Abstract
The innate immune system provides rapid and effective host defense against microbial invasion in a manner that is independent of prior exposure to a given pathogen.1 It has long been appreciated that the blood contains important elements that mediate rapid responses to infection. Thus, anatomic compartments with ample blood supply are less frequently infected and recover more readily once infected, whereas regions with poor perfusion are prone to severe infection and may require surgical débridement. Blood-borne innate immune mediators are either carried in circulating blood cells (ie, leukocytes and platelets) or in plasma after release from blood cells or on secretion by the liver.
Introduction
A growing body of research has revealed the mechanisms by which blood cells exert microbicidal activity. Activated neutrophils, eosinophils, macrophages, and, to a more limited extent, lymphocytes increase oxygen consumption during phagocytosis in what has been termed the “respiratory burst.” This oxidative response is now known to be mediated by a multicomponent leukocyte oxidase that transfers electrons to molecular oxygen.2 These oxygen radicals are converted to hydrogen peroxide and, by the action of myeloperoxidase, to hypochlorous acid—microbicidal agents that have long been used as commercial and household antimicrobials.3 Chronic granulomatous disease (CGD) is caused by genetically acquired defect(s) in the phagocyte oxidase and is characterized by increased frequency of infections with certain microbial pathogens.4
Nitric oxide is another small, rapidly diffusable antimicrobial mediator, whose production by inducible nitric oxide synthase (iNOS) contributes to mammalian host defense against intracellular pathogens.5 Although most readily demonstrable in murine macrophages, iNOS expression and function have been detected in human macrophages derived from patients with infection or inflammatory disorders5 as well as in human neutrophils activated by bacterial infection.6
Studies of murine macrophages have identified the natural resistance-associated macrophage protein (NRAMP) as an important mediator of innate defense against certain intracellular pathogens (eg, Salmonella, Mycobacteria, andLeshmania).7 Based on homology to proteins of known function as well as direct experimental evidence, it has been argued that NRAMP modulates ion flux across the phagolysosomal membrane.8 Genetic polymorphism of the NRAMP1gene has been associated with variable human susceptibility to mycobacterial infections.9
Several observations have suggested that eukaryotic organisms also use peptide-based oxygen-independent antimicrobial mechanisms. Both leukocytes from patients with CGD and normal leukocytes deprived of oxygen in vitro are capable of killing a variety of microorganisms.10 Moreover, crude acid extracts of leukocytes possess direct microbicidal activity that is oxygen independent.11 Over the past 20 years, a growing number of cationic proteins and peptides with direct microbicidal activity demonstrable in vitro have been isolated and characterized.12 It is increasingly appreciated that an important mechanism by which blood exerts antimicrobial activity is by the mobilization of these cytotoxic proteins and peptides to sites of infection. With few exceptions, cell-associated agents are carried in the cytosolic granules of leukocytes and platelets, whereas extracellular agents are either the product of cellular degranulation or of secretion from the liver into acute-phase plasma. In addition to direct microbicidal activity, many of these agents are also capable of neutralizing the proinflammatory effects of microbial surface components.13-15
The neutrophil granule antibiotics are generally membrane-active cationic proteins and peptides whose affinity for the negatively charged microbial surface depends not only on electrostatic interactions but also on their tertiary (3-dimensional) structure. Despite having similar net positive charge, these agents vary markedly in size, structure, and mechanisms of action, as well as in the selectivity of their cytotoxic effects. Antimicrobial proteins and peptides often demonstrate relative selectivity toward microbial cells, which has been attributed to their relatively higher affinity for the surface lipids of microbial as opposed to eukaryotic cells.16,17 Although the focus of this review is on the antimicrobial properties of such cytotoxic proteins and peptides, many of these agents manifest additional activities relating to immune modulation and wound healing.12 Similar proteins and peptides have also been identified in mucosal epithelial cells.18
Innate defense molecules: a phylogenetic perspective
Although the focus of this review is on the antimicrobial proteins and peptides of mammalian blood, it is instructive to consider the strong similarities of this arm of the innate immune system to corresponding mediators of nonmammalian vertebrate and invertebrate animals as well as plants. The expression of antimicrobial proteins and peptides in highly divergent species reflects the common need of multicellular organisms to defend against microbial invasion (Table1).19,20 In particular, great progress has been made in characterizing the innate responses of insects to infection and septic injury.19-21 Innate responses to microbial surface components by both insects and mammals are mediated by structurally homologous signaling molecules, including Toll-like receptors (TLRs), cytosolic kinases, nuclear factor (NF)-κB transcription factors, and NF-κB response elements.22 By such pathways, septic injury to insects induces rapid (12-48 hours) expression of multiple genes encoding antimicrobial proteins and peptides in both blood cells (hemocytes) and the insect fat body (liver equivalent).19,20,23 Approximately 100 such peptide antibiotics, many of which reach high micromolar concentrations in insect blood on maximal induction, have been described. Inducibility of antimicrobial proteins and peptides is also a feature of innate defense both systemically and at epithelial sites in plants,24insects, and amphibians (eg, frogs25), as well as mammals.26 27 Although generally cationic, these peptides vary markedly in structure, with diverse mechanisms of action (Table 1) that continue to be investigated.
