Abstract
Kaposi sarcoma (KS) is responsive to a number of different steroid hormones, such as glucocorticoids and retinoids. An active metabolite of vitamin D, 1α,25 dihydroxyvitamin D3, was used to study the effect of this steroid hormone in KS. Steroid hormones exert their effect through their cognate nuclear receptors, which for vitamin D metabolites is the vitamin D receptor (VDR). It was first shown that KS cell lines and primary tumor tissue express high levels of VDR, whereas endothelial cells had minimal expression and fibroblasts had no expression. Second, KS cell growth was inhibited by VDR agonist 1α,25 dihydroxyvitamin D3 with a 50% inhibitory concentration of 5 × 10 −8 mol/L, whereas endothelial cells and fibroblast cells showed no response. Studies on the mechanism of KS tumor growth inhibition by 1α,25 dihydroxyvitamin D3 showed that production of autocrine growth factors interleukin (IL)-6 and IL-8 was reduced in a dose-dependent manner, whereas no effect was observed on vascular endothelial growth factor and basic fibroblast growth factor. Transcription initiated at the IL-6 promoter was repressed by VDR agonist. The DNA sequences required to mediate this repression were localized to nucleotides −225/−110 in the 5′-flanking region. The antitumor activity of VDR agonists was also confirmed in KS tumor xenograft and after topical application in patients with KS. 1α,25 Dihydroxyvitamin D3 and its analogs may thus be candidates for clinical development in KS.
Introduction
Kaposi sarcoma (KS) occurs predominantly in men with human immunodeficiency virus (HIV) infection at a rate of about 100 000 times that of the general population and occurs in approximately 15% of patients with acquired immune deficiency syndrome (AIDS).1 KS is a highly vascular tumor, and the tumor cells display features of activated endothelial cells, including high expression of several phenotypic markers, such as CD34, PAL-E, and UEA binding,2 as well as various tyrosine kinases, such as Flt-1, Flk-1/KDR,3 Flt-4,4 Tie-1, and Tie-2 (R. Masood, unpublished data), otherwise only expressed in endothelial cells. Biological studies have shown that the tumor cells produce and respond to several factors, including interleukin (IL)-1,5 IL-6,6 IL-8,7Oncostatin-M,8 vascular endothelial growth factor (VEGF),3 and basic fibroblast growth factor (bFGF).5 HIV proteins also up-regulate some of these factors, in addition to the mitogenic effects of HIV tat protein.9
Glucocorticoids have been shown to be associated with the development of KS in several clinical settings, including skin disorders, lymphoproliferative disorders, HIV infection, and renal transplant recipients.10-12 Furthermore, withdrawal of glucocorticoids can lead to regression of KS in nearly a quarter of the cases. The effect appears to be directly through the blockage in transforming growth factor-β (an autocrine growth inhibitory factor) activation.13 Retinoids, however, have been shown to inhibit KS cell growth in vitro14 and to demonstrate activity in human trials.15,16 The therapeutic activity of retinoids is mediated by the inhibition of IL-6,17 an autocrine growth factor for KS.6Retinoids exert this effect by repressing the enhancer action of the transcription factor NF-IL-6 on the IL-6 promoter.18 The biological effects of retinoids in KS are mediated by retinoic acid (RA) receptors and retinoid X receptors (RXRs), which belong to the superfamily of steroid/thyroid hormone nuclear receptors.19
The nuclear receptor for the active form of vitamin D3, 1α,25 dihydroxyvitamin D3, the vitamin D3 receptor (VDR), also belongs to the steroid/thyroid hormone nuclear receptor superfamily. After ligand binding, VDR binds to specific DNA sequences as a heterodimer with RXR to regulate the expression of target genes.20 1α,25 Dihydroxyvitamin D3 is an important regulator of cell growth and differentiation of various cell types.21-23 Further, 1α,25 dihydroxyvitamin D3 inhibits the production of IL-6 and IL-8 in cultured keratinocytes24-27 as well as in vivo in psoriatic lesions,28,29 osteoclasts,30peripheral blood mononuclear cells (PBMCs) of psoriasis patients,27,31 melanoma,32 and fibroblasts.27,33 Because both IL-6 and IL-8 are autocrine growth factors for KS cells6 (R. Masood, unpublished data), we sought to determine whether a VDR agonist would inhibit the growth of KS cells in vitro and in vivo. Here we describe that 1α,25 dihydroxyvitamin D3 inhibits the growth of KS cells in vitro by inhibiting the production of both IL-6 and IL-8. Furthermore, we demonstrate that, unlike RA, 1α,25 dihydroxyvitamin D3 does not induce the expression of the angiogenic factors VEGF and bFGF in KS cells.18 These results may have important implications in the use of these agents in cancer therapy. Finally, we demonstrate that a synthetic VDR agonist, calcipotriol, has antitumor effects in patients with KS.
Materials and methods
Chemicals
All chemicals were of analytical grade. 1α,25 Dihydroxyvitamin D3 was purchased from Calbiochem (La Jolla, CA). The rat anti-VDR monoclonal antibody was obtained from Chemicon International (Temecula, CA). The sheep antimouse immunoglobulin G (IgG)-fluorescein isothiocyanate conjugate, the biotinylated sheep antimouse IgG, antirat IgG, and fluorescein-streptavidin were obtained from Sigma Immunochemicals (St. Louis, MO). The universal biotinylated secondary antibody was purchased from Ventana (Tucson, AZ). The avidin biotinylated horseradish peroxidase H complex kit was obtained from Vector Laboratories (Burlinghame, CA).
