some of the effects of heparin on platelet-thrombin interactions.38 On the other hand, evidence suggests that heparin can also bind to GPIb and thus could block in the other direction as well.39
Several recent papers have addressed the mechanism of activation of platelets involving GPIb. Béguin and coworkers40observed a requirement for GPIb in procoagulant enhancement by fibrin in tissue factor-initiated thrombin generation. In this case vWf was also necessary, which could be explained by vWf binding to GPIb providing additional linking and signaling interactions between platelets. The vWf-fibrin-GPIb interactions might play a role in flip-flop events. Hayes and Tracy41 tried to identify the platelet high-affinity thrombin receptor by preparing polyclonal antibodies against a hirudin-like sequence similar to the thrombin anionic exosite I binding sequences present in hirudin, GPIbα, and PAR-1. Platelets treated with these antibodies show an enhanced response to thrombin. Hayes and Tracy41 argue against a role for GPIbα in thrombin activation of platelets, despite support for this from an extensive body of literature16,18,24,42,43because an antibody to GPIbα did not enhance calcium release in response to thrombin. However, antibodies to GPIbα have never been shown to enhance calcium signaling but rather have been shown many times to inhibit it.16,42 44 It is difficult to see how inhibition of signaling to thrombin by blocking the thrombin-binding site on GPIbα can be explained by a platelet thrombin response not involving GPIbα.
In this paper we present results supporting a unique role for GPIb in thrombin-platelet interactions in the development of platelet procoagulant activity in response to thrombin activation.
Materials and methods
Materials
Bovine serum albumin (BSA), apyrase grade VIII, EDTA, fluorescein isothiocyanate (FITC)-isomer I on celite 10%, mouse IgG-FITC, and α-thrombin were from Sigma (St Louis, MO). Sepharose CL-2B was from Amersham Pharmacia Biotech (Freiburg, Germany). Ristocetin was from WAK-Chemie Medical GmbH (Bad Homburg, Germany). Quantum 26 p FITC-standard beads were from Flow Cytometry Standards (Leiden, Netherlands). Annexin V-FITC (FITC to protein (F/P) molar ratio, 1.0) was from Bender Med Systems (Vienna, Austria). Human γ-thrombin was from Kordia (Leiden, Netherlands). The peptides SFLLRN (single-letter amino acid code; Ser-Phe-Leu-Leu-Arg-Asn, TRAP) and YFLLRNP (Tyr-Phe-Leu-Leu-Arg-Asn-Pro) were from Bachem Biochemica GmbH (Heidelberg, Germany). The peptides GRGDSP (Gly-Arg-Gly-Asp-Ser-Pro), GPRP (Gly-Pro-Arg-Pro), and bradykinin were from Calbiochem-Novabiochem GmbH (Bad Soden/Ts, Germany). Human fibrinogen (vWf and plasminogen free) was from Enzyme Research Labs (South Bend, IN). Monoclonal antibodies (MoAbs) anti-GPIb (SZ2), anti-CD62P-FITC (CLB-thromb/6), and anti-vWf (4F9) were from Coulter-Immunotech Diagnostics (Hamburg, Germany) and MoAb 2128 was produced in our own laboratory. Both antibodies directed against GPIb recognize an epitope on the GPIbα chain involved in the vWf- and thrombin-binding sites. MoAb 4F9 was conjugated to FITC by the method of Rinderknecht.45Fibrinogen was conjugated to FITC as described earlier.46Anti-vWf MoAbs AVW1 and AVW3 were from GTI (Brookfield, WI). Anti-vWf MoAb 9 to the GPIIb-IIIa-binding site on vWf was a kind gift from Dr D. Meyer. The Fab fragments of MoAb IV.3 to FcγRII were from Medarex (Annandale, NJ). The MoAb VM16d to the thrombin-binding site on GPIbα was a kind gift from Dr A.V. Mazarov and the MoAb 7E3 to the fibrinogen-binding site on GPIIb-IIIa was a kind gift from Dr B. Coller. The polyclonal antifibrinogen antibody (Ab) was from DAKO A/S (Glostrup, Denmark) and the MoAb IIaR-A against the PAR-1 thrombin receptor was from Dunn Labortechnik GmbH (Asbach, Germany). Glycocalicin, the soluble extracellular domain of GPIbα, was prepared from washed platelets as previously described.23Heparin-Na was from Braun Melsungen AG (Melsungen, Germany) and Fragmin (low molecular weight heparin) from Pharmacia GmbH (Erlangen, Germany). The p-nitroanilide chromogenic substrate for thrombin, Tos-Gly-Pro-Arg-pNA.AcOH was from Pentapharm Ltd (Basle, Switzerland). Human prothrombin and human factor Xa were from American Diagnostica Inc (Greenwich, CT).
Preparation of platelets
Venous blood from healthy adult volunteers and from patient a.m. with Glanzmann thrombasthenia, described earlier,46 who had not taken any medication affecting platelet function for at least 2 weeks before the study, was taken by venipuncture of the antecubital vein. Blood was anticoagulated with trisodium citrate (9 parts of blood, 1 part of trisodium citrate 0.108 mol/L). Platelet-rich plasma (PRP) was prepared by centrifugation at 250g for 10 minutes at room temperature. Platelets were gel-filtered on Sephadex CL-2B equilibrated in solution I (127 mmol/L NaCl, 2.7 mmol/L KCl, 0.42 mmol/L NaH2PO4, 12 mmol/L NaHCO3, 1 mmol/L MgCl2, 5.5 mmol/L glucose, 3.5% BSA, pH 7.35) containing 0.1 U/mL apyrase.47
Platelet aggregation
Platelet aggregation studies were performed in an aggregometer (Chrono-Log, Haverton, PA) within 3 hours of venipuncture using gel-filtered platelets at 1 × 108 platelets/mL.
Thrombin generation on gel-filtered platelets
Gel-filtered platelets were diluted to 2.5 × 107platelets/mL with TBS (50 mmol/L Tris, 175 mmol/L NaCl, 6 mmol/L CaCl2, pH 7.5). Platelets (200 μL) were activated with thrombin (0.5 U/mL) or TRAP (50 μmol/L) for 25 minutes (room temperature, stirring). Factor Xa (0.4 U/mL) was added to activated or control platelets and thrombin generation by the prothrombinase complex was started by adding prothrombin (10 μg/mL). Aliquots (10 μL) were removed at set times and added to microtiter-plate wells containing 140 μL TBS, 50 mmol/L Tris,175 mmol/L NaCl, 20 mmol/L EDTA, pH 7.9, which stops further prothrombin cleavage. The diluted samples were then assayed for thrombin activity after adding the p-nitroanilide thrombin substrate (2 mmol/L). Absorbance at 405 nm was measured at 30-second intervals, and the data were calculated as the maximum slope (mOD/min) in each well. Thrombin concentrations in each sample were obtained by comparison to a reference thrombin preparation.
Flow cytometry
Samples were analyzed using a Becton Dickinson FACScan flow cytometer (Heidelberg, Germany) with excitation by an argon laser at 488 nm. The FACScan was used in a standard configuration with a 530-nm bandpass filter. Standard beads containing specific amounts of “mean equivalent soluble fluorescein molecules” were used for calibration. Standard beads or platelets were gated and data were obtained from fluorescence channels in a logarithmic mode. A total of 5000 events were analyzed. Specific binding of MoAbs was calculated by subtracting nonspecific binding as determined with a FITC-labeled mouse isotype-specific IgG.
Annexin V-FITC binding
Gel-filtered platelets were diluted to 2.5 × 107platelets/mL with solution II (127 mmol/L NaCl, 2.7 mmol/L KCl, 0.42 mmol/L NaH2PO4, 12 mmol/L NaHCO3, 1 mmol/L MgCl2, 4 mmol/L CaCl2, 5.5 mmol/L glucose, 3.5% BSA, pH 7.35). Platelets (200 μL) were activated with thrombin (0.05-2 U/mL) or TRAP (1-200 μmol/L) for 25 minutes (room temperature, with gentle shaking) in a total volume of 250 μL in solution II. Thrombin- or TRAP-treated or control platelets were incubated with annexin V-FITC (1.5 μg/mL) in the dark (room temperature, shaken gently). After 15 minutes, 0.5 mL of solution II was added and after another 15 minutes samples were analyzed by flow cytometry. Inhibitors (antibodies, peptides, heparin, or low molecular weight heparin) were added directly to the platelets after gel filtration. After 5 minutes, platelets were activated with thrombin and treated as described above. Specific binding was calculated by subtracting nonspecific binding as determined in the presence of 20 mmol/L EDTA.