Antimicrobial proteins and peptides of mammalian blood
Neutrophils
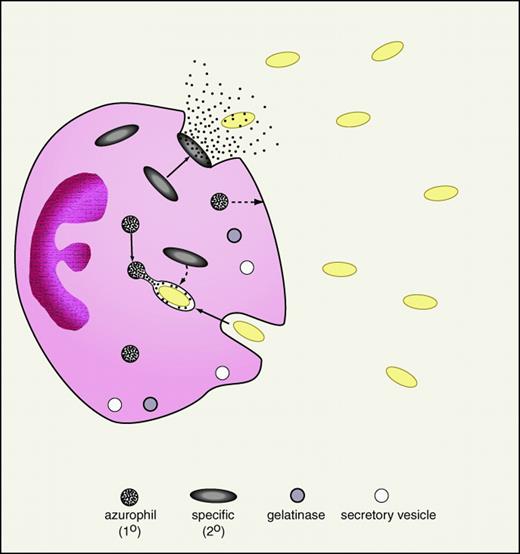
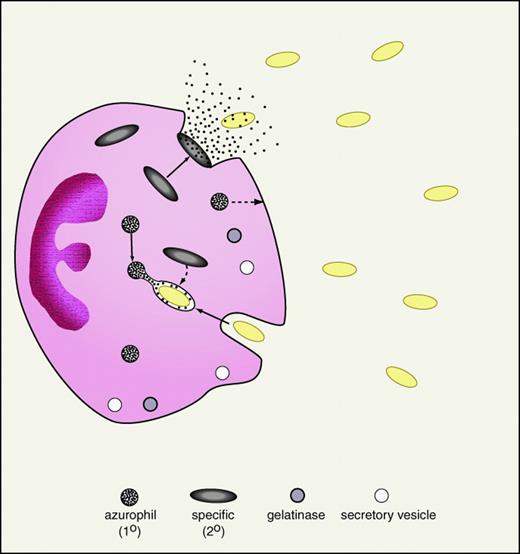
The neutrophil or polymorphonuclear leukocyte is a central cellular effector of the innate immune system. Thus patients with defects in neutrophil quantity or quality experience increased frequency and severity of infections.28 The relevance of neutrophils in the clinical arena is also implicit in the use of the neutrophil count and the percentage of band forms in gauging the likelihood of bacterial sepsis. Neutrophils are formed in the bone marrow where their cytoplasmic granules are synthesized in an orderly progression: primary (or azurophilic) granules then secondary (or specific) granules (Figure 1). More recently, a greater diversity of neutrophil granule subpopulations has been defined, including gelatinase granules and secretory vesicles.29 However, the antimicrobial proteins and peptides appear to be largely confined to the primary and secondary granules (Table 2). Activated neutrophils migrate to sites of infection where they deploy their granule-associated arsenal. The primary granules of neutrophils contain a variety of antimicrobial proteins and peptides as well as hydrolytic enzymes and are largely degranulated into the phagosome thereby exposing ingested microorganisms to high concentrations of granule contents (Figure 1). Secondary granules contain distinct antimicrobial proteins and peptides are deployed toward the leading edge of the chemotaxing neutrophil from which they are readily degranulated extracellularly. Finally, antimicrobial components of the neutrophil cytosol may be released into inflammatory fluids following neutrophil cell death by “holocrine secretion.”30
Neutrophil degranulation of antibiotic proteins and peptides.
An activated neutrophil in the process of phagocytosis of gram-negative bacteria (yellow ovals) is demonstrated. As shown, specific (secondary) granules are more prone to degranulate their contents (including lactoferrin and cathelicidins) into the extracellular space. In contrast, azurophil (primary) granules, containing BPI and defensins, are predominantly degranulated into the phagolysosome. To a lesser extent, specific granules also degranulate into the phagolysosome and primary granules to the extracellular space (broken arrows). Neutrophil granule populations, including gelatinase granules and secretory vesicles, are demonstrated at the bottom of the figure.
Neutrophil degranulation of antibiotic proteins and peptides.
An activated neutrophil in the process of phagocytosis of gram-negative bacteria (yellow ovals) is demonstrated. As shown, specific (secondary) granules are more prone to degranulate their contents (including lactoferrin and cathelicidins) into the extracellular space. In contrast, azurophil (primary) granules, containing BPI and defensins, are predominantly degranulated into the phagolysosome. To a lesser extent, specific granules also degranulate into the phagolysosome and primary granules to the extracellular space (broken arrows). Neutrophil granule populations, including gelatinase granules and secretory vesicles, are demonstrated at the bottom of the figure.