Recombinant plasmids
The reporter constructs pIC225 and pIC596 were obtained from Dr P. B. Seghal (New York Medical College, Valhalla, NY). These reporter constructs contain the IL-6 5′-flanking nucleotides −225/+13 and −596/+13, respectively.34 Expression vector for vitamin D3 receptor was obtained from Dr J. White (McGill University, Montreal, Canada).
Cell culture
Long-term spindle isolate KS-59 was established from a KS lesion of an AIDS-KS patient and propagated in gelatin-coated flasks in culture medium that consisted of RPMI 1640 supplemented with 10% charcoal-stripped, heat-inactivated fetal calf serum (FCS), 1% sodium pyruvate, 1% Nutridoma HU (Boehringer Mannheim, Indianapolis, IN), 1% essential and nonessential amino acids, 2 mmol/L glutamine, 1% penicillin and streptomycin (Gibco/BRL, Gaithersburg, MD)3,13 in the absence of conditioned medium of transformed T-cell lines. Immortalized KS cell line KS Y-1 was established from the pleural effusion of an AIDS-KS patient without the use of exogenous growth factors as previously reported.2It is monoclonal and has been propagated for more than 100 passages. KS Y-1 was propagated on gelatin-coated plates in RPMI 1640 culture medium supplemented with 2% FCS; 1% each sodium pyruvate, essential amino acids, and nonessential amino acids; 1 mmol/L glutamine and antibiotics as above. Human umbilical vein endothelial cells (HUVECs), human aortic smooth muscle cells, and human skin fibroblasts (Clonetics, San Diego, CA) were cultured in the media as recommended by the manufacturer.
Tumor tissues
KS tumor tissue and normal adjoining skin from the same patient were collected after informed consent and were snap frozen until analysis. Samples of the biopsies were fixed in formalin, and both frozen sections and the formalin-fixed tissues were sectioned for immunocytochemistry for VDR.
Immunocytochemistry
Cultured human KS isolate (KS-59) and cell line (KS Y-1), HUVECs, and fibroblast cells were trypsinized and collected onto glass slides with the use of a cytospin centrifuge (Shandon, Astmoor, England), and fixed. Slides were treated with monoclonal antibody to rat VDR with 1:50 dilution in phophate-buffered saline (PBS) and 10% FCS as the primary antibody. The receptor was visualized by immunoperoxidase stain by the method of Berger et al.35Tissue sections of KS biopsy material were also stained with the use of the same antibodies.
Northern analysis
KS Y-1 and HUVEC cells were grown to near confluence and treated with various concentrations of vitamin D3, ranging from 10−9 to 10−6 mol/L for 24 hours. Total RNA was extracted from washed cell pellets by the guanidium thiocyanate method (RNAzol, Tel-Test Inc, Friendswood TX) and separated on a 1% agarose formaldehyde gel, followed by transfer onto a nylon membrane and cross-linking with UV-light (Stratagene, La Jolla, CA). The transferred RNA was prehybridized at 68°C for at least 30 minutes in Quick Hyb solution (Stratagene, San Diego, CA) that contained 100 μg of salmon sperm DNA. The filters were then hybridized with32P-nick translated full-length complementary DNA (cDNA) probes for IL-6, IL-8, VEGF, bFGF (generous gift from J. Abraham, Scios Nova, Mountain View, CA), VDR, and β-actin at 68°C for 2 hours. Signal was visualized by overnight exposure to autoradiography film. The membranes were hybridized with 1 probe at a time, stripped of the radiolabeled probe, and reprobed. The level of β-actin messenger RNA (mRNA) was used to normalize for the quantity of total mRNA. The quantitative difference in the mRNA for the target genes was calculated relative to the β-actin levels with the use of a Molecular Dynamics Phosphor Imager model 445SI.
Cell proliferation studies
Early passage AIDS-KS spindle cell isolates (KS-59) and neoplastic KS cell line (KS Y-1) were seeded at a density of 1.0 × 104 cells/well in a 24-well plate in culture medium. T1 fibroblasts (ATCC, Manassas, VA) were seeded at the same density in DMEM supplemented with 10% FCS and antibiotics. The cells were allowed to attach overnight and were treated with varying concentrations of 1α,25 dihydroxyvitamin D3 on days 1 and 3. Cells were counted on day 6 with the use of a Coulter Particulate Counter (Hialeah, FL). Cell proliferation assays were performed on HUVECs and on human skin fibroblasts in a similar manner.
Enzyme-linked immunosorbent assay
Early passage AIDS-KS spindle cell isolates (KS-59) and neoplastic KS cell line (KS Y-1) were seeded at a density of 5 × 104 cells/well on 24-well plates coated with gelatin. Supernatants were collected from KS cells treated with various concentrations of 1α,25 dihydroxyvitamin D3(10−6 mol/L to 10−9 mol/L) for 24 hours. The supernatants were centrifuged to remove cell debris and were stored at −70°C until analysis. IL-6 and IL-8 levels were measured with the use of commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN), using the manufacturer's recommended procedure. The cytokine levels were corrected for the cell numbers present at the time of supernatant collection.