Analysis of ristocetin-induced vWf binding to platelets
The PRP was diluted to 2.5 × 107 platelets/mL with solution II. Platelets (200 μL) were treated with ristocetin (0.1-1.0 mg/mL) for 3 minutes and fixed with formaldehyde. After washing platelets and resuspending in solution II, they were incubated with FITC-conjugated anti-vWf MoAb (4F9, 10 μg/mL). After 1 hour the platelets were washed and then analyzed by flow cytometry.
Analysis of CD62 expression on platelets
The PRP was diluted to 2.5 × 107 platelets/mL with solution II. Platelets (200 μL) were activated with α-thrombin (0.5-20 nmol/L) or γ-thrombin (5-200 nmol/L) in the presence of GPRP (1.25 mmol/L) for 3 minutes and fixed with formaldehyde. After washing platelets and resuspending in solution II, they were incubated with anti-CD62P-FITC MoAb (10 μg/mL). After 1 hour platelets were again washed and analyzed by flow cytometry.
Fibrinogen-FITC binding
In the presence of GPRP (1.25 mmol/L), gel-filtered platelets (200 μL, 2.5 × 107 platelets/mL in solution II) were incubated with fibrinogen-FITC (100 μg/mL), which was preincubated with an antifibrinogen Ab (100 μg-1 mg/mL, control 0 μg/mL, 30 minutes), for 10 minutes. Platelets were activated with 1 U/mL thrombin or 50 μmol/L TRAP for 3 minutes and fixed with formaldehyde. After washing platelets and resuspending them in solution II, they were analyzed by flow cytometry. Fab fragments of MoAb IV.3 (20 μg/mL) against FcγRII were first added to prevent possible platelet activation by antibody binding to FcγRII.
Measurement of annexin V-FITC binding sites/platelet
The bead standard containing beads with 5 different levels of fluorescence was analyzed on the flow cytometer. The beads were treated as the platelets. All samples using microbeads were analyzed in triplicate. A linear regression curve plotted the mean fluorescence intensity channel for each bead peak against the known mean equivalent soluble fluorescein molecules level for each bead population. To determine the mean number of annexin V-FITC molecules bound per platelet, the mean equivalent soluble fluorescein molecules per cell was first determined by comparison of the cellular mean fluorescence intensity with the standard curve, and then dividing the difference in the number of FITC molecules for each cell and negative control by the F/P molar ratio for the marker. Specific binding was calculated by subtracting nonspecific binding as determined in the presence of 20 mmol/L EDTA.
Results
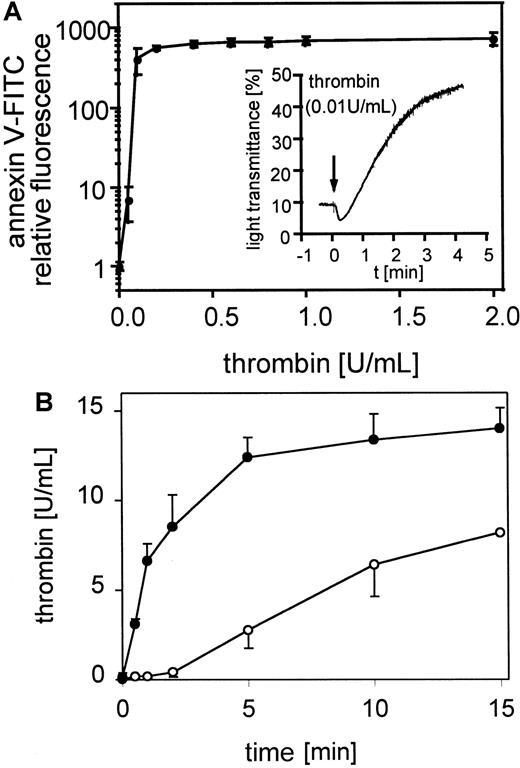
Thrombin induced procoagulant activity on platelets
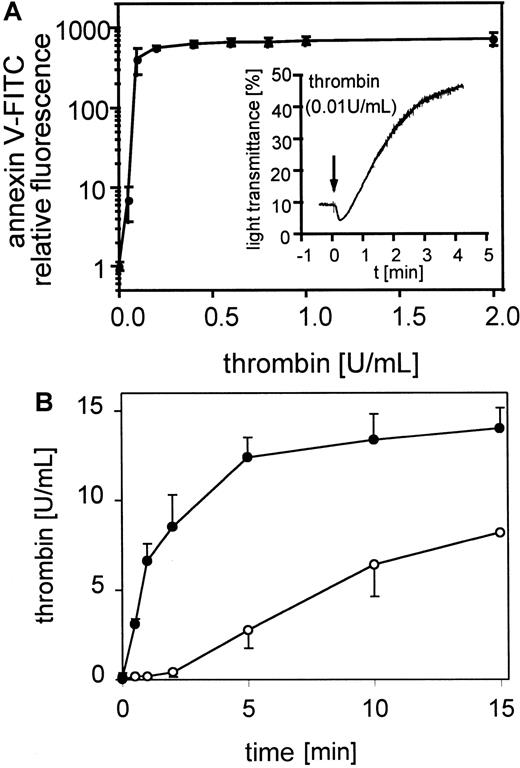
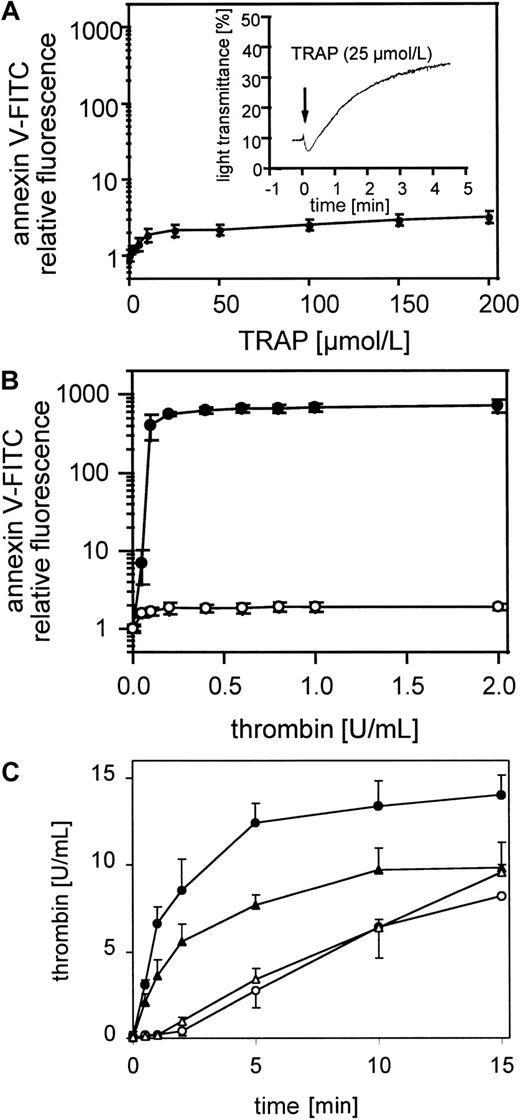
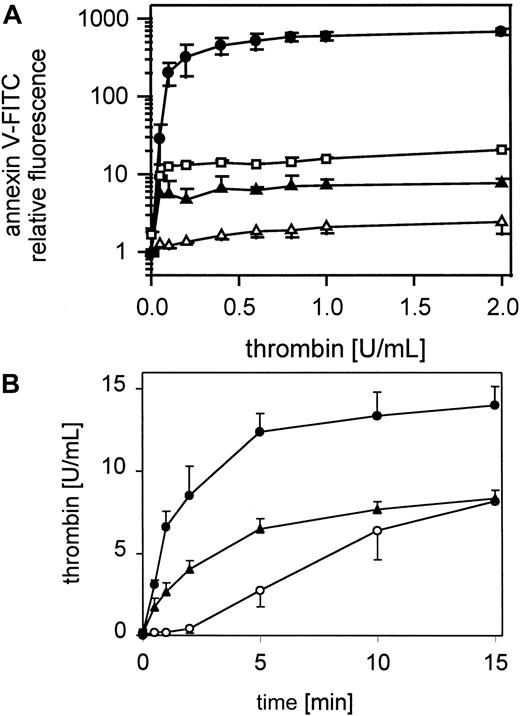
The exposure of negatively charged phospholipids on gel-filtered thrombin-stimulated platelets (0.05-2 U/mL) detected by annexin V-FITC is shown in Figure 1A. The expression of negatively charged aminophospholipids depended on the thrombin concentration. Maximal expression of negatively charged phospholipids was reached at approximately 0.2 to 0.4 U/mL thrombin, whereas these platelets aggregated to 0.01 U/mL thrombin (Figure 1A, insert). In all experiments 60% to 80% of the platelets measured are procoagulant after thrombin activation (data not shown). The activation of prothrombin by the prothrombinase complex in the presence of 0.4 U/mL factor Xa and 6 mmol/L CaCl2 plus resting or thrombin-activated gel-filtered platelets (0.5 U/mL) was measured after 30 seconds and 1, 2, 5, 10, and 15 minutes (Figure 1B). Under these conditions activated platelets provide the required cofactors, factor Va and the negatively charged phospholipid membrane. On thrombin-activated platelets the added prothrombin was completely converted to thrombin after 15 minutes. In the absence of prior activation of the platelets, thrombin generation shows a characteristic lag phase, indicating a requirement for feedback activation of the platelets during the incubation, by low amounts of thrombin generated from prothrombin cleavage by factor Xa.