Lactoferrin.
A member of the transferrin family of iron-binding proteins, lactoferrin is an 80-kd protein with 2 iron-binding sites arranged in a bilobed structure.31 Lactoferrin is localized in the secondary granules of neutrophils as well as tear fluid, saliva, and breast milk. In addition to depriving microorganisms of an essential nutrient by binding iron,32 lactoferrin can also exert a directly microbicidal effect, presumably via membrane disruption.33 Lactoferricin is a naturally occurring non–iron-binding microbicidal peptide derived from the N-terminus of lactoferrin.34 Several studies have also documented antiviral effects of lactoferrin against multiple viral pathogens including human immunodeficiency virus.35
In addition to its antibacterial properties, lactoferrin has also been shown to bind to the lipid A moiety of gram-negative bacterial lipopolysaccharide (LPS) thereby neutralizing its endotoxic activity. However, the endotoxin-neutralizing properties of lactoferrin are limited in the presence of the plasma lipopolysaccharide-binding protein (LBP),36 an activator of endotoxic activity that delivers LPS to the macrophage CD14/TLR.37 Nevertheless, lactoferrin administered orally is apparently capable of reducing mortality in a porcine endotoxin shock model.38
Bactericidal/permeability-increasing protein (BPI).
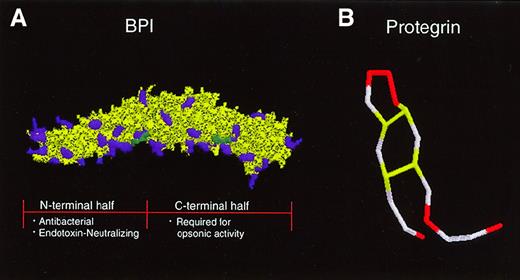
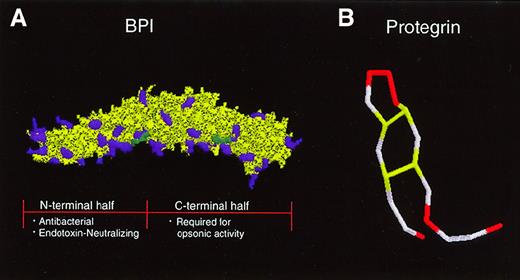
The 55-kd BPI selectively exerts multiple activities against gram-negative bacteria: (1) cytotoxicity, via sequential effects on outer and inner lipid membranes, (2) opsonization to enhance phagocytosis by neutrophils, and (3) neutralization of gram-negative bacterial LPS or endotoxin.39 The crystal structure of BPI reveals a symmetric bipartite structure characterized by N- and C-terminal regions each of which contain lipid-binding apolar pockets40 (Figure 2). Whereas the N-terminal region of BPI is highly cationic and contains both antibacterial and endotoxin-neutralizing properties, its C-terminal end contributes to the ability of BPI to opsonize gram-negative bacteria.41
Tertiary structures of a neutrophil-derived antimicrobial protein and peptide in clinical trials.
(A) BPI has a bipartite structure characterized by 2-fold symmetry that gives the molecule a “boomerang” shape. Cationic amino acid residues (purple) are concentrated in the N-terminal half of the molecule, which carries the endotoxin-neutralizing and bactericidal activities of the protein. A recombinant N-terminal BPI fragment is currently in clinical trials. The C-terminal half of BPI is required for opsonic activity. Two apolar sites thought to be important for interaction with lipids are indicated in green. (B) The protegrin peptide, which is derived from a cathelicidin precursor, is composed of 18 amino acids. Four cysteine residues form 2 disulfide bonds (yellow), giving the peptide a hairpin structure. Six cationic arginine residues are indicated in red.
Tertiary structures of a neutrophil-derived antimicrobial protein and peptide in clinical trials.
(A) BPI has a bipartite structure characterized by 2-fold symmetry that gives the molecule a “boomerang” shape. Cationic amino acid residues (purple) are concentrated in the N-terminal half of the molecule, which carries the endotoxin-neutralizing and bactericidal activities of the protein. A recombinant N-terminal BPI fragment is currently in clinical trials. The C-terminal half of BPI is required for opsonic activity. Two apolar sites thought to be important for interaction with lipids are indicated in green. (B) The protegrin peptide, which is derived from a cathelicidin precursor, is composed of 18 amino acids. Four cysteine residues form 2 disulfide bonds (yellow), giving the peptide a hairpin structure. Six cationic arginine residues are indicated in red.