Transfection and chloramphenicol acetyltransferase assay
KS Y-1 cells were transfected by the cationic liposome-mediated transfection procedure. Cells were plated 18 hours before transfection at about 50% confluence (about 60 000 cells/well) in 12-well plates. The cells were transfected with either pIC225 or pIC596 reporter constructs (1.0 μg), along with VDR expression vector (0.2 μg), using 4 μg of Lipofectamine (Life Technologies) for each well in a total volume of 500 μL. All of the transfections were performed in triplicate. 1α,25 Dihydroxyvitamin D3 was added 18 hours after transfection, and 6 hours later the cells were treated with 12-O-tetradecanoyl-13-acetate (TPA; 50 ng/mL). The cells were harvested on the following day and lysed in a hypotonic buffer (100 μL/well) that contained DNase I, Triton X-100, Tris-HCl, and EDTA. Chloramphenicol acetyltransferase (CAT) activity was assayed in a total volume of 50 μL that contained 25 μL of the lysed cell extract with 5 μL of 4 mmol/L acetyl coenzyme A and 0.1 μCi of [14C]-chloramphenicol for 2 hours at 37°C. Chloramphenicol, mono-, and di-acetylated chloramphenicol were extracted with ethyl acetate and resolved by thin-layer chromatography on silica gel plates in a chloroform-to-methanol ratio of 19:1 (v:v). [14C]-labeled products were visualized by autoradiography, and the conversion of [14C] chloramphenicol to [14C] acetyl chloramphenicol was quantitated with the use of a model 445SI phosphorimager.
In vivo studies
KS Y-1 cells (5 × 106) were inoculated subcutaneously into the right front armpit of 12 male Balb/c NU/nu mice at 5 weeks of age (day 0). Starting on the following day, half of the mice received 1α,25 dihydroxyvitamin D3 orally at a dose of 5 mg/kg body weight, and the other half received diluent alone. The treatment was carried out every day for 12 consecutive days. Mice were killed after the final measurements on day 12. Tumor sizes were measured externally with calipers to determine the length (L) and width (W) of the tumor nodule. Tumor volume was calculated according to the formula V = L × W2 × 0.52. Animals were treated in accordance with the guidelines of the Animal Use and Care Committee at the University of Southern California.
Results
Vitamin D3 receptor is expressed in KS cells at high levels
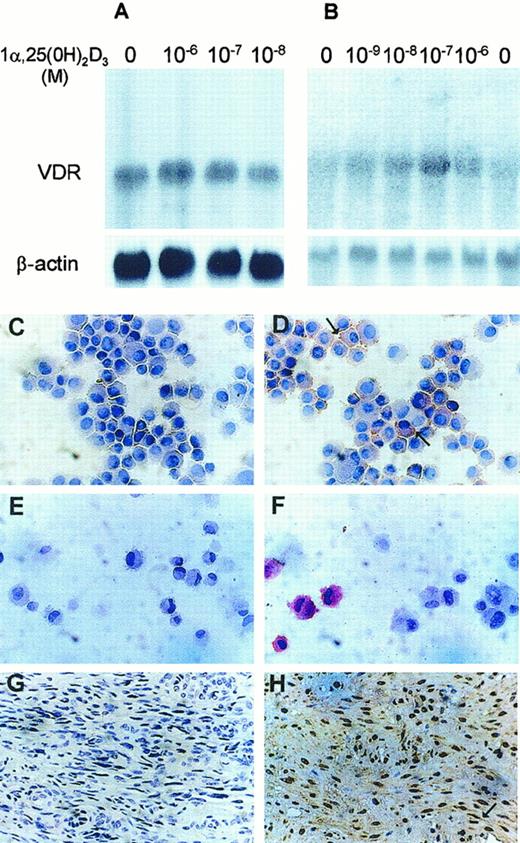
We examined the expression of VDR in KS cell lines by Northern blot and immunocytochemistry. High levels of a single species of VDR mRNA (4.6 kilobase [kb]) were expressed in KS Y-1 (Figure1A). All KS cell lines examined showed similar expression (data not shown). Very low-level expression was observed in endothelial cells (Figure 1B), whereas human skin fibroblasts had no expression (data not shown). Interestingly, VDR mRNA levels did not change significantly after treatment of the KS Y-1 cells with 1α,25 dihydroxyvitamin D3 (Figure 1A). Constitutive and nonregulatable expression of VDR in KS Y-1 cells may reflect cellular changes associated with transformation. VDR localization studies by immunocytochemical analysis showed specific cytoplasmic and nuclear staining of both KS Y-1 cells (Figure 1D) and HUVECs (Figure1F) in comparison to the lack of specific staining in both these cell types obtained with isotype specific antibodies (Figure 1C,E; KS Y-1 and HUVECs, respectively). Consistent with the Northern blot data, the amount of VDR-antibody-specific staining in endothelial cells was far lower than that in KS cells. No staining was observed in human fibroblasts (data not shown). Tumor tissues obtained from the KS lesion biopsy were also studied for VDR protein localization. Protein expression was noted in the spindle cells and the cells lining the small vascular structures in the tumors (Figure 1H). No staining of the KS tumor was observed with control rat immunoglobulin (Figure 1G). This finding is in contrast to the lack of expression in vessels of normal tissues.
Expression of vitamin D3 receptor in KS Y-1, HUVEC, and KS tumor tissue.
