Abstract
Deficiency of folate or vitamin B12 (cobalamin) causes megaloblastic anemia, a disease characterized by pancytopenia due to the excessive apoptosis of hematopoietic progenitor cells. Clinical and experimental studies of megaloblastic anemia have demonstrated an impairment of DNA synthesis and repair in hematopoietic cells that is manifested by an increased percentage of cells in the DNA synthesis phase (S phase) of the cell cycle, compared with normal hematopoietic cells. Both folate and cobalamin are required for normal de novo synthesis of thymidylate and purines. However, previous studies of impaired DNA synthesis and repair in megaloblastic anemia have concerned mainly the decreased intracellular levels of thymidylate and its effects on nucleotide pools and misincorporation of uracil into DNA. An in vitro model of folate-deficient erythropoiesis was used to study the relationship between the S-phase accumulation and apoptosis in megaloblastic anemia. The results indicate that folate-deficient erythroblasts accumulate in and undergo apoptosis in the S phase when compared with control erythroblasts. Both the S-phase accumulation and the apoptosis were induced by folate deficiency in erythroblasts fromp53 null mice. The complete reversal of the S-phase accumulation and apoptosis in folate-deficient erythroblasts required the exogenous provision of specific purines or purine nucleosides as well as thymidine. These results indicate that decreased de novo synthesis of purines plays as important a role as decreased de novo synthesis of thymidylate in the pathogenesis of megaloblastic anemia.
Introduction
Deficiency of folate or cobalamin causes megaloblastic anemia, a disease in which pancytopenia results from differentiating hematopoietic cells dying before reaching maturity. This effect is most prominent in the erythroid lineage and is termed ineffective erythropoiesis. The differentiation of erythroid cells is ineffective in that the anemia resulting from the failure to produce new erythrocytes stimulates erythropoietin (EPO) production, which, in turn, increases the proportion of erythroid cells at the colony-forming unit erythroid (CFU-E) and proerythroblast stages of development.1 Most of the progeny of these increased populations of CFU-E and proerythroblasts never reach the reticulocyte stage because they undergo apoptosis during the later stages of erythroid differentiation.2 Apoptosis in megaloblastic anemia appears to be linked to an inability of hematopoietic cells to maintain the integrity of their DNA because of decreased replication and repair capacities. The inability to maintain DNA integrity in megaloblastic anemia has been demonstrated in cytogenetic studies in which the hematopoietic cells of these patients were found to have increased chromosomal breakage.3 4
Folate, in the form of tetrahydrofolate (THF) coenzymes, plays a role in the synthesis of thymidylate and purines and is indirectly involved in the methylation of cytosines in DNA (reviewed by Shane and Stokstad5). Whereas folate deficiency directly limits the intracellular levels of THF coenzymes, cobalamin deficiency limits the intracellular supply of THF coenzymes by “trapping” folate in the 5-methyltetrahydrofolate (methyl-THF) form.6 Thus, folate or cobalamin deficiency can lead to DNA alterations by limiting intracellular thymidylate, purine deoxynucleotides, or methyl groups for cytosine methylation. Of these reactions, the one that is most closely associated with megaloblastic anemia is the methylation of deoxyuridylate (dUMP), yielding thymidylate (dTMP), in which the coenzyme 5,10-methylenetetrahydrofolate (methylene-THF) supplies a methylene group, and reducing equivalents. A biochemical assay for cobalamin or folate deficiency, the deoxyuridine suppression test, is based on the decreased conversion of dUMP to dTMP in cells of megaloblastic anemia patients.7,8 Also, assays for increased uracil misincorporation into DNA in patients with cobalamin or folate deficiency are based on the increased ratio of dUMP/dTMP in hematopoietic cells.9-11 However, the lack of DNA integrity and accompanying apoptosis in megaloblastic anemia may be due to decreased purine synthesis as well as decreased thymidylate synthesis.
Because impaired DNA synthