Abstract
Dendritic cells (DCs) disappear from lymph nodes 1 to 2 days after antigen presentation, presumably by apoptosis. To evaluate the role of death ligands in elimination of DCs, we analyzed the sensitivity of human DCs to CD95 ligand (CD95L) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). We found mature DCs to be resistant to killing via CD95L or TRAIL, whereas only immature DCs were partially sensitive. However, all DC populations expressed CD95, TRAIL-R2, and TRAIL-R3 at comparable levels, suggesting that sensitivity to death ligand-induced DC apoptosis is not regulated at the receptor level. Interestingly, mature DCs highly expressed the caspase 8 inhibitory protein cFLIP, whereas only low levels were detected in immature DCs. Thus, death ligand sensitivity proved to be dependent on DC maturation and inversely correlated with expression levels of cFLIP. Induction of apoptosis by TRAIL or CD95L does not seem to play a role in the elimination of mature DCs, but instead might serve to regulate immature DC populations.
Introduction
Dendritic cells (DCs), a class of leukocytes found in most tissues in trace amounts are able to present antigen to T cells in a potent manner. Immature DCs reside in peripheral tissues, such as the skin, capture antigen, and then migrate to the draining lymphoid organs, where they may prime both CD4+ as well as CD8+ T cells (for review by Banchereau and Steinman1). During this process, DCs mature with concomitant increase of the expression of costimulatory molecules and decreased capacity to process antigen. The knowledge of the fate of DCs after activation of cognate T cells is sparse. One to 2 days after antigen presentation, DCs disappear from the lymph node, presumably by apoptosis.2 Therefore, revealing the mechanisms regulating DC survival and cell death is crucial for a better understanding of the regulation of antigen presentation in a physiologic as well as a therapeutic context.
Members of the tumor necrosis factor (TNF) receptor/ligand family play a critical role in the regulation of the immune system. An important physiologic effect of a subgroup of this family, the death receptors, is the induction of apoptosis after binding their specific ligand. Death ligands such as CD95 ligand (CD95L) play an important role in autoimmune disorders, immune privilege, and tumor escape.3-6 In contrast to CD95L, the TNF-related apoptosis-inducing ligand (TRAIL) may bind to 5 different receptors. Two of these, TRAIL-R1 and TRAIL-R2, as well as the CD95 receptor, are characterized by functional cytoplasmic death domains. In contrast, TRAIL-R3 is a membrane-anchored truncated receptor and TRAIL-R4 lacks a functional death domain.7-9 It was suggested that they might act as decoy receptors by competition for limited amounts of the ligand.7,8 Cell death via death receptors can be regulated at different levels, including altered surface expression or by inhibition of intracellular signaling events. A recently identified molecule able to inhibit death receptor-mediated apoptosis is c-FLIP. The long form of cFLIP (cFLIPL) appears to block death receptor signaling by preventing caspase 8 activation10 at the death-inducing signaling complex (DISC).11
Two previous reports have suggested a role for CD95 for the induction of DC apoptosis.12,13 We have reevaluated this issue in more detail and show here that only immature DCs are susceptible to death receptor-mediated apoptosis. Mature DCs up-regulate cFLIP and are resistant to CD95- and TRAIL-induced cell death. Thus, in contrast to a recently published report,14our results suggest that fully mature human DCs are largely resistant to TRAIL- and CD95L-mediated apoptosis. We therefore conclude that these apoptosis-inducing receptor-ligand systems may be of functional importance at the level of immature rather than mature DCs.
Materials and methods
Materials
The protease inhibitor z-Val-Ala-Asp-fluoromethyl ketone (ZVAD-fmk) was obtained from Bachem (Heidelberg, Germany). Monoclonal antibodies (mAb) to cFLIP were generated and used as described.11 mAb to TRAIL-R1 (clone M 271), TRAIL-R2 (clone M 413), TRAIL-R3 (clone M 430), and TRAIL-R4 (clone M 444) for analysis of TRAIL-R1-4 surface expression were generated as described15 and applied as recently published.16 Recombinant leucine zipper (LZ)-TRAIL and LZ-CD95L were produced and purified as described.17 18TRAIL reagents and recombinant LZ-CD40L were kindly provided by Immunex Corp, Seattle, WA. CD95 Ab and anti-CD83 Ab were obtained from Dianova, Hamburg, Germany. CD25-PE, CD83-PE, and CD80 mAb were from Pharmingen, Hamburg, Germany. CD1a Ab (OKT6) was from American Type Culture Collection (Rockville, MD). Horseradish peroxidase (HRP)-tagged and FITC-conjugated goat-antimouse IgG were from Pharmingen (Hamburg, Germany).