Platelet procoagulant activity in response to thrombin.
(A) Annexin V-FITC binding to gel-filtered platelets activated with 0.05 to 2 U/mL thrombin (n = 4); ● indicates 25 000 platelets/μL. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. Insert: aggregation of gel-filtered platelets induced by 0.01 U/mL thrombin. The platelet counts were adjusted to 1 × 108/mL. The data shown are representative of 3 similar experiments. (B) Thrombin generation by prothrombinase complex was measured on resting and thrombin-activated (0.5 U/mL) gel-filtered platelets in the presence of 0.4 U/mL factor Xa and 6 μmol/L CaCl2 (n = 3); ○ indicates 25 000 platelets/μL, ●, 25 000 platelets/μL activated with thrombin. Data are the mean of the indicated number (n) of experiments using platelets from different donors.
Platelet procoagulant activity in response to thrombin.
(A) Annexin V-FITC binding to gel-filtered platelets activated with 0.05 to 2 U/mL thrombin (n = 4); ● indicates 25 000 platelets/μL. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. Insert: aggregation of gel-filtered platelets induced by 0.01 U/mL thrombin. The platelet counts were adjusted to 1 × 108/mL. The data shown are representative of 3 similar experiments. (B) Thrombin generation by prothrombinase complex was measured on resting and thrombin-activated (0.5 U/mL) gel-filtered platelets in the presence of 0.4 U/mL factor Xa and 6 μmol/L CaCl2 (n = 3); ○ indicates 25 000 platelets/μL, ●, 25 000 platelets/μL activated with thrombin. Data are the mean of the indicated number (n) of experiments using platelets from different donors.
Thrombin but not TRAP induced procoagulant activity on platelets
There was a slight increase in annexin V-FITC binding, that is, exposure of aminophospholipids, after activation of gel-filtered platelets with TRAP at concentrations up to 10 μmol/L, which was maintained as a plateau up to 200 μmol/L (Figure2A). This increase was insignificant compared to that obtained with thrombin (Figure 2B). However, aggregation was induced at a concentration of 25 μmol/L TRAP (Figure2A, insert). Thrombin failed to stimulate annexin V-FITC binding to gel-filtered platelets after pretreatment with 50 μmol/L TRAP for 10 minutes (Figure 2B). No annexin V-FITC binding could be detected even when platelets were activated with high thrombin concentrations, up to 2 U/mL. Thus, pretreatment with 50 μmol/L TRAP prevented expression of negatively charged phospholipids on platelets in response to thrombin. Pretreatment of gel-filtered platelets with 50 μmol/L TRAP for 10 minutes also reduced thrombin generation by the prothrombinase complex on thrombin-activated platelets (0.5 U/mL) as shown in Figure2C. Thrombin generation on TRAP-activated platelets (50 μmol/L) was similar to that on resting platelets (Figure 2C).
Platelet procoagulant activity in response to thrombin and TRAP.
(A) Annexin V-FITC binding to gel-filtered platelets activated with 1 to 200 μmol/L TRAP (n = 4); ● indicates 25 000 platelets/μL. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. Insert: aggregation of gel-filtered platelets induced by 25 μmol/L TRAP. The platelet counts were adjusted to 1 × 108/mL. The data shown are representative of 3 similar experiments. (B) After pretreatment with 50 μmol/L TRAP, platelets were activated with 0.05 to 2 U/mL thrombin (n = 3); ● indicates 25 000 platelets/μL, ○, 25 000 platelets/μL after preactivation with TRAP. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. (C) Thrombin generation by the prothrombinase complex was measured on resting, thrombin-activated (0.5 U/mL) gel-filtered platelets, TRAP-activated (50 μmol/L) gel-filtered platelets, as well as thrombin-activated (0.5 U/mL) gel-filtered platelets after pretreatment with 50 μmol/L TRAP, in the presence of 0.4 U/mL factor Xa and 6 μmol/L CaCl2 (n = 3); ○ indicates 25 000 platelets/μL; ●, 25 000 platelets/μL activated with thrombin; ▵, 25 000 platelets/μL activated with 50 μmol/L TRAP; ▴, 25 000 platelets/μL pretreated with TRAP, activated with thrombin. Data are the mean of the indicated number (n) of experiments using platelets from different donors.
Platelet procoagulant activity in response to thrombin and TRAP.
(A) Annexin V-FITC binding to gel-filtered platelets activated with 1 to 200 μmol/L TRAP (n = 4); ● indicates 25 000 platelets/μL. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. Insert: aggregation of gel-filtered platelets induced by 25 μmol/L TRAP. The platelet counts were adjusted to 1 × 108/mL. The data shown are representative of 3 similar experiments. (B) After pretreatment with 50 μmol/L TRAP, platelets were activated with 0.05 to 2 U/mL thrombin (n = 3); ● indicates 25 000 platelets/μL, ○, 25 000 platelets/μL after preactivation with TRAP. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. (C) Thrombin generation by the prothrombinase complex was measured on resting, thrombin-activated (0.5 U/mL) gel-filtered platelets, TRAP-activated (50 μmol/L) gel-filtered platelets, as well as thrombin-activated (0.5 U/mL) gel-filtered platelets after pretreatment with 50 μmol/L TRAP, in the presence of 0.4 U/mL factor Xa and 6 μmol/L CaCl2 (n = 3); ○ indicates 25 000 platelets/μL; ●, 25 000 platelets/μL activated with thrombin; ▵, 25 000 platelets/μL activated with 50 μmol/L TRAP; ▴, 25 000 platelets/μL pretreated with TRAP, activated with thrombin. Data are the mean of the indicated number (n) of experiments using platelets from different donors.
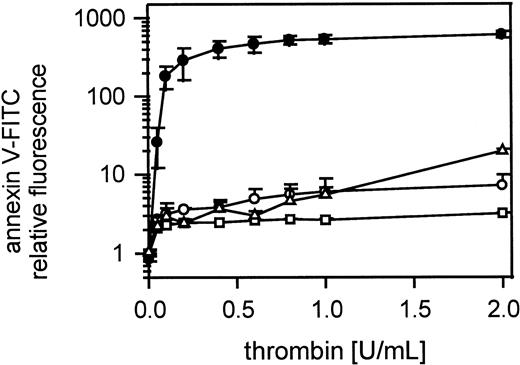
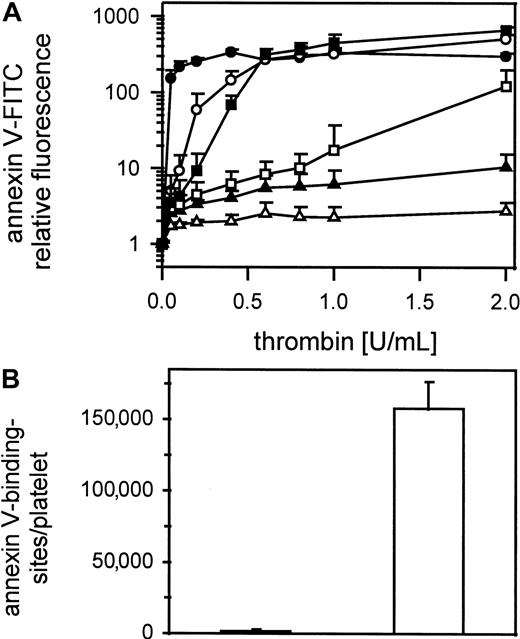
Cell-cell contact is necessary for expression of platelet procoagulant activity
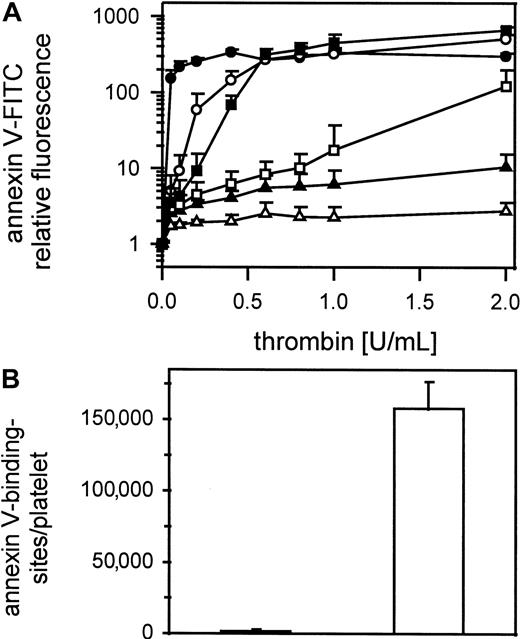
Figure 3A shows thrombin-induced expression of negatively charged phospholipids on gel-filtered platelets detected by annexin V-FITC binding in samples containing 1000 to 100 000 platelets/μL. Samples were shaken gently during thrombin activation (0.05-2 U/mL). Only in platelet suspensions containing more than 12 500 platelets/μL did annexin V-FITC binding reach saturating concentrations, meaning maximum expression of procoagulant activity. Unshaken platelets (25 000/μL) stimulated with 1 U/mL thrombin did not bind annexin V-FITC (Figure 3B). In contrast, shaken thrombin-stimulated platelets (1 U/mL) bound 160 000 ± 19 000 molecules of annexin V/platelet.