Bactericidal/permeability-increasing protein is bactericidal at nanomolar concentrations toward certain species of gram-negative bacteria (eg, the serum-resistant encapsulated clinical isolateEscherichia coli K1/r but not certain isolates ofSerratia marcesens or Enterobacter cloaceae42). The selectivity of the action of BPI toward gram-negative bacteria has been attributed to its high affinity (nmol/L) for the lipid A moiety of LPS (or “endotoxin”).43 Studies of the effects of BPI on the biophysical properties of model membranes composed of LPS or phosphatidylglycerol suggest that intercalation of BPI into membrane lipids by interaction with lipid phosphate groups and acyl chains perturbs higher order lipid structure leading to outer and inner membrane lysis.44,45 Gram-negative bacteria with longer LPS chain length are more resistant to BPI action, presumably due to steric hindrance of BPI penetration to target site(s).46 Although BPI is active against membrane vesicles and l-forms of both gram-negative and gram-positive bacteria, the cell walls of gram-positive bacteria render these organisms refractory to the antibacterial activity of BPI.47
Activity of BPI is manifest not only in artificial laboratory media, but also in blood, plasma, and serum. Activated neutrophils release BPI into inflammatory fluids where it is potently bactericidal. BPI acts in synergy with the members of the cathelicidin and defensin peptide families (discussed below) as well as the complement system.48 Addition of a neutralizing anti-BPI serum blocks the bactericidal activity of rabbit inflammatory (ascitic) fluid against encapsulated gram-negative bacteria suggesting that such activity is BPI dependent.49
The ability of BPI to potently inhibit endotoxin of isolated LPS (regardless of chemotype) and of whole bacteria (including strains resistant to the antibacterial action of BPI42,50) is opposite to that of its structural homolog the LBP, which is an acute-phase reactant that greatly amplifies LPS proinflammatory signaling.51 Whereas LBP disaggregates LPS, delivering LPS monomers to the cellular CD14/TLR complex, BPI enhances LPS aggregation, thereby preventing LBP action.52 Although multiple cationic proteins and peptides have demonstrated anti-endotoxic activity when tested in artificial media in vitro,15 BPI is notable for its ability to neutralize endotoxin in biologic fluids even in the presence of LBP,53 suggesting that BPI may contribute to down-regulating the proinflammatory effects of gram-negative bacteria and endotoxin in vivo.
Serprocidins.
The serprocidins are 25- to 37-kd serine proteases with cytotoxic activity that are localized in neutrophil primary granules.54 The serprocidin family includes neutrophil elastase, cathepsin G, proteinase 3, and azurocidin/CAP37. The serprocidins are structurally related to the granzymes of cytotoxic T cells and manifest microbicidal activity both via direct membrane perturbation as well as by proteolytic action. Whereas elastase and cathepsin G are catalytically active, the divergent primary structure of azurocidin in the region corresponding to the active site render it enzymatically inactive.55 Nonenzymatic killing by serprocidins is believed to depend on the membrane perturbing effects of these cationic proteins. Serprocidins manifest broad cytotoxic activity against gram-negative (eg, E coli) and gram-positive bacteria (Streptococcus faecalis), fungi (Candida albicans), and protozoa as well as mammalian cells.54 Azurocidin acts in synergy with either elastase or cathepsin G to kill the bacterium Capnocytophaga sputigena.56 Elastase also participates in generating antimicrobial activity during inflammatory responses by cleaving cathelicidin proforms (see below) to generate active antimicrobial peptides.57 Of note, mice rendered deficient in neutrophil elastase by genetic engineering are more susceptible to sepsis and death despite normal accumulation of leukocytes, suggesting an impairment in bacterial killing.58 Unlike most of the other cationic proteins and peptides, azurocidin actually enhances cellular responses to endotoxin by a mechanism that has yet to be defined.59
Cathelicidins.
The cathelicidins are a structurally diverse group of antimicrobial peptides that are expressed at the C-terminus of 11- to 20-kd inactive proforms in the neutrophil secondary granules of humans and other mammalian species.60 Because neutrophil secondary granules are readily degranulated to the extracellular space, cathelicidins are released into inflammatory fluids where they are found at relatively high concentrations.49 Some cathelicidin genes possess upstream DNA motifs (eg, NF-κB, interleukin [IL]-6, granulocyte/macrophage colony-stimulating factor [GM-CSF], and NF-1) predicted to confer inducibility during acute-phase responses.61,62 The human cathelicidin peptide FALL-39/hCAP-18 is expressed in neutrophil precursors, is induced in keratinocytes of inflamed skin,63 and is found in high concentrations in the lipoprotein fraction of plasma.64Derived from porcine neutrophils, protegrin is a 2-kd peptide composed of 18 amino acids with 2 internal disulfide bonds (Figure 2B). The broad spectrum microbicidal activity of protegrin against bacterial and fungal pathogens requires intact disulfide bonds and is believed to be mediated by the formation of multimeric pores in the microbial membrane.65,66 The amphipathic rabbit cathelicidin peptide CAP-18 is not only microbicidal but also binds and neutralizes endotoxin.67 The rabbit p15s, which apparently do not undergo cleavage, act synergistically with BPI against gram-negative bacteria.68 Solid-phase peptide synthesis has been used to create a carboxymethylated congener of the cathelicidin peptide indolicidin with increased cationicity thereby enhancing its activity against gram-negative bacteria.69
Lysozyme.