Cell culture and preparation of human dendritic cells
Human DCs were prepared according to Romani et al.19 Briefly, peripheral blood mononuclear cells were isolated from heparinized buffy coats of healthy adult donors by adherence to plastic cell culture dishes in RPMI 1640 medium, supplemented with 0.5% autologous plasma, 2 mmol/L L-glutamine, 50 μg/mL gentamicin, and 100 IU/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) for 60 minutes. The nonadherent cells were removed by 2 × gentle washing with warm phosphate-buffered saline (PBS). Adherent monocytes were cultured for 7 days in RPMI 1640 medium (Gibco, Eggenstein, Germany), supplemented with 3% autologous serum, 2 mmol L-glutamine and 50 μg/mL gentamicin, 500 IU/mL rhIL-4 (Strathmann, Hannover, Germany), and 800 IU/mL rhGM-CSF (Leukomax; Sandoz, Basel, Switzerland). For the maturation of DCs, day 7 cells were cultured in a cytokine cocktail of IL-1α (500 U/mL), IL-6, TNF-α, IL-1β (all 1000 IU/mL; Strathmann Biotech GmbH, Hannover, Germany), and PGE2 (10−8 mol/L, Sigma, Deisenhofen, Germany) for another 3 days.20 For comparison to more mature DC populations, floating vital cells (as determined by trypan blue exclusion) were harvested after 2 days of culture (day 3) and incubated overnight with death ligands as described below. For the comparison of maturation with cytokine cocktail and LZ-CD40L, day 7 DCs were cultured for another 3 days in GM-CSF/IL-4 alone, with cytokine cocktail, or with 1 μg/mL recombinant LZ-CD40L. Mature DCs were defined by morphologic (eg, motile cytoplasmic processes), structural (eg, expression of CD83 and major histocompatibility complex [MHC] at high levels), and functional (allo–T-cell stimulatory capacity) characteristics. Human Jurkat T cells were cultured in RPMI 1640 (Gibco, Eggenstein, FRG) containing 5% fetal calf serum (FCS).
FACS analysis
FACS analysis was essentially performed as described elsewhere21 with the above described antibodies. The 104 cells were analyzed by FACScan (Becton Dickinson, San Jose, CA).
Induction of cell death
Precursor cells (day 3), immature DCs (day 7), as well as mature DCs (day 10), were seeded at a density of 1 × 105/mL and either LZ-TRAIL or LZ-CD95L was added to cells at indicated concentrations. Initial set-up experiments demonstrated that increasing the cytokine concentrations up to 10 μg/mL of LZ-Trail or 200 ng/mL of LZ-CD95L was equally unable to induce apoptosis in fully mature DCs (data not shown). Therefore, we used 1 μg/mL of LZ-Trail or 100 ng/mL of LZ-CD95L in all subsequent experiments unless otherwise stated. Apoptosis was detected 8 to 16 hours later by Annexin-FITC/propidium iodide double staining.
Western blot analysis
Total cellular protein was collected as described22with the exception that Complete Protease Inhibitor CocktailR (Boehringer, Mannheim, Germany) was used. Seventy-five micrograms of protein was electrophoresed on SDS-PAGE gels and transferred to nitrocellulose membranes. Membranes were blocked and hybridization was performed with antibodies to cFLIP. After incubation with appropriate secondary antibodies, bands were visualized using the ECL detection kit (Amersham, Arlington Heights, IL). Blots were subsequently incubated with Anti-tubulin AB (Sigma) to control for equal loading of the gel.