VDR mRNA in KS Y-1 (A) and in HUVECs (B). Cells were treated with various concentrations of vitamin D3 for 24 hours, and total RNA was extracted. Total RNA (15 μg) was electrophoresed, blotted, and hybridized to32P-labeled full-length human VDR cDNA (upper panels) and β-actin (lower panels), and exposed to x-ray film until signal was detectable (16 hours for VDR and β-actin in panel A, 3 days for VDR in panel B, and 4 hours for β-actin in panel B). Both cell types express the 4.6 kb mRNA for VDR. Immunocytochemical analysis for VDR expression. KS cell line (KS Y-1) and HUVECs were stained for VDR by using monoclonal antibody. KS cells (D) and HUVECs (F) show positive signal, whereas cells stained with isotype-specific antibodies were negative (C and E, KS and HUVEC, respectively). Antibody was used at 1:50 dilution for the KS Y-1 cells and 1:25 for the HUVECs. KS tumor tissue showed strong VDR-specific signal in spindle cells (H), whereas no staining was observed in KS tumor tissue stained with isotype-specific control (G).
Expression of vitamin D3 receptor in KS Y-1, HUVEC, and KS tumor tissue.
VDR mRNA in KS Y-1 (A) and in HUVECs (B). Cells were treated with various concentrations of vitamin D3 for 24 hours, and total RNA was extracted. Total RNA (15 μg) was electrophoresed, blotted, and hybridized to32P-labeled full-length human VDR cDNA (upper panels) and β-actin (lower panels), and exposed to x-ray film until signal was detectable (16 hours for VDR and β-actin in panel A, 3 days for VDR in panel B, and 4 hours for β-actin in panel B). Both cell types express the 4.6 kb mRNA for VDR. Immunocytochemical analysis for VDR expression. KS cell line (KS Y-1) and HUVECs were stained for VDR by using monoclonal antibody. KS cells (D) and HUVECs (F) show positive signal, whereas cells stained with isotype-specific antibodies were negative (C and E, KS and HUVEC, respectively). Antibody was used at 1:50 dilution for the KS Y-1 cells and 1:25 for the HUVECs. KS tumor tissue showed strong VDR-specific signal in spindle cells (H), whereas no staining was observed in KS tumor tissue stained with isotype-specific control (G).
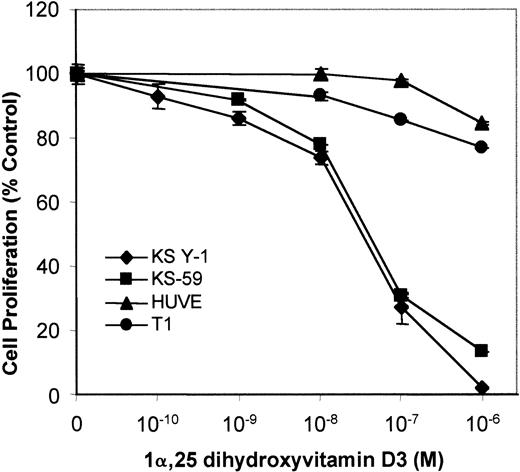
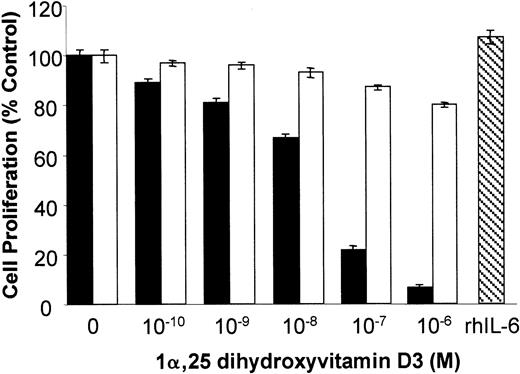
1α,25 Dihydroxyvitamin D3 inhibits KS cell growth in vitro
To test the growth regulatory effects of VDR agonists on KS cells, KS Y-1 and KS-59 were treated with 1α,25 dihydroxyvitamin D3, and cell proliferation was measured. 1α,25 Dihydroxyvitamin D3 inhibited proliferation of both KS cell lines in a dose-dependent manner. The concentration of 1α,25 dihydroxyvitamin D3 required for 50% inhibition of cell proliferation (IC50) was 5 × 10−8 mol/L (Figure 2). In contrast, 1α,25 dihydroxyvitamin D3 did not significantly inhibit the proliferation of endothelial cells and human T1 fibroblasts.
Effect of vitamin D3 on cell growth of KS Y-1, KS-59, HUVECs, and T1 human fibroblasts.
KS cell lines were seeded at a density of 1 × 104 cells per well in gelatinized 24-well plates. Cells were treated at the indicated concentrations of 1α,25 dihydroxyvitamin D3 on days 1 and 3, and the cell count was performed on day 6. Cell numbers are expressed relative to control untreated. Data from each experiment represents the mean and SD of the experiments done in quadruplicate.
Effect of vitamin D3 on cell growth of KS Y-1, KS-59, HUVECs, and T1 human fibroblasts.
KS cell lines were seeded at a density of 1 × 104 cells per well in gelatinized 24-well plates. Cells were treated at the indicated concentrations of 1α,25 dihydroxyvitamin D3 on days 1 and 3, and the cell count was performed on day 6. Cell numbers are expressed relative to control untreated. Data from each experiment represents the mean and SD of the experiments done in quadruplicate.