Influence of platelet count on thrombin-induced procoagulant activity.
(A) Gel-filtered platelets at different concentrations were activated with 0.05 to 2 U/mL thrombin; ● indicates100 000 platelets/μL; ○, 50 000 platelets/μL; ▪, 25 000 platelets/μL; ■, 12 500 platelets/μL; ▴, 5000 platelets/μL; ▵, 1000 platelets/μL (n = 3). (B) Annexin V-FITC-binding sites were determined on thrombin activated (1 U/mL) shaken (white column) and unshaken (black column) gel-filtered platelets (n = 6). The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. Data are the mean of the indicated number (n) of experiments using platelets from different donors.
Influence of platelet count on thrombin-induced procoagulant activity.
(A) Gel-filtered platelets at different concentrations were activated with 0.05 to 2 U/mL thrombin; ● indicates100 000 platelets/μL; ○, 50 000 platelets/μL; ▪, 25 000 platelets/μL; ■, 12 500 platelets/μL; ▴, 5000 platelets/μL; ▵, 1000 platelets/μL (n = 3). (B) Annexin V-FITC-binding sites were determined on thrombin activated (1 U/mL) shaken (white column) and unshaken (black column) gel-filtered platelets (n = 6). The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. Data are the mean of the indicated number (n) of experiments using platelets from different donors.
Blockage of GPIIb-IIIa or its absence prevents thrombin-induced expression of platelet procoagulant activity
Figure 4A shows that incubation of gel-filtered platelets with the GPIIb-IIIa inhibiting peptide GRGDSP (1 mmol/L), which prevents RGD-containing ligands of GPIIb-IIIa such as fibrinogen binding to GPIIb-IIIa, for 10 minutes before activation with thrombin (0.05-2 U/mL), strongly inhibited annexin V-FITC binding. Incubation of gel-filtered platelets with the GPIIb-IIIa blocking MoAb 7E3 (20 μg/mL) also completely prevented exposure of procoagulant activity in response to thrombin (Figure 4A). Similarly, gel-filtered platelets from a patient with Glanzmann thrombasthenia did not develop procoagulant activity in response to thrombin (Figure 4A) compared to a normal control. Procoagulant activity, measured as thrombin generation by the prothrombinase complex, was also reduced after blockage of GPIIb-IIIa with MoAb 7E3 (20 μg/mL) as shown in Figure 4B.
Role of GPIIb-IIIa in thrombin-induced platelet procoagulant activity.
(A) Gel-filtered platelets were preincubated with 1 μmol/L GRGDS or with 20 μg/mL 7E3 for 10 minutes and activated with thrombin (0.05-2 U/mL), and gel-filtered platelets from a patient with Glanzmann thrombasthenia were activated with thrombin (0.05-2 U/mL); ● indicates 25 000 control platelets/μL; ○, 25 000 platelets/μL in presence of GRGDS; ■, 25 000 platelets/μL in presence of 7E3; ▵, 25 000 platelets/μL from a patient with Glanzmann thrombasthenia. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. (B) Thrombin generation by the prothrombinase complex on resting and thrombin-activated (0.5 U/mL) gel-filtered platelets and thrombin-activated (0.5 U/mL) gel-filtered platelets after pretreatment with 20 μmol/L 7E3 in the presence of 0.4 U/mL factor Xa and 6 μmol/L CaCl2; ○ indicates 25 000 platelets/μL; ●, 25 000 platelets/μL activated with thrombin; ■, 25 000 platelets/μL, pretreated with 7E3, activated with thrombin. Data are the mean of 3 experiments using platelets from different donors.
Role of GPIIb-IIIa in thrombin-induced platelet procoagulant activity.
(A) Gel-filtered platelets were preincubated with 1 μmol/L GRGDS or with 20 μg/mL 7E3 for 10 minutes and activated with thrombin (0.05-2 U/mL), and gel-filtered platelets from a patient with Glanzmann thrombasthenia were activated with thrombin (0.05-2 U/mL); ● indicates 25 000 control platelets/μL; ○, 25 000 platelets/μL in presence of GRGDS; ■, 25 000 platelets/μL in presence of 7E3; ▵, 25 000 platelets/μL from a patient with Glanzmann thrombasthenia. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. (B) Thrombin generation by the prothrombinase complex on resting and thrombin-activated (0.5 U/mL) gel-filtered platelets and thrombin-activated (0.5 U/mL) gel-filtered platelets after pretreatment with 20 μmol/L 7E3 in the presence of 0.4 U/mL factor Xa and 6 μmol/L CaCl2; ○ indicates 25 000 platelets/μL; ●, 25 000 platelets/μL activated with thrombin; ■, 25 000 platelets/μL, pretreated with 7E3, activated with thrombin. Data are the mean of 3 experiments using platelets from different donors.
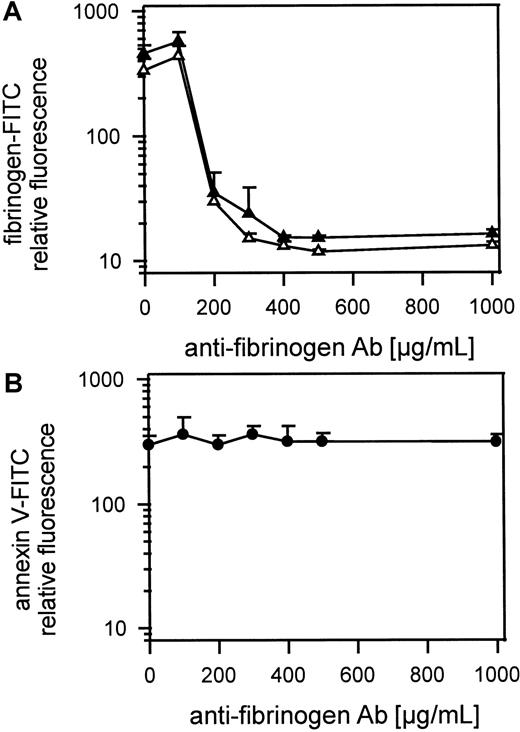
Preventing fibrinogen binding to platelets does not inhibit the exposure of procoagulant activity
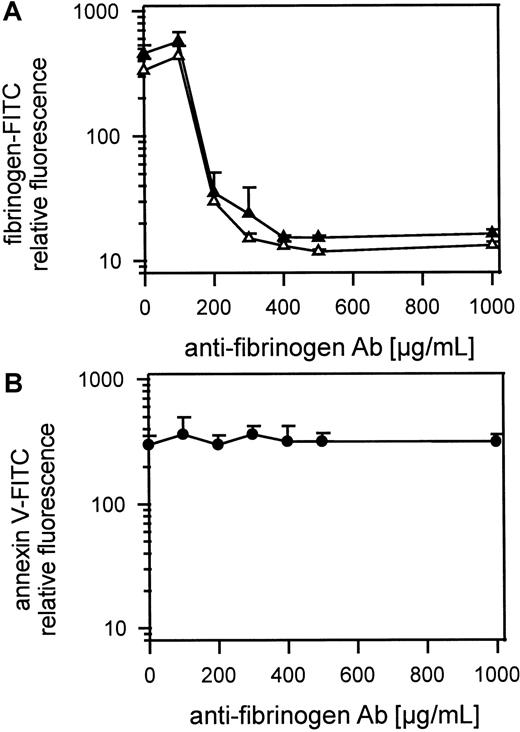
Figure 5A shows gel-filtered platelets activated with 1 U/mL thrombin or 50 μmol/L TRAP with or without antifibrinogen Ab (100 μg-1 mg/mL in the presence of 1.25 mmol/L GPRP and 100 μg/mL fibrinogen-FITC). FITC-fibrinogen and endogenous fibrinogen binding to platelets was inhibited by a large excess of antifibrinogen Ab and possible platelet aggregation by cross-linking fibrin monomers was inhibited by the peptide GPRP. Maximal inhibition of fibrinogen-FITC binding was reached at approximately 400 μg/mL Ab. The exposure of procoagulant activity detected by annexin V-FITC binding was not influenced by preventing fibrinogen binding to platelets (Figure 5B). Gel-filtered platelets were preincubated with high amounts of antifibrinogen Ab (100 μg-1 mg/mL) and were activated with 1 U/mL thrombin. Because 400 μg/mL antifibrinogen Ab totally blocked binding of 100 μg/mL of added FITC-fibrinogen, in the presence of all endogenous fibrinogen from the platelets, we were sure that all released fibrinogen was also inhibited. To prevent fibrin monomers binding to each other or cross-linking platelets, the peptide GPRP (1.25 mmol/L) was added to each sample. In all samples Fab fragments of MoAb IV.3 (20 μg/mL) against FcγRII were first added to platelets to prevent platelet activation by antibody binding to FcγRII.