Results and discussion
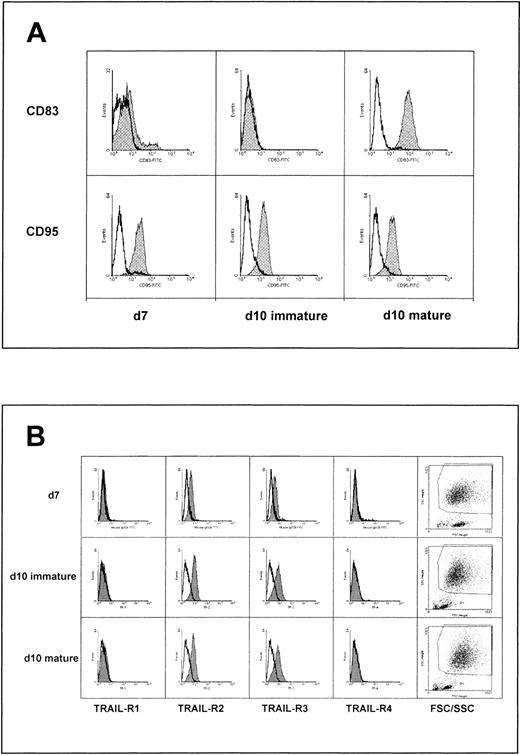
To analyze the expression levels of death receptors in relation to DC maturation, human monocyte-derived DCs were analyzed for the surface expression of CD83, CD95, as well as TRAIL-R1 to -R4, at day 7 as well as after cytokine cocktail activation at day 10. Immature DCs exhibited typical DC morphology and expressed CD1a, a marker for Langerhans cells.23 As shown in Figure1A, immature, CD83-negative DCs cultured for 7 or 10 days in the presence of GM-CSF and IL-4 clearly expressed CD95. When a mature phenotype was induced by addition of the indicated concentrations of IL-1 alpha, IL-1 beta, IL-6, TNF alpha, and PGE2, mature DCs expressed CD83 with no significant change in CD95 receptor expression (Figure 1A). Similarly, immature as well as mature DCs were stained for TRAIL-R1-4 surface expression. DCs expressed TRAIL-R2 as well as TRAIL-R3 on the surface with no significant change in expression levels during maturation. No significant staining was detectable with TRAIL-R1– or TRAIL-R4–specific mAb in all DC populations (Figure 1B).
Death receptor expression during maturation of human monocyte– derived dendritic cells.
(A) Immature DCs cultured for 7 days (d7) or 10 days (d10 immature) as well as mature DCs treated with IL-1α (500 IU/mL), IL6, TNF-α, IL-1β (all at 1000 IU/mL), and PGE2 (10−8mol/L) from day 7 to day 10 (d10) were incubated with 5 μg/mL of monoclonal Ab to CD95 (DX2) or CD83, followed by FITC-conjugated secondary Ab and analyzed by FACS. (B) Cells were treated as described above and analyzed for the presence of TRAIL receptors (TRAIL-R) 1 to 4.
Death receptor expression during maturation of human monocyte– derived dendritic cells.
(A) Immature DCs cultured for 7 days (d7) or 10 days (d10 immature) as well as mature DCs treated with IL-1α (500 IU/mL), IL6, TNF-α, IL-1β (all at 1000 IU/mL), and PGE2 (10−8mol/L) from day 7 to day 10 (d10) were incubated with 5 μg/mL of monoclonal Ab to CD95 (DX2) or CD83, followed by FITC-conjugated secondary Ab and analyzed by FACS. (B) Cells were treated as described above and analyzed for the presence of TRAIL receptors (TRAIL-R) 1 to 4.
We next analyzed the sensitivity to TRAIL- or CD95L-induced cell death. As shown in Figure 2, the earliest nonadherent population of monocyte-derived DCs (day 3) was highly susceptible to apoptosis mediated by CD95 and, albeit to a lesser extent, by TRAIL (Figure 2A, B, upper panel). Day 7 cells, expressing typical DC markers, such as CD1a (not shown), were less sensitive, but still showed partial sensitivity to CD95 and TRAIL (Figure 2A,B, middle panel). In marked contrast, fully mature day 10 DCs were completely resistant to CD95L and TRAIL alone or a combination of the 2 apoptosis-inducing ligands, even at the highest concentrations tested (Figure 2A,B). When compared with death ligand–induced apoptosis in Jurkat cells, 100-fold higher concentrations of death ligands were used but were unable to kill fully mature DCs (Figure 2B). Irrespective of whether maturation was induced by cytokine cocktail or CD40L alone, fully mature DCs were resistant to death ligand–induced apoptosis (Figure 2C). Therefore, our study clearly demonstrates that sensitivity to death ligands decreases during cytokine or CD40L-induced maturation of monocyte-derived human dendritic cells with fully matured DCs being completely resistant to CD95L or TRAIL. In the TRAIL system, it was proposed that the receptors TRAIL-R3, TRAIL-R4, and osteoprotegerin (OPG) might provide resistance to TRAIL by competing for extracellular TRAIL.3 Our surface expression data expand recent results in human monocytes, demonstrating that also in monocyte-derived human DCs, the only detectable surface expressed TRAIL-Rs are TRAIL-R2 and TRAIL-R3.15 However, surface expression in DCs could not explain differential sensitivity, since expression levels of TRAIL-R2, -R3, and CD95 did not change during the course of maturation.