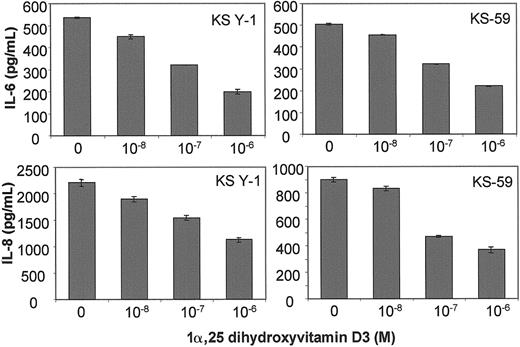
1α,25 Dihydroxyvitamin D3 inhibits IL-6 and IL-8 protein levels in KS cells
KS cells in culture were treated with various concentrations of 1α,25 dihydroxyvitamin D3 for 24 hours. ELISA assays of the supernatants showed that 1α,25 dihydroxyvitamin D3inhibited IL-6 and IL-8 protein levels in KS Y-1 (Figure3, upper left, lower left) and KS-59 (Figure 3, upper right, lower right) cells in a dose-dependent manner. The IC50 for IL-6 and IL-8 inhibition was between 0.1 and 1 μmol/L for both cell lines examined. It should be mentioned that RA, which also inhibits KS cell growth by down-regulating IL-6, showed a similar IC50 for inhibition of IL-6 protein in KS cell lines.18
IL-6 and IL-8 protein production in KS Y-1 and KS-59 in response to treatment with 1α,25 dihydroxyvitamin D3.
KS cell lines were seeded at a density of 5 × 104 and treated with the indicated concentrations of 1α,25 dihydroxyvitamin D3. Supernatants were collected after 24 hours and analyzed for IL-6 and IL-8 levels by ELISA. The levels were corrected for the cell number and presented as mean ± SD of 2 separate experiments done in triplicate.
IL-6 and IL-8 protein production in KS Y-1 and KS-59 in response to treatment with 1α,25 dihydroxyvitamin D3.
KS cell lines were seeded at a density of 5 × 104 and treated with the indicated concentrations of 1α,25 dihydroxyvitamin D3. Supernatants were collected after 24 hours and analyzed for IL-6 and IL-8 levels by ELISA. The levels were corrected for the cell number and presented as mean ± SD of 2 separate experiments done in triplicate.
IL-6 abrogates the inhibitory effect of 1α,25 dihydroxyvitamin D3 on KS cell growth
To determine if the 1α,25 dihydroxyvitamin D3-mediated inhibition of KS cell growth was secondary to the effect on IL-6 expression, we tested whether addition of exogenous recombinant human IL-6 (rhIL-6) was able to compensate for cell growth inhibition in the presence of 1α,25 dihydroxyvitamin D3. As illustrated in Figure 4, 1α,25 dihydroxyvitamin D3 produced a dose-dependent inhibition of KS Y-1 proliferation. Addition of exogenous rhIL-6 (10 ng/mL) effectively counteracted the growth inhibitory effect of 1α,25 dihydroxyvitamin D3, despite having no effect on cell proliferation on its own (Figure 4). This finding indicates that 1α,25 dihydroxyvitamin D3 inhibits KS cell growth through inhibition of IL-6 expression.
Exogenous IL-6 abrogates 1α,25 dihydroxyvitamin D3-mediated growth inhibition of KS cells.
Inhibition of growth of KS Y-1 cells was determined by treatment of the cells with the indicated concentrations of 1α,25 dihydroxyvitamin D3 for 72 hours in the presence (■) or absence (▪) of rhIL-6 (10 ng/mL). Cell counts were determined with a Coulter Counter. The right-hand side of the graph (▧) shows the effect of rhL-6 alone on cell proliferation. Each value is the mean ± SEM of the assays performed in triplicate.
Exogenous IL-6 abrogates 1α,25 dihydroxyvitamin D3-mediated growth inhibition of KS cells.
Inhibition of growth of KS Y-1 cells was determined by treatment of the cells with the indicated concentrations of 1α,25 dihydroxyvitamin D3 for 72 hours in the presence (■) or absence (▪) of rhIL-6 (10 ng/mL). Cell counts were determined with a Coulter Counter. The right-hand side of the graph (▧) shows the effect of rhL-6 alone on cell proliferation. Each value is the mean ± SEM of the assays performed in triplicate.
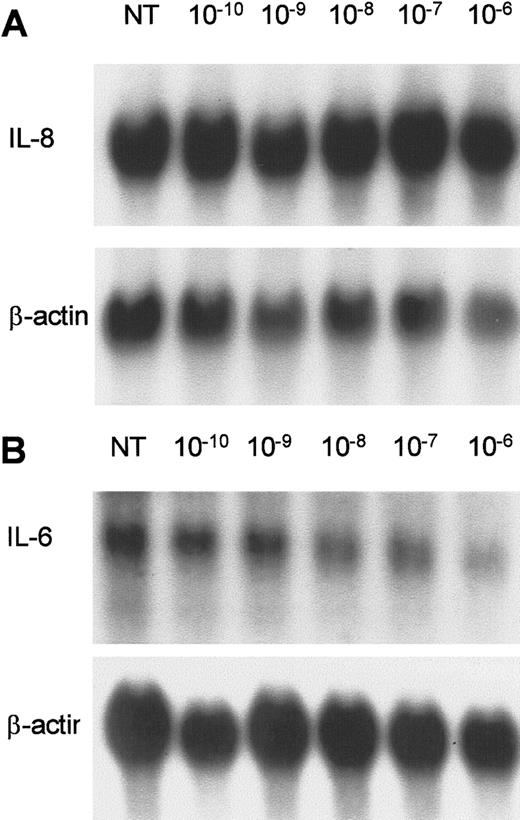
1α,25 Dihydroxy vitamin D3 inhibits IL-6 mRNA levels and IL-6 promoter activity
Northern analysis of KS Y-1 cells treated with 1 μmol/L 1α,25 dihydroxyvitamin D3 showed a decrease in IL-6 but not in IL-8 mRNA levels at 24 hours (Figure5A,B). VEGF and bFGF mRNA levels were unchanged in KS cells after 1α,25 dihydroxyvitamin D3treatment for 24 hours (data not shown). To determine if 1α,25 dihydroxyvitamin D3 regulates the IL-6 promoter, we performed transient transfection assays. Two different constructs, containing either 596 or 225 base pairs (bp) of IL-6 5′-flanking DNA upstream of the transcription start site fused to the CAT reporter gene were studied in cotransfection experiments with the VDR expression vector in KS Y-1 cells. Because of low basal activity of the IL-6 promoter in KS Y-1 cells (Figure 6, lane 1), TPA was added to induce the reporter expression. The activity from both constructs declined in a dose-dependent manner, but the effect was more marked in the −225 construct (shown), with more than 70% inhibition of activity at a dose of 10−6 mol/L 1α,25 dihydroxyvitamin D3 (Figure 6). These data show that ligand-activated VDR regulates the IL-6 promoter, and the active site lies within the first 225 nucleotides of the promoter upstream of the transcription start site. We have shown previously that pIC110, which contains nucleotides −110/+13 had no activity in KS Y-1 cells.18 The response elements thus must reside between positions −225 and −110.