Role of fibrinogen binding in thrombin-induced platelet procoagulant activity.
(A) Inhibition of thrombin- or TRAP-induced fibrinogen binding to gel-filtered platelets by antifibrinogen Ab detected by fibrinogen-FITC binding. Gel-filtered platelets were incubated with fibrinogen-FITC (100 μg/mL), GPRP (1.25 μmol/L), and IV.3 (20 μg/mL) and antifibrinogen Ab (100 μg-1 mg/mL). Platelets were activated with 1 U/mL thrombin or with 50 μmol/L TRAP; ▴ indicates 25 000 thrombin-activated platelets/μL; ▵, 25 000 TRAP-activated platelets/μL. (B) The influence of antifibrinogen Ab on thrombin-induced platelet procoagulant activity detected by annexin V-FITC binding; gel-filtered platelets were preincubated with IV.3 (20 μg/mL) for 5 minutes, were incubated with the antifibrinogen Ab (100 μg-1 mg/mL) for 10 minutes and activated with thrombin (1 U/mL); ● indicates 25 000 thrombin-activated platelets/μL. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. Data are the mean of 3 experiments using platelets from different donors.
Role of fibrinogen binding in thrombin-induced platelet procoagulant activity.
(A) Inhibition of thrombin- or TRAP-induced fibrinogen binding to gel-filtered platelets by antifibrinogen Ab detected by fibrinogen-FITC binding. Gel-filtered platelets were incubated with fibrinogen-FITC (100 μg/mL), GPRP (1.25 μmol/L), and IV.3 (20 μg/mL) and antifibrinogen Ab (100 μg-1 mg/mL). Platelets were activated with 1 U/mL thrombin or with 50 μmol/L TRAP; ▴ indicates 25 000 thrombin-activated platelets/μL; ▵, 25 000 TRAP-activated platelets/μL. (B) The influence of antifibrinogen Ab on thrombin-induced platelet procoagulant activity detected by annexin V-FITC binding; gel-filtered platelets were preincubated with IV.3 (20 μg/mL) for 5 minutes, were incubated with the antifibrinogen Ab (100 μg-1 mg/mL) for 10 minutes and activated with thrombin (1 U/mL); ● indicates 25 000 thrombin-activated platelets/μL. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. Data are the mean of 3 experiments using platelets from different donors.
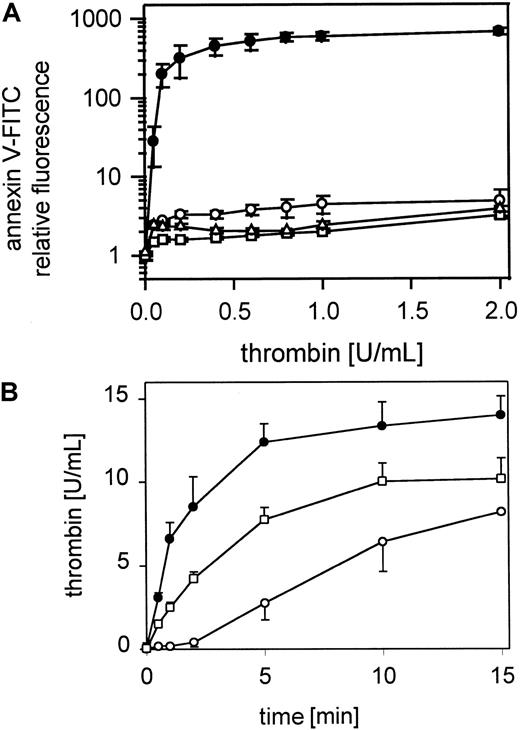
Platelet procoagulant activity exposure is inhibited by monoclonal antibodies to GPIbα, by glycocalicin, by heparin, or by low molecular weight heparin
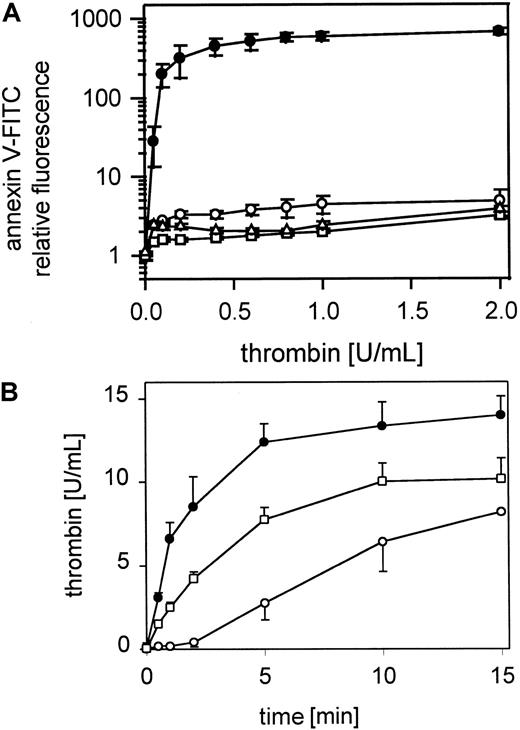
Annexin V-FITC binding to gel-filtered thrombin-stimulated platelets was inhibited by anti-GPIbα MoAb SZ2 (Figure6A) or anti-GPIbα MoAb 2128 (data not shown), which prevent both thrombin and vWf binding to GPIbα. The antibodies were used at 20 μg/mL and were incubated with gel-filtered platelets for 10 minutes before thrombin activation (0.05-2 U/mL). Incubation of gel-filtered platelets with glycocalicin (0.4 μmol/L) led to a marked reduction in platelet procoagulant activity over the total range of thrombin concentrations (0.05-2 U/mL) (Figure 6A). Heparin-Na (400 IE) (data not shown) or Fragmin (40 IE) (Figure 6A) added to the platelet suspension for 10 minutes before thrombin activation (0.05-2 U/mL) also led to a total inhibition of platelet procoagulant activity across the full range of thrombin concentrations. The optimal antibody, glycocalicin, heparin, and Fragmin concentrations were determined separately. Thrombin generation by the prothrombinase complex was also clearly inhibited after blocking GPIb with the MoAb SZ2 (Figure 6B).
Effect of blocking GPIb-thrombin binding on thrombin-induced platelet procoagulant activity.
(A) Gel-filtered platelets were incubated with MoAb SZ2 (20 μg/mL) or glycocalicin (0.4 μmol/L) or Fragmin (40 IU/mL) and activated with thrombin (0.05-2 U/mL); ● indicates 25 000 platelets/μL; ▴, 25 000 platelets/μL in the presence of MoAb SZ2; ■, 25 000 platelets/μL in the presence of glycocalicin; ▵, 25 000 platelets/μL in the presence of Fragmin. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. (B) Thrombin generation by the prothrombinase complex on resting and thrombin-activated (0.5 U/mL) gel-filtered platelets and thrombin-activated (0.5 U/mL) gel-filtered platelets after pretreatment with 20 μmol/L SZ2 in the presence of 0.4 U/mL factor Xa and 6 μmol/L CaCl2; ○ indicates 25 000 platelets/μL; ●, 25 000 platelets/μL activated with thrombin; ▴, 25 000 platelets/μL, pretreated with SZ2, activated with thrombin. Data are the mean of 3 experiments using platelets from different donors.
Effect of blocking GPIb-thrombin binding on thrombin-induced platelet procoagulant activity.