Mature DCs, but not immature DCs are resistant to death ligand–induced apoptosis.
(A) d3 cells (upper panel), d7 cells (middle panel), or d10 cells (lower panel) were incubated in the presence of 100 ng/mL LZ-CD95L, 1 μg/mL LZ-TRAIL, or the combination of both for 16 hours and survival of cells was determined by Annexin/propidium iodide staining and FACS. (B) Dose response curves of different populations of DCs (d3, d7, and d10). Cells were treated with the indicated concentrations of LZ-CD95L (square), LZ-TRAIL (triangle), or the combination of both death ligands (rhombs), and survival was determined 16 hours later by Annexin/propidum iodide staining by FACS (shown is a representative experiment [percentage of control survival] of a total of 4 independent experiments with similar results). (C) Cytokine cocktail-induced maturation of DCs is comparable to CD40L-induced maturation, but necessary to induce resistance to CD95L or TRAIL. The d7 DCs were cultured for 3 days either in GM-CSF and Il-4 alone, with addition of cytokine cocktail (see “Materials and methods”) or recombinant LZ-CD40L (1 μg/mL) and analyzed by surface analysis for CD1a, CD25, CD80, and CD83 (upper panel). Cells were subsequently treated with the LZ-CD95L (200 ng/mL), LZ-TRAIL (10 μg/mL), or the combination of both death ligands, and survival was determined 16 hours later by Annexin/propidum iodide staining by FACS as percentage of control (shown is a representative result of a total of 2 independent experiments with similar results). (D) cFLIP expression during maturation of DCs. Seventy-five micrograms of protein of monocyte-derived dendritic cells cultured for 4 days (d4) or 7 days (d7) in the presence of GM-CSF and IL-4 or subsequent 3 days in the presence of IL-1 α (500 IU/mL), IL-6, TNF-α, IL-1 β (all 1000 IU/mL), and PGE2 (10−8 mol/L) were analyzed by Western blotting with cFLIP specific antibodies. Low levels of cFLIPL are expressed in d4 and d7 immature DCs, whereas fully matured DCs (d10) show strong expression of cFLIPL. Reprobing of the membrane with tubulin Ab demonstrates comparable loading of protein.
Mature DCs, but not immature DCs are resistant to death ligand–induced apoptosis.
(A) d3 cells (upper panel), d7 cells (middle panel), or d10 cells (lower panel) were incubated in the presence of 100 ng/mL LZ-CD95L, 1 μg/mL LZ-TRAIL, or the combination of both for 16 hours and survival of cells was determined by Annexin/propidium iodide staining and FACS. (B) Dose response curves of different populations of DCs (d3, d7, and d10). Cells were treated with the indicated concentrations of LZ-CD95L (square), LZ-TRAIL (triangle), or the combination of both death ligands (rhombs), and survival was determined 16 hours later by Annexin/propidum iodide staining by FACS (shown is a representative experiment [percentage of control survival] of a total of 4 independent experiments with similar results). (C) Cytokine cocktail-induced maturation of DCs is comparable to CD40L-induced maturation, but necessary to induce resistance to CD95L or TRAIL. The d7 DCs were cultured for 3 days either in GM-CSF and Il-4 alone, with addition of cytokine cocktail (see “Materials and methods”) or recombinant LZ-CD40L (1 μg/mL) and analyzed by surface analysis for CD1a, CD25, CD80, and CD83 (upper panel). Cells were subsequently treated with the LZ-CD95L (200 ng/mL), LZ-TRAIL (10 μg/mL), or the combination of both death ligands, and survival was determined 16 hours later by Annexin/propidum iodide staining by FACS as percentage of control (shown is a representative result of a total of 2 independent experiments with similar results). (D) cFLIP expression during maturation of DCs. Seventy-five micrograms of protein of monocyte-derived dendritic cells cultured for 4 days (d4) or 7 days (d7) in the presence of GM-CSF and IL-4 or subsequent 3 days in the presence of IL-1 α (500 IU/mL), IL-6, TNF-α, IL-1 β (all 1000 IU/mL), and PGE2 (10−8 mol/L) were analyzed by Western blotting with cFLIP specific antibodies. Low levels of cFLIPL are expressed in d4 and d7 immature DCs, whereas fully matured DCs (d10) show strong expression of cFLIPL. Reprobing of the membrane with tubulin Ab demonstrates comparable loading of protein.