Effect of 1α,25-dihydroxyvitamin D3 on IL-6 and IL-8 expression in AIDS-KS cells.
KS Y-1 cultures were treated with 1α,25-dihydroxyvitamin D3 at concentrations ranging from 1 × 10−10to 1 × 10−6 mol/L for 24 hours. (A) IL-8 and (B) IL-6. The lower panel in each shows the β-actin signal obtained on the same filter after stripping and rehybridization.
Effect of 1α,25-dihydroxyvitamin D3 on IL-6 and IL-8 expression in AIDS-KS cells.
KS Y-1 cultures were treated with 1α,25-dihydroxyvitamin D3 at concentrations ranging from 1 × 10−10to 1 × 10−6 mol/L for 24 hours. (A) IL-8 and (B) IL-6. The lower panel in each shows the β-actin signal obtained on the same filter after stripping and rehybridization.
1α,25 Dihydroxyvitamin D3-mediated repression of IL-6 promoter activity in KS cells.
Shown is a representative phosphorimager analysis of pIC225 activity cotransfected with VDR expression vector. The IL-6 promoter construct, containing 225 bp of IL-6 gene 5′-flanking DNA sequence fused to the CAT reporter gene, was cotransfected with VDR expression vector. The CAT activity of this construct in the presence of increasing amounts of 1α,25 dihydroxyvitamin D3 is shown. The IL-6 promoter is minimally inactive in KS Y-1 cells (left-most lane) in the absence of TPA. Numbers above the image were obtained by phosphorimager quantitation of CAT activity in which acetylated products (upper spots) are expressed as a percentage of the total [14C].
1α,25 Dihydroxyvitamin D3-mediated repression of IL-6 promoter activity in KS cells.
Shown is a representative phosphorimager analysis of pIC225 activity cotransfected with VDR expression vector. The IL-6 promoter construct, containing 225 bp of IL-6 gene 5′-flanking DNA sequence fused to the CAT reporter gene, was cotransfected with VDR expression vector. The CAT activity of this construct in the presence of increasing amounts of 1α,25 dihydroxyvitamin D3 is shown. The IL-6 promoter is minimally inactive in KS Y-1 cells (left-most lane) in the absence of TPA. Numbers above the image were obtained by phosphorimager quantitation of CAT activity in which acetylated products (upper spots) are expressed as a percentage of the total [14C].
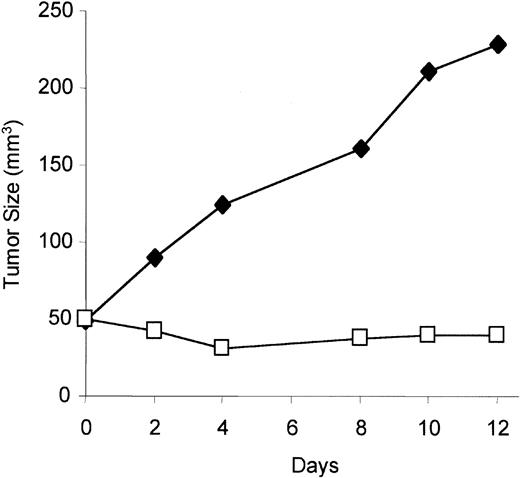
1α,25 Dihydroxyvitamin D3 inhibits KS tumor growth in vivo
To test if VDR agonist has activity in vivo against KS tumor growth, the KS cell line (KS Y-1) was implanted subcutaneously in immunodeficient mice, which were treated with 1α,25 dihydroxyvitamin D3, or vehicle alone, daily for 12 days. Mice treated with 1α,25 dihydroxyvitamin D3 showed significant retardation of tumor growth compared with mice treated with the vehicle alone (Figure 7).
Effect on tumor growth of vitamin D3 in immunodeficient mice.
Equal numbers of KS Y-1 cells (5 × 106) were inoculated in mice, and 1α,25 dihydroxyvitamin D3 was administered intraperitoneally daily for the duration of the experiment. Tumor measurements were done every other day until day 12. The results represent the mean tumor volume of 6 mice in each group. Results from treated mice, ■; results from mice treated with diluent alone, ♦.
Effect on tumor growth of vitamin D3 in immunodeficient mice.