(A) Gel-filtered platelets were incubated with MoAb SZ2 (20 μg/mL) or glycocalicin (0.4 μmol/L) or Fragmin (40 IU/mL) and activated with thrombin (0.05-2 U/mL); ● indicates 25 000 platelets/μL; ▴, 25 000 platelets/μL in the presence of MoAb SZ2; ■, 25 000 platelets/μL in the presence of glycocalicin; ▵, 25 000 platelets/μL in the presence of Fragmin. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. (B) Thrombin generation by the prothrombinase complex on resting and thrombin-activated (0.5 U/mL) gel-filtered platelets and thrombin-activated (0.5 U/mL) gel-filtered platelets after pretreatment with 20 μmol/L SZ2 in the presence of 0.4 U/mL factor Xa and 6 μmol/L CaCl2; ○ indicates 25 000 platelets/μL; ●, 25 000 platelets/μL activated with thrombin; ▴, 25 000 platelets/μL, pretreated with SZ2, activated with thrombin. Data are the mean of 3 experiments using platelets from different donors.
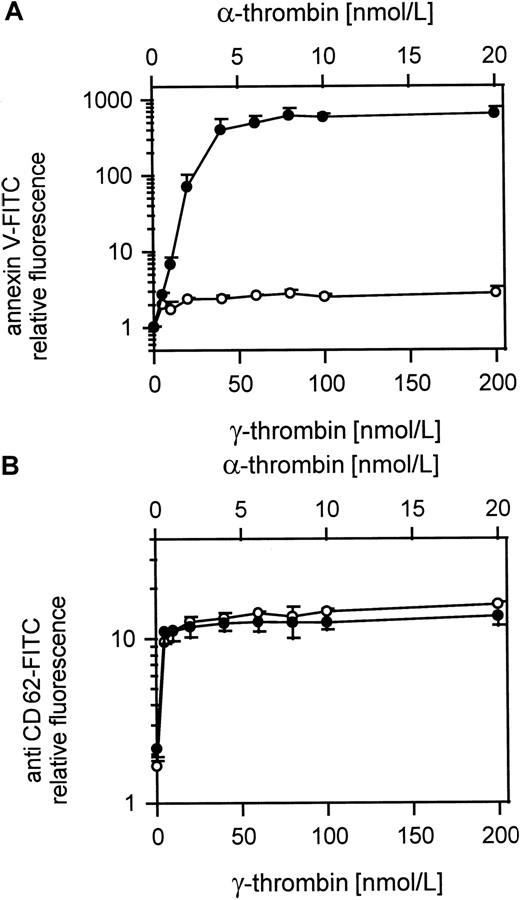
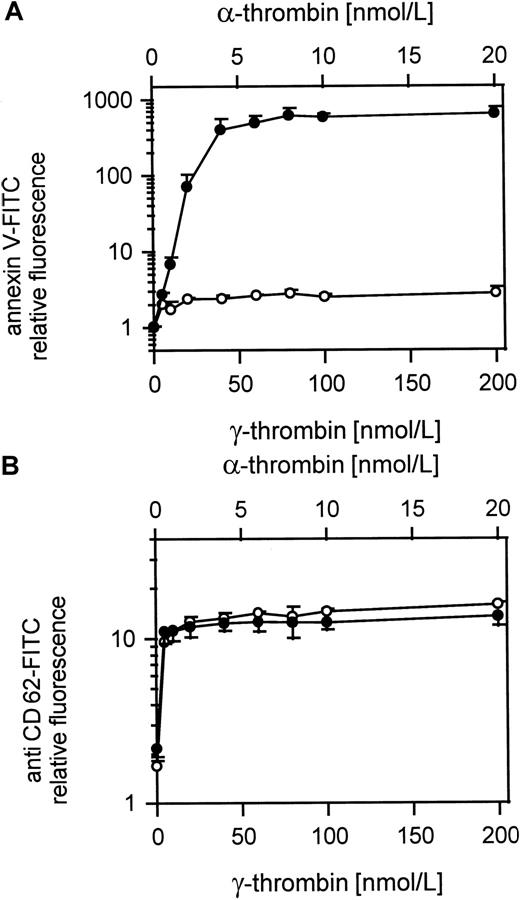
Comparison of platelet procoagulant activity and CD62 exposure in response to α- and γ-thrombin
Figure 7A shows the procoagulant response to γ-thrombin compared to α-thrombin at a 10-fold lower dose. Whereas the α-thrombin gives a normal procoagulant activity, the γ-thrombin, like TRAP, barely induces a procoagulant response. However, both forms of thrombin gave equivalent surface exposure of CD62 at these relative doses (Figure 7B) showing that the platelets were otherwise activated to an equivalent extent.
Comparison of α- and γ-thrombin–induced platelet procoagulant activity.
(A) Platelet procoagulant activity induced by α- or γ-thrombin; ● indicates 25 000 platelets/μL activated with 5 to 20 nmol/L α-thrombin; ○, 25 000 platelets/μL activated with 50 to 200 nmol/L γ-thrombin. (B) CD62 expression on α- and γ-thrombin–activated gel-filtered platelets; ● indicates 25 000 platelets/μL activated with 5 to 20 nmol/L α-thrombin; ○, 25 000 platelets/μL activated with 50 to 200 nmol/L γ-thrombin. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. Data are the mean of 3 experiments using platelets from different donors.
Comparison of α- and γ-thrombin–induced platelet procoagulant activity.
(A) Platelet procoagulant activity induced by α- or γ-thrombin; ● indicates 25 000 platelets/μL activated with 5 to 20 nmol/L α-thrombin; ○, 25 000 platelets/μL activated with 50 to 200 nmol/L γ-thrombin. (B) CD62 expression on α- and γ-thrombin–activated gel-filtered platelets; ● indicates 25 000 platelets/μL activated with 5 to 20 nmol/L α-thrombin; ○, 25 000 platelets/μL activated with 50 to 200 nmol/L γ-thrombin. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. Data are the mean of 3 experiments using platelets from different donors.
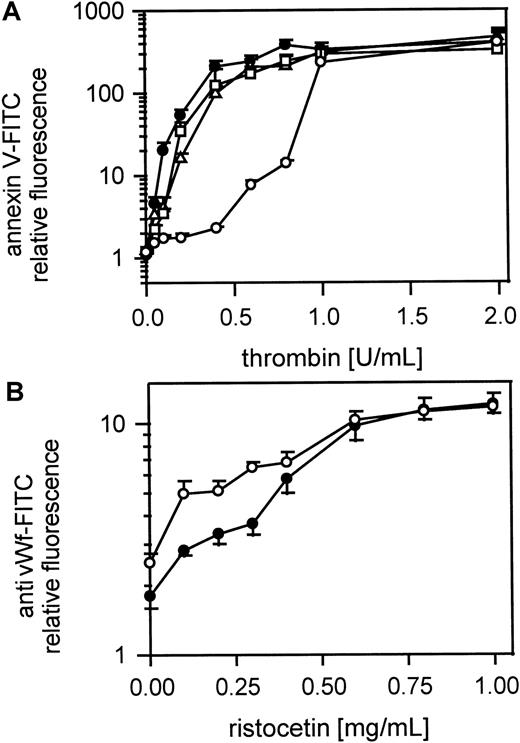
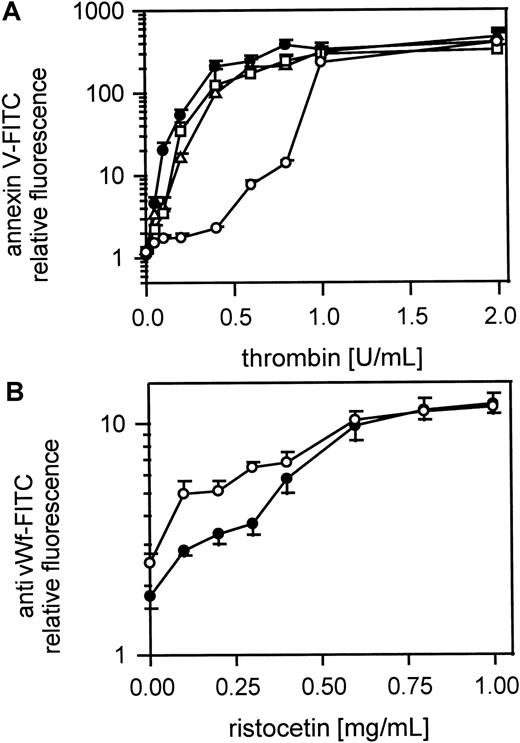
The thrombin- but not the vWf-binding site on GPIbα is implicated in the procoagulant response to thrombin
Figure 8A shows the platelet procoagulant response of gel-filtered platelets to thrombin in the presence of MoAbs to GPIbα and vWf. Fab fragments of MoAb IV.3 (20 μg/mL) against FcγRII were first added to the platelet suspension to prevent possible platelet activation by antibody binding to FcγRII. A MoAb to GPIbα, which inhibits thrombin binding but not vWf binding (VM16d), inhibited the procoagulant response of platelets to low but not to high doses of thrombin. MoAbs to vWf that either block (AVW3) or do not affect (AVW1) vWf binding to GPIbα induced by ristocetin did not affect procoagulant activity after thrombin activation. Figure 8B shows that ristocetin-induced vWf binding to platelets was not affected by the MoAb (VM16d) to the thrombin-binding site on GPIbα. The optimal antibody concentrations were determined separately. To test whether vWf binding to GPIIb-IIIa plays a role in thrombin-induced platelet procoagulant activity we used a MoAb 9 (20 μg/mL) against the GPIIb-IIIa binding site of vWf. MoAb 9 did not inhibit procoagulant activity, whereas it clearly inhibited TRAP induced vWf binding to GPIIb-IIIa (data not shown).