We therefore investigated whether intracellular proteins might be involved in the regulation of sensitivity and resistance to death ligands. Recent reports demonstrated that death ligand-induced apoptosis can be inhibited by overexpression of cFLIP, making it a likely candidate for intracellular inhibition of death receptor signaling.24 Thus, we analyzed cFLIP expression, known to interfere with caspase 8 activation,10,11,24 at different stages of DC maturation. Western blot analysis showed that only the long form of cFLIP, cFLIPL, (55 kd), but not the short form of cFLIP (cFLIPS) was detectable at low levels in immature day 4 DCs and day 7 DCs. In contrast, fully matured DCs showed strong up-regulation of cFLIPL (Figure 2D). Thus, our data indicate that the up-regulation of cFLIPL may act as regulator of death ligand sensitivity in fully mature DCs. However, cFLIP levels cannot be causative for the marked decrease in sensitivity between day 3 and day 7 DCs. This suggests that in addition to cFLIP, other regulatory mechanisms might also contribute to the detectable resistance of DCs to death ligands. A similar correlation between resistance to TRAIL and cFLIP expression were recently reported for melanoma cells25,26 and primary human keratinocytes.16 Our data demonstrating resistance of fully mature DCs to both TRAIL and CD95L are in contrast to the recent report by Wang et al14 that demonstrated TRAIL-mediated killing of mature DCs. The authors detected a marked increase of the number of DCs in patients with inherited caspase 10 defects as well as a marked sensitivity of CD83+ DCs to TRAIL and to CD95L.14 Our data clearly show that only immature DCs are susceptible to TRAIL- and CD95L-mediated apoptosis in an autologous system. In contrast to Wang et al,14 we show that CD83+ mature DCs are completely resistant to TRAIL- and CD95L-mediated apoptosis in vitro. Thus, our data suggest that the detectable increase of DCs in lymph nodes of patients with angiolymphoproliferative syndrome (ALPS) type II could be explained by the failure of TRAIL or CD95L to kill immature DCs at some stage before lymph node entry rather than mature DCs at a later stage.
Acknowledgments
We thank E. Horn, C. Kurzmann, and D. Süss for expert technical assistance.
A.M. was supported by IZKF grant A1 (BMBF 01 KS 9603). W.F. was supported by the DFG Sonderforschungsbereich 465. H.W. was supported by the AIDS Stipend Program of the Bundesministerium für Bildung und Forschung.
M.L. and H.W. contributed equally to this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Eckhart Kämpgen, University of Würzburg Medical School, Department of Dermatology, Josef-Schneider-Str. 2, 97080 Würzburg, Germany.

![Fig. 2. Mature DCs, but not immature DCs are resistant to death ligand–induced apoptosis. / (A) d3 cells (upper panel), d7 cells (middle panel), or d10 cells (lower panel) were incubated in the presence of 100 ng/mL LZ-CD95L, 1 μg/mL LZ-TRAIL, or the combination of both for 16 hours and survival of cells was determined by Annexin/propidium iodide staining and FACS. (B) Dose response curves of different populations of DCs (d3, d7, and d10). Cells were treated with the indicated concentrations of LZ-CD95L (square), LZ-TRAIL (triangle), or the combination of both death ligands (rhombs), and survival was determined 16 hours later by Annexin/propidum iodide staining by FACS (shown is a representative experiment [percentage of control survival] of a total of 4 independent experiments with similar results). (C) Cytokine cocktail-induced maturation of DCs is comparable to CD40L-induced maturation, but necessary to induce resistance to CD95L or TRAIL. The d7 DCs were cultured for 3 days either in GM-CSF and Il-4 alone, with addition of cytokine cocktail (see “Materials and methods”) or recombinant LZ-CD40L (1 μg/mL) and analyzed by surface analysis for CD1a, CD25, CD80, and CD83 (upper panel). Cells were subsequently treated with the LZ-CD95L (200 ng/mL), LZ-TRAIL (10 μg/mL), or the