Equal numbers of KS Y-1 cells (5 × 106) were inoculated in mice, and 1α,25 dihydroxyvitamin D3 was administered intraperitoneally daily for the duration of the experiment. Tumor measurements were done every other day until day 12. The results represent the mean tumor volume of 6 mice in each group. Results from treated mice, ■; results from mice treated with diluent alone, ♦.
1α,25 Dihydroxyvitamin D3 inhibits KS tumor growth in humans
To test if VDR agonist has any antiproliferative activity in patients with KS, we treated 8 male patients with a synthetic VDR agonist, calcipotriene (Dovonex) 0.005% ointment that was applied topically twice a day to selected lesions. The treatment was administered until tumor response or until tumor progression at the site of application required systemic treatment. Characteristics of the patients are shown in Table 1. The median duration of treatment was 4 weeks (range, 2 to 104). Four patients showed 50% or greater reduction in their treated lesions, after 3, 4, 10, and 13 weeks. All 4 responders had relatively low tumor burden (<10 lesions), and none had prior exposure to a systemic chemotherapeutic agent. One patient has been treated over a period of 2 years with no side effects reported and with gradual disappearance of the treated lesion (Figure 8). The response occurred within 24 to 63 days of initiation of treatment. The remaining 4 patients showed no response to this treatment. Thus, topical treatment with a VDR agonist shows antitumor activity in patients with KS.
Effect of calcipotriene on a KS lesion of an HIV-negative patient.
Calcipotriene ointment (0.005%) was applied twice daily to the lesion. (A) The tumor before initiation of treatment. (B) After 2 months. (C) After 18 months. (D) After 39 months. Tumor response is apparent at 2 months, with marked reduction after 18 months and complete resolution by 39 months.
Effect of calcipotriene on a KS lesion of an HIV-negative patient.
Calcipotriene ointment (0.005%) was applied twice daily to the lesion. (A) The tumor before initiation of treatment. (B) After 2 months. (C) After 18 months. (D) After 39 months. Tumor response is apparent at 2 months, with marked reduction after 18 months and complete resolution by 39 months.
Discussion
KS is a disease with strong angiogenic and inflammatory aspects. Inflammatory cytokines that are of special interest in KS are IL-6 and IL-8, both of which are autocrine growth factors for KS cells6 (R. Masood, unpublished data). Because IL-8 is an angiogenic factor and chemokine for inflammatory cells, it is likely that large amounts of IL-8 production by KS cells is at least in part responsible for exuberant angiogenesis and abundance of mononuclear cells in the tumor lesions.
The differentiation and antiproliferative activities of 1α,25 dihydroxyvitamin D3 and calcipotriol, apart from their widely acknowledged role in calcium homeostasis, are well established.21-23 The antiproliferative effect of VDR agonists is evident from the inhibition of keratinocytes,24 endothelial cells,36 and monocyte/macrophage lineage cells37-39 in culture. The prodifferentiation effect of VDR agonists is exemplified by their ability to inhibit proliferation and to induce irreversible phenotypic change in various myeloid cell lines of monocyte/macrophage lineage.38-40 Apart from their antiproliferative and cell differentiation activities, VDR agonists are being increasingly recognized as immunomodulators because of their ability to inhibit proliferation of activated T lymphocytes and production of IL-2, IL-1α, IL-6, TNF-α, IL-8, and interferon-γ in culture systems of mononuclear cells or T lymphocytes.37-44 Adding to the list of immunoregulatory functions, it has also recently been shown to inhibit IL-12 production in activated macrophages and dendritic cells.45 However, 1α,25 dihydroxyvitamin D3increased the production of IL-1β, TNF-α, and IL-6 in myeloid cell lines U937, HL-60, and THP-1.44 Therefore, the down-regulation or induction of various cytokines by VDR agonists appears to be cell-context dependent. Yet another activity of vitamin D3 analogs is inhibition of angiogenesis. In a number of in vivo systems, the number and branching of blood vessels was reduced in the presence of vitamin D3 analogs.46-50
This study showed that KS responds to a vitamin D analog (1α,25 dihydroxyvitamin D3), that KS cell growth rate in culture is inhibited in a dose-dependent manner by 1α,25 dihydroxyvitamin D3, that KS cell tumor xenograft growth in nude mice are inhibited by oral dosing with 1α,25 dihydroxyvitamin D3, and that KS lesions in patients respond to treatment with a topical preparation of 1α,25 dihydroxyvitamin D3. In addition to demonstrating the inhibitory effects of 1α,25 dihydroxyvitamin D3 on KS cell and tumor growth, we identified a potential mechanism of action.
We show that KS cells, and to a much lesser extent endothelial cells, express the VDR and so are capable of responding directly to active vitamin D metabolites. We observed a marked up-regulation of VDR in KS tumor biopsy tissue compared with normal skin from the same patient. In the tumor, both the tumor (spindle) cells and endothelial cells lining the vascular spaces showed strong nuclear staining for VDR. A significant difference between vascular endothelial cells in the tumor compared with normal vessels is that these cells are proliferating rapidly to sustain neo-angiogenesis stimulated by the tumor. Endothelial cells lining vessels in normal skin are essentially quiescent. Added to this situation in KS is the nature of the tumor cells themselves. KS tumor cells share so many of the phenotypic markers and morphological characteristics of activated endothelial cells that they might be considered to be neoplastic (and thus rapidly dividing) endothelial cells. In this light, it is interesting to note that a 4.5-fold up-regulation of VDR number has previously been noted in rapidly growing compared with growth-arrested endothelial cells.36 The mechanism whereby the tumor cells up-regulate VDR expression in the tumor vasculature is unknown. However, it is likely related to the changes in endothelial cell growth rate integral to neo-angiogenesis. It is possible that VDR is up-regulated by angiogenic factors, such as VEGF, bFGF, and IL-6 produced by the KS tumor cells.