Role of vWf in thrombin-induced platelet procoagulant activity.
(A) Effect of MoAb against vWf (AVW1 and AVW3) and MoAb VM16d against the thrombin-binding site of GPIb; gel-filtered platelets were preincubated with Fab fragments of the MoAb IV.3 (20 μg/mL), treated with the MoAbs AVW1, AVW3, or VM16d (20 μg/mL) and were then activated with thrombin (0.05-2 U/mL); ● indicates 25 000 platelets/μL; ○, 25 000 platelets/μL in the presence of VM16d; ▵, 25 000 platelets/μL in presence of AVW1; ■, 25 000 platelets/μL in presence of AVW3. (B) vWf binding to platelets in the presence of MoAb VM16d, blocking the thrombin-binding site on GPIb; platelets from PRP were treated with ristocetin (200 μg-1 mg/mL) and vWf binding was measured with the FITC-labeled MoAb 4F9; ● indicates 25 000 platelets/μL; ○, 25 000 platelets/μL in the presence of MoAb VM16d. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. Data are the mean of 3 experiments using platelets from different donors.
Role of vWf in thrombin-induced platelet procoagulant activity.
(A) Effect of MoAb against vWf (AVW1 and AVW3) and MoAb VM16d against the thrombin-binding site of GPIb; gel-filtered platelets were preincubated with Fab fragments of the MoAb IV.3 (20 μg/mL), treated with the MoAbs AVW1, AVW3, or VM16d (20 μg/mL) and were then activated with thrombin (0.05-2 U/mL); ● indicates 25 000 platelets/μL; ○, 25 000 platelets/μL in the presence of VM16d; ▵, 25 000 platelets/μL in presence of AVW1; ■, 25 000 platelets/μL in presence of AVW3. (B) vWf binding to platelets in the presence of MoAb VM16d, blocking the thrombin-binding site on GPIb; platelets from PRP were treated with ristocetin (200 μg-1 mg/mL) and vWf binding was measured with the FITC-labeled MoAb 4F9; ● indicates 25 000 platelets/μL; ○, 25 000 platelets/μL in the presence of MoAb VM16d. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. Data are the mean of 3 experiments using platelets from different donors.
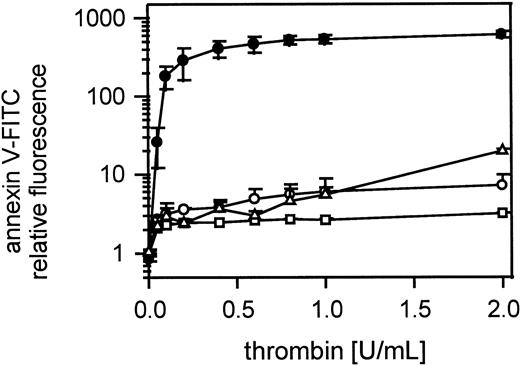
Inhibition of platelet procoagulant activity by blocking PAR-1
Incubation of gel-filtered platelets with the PAR-1 partial agonist peptide YFLLRNP (100 μmol/L, 10 minutes), which induces platelet shape change but does not cause further activation or aggregation, nearly completely inhibited annexin V-FITC binding to thrombin-stimulated (0.05-2 U/mL) platelets (Figure9). The peptide bradykinin, which acts as a total antagonist of PAR-1, gave similar results (Figure 9). Gel-filtered platelets were incubated with bradykinin at 1 mmol/L for 10 minutes. Blocking PAR-1 with MoAb IIaR-A (20 μg/mL) inhibited annexin V-FITC binding at thrombin concentrations up to 1 U/mL (Figure9). In this experiment Fab fragments of MoAb IV.3 (20 μg/mL) against FcγRII were also added to the platelet suspension to prevent possible platelet activation by cross-linking through antibody Fc domain binding to FcγRII.
Role of PAR-1 in thrombin-induced platelet procoagulant activity.
Inhibition of thrombin-induced procoagulant activity by the partial PAR-1 agonist YFFLRNP, by the peptide bradykinin, and by MoAb IIaR-A against the PAR-1 receptor; gel-filtered platelets were incubated with YFLLRNP (100 μmol/L) or with bradykinin (1 μmol/L) or with MoAb IIaR-A (20 μg/mL) and activated with thrombin (0.05-2 U/mL); ● indicates 25 000 platelets/μL; ○, 25 000 platelets/μL in presence of YFLLRNP; ■, 25 000 platelets/μL in the presence of bradykinin; ▵, 25 000 platelets/μL in the presence of MoAb IIaR-A. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. Data are the mean of 3 experiments using platelets from different donors.
Role of PAR-1 in thrombin-induced platelet procoagulant activity.
Inhibition of thrombin-induced procoagulant activity by the partial PAR-1 agonist YFFLRNP, by the peptide bradykinin, and by MoAb IIaR-A against the PAR-1 receptor; gel-filtered platelets were incubated with YFLLRNP (100 μmol/L) or with bradykinin (1 μmol/L) or with MoAb IIaR-A (20 μg/mL) and activated with thrombin (0.05-2 U/mL); ● indicates 25 000 platelets/μL; ○, 25 000 platelets/μL in presence of YFLLRNP; ■, 25 000 platelets/μL in the presence of bradykinin; ▵, 25 000 platelets/μL in the presence of MoAb IIaR-A. The fluorescence of 5000 platelets per measuring point was determined by flow cytometry. Data are the mean of 3 experiments using platelets from different donors.
Discussion
The differences in platelet responses to thrombin compared to the thrombin receptor peptide SFLLRN (TRAP) have been the object of several studies48-51 but have not been completely elucidated. It seems clear that thrombin gives a more extensive platelet activation than TRAP. Recently, the discovery that platelets have more than one protease-activated receptor suggested a possible solution to this problem; however, the other thrombin-sensitive receptor, PAR-4, requires higher levels of thrombin than PAR-1.26,27 The earliest platelet thrombin receptor described was GPIb,52but it is not cleaved by thrombin and because proteolytic activity is essential for thrombin-induced platelet activation, following the discovery of the PAR-153 there has been a tendency to neglect these earlier findings. Nevertheless, considerable evidence supports a role for GPIb in platelet activation by thrombin. In particular, it is well established that reagents interacting with the thrombin-binding site on GPIbα such as specific MoAbs54and snake C-type lectins such as echicetin55 do affect the platelet response to thrombin, particularly to low doses (< 0.5 U/mL). Inhibition by these reagents is specific because other anti-GPIb antibodies or other C-type lectins56 binding to GPIbα do not produce this effect. Platelets lacking GPIb from patients with the Bernard-Soulier syndrome also show reduced sensitivity to thrombin.57 On the other hand, there is increasing evidence that the GPIb-V-IX complex is able to signal to the platelet interior when another ligand, vWf, binds to it.9-13
One major difference between platelet activation by thrombin, which can use all the thrombin receptors, and TRAP, which only activates the PAR-1, is the exposure of procoagulant activity48 (Figures1 and 2). Development of procoagulant activity involves flip-flop of lipids within the platelet plasma membrane so that negatively charged aminophospholipids, phosphatidylserine, ethanolamine, and inositol are exposed on the outer surface of the plasma membrane. Exposure of these phospholipids can be conveniently measured by using the property of annexin V to bind to negatively charged phospholipid surfaces but not to the neutral phospholipids of the resting platelet surface. To ensure that annexin V binding was representative of platelet procoagulant activity, basic experiments were also duplicated measuring thrombin generation by a chromogenic method.
It was shown previously58 that thrombin desensitizes platelets to a subsequent challenge by TRAP and that preactivation by TRAP desensitizes to a later treatment with thrombin. In the case of procoagulant activity we show (Figure 2B,C) that activation of platelets by TRAP desensitizes them to a later dose of thrombin, even when this was high (2 U/mL). This shows that preactivation of PAR-1 also down-regulates the mechanisms leading to procoagulant expression.