Lysozyme is a 14-kd enzyme that degrades bacterial peptidoglycans by cleaving the glycosidic bond of N-acetyl glucosamine. This enzyme is stored in both primary and secondary neutrophil granules. Gram-positive bacteria with minimally cross-linked peptidoglycan (eg,Bacillus subtilis) allow access of lysozyme to its substrate, are rapidly lysed and killed by this enzyme, and are generally (consequently?) nonpathogenic. Most gram-positive clinical pathogens possess highly cross-linked peptidoglycan and are thereby resistant to the action of lysozyme. Moreover, gram-negative bacteria are generally resistant to the action of lysozyme by virtue of their hydrophobic outer membrane. The spectrum of action of native lysozyme may be broadened by synergistic action with other antibiotic proteins of neutrophils including lactoferrin70 and defensins.71 Use of recombinant DNA technology to create a novel lysozyme congener possessing a hydrophobic N-terminal peptide enhances activity against gram-negative bacteria.72Transgenic expression of human lysozyme in mice is associated with production of milk that is antimicrobial toward cold spoilage pathogens such as Lactobacillus viscus.73
Phospholipases A2.
The 14-kd phopholipases A2 (PLA2) are disulfide-rich enzymes that hydrolyze phospholipids at the 2-acyl position. Group II PLA2, which are structurally defined by a unique disulfide arrangement, are found in neutrophil granules and are also secreted by the liver into plasma as acute-phase reactants.18 Neutrophil-derived antibiotics such as BPI and certain cathelicidins (eg, rabbit p15s) can render the membrane phospholipids of E coli susceptible to PLA2-mediated enzymatic cleavage.74 The degree of destruction of gram-negative bacteria ingested by neutrophils is related to the magnitude of phospholipolysis.75 More recent work has focused on the potent (nmol/L) and selective direct bactericidal action of PLA2 against gram-positive bacteria including Staphylococcus aureus, Listeria monocytogenes, and vancomycin-resistant Enterococcus. PLA2 is found in murine intestinal Paneth cells, rabbit inflammatory ascitic fluid, human tear fluid,76 and in the acute-phase plasma of baboons challenged with intravenous bacteria.27 In all of these settings, PLA2 has been shown to function enzymatically as the primary microbicide active against gram-positive bacteria. Consistent with these results, mice that express PLA2 are relatively protected against S aureus infection when compared to their PLA2−/− counterparts.77
Calprotectin.
The calprotectin complex is composed of 8- and 14-kd members of the S-100 family of calcium-binding proteins and is particularly abundant in the cytosol of neutrophils where it represents about 30% of total cytosolic protein.78 Calprotectin manifests antimicrobial activity in the μmol/L range against bacteria and fungi by zinc chelation that is mediated by histidine-rich protein regions.79 Both neutrophil lysates and abscess fluids possess zinc-reversible antimicrobial activity suggesting that “holocrine secretion” of calprotectin from neutrophils undergoing cell death might represent another mechanism by which these cells combat infection.80
Defensins.
The defensins are a family of 4-kd peptides with broad cytotoxic activity against bacteria, fungi, parasites, viruses, and host cells.81 The activity of defensins depends on both their cationicity as well as their 3-dimensional structure.82Defensins form multimeric voltage-dependent pores that permeabilize cellular membranes. Whereas classical (α-) defensins are characterized by 6 invariant cysteine residues forming 3 disulfide bonds, β-defensins contain a distinct disulfide arrangement. Humans express α-defensins in neutrophils and β-defensins in intestinal Paneth cells, as well as pulmonary71 and reproductive epithelia.83 The isolation of a salt-sensitive pulmonary β-defensin has suggested that impairment of this innate defense is an important contributor to chronic pulmonary infections in patients with cystic fibrosis.84 However, the ion composition of airway surface fluid is apparently similar in patients with cystic fibrosis and normal controls raising doubts about the importance of airway liquid tonicity to the impairment of antibacterial defense in patients with cystic fibrosis.85
The activity of defensins is limited by monovalent and divalent cations as well as by plasma and serum proteins suggesting that the action of these peptides is limited in the extracellular environment to prevent indiscriminate cytotoxicity. Thus, whereas β-defensins act at mucosal surfaces, α-defensins may be most active intracellularly in the phagolysosome.
Of note, a recently described cyclic antimicrobial peptide found in the neutrophils and monocytes of rhesus macaques is an index member of a novel family named “theta” (θ)-defensins.86 Elegant biochemical analysis reveals that rhesus θ-defensin-1 (RTD-1) is naturally produced by a unique ligation of 2 truncated α-defensins. The cyclic conformation of θ-defensins confers relative salt insensitivity allowing this peptide to exert its microbicidal effect in physiologic saline.