KS cells produce the cytokines IL-6 and IL-8,7,8 which are autocrine growth factors6 (R. Masood, unpublished data). Vitamin D analogs have been shown to down-regulate the production and/or expression of these 2 cytokines in a number of cell types. IL-6 is down-regulated in fibroblasts,33,51 in PBMCs from psoriatic patients,31 and in monocytic and thymocytic cells.39,40 IL-8 is down-regulated in fibroblasts,33,51,52 keratinocytes,24-26osteoclasts,30 and A3 human melanoma cells.32In KS cells, we found that the VDR agonist inhibited IL-6 secretion that could be accounted for by a reduction in activity of the IL-6 promoter, whereas it reduced IL-8 protein secretion, but not mRNA levels, implying posttranscriptional effects. In addition, we showed that the 1α,25 dihydroxyvitamin D3 induced growth inhibition in KS could largely be attributed to the loss of IL-6 expression because addition of exogenous rhIL-6 in the presence of even high concentrations of 1α,25 dihydroxyvitamin D3(10−7 mol/L) restored KS cell growth. In this respect, the action of 1α,25 dihydroxyvitamin D3 on KS cells is similar to results we previously obtained for retinoids.18Retinoids inhibit the proliferation of KS cells in culture by down-regulating IL-6 production; however, RA inhibited only the production of IL-6 but not IL-8. The level of IL-8 protein was, in fact, induced by RA in KS cells.
Transient expression analysis revealed that the response element that transduces the down-regulation of the IL-6 promoter by 1α,25 dihydroxyvitamin D3 is localized to the region −225/−110 with respect to the transcription start site. This region is devoid of the VDR element to which a heterodimer of the VDR and RXR bind to modulate expression of target genes.20 We localized RA inhibition of the IL-6 promoter to this same region. RA inhibited IL-6 mRNA expression indirectly by antagonizing the enhancer effect of NF-IL-6 on the IL-6 promoter.18 The precise mechanism of IL-6 regulation is currently under investigation. Vitamin D analogs and retinoids have cooperative effects that either enhance or repress gene expression in a number of systems.47 53-58 One avenue of enquiry would be to investigate the individual and combined effects of these factors on NF-IL-6 gene expression.
Because retinoids inhibit KS growth in vitro and in vivo, the next obvious question was whether a VDR agonist would work in vivo in KS patients. Calcipotriene (Dovonex), a VDR agonist, is available commercially as a topical preparation. Calcipotriene was thus tested in patients with KS once a day. Eight patients were treated, with significant tumor regression noted in 4 patients within a period of 4 to 9 weeks. A larger prospective trial of calcipotriene is under development.
As observed for KS cells, recombinant IL-6 acts as a growth factor for primary myeloma cells.59 Thus, VDR agonists also hold promise in the treatment of multiple myeloma as well as other proliferative and inflammatory diseases in which IL-6 and IL-8 production contributes to the pathogenesis of the disease. In psoriatic skin lesions, an increase in IL-6 and IL-8 has been reported,28,31 and these cytokines could theoretically result in proliferation of keratinocytes. Therefore, inhibition of IL-6 and IL-8 production might be the mechanism of action of VDR agonist in psoriasis. Similarly, rheumatoid arthritis, an inflammatory condition with increased IL-6 and IL-8 levels in synovial fluid,60 61 might be therapeutically responsive to VDR agonists.
Supported in part by the Bridges & Larson Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Parkash S. Gill, Norris Cancer Hospital and Research Institute, Rm 3438, 1441 Eastlake Ave, Los Angeles, CA 90089; e-mail: parkashg@hsc.usc.edu.

![Fig. 6. 1α,25 Dihydroxyvitamin D3-mediated repression of IL-6 promoter activity in KS cells. / Shown is a representative phosphorimager analysis of pIC225 activity cotransfected with VDR expression vector. The IL-6 promoter construct, containing 225 bp of IL-6 gene 5′-flanking DNA sequence fused to the CAT reporter gene, was cotransfected with VDR expression vector. The CAT activity of this construct in the presence of increasing amounts of 1α,25 dihydroxyvitamin D3 is shown. The IL-6 promoter is minimally inactive in KS Y-1 cells (left-most lane) in the absence of TPA. Numbers above the image were obtained by phosphorimager quantitation of CAT activity in which acetylated products (upper spots) are expressed as a percentage of the total [14C].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/9/10.1182_blood.v96.9.3188/5/m_h82100324006.jpeg?Expires=1767737420&Signature=oqlsRkXlfj2J66YRK6Bs5l9PVxyY0RSeJ5zOVw7koM8chyi0QVcY36zUlMyZo~mwDaIMWA8vau4mlih1ZUIKF0M5Vz9-uj6vRvoVur8EMJ3VydrKru361FiDDouJuMUeWrUCTS7-YGZxM2pyBPIoCdJiSuy~iM7Oy2-IN5nkytev6ZWmrR~HZJ~7csYvMbX3GBeP3SRV54LigHuAOlBT4tzQgxe-mdkxBSgFjfriPvyUe3gNhUUtw0qfwjBWC9NJrNnrEk4v--h5QzH6kE-vOGla~V2PXRvkxmkbHJhONNSSBzIcTJfTSFJohaubGnuRoFYH0k~smDm9vBopPV5FmQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)