combination of both death ligands, and survival was determined 16 hours later by Annexin/propidum iodide staining by FACS as percentage of control (shown is a representative result of a total of 2 independent experiments with similar results). (D) cFLIP expression during maturation of DCs. Seventy-five micrograms of protein of monocyte-derived dendritic cells cultured for 4 days (d4) or 7 days (d7) in the presence of GM-CSF and IL-4 or subsequent 3 days in the presence of IL-1 α (500 IU/mL), IL-6, TNF-α, IL-1 β (all 1000 IU/mL), and PGE2 (10−8 mol/L) were analyzed by Western blotting with cFLIP specific antibodies. Low levels of cFLIPL are expressed in d4 and d7 immature DCs, whereas fully matured DCs (d10) show strong expression of cFLIPL. Reprobing of the membrane with tubulin Ab demonstrates comparable loading of protein.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/7/10.1182_blood.v96.7.2628/5/m_h81700080002.jpeg?Expires=1769163870&Signature=OW1rSpK3eC6zwfy3UrmumcQU7kRYoTY6xTlTiyjcrfX-c9OrTW2ZAzDsdk~zeSyM2e4IFW8Hrb7QxaRAVXD9U9tRMpqHUZl~cSKYkJzo4NCvRLX~cUypcntMB~aTgUwBEfhkRF5Fxln5xnl7buOPut24BltJ8fZPZPiN-QgwYLEKQSxbBNIiHqisT7uvO-4ySv8QHtQ6YhV5SjalWfqBV5FBXJwp2UbxwdP95BG5N2xnRSvfczxLq778jgazpM72yf~HPBAbx2PQSybUkVT5rr37qTYwQw1D2XjOO8VOa7x~jL~0zrRH~qFIvV~jI5zj3QM7EuPgSvWVaR5GE8ZMpw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 2. Mature DCs, but not immature DCs are resistant to death ligand–induced apoptosis. / (A) d3 cells (upper panel), d7 cells (middle panel), or d10 cells (lower panel) were incubated in the presence of 100 ng/mL LZ-CD95L, 1 μg/mL LZ-TRAIL, or the combination of both for 16 hours and survival of cells was determined by Annexin/propidium iodide staining and FACS. (B) Dose response curves of different populations of DCs (d3, d7, and d10). Cells were treated with the indicated concentrations of LZ-CD95L (square), LZ-TRAIL (triangle), or the combination of both death ligands (rhombs), and survival was determined 16 hours later by Annexin/propidum iodide staining by FACS (shown is a representative experiment [percentage of control survival] of a total of 4 independent experiments with similar results). (C) Cytokine cocktail-induced maturation of DCs is comparable to CD40L-induced maturation, but necessary to induce resistance to CD95L or TRAIL. The d7 DCs were cultured for 3 days either in GM-CSF and Il-4 alone, with addition of cytokine cocktail (see “Materials and methods”) or recombinant LZ-CD40L (1 μg/mL) and analyzed by surface analysis for CD1a, CD25, CD80, and CD83 (upper panel). Cells were subsequently treated with the LZ-CD95L (200 ng/mL), LZ-TRAIL (10 μg/mL), or the combination of both death ligands, and survival was determined 16 hours later by Annexin/propidum iodide staining by FACS as percentage of control (shown is a representative result of a total of 2 independent experiments with similar results). (D) cFLIP expression during maturation of DCs. Seventy-five micrograms of protein of monocyte-derived dendritic cells cultured for 4 days (d4) or 7 days (d7) in the presence of GM-CSF and IL-4 or subsequent 3 days in the presence of IL-1 α (500 IU/mL), IL-6, TNF-α, IL-1 β (all 1000 IU/mL), and PGE2 (10−8 mol/L) were analyzed by Western blotting with cFLIP specific antibodies. Low levels of cFLIPL are expressed in d4 and d7 immature DCs, whereas fully matured DCs (d10) show strong expression of cFLIPL. Reprobing of the membrane with tubulin Ab demonstrates comparable loading of protein.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/7/10.1182_blood.v96.7.2628/5/m_h81700080002.jpeg?Expires=1769163871&Signature=ddztwgCoxTPlpKfMXOJx26vLxqZkIJXQaEiC1LuVTJmtfSrnPkQ0ewCleaIacHSbSKSUdX0q7H5pfLQoe5A~gM90wXg2cNqKtllf2~8HfsNaOwU24KgjZaFjU0DxU2yY~xJP5o8nhtAlVzR0hwIg0HVi7f6giVVshmTTtxSphNT6WXrmGyHQbm7rTM6bU8lUlBFKYAZp7UV-3IG23dYYHa4hC7WzqER0TD86dHzu80MOU05JEv~o3E0FkjicFwtVZxKG0M1MXzPlKsc5zi3tj4rdDMZNHuz3opuKLDLFI8LebPJ8Z4CXy7L8zG3VwCX2r8rSnD0eLkV~IyrLvflrog__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)