Eosinophils
An important role for eosinophils in antiparasitic defense has been suggested by several observations: (1) parasitic infection is associated with marked eosinophilia mediated by IL-5, (2) depletion of eosinophils with selective antisera has been associated with increased pathogen burden in some animal models of parasitic infection,87 and (3) eosinophil-derived cytotoxic proteins demonstrate antiparasitic activity in vitro. Eosinophils also possess antimicrobial activity against bacteria and fungi. Although eosinophils have been shown to have lesser bactericidal activity toward E coli and S aureus than neutrophils, it is not known whether this might relate to relative deficiency of oxygen-dependent (eg, peroxidase-H2O2-Cl system) or oxygen-independent (eg, antimicrobial protein/peptide) mechanisms.88 89
Eosinophils contain at least 2 granule populations—the primary granules, containing the Charcot-Leyden crystal protein, and the secondary granules that carry cytotoxic proteins.90 Major basic protein (MBP), which accounts for about 50% of eosinophil granule protein content, is a 10- to15-kd arginine-rich polypeptide that aggregates to form the crystalline core of eosinophil secondary granules. MBP demonstrates antihelminthic (Trichinella spiralis,91,Schistosoma mansoni,92,Brugia species91), antiprotozoal (Trypanosoma cruzi93), and antibacterial (E coli, S aureus)94 activities and is deposited on the surface of the fungus Paracoccidioidomycosis brasiliensis in biopsy samples from patients.95 A divergent anionic homolog of MBP, termed MBPH, has recently been cloned from maturing eosinophils and has reduced cytotoxic activity.96 Eosinophil cationic protein (ECP) and eosinophil-derived neurotoxin (EDN) are 17- to 21-kd cationic proteins with homology to ribonucleases, which can exert cytotoxic activity by both catalytic and noncatalytic mechanisms.97 The arginine-rich ECP is highly cationic and is thought to mediate cytotoxicity by membrane-disruptive effects that are also manifest against metabolically active bacteria94and that may be mediated by formation of voltage-gated ion channels in lipid bilayers.98 Like MBP, ECP demonstrates bactericidal activity by sequential permeabilization of the outer and inner membranes of E coli.94 EDN is less cationic than ECP and may mediate cytotoxic effects by catalytic mechanism as suggested by the ability of RNase inhibitors to block the cytotoxicity of EDN toward T cruzi.93 Both ECP and EDN have recently been detected in neutrophils as well.99
Eosinophils share certain members of the neutrophil armamentarium. Lysozyme has been localized to eosinophil secondary granules.100 It has been suggested that the presence of relatively low amounts of BPI in the secondary granules of human eosinophils might reflect a role for these cells in antibacterial host defense or a role for BPI in antiparasitic activity.101
Macrophages
In addition to oxidase and nitric oxide synthase activity, macrophages also possess oxygen-independent microbicidal activity.102 Macrophages express multiple components of the classic and alternative pathways of the complement system, as well as several of the antimicrobial proteins and peptides described in neutrophils.103 Multiple studies have demonstrated that the enzymes elastase and lysozyme are expressed by activated macrophages. Rabbit pulmonary macrophages express defensin peptides.104 The origin of cell surface BPI expressed on human peripheral blood monocytes remains to be determined.105 The cytosolic calprotectin protein that is abundant in neutrophil cytosol is also found in macrophages.78
Studies have attempted to identify antimicrobial proteins and peptides in macrophages or in macrophage-like cell lines activated by interferon-γ, which enhances macrophage microbicidal activity. Several cationic murine microbicidal proteins (MUMPS), which are members of the cationic histone family, have been isolated from macrophages.106 MUMPS possess broad-spectrum antimicrobial activity against gram-negative, gram-positive, mycobacterial, and fungal pathogens and may contribute to the microbicidal activity of the macrophage lysosomal apparatus. Ubiquicidin is a 6.6-kd cationic peptide derived from the cytosolic fraction of interferon-activated RAW 264.7 cells with activity toward gram-negative (E coli,Salmonella typhimurium, Yersinia enterocolitica) and gram-positive (L monocytogenes, S aureus) bacteria.107
Transgenic techniques have been used to enhance the antimicrobial activity of macrophages. A preliminary effort to enhance intracellular microbicidal activity by xenogenic expression of the defensin HNP-1 in murine macrophages enhanced growth inhibition of Histoplasma capsulatum.108 Macrophages transfected with a BPI-IgG fusion protein are better equipped to neutralize endotoxin and have diminished tumor necrosis factor (TNF)-α release in response to LPS.109
Cytotoxic T lymphocytes and natural killer cells