Examination of the procoagulant activity in different platelet concentrations treated with the same amount of thrombin clearly showed that there was a concentration-dependent response (Figure 3A). This suggested that the exposure of procoagulant surface in response to thrombin was dependent on platelet-platelet contact. This was confirmed by comparing the exposure of procoagulant surface in unshaken versus shaken platelets at the same concentration treated with the same amount of thrombin (Figure 3B). In unshaken platelets there was no exposure of procoagulant activity, whereas in the shaken platelets this was exposed normally. This role of activated platelet-platelet contact suggested that GPIIb-IIIa might also be implicated in the procoagulant activity because it is well known that linkage of GPIIb-IIIa on different platelets by fibrinogen is the basis for platelet aggregation.59 Blockage of GPIIb-IIIa by a specific peptide antagonist GRGDSP60 (Figure 4A), or by a specific MoAb 7E3 directed to GPIIb-IIIa61 (Figure 4), also inhibited exposure of procoagulant phospholipids. This involvement of GPIIb-IIIa in exposure of procoagulant activity in response to thrombin was confirmed using platelets from a patient with Glanzmann thrombasthenia where GPIIb-IIIa is missing and there was no procoagulant response to thrombin (Figure 4A). This effect of blockage or absence of GPIIb-IIIa could have been due to prevention of the best known ligand, fibrinogen, binding to GPIIb-IIIa and therefore of platelet aggregation via this mechanism. To check whether this was the case, fibrinogen binding to activated GPIIb-IIIa was blocked by a large excess of polyclonal antibodies to fibrinogen. Although, as measured by FITC-fibrinogen binding, fibrinogen binding was successfully prevented (Figure 5A), this did not affect expression of procoagulant activity (Figure 5B). This suggests that GPIIb-IIIa has an additional function in expression of procoagulant activity and also that the platelet-platelet contact, necessary for procoagulant expression might involve receptor-protein interactions other than GPIIb-IIIa/fibrinogen such as GPIb/thrombin/PAR-1 or GPIb/thrombin/prothrombin. Byzova and Plow62 showed that prothrombin binding to GPIIb-IIIa was necessary for normal procoagulant activity. It should be noted that in Bernard-Soulier syndrome where GPIIb-IIIa and fibrinogen are present normally there is still a defect in procoagulant activity.30
The role of GPIb in exposure of procoagulant activity was tested using MoAbs known to block thrombin-binding sites on GPIb. One was SZ2 where the epitope lies in the middle of the highly negatively charged domain of GPIbα containing 3 sulfated tyrosine residues54 and implicated in both thrombin and vWf binding. The other was 2128 produced in our own laboratory where the epitope is not yet established but which also blocks thrombin and vWf binding. Both of these antibodies (data shown for SZ2 only) blocked the exposure of procoagulant activity on platelets over the whole range of thrombin concentrations used (Figure 6). This provides strong evidence that GPIb is indeed involved in thrombin-induced exposure of procoagulant activity.
This role of GPIb was confirmed by some alternative approaches. Glycocalicin, the soluble extracellular domain of GPIbα, which contains the thrombin- and vWf-binding sites, inhibited thrombin-induced exposure of platelet procoagulant activity by competitively binding to thrombin and preventing it from interacting with GPIbα on platelets (Figure 6A). This effect was found for all thrombin concentrations tested (up to 2 U/mL).
Heparin inhibits thrombin-induced platelet activation via GPIb63 either by blocking the GPIbα-binding site on thrombin, thought to be the anionic exosite II,38 or by blocking the thrombin-binding site on GPIbα.39 Both heparin and low molecular weight heparin (Fragmin) inhibited procoagulant expression on thrombin-treated platelets (Figure 6A). The effect of heparin and low molecular weight heparin on thrombin-induced platelet procoagulant activity provides an additional explanation for their antithrombotic effects.
The differences in platelet responses to α- and γ-thrombin were examined. Although α-thrombin is the normal form and induces the procoagulant response described above, γ-thrombin is a proteolytic cleavage product from α-thrombin, which is still able to activate platelets (as measured by aggregation and release) albeit to a lower level than α-thrombin.64 However, because the proteolysis removes the parts of the molecule that form the anionic exosite II, γ-thrombin is unable to bind to GPIb.65 At a 10-fold higher concentration than α-thrombin, γ-thrombin did not induce platelet procoagulant activity (Figure 7A). However, these relative concentrations of α-thrombin and γ-thrombin were equally effective in causing surface exposure of CD62 antigen by release from platelet granules (Figure 7B). These results again show that thrombin needs to interact with GPIb to produce a platelet procoagulant surface.
Because none of the above experiments can completely exclude a role for GPIb in thrombin-induced platelet procoagulant activity via an indirect mechanism, it was important to rule out a possible role for vWf-GPIb interactions. MoAbs to vWf were incubated with platelets before adding thrombin. MoAb AVW1 with an epitope on the C-terminal part of vWf does not affect function, whereas the other (AVW3), with an epitope on the A1 domain66 of vWf, blocks vWf binding to GPIb. Neither affected thrombin-induced platelet procoagulant activity (Figure 8A). Fab fragments of FcγRII-blocking antibody (IV.3, 20 μg/mL) were added to avoid platelet activation via the Fc domain of these antibodies. Similarly, an antibody (MoAb9) against the GPIIb-IIIa binding site on vWf had no effect on thrombin-induced procoagulant activity (data not shown).
VM16d, a MoAb against GPIb, which blocks thrombin but not vWf binding to GPIb,67 only inhibited the procoagulant response to thrombin at lower concentrations (< 0.5 U/mL) (Figure 8A). This difference compared to SZ2 and 2128 may be due either to the position of the epitope or to their relative avidity for GPIb compared to thrombin. Both SZ254 and 2128 (unpublished) have epitopes in or near the highly negatively charged sulfated tyrosine-containing peptide of GPIbα probably interacting with the anionic exosite II.68 This sequence is also involved in vWf binding to GPIbα.54,69 However, the VM16d epitope lies outside this domain, on the double loop domain of GPIbα,70 on a sequence specific for thrombin but possibly less crucial for its binding. Therefore, higher concentrations of thrombin can still interact with GPIb and cause procoagulant expression. VM16d does not prevent vWf binding to platelet GPIb in the presence of ristocetin as shown in Figure 8B.
Although cleavage of PAR-1 is essential to thrombin activation of platelets, its role in procoagulant expression was unclear. As noted above, preactivation of platelets with TRAP desensitized the procoagulant response to thrombin most likely via down-regulation of signaling via PAR-1. Two peptides block the effect of thrombin on PAR-1. YFLLRNP behaves as a partial agonist, inducing shape change but no release or aggregation.71 It may block PAR-1 or cause the same kind of desensitization as TRAP. It prevented procoagulant expression at all concentrations of thrombin (Figure 9). Bradykinin, which may block PAR-1,72 also completely inhibited thrombin-induced procoagulant expression (Figure 9). Blocking PAR-1 with MoAb IIaR-A prevented expression of procoagulant activity by up to 1 U/mL thrombin (Figure 9).
These results show that thrombin binding to GPIb, activation of PAR-1, platelet-platelet contact and GPIIb-IIIa are all essential for procoagulant expression in response to thrombin but that vWf is not involved and fibrinogen binding to GPIIb-IIIa may not be essential. Although signaling via PAR-1 alone is enough for several aspects of platelet activation, it is not adequate for expression of procoagulant activity assessed by annexin V binding or thrombin generation. Several studies found differences in platelet responses to thrombin and TRAP48-51; however, others showed that TRAP could stimulate platelets as strongly as thrombin.73 Because vWf binding to GPIb induces tyrosine phosphorylation and other signals, either by clustering or shear-stress mechanisms,9,10,13 binding of thrombin to GPIb might cause similar ancillary signals. Blockage of GPIb affects platelet procoagulant activity across a wide range of thrombin concentrations but only affected calcium or aggregation responses at low levels.16 Either the signals via GPIb have a particular role in the development of procoagulant activity or the thrombin-binding site on GPIb has supplementary roles in procoagulant activity in addition to signaling. Our results clearly show that GPIbα has a specific role in the thrombin-induced platelet procoagulant response. Whether this is only via specific signals from GPIb to the platelet cytoplasm or by affecting the activation of components of the coagulation cascade or both is under active investigation.
Acknowledgments
We thank Dr B. Coller, Dr A.V. Mazurov, and Dr D. Meyer for 7E3, VM16d, and MoAb9 antibodies, and the blood donors and patienta.m. with Glanzmann thrombasthenia type I for giving fresh blood.
K.J.C. was supported by grants from the Swiss National Science Foundation, no. 31-52396.97, and from Hoffmann-LaRoche Ltd., Basle.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Beate E. Kehrel, Mendelstrasse 11, D 48149, Münster, Germany; e-mail: kehrel@uni-muenster.de.