Both cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells possess granule-associated cytotoxic proteins and peptides that can be directed toward microbial pathogens as well as host cells altered by infection or tumorigenesis. CTLs and NK cells contain perforin, a monomeric pore-forming protein with homology to the C9 component of complement.110 The granzymes are a family of 25- to 30-kd serine proteases found in the granules of cytotoxic T cells that are homologous to the neutrophil-derived serprocidins.111Granzymes, which are expressed in keratinocytes as well, participate in triggering apoptosis in target host cells and in bactericidal activity.112,113 CTLs and NK cells are also the repository of members of the saposin class of cytotoxic peptides, including porcine NK lysin and human granulysin. NK lysin is an IL-2–inducible 78 amino acid cationic cytotoxic peptide with activity against bacteria (including E coli and Bacillus megaterium) and tumor cells.114 NK lysin possesses 3 intramolecular disulfide bonds that are required for potent cytotoxic activity and whose reduction by host thioredoxin reductase may serve as a means of limiting the cytotoxic effect of this peptide.115 In addition to direct cytotoxic effects, NK lysin is capable of binding and neutralizing LPS.116Granulysin, a peptide of CTL granules, is a broad-spectrum microbicide that is required for intracellular killing of Mycobacterium tuberculosis.117
Platelets
Although principally thought to have a role in hemostasis and wound healing, platelets are increasingly appreciated as antimicrobial effectors.118 From an evolutionary perspective, the expression of coagulation, wound healing, and antimicrobial defense by a blood-borne cell is well described in the hemocytes of the horseshoe crab Limulus polyphemus.119 In an animal model of Streptococcus sanguis endocarditis, rabbits rendered thrombocytopenic by administration of antiplatelet serum developed a higher bacterial burden at aortic valve vegetations, suggesting an antimicrobial role for platelets.120
Over the past decade, work by Yeaman and colleagues121,122has demonstrated that platelet α granules contain a number of distinct 6- to 9-kd cationic antimicrobial peptides derived from either platelet acid extracts (PMPs) or extracellular medium of platelets activated with thrombin (tPMPs). Whereas PMPs contain 3 to 4 cystine residues, tPMPs contain few or no cysteine residues. PMPs possess broad-spectrum antimicrobial activity against gram-negative (E coli), gram-positive (S aureus and Staphlococcus epidermidis as well as viridans streptococci) and fungal pathogens (C albicans and Cryptococcus neoformans). Electron microscopy and studies of membrane transmembrane potential suggest that PMPs target the microbial cytoplasmic membrane. The effects of PMPs on the cell membrane of S aureus are distinct from those of defensin peptides, demonstrating enhanced activity against strains with larger transmembrane potential.123
Pathogenic strains of S aureus and S epidermidisderived from patients with endocarditis are often resistant to tPMP, raising the possibility that such resistance might be an important virulence factor in such infections.124
Clinical application
The effort to develop blood cell-derived proteins and peptides as novel antibiotics is driven by at least 2 considerations: (1) the rising prevalence of multidrug-resistant microorganisms, and (2) a growing population of immunocompromised patients. In terms of immunocompromised patients, clinical conditions associated with impaired leukocyte or platelet function include newborn status, overwhelming sepsis, leukemia, exposure to chemotherapy, congenital hematopoietic defects, and cystic fibrosis.125 However, the ability to enhance hematopoietic cell defense has been limited. Granulocyte transfusions are associated with potential side effects, including alloimmune reactions126 and induction of pulmonary inflammation. Administration of the cytokines such as granulocyte colony-stimulating factor and GM-CSF has been shown to raise neutrophil counts in patients undergoing chemotherapy but has not demonstrated improved survival or clinical outcome.127Whether the leukocytes generated by the administration of such growth factors are fully functional and contain all of the aforementioned antimicrobial apparatus has not been fully defined. Thus, considerations relating to both microbe and host have generated considerable enthusiasm for evaluating host-derived antimicrobial proteins and peptides as potential novel antibiotics.
Topical/aerosol formulations
The magainin peptides derived from frog skin were the first of the animal-derived antimicrobial peptides to reach clinical trial. Phase III evaluation of a topical magainin preparation for treatment of impetigo and polymicrobial infections characteristic of ulcers in patients with diabetes128 reportedly demonstrated insufficient activity. The wide variety of insect-derived antimicrobial peptides (Table 1) are currently in early stages of development as potential novel antibiotics.129
The porcine neutrophil-derived peptide protegrin (Figure 2B) is the first of the mammalian cathelicidin peptides to reach clinical trial. Topical administration of protegrin in a hamster model of chemotherapy-induced oral mucositis was associated with decreased microbial burden at mucosal lesions, decreased lesion severity, and accelerated recovery.130 A phase I study demonstrated that this peptide can be safely delivered topically to humans who develop mucositis in the context of myeloablative chemotherapy. A recently completed phase II study of topical (oral) protegrin involving 177 patients undergoing bone marrow transplantation indicated that administration of this peptide is associated with a statistically significant reduction in mucositis after transplantation and a trend toward a reduced number of febrile days.131 A phase III study of protegrin for the prevention of mucositis associated with myeloablative chemotherapy is now underway. Protegrin is also being evaluated as an aerosolized antimicrobial therapy for the chronic respiratory infections of patients with cystic fibrosis.
Systemic administration
The selective action of BPI (Figure 2A) toward gram-negative bacteria and their endotoxin, which has been documented in vitro, has recently been demonstrated in animal models and in humans as well. A recombinant 21-kd N-terminal modified fragment (rBPI21) of human BPI expresses both the antibacterial and anti-endotoxic activities of the holoprotein and has demonstrated beneficial effects, either alone or in synergistic combination with conventional antibiotics, in animal models of sepsis, pneumonia, endotoxemia, and burns.48 Phase I studies in humans indicate that rBPI21 is well tolerated and nonimmunogenic. rBPI23 (another recombinant N-terminal fragment of BPI) given intravenously to subjects who have received endotoxin is able to markedly inhibit LPS-induced cytokine release,132coagulant responses, and pathophysiologic changes such as alteration of cardiac index.133
Several potential indications for rBPI21 have been selected for phase II studies, based on the presence of gram-negative bacteria or endotoxemia or both: (1) meningococcemia and intra-abdominal infections (endotoxin on or released from invading bacteria), (2) hemorrhagic trauma (endotoxin translocation secondary to decreased intestinal barrier integrity), and (3) liver resection (decreased endotoxin clearance). A phase II study demonstrated that open-label administration of rBPI21 to 26 children with fulminant meningococcemia, admitted to a pediatric intensive care unit, was associated with reduced mortality relative to that predicted by clinical prognostic scores, IL-6 levels, and historical control.134 Two phase III double-blinded placebo-controlled trials of rBPI21 for the treatment of hemorrhagic trauma and of fulminant meningococcemia have been completed recently. Although the former trial was stopped due to insufficient activity, the treatment group in the meningococcemia study reportedly had lower rates of mortality and morbidity. Results of this study are currently under evaluation.
Potential future indications for rBPI21 may include conditions characterized by a relative deficiency of endogenous BPI. Newborns are relatively deficient in BPI by virtue of the relatively low amounts of BPI protein in their neutrophils135 as well as their well known tendency for leukopenia in the face of overwhelming sepsis. Supplementation of newborn cord blood with rBPI21enhances its antibacterial activity against certain gram-negative clinical pathogens and inhibits release of TNF-α induced by such bacteria,42 suggesting that supplementing rBPI21 might be of benefit to newborns suffering from gram-negative bacterial infections or endotoxemia. Another population at risk for bacteremia or endotoxemia in the setting of limited endogenous BPI stores are oncology patients who become profoundly neutropenic during chemotherapy. In this regard, it is notable that in an animal model of graft-versus-host disease (GVHD), chemotherapy-related mucosal injury allowed endotoxin translocation across the gut causing cytokine release and thereby triggering GVHD.136 This study raises the possibility that neutralizing endotoxin might reduce the risk of GVHD.
It is likely that additional leukocyte- and platelet-derived antibiotics will reach clinical trial as systemic agents. The peptide protegrin reduces mortality of leukopenic mice injected with vancomycin-resistant Enterococcus faecium65 and a peptide derived from CAP18 reduces cytokine release and mortality when administered with aztreonam in a mouse model of Pseudomonas aeruginosa infection.137 Adenovirus-mediated gene transfer of the human cathelicidin peptide LL-37/hCAP-18 was found to restore bacterial killing in a cystic fibrosis xenograft model,138 raising the possibility that enhancing such innate immune mechanisms might someday be of clinical benefit.
Conclusion
The growing problem of microbial resistance has placed increasing emphasis on developing novel antibiotics. Moreover, a growing number of patients have impaired leukocyte- or platelet-mediated defense either due to their primary disease or its treatment. Blood-derived antibiotic proteins and peptides represent a source of innate defense molecules that target the microbial membrane leading to growth arrest and in some instances, neutralization of proinflammatory surface components (eg, LPS). These natural antibiotics and their synthetic congeners have activity that can be demonstrated in biologic fluids and animal models and have proven safe in preliminary clinical trials. Judicious enhancement of this arm of innate immunity may eventually prove of clinical benefit to selected patients.
Acknowledgments
The advice and support of Drs Philip Pizzo and Donald Goldmann are gratefully appreciated.
Supported by the Medical Scientist Training Program, as part of National Institutes of Health Training Grant 5T32GM07308 from the National Institute of General Medical Science; NIH/National Center for Research Resources/ General Clinical Research Center Grant M01RR02172, The Children's Hospital of Boston; an American Academy of Pediatrics Resident Research Award; The Dana Farber Cancer Research Institute; and by a grant from XOMA (US) LLC.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ofer Levy, Division of Infectious Disease, The Children's Hospital, 300 Longwood Ave, Boston, MA 02115; e-mail:levy_o@a1.tch.harvard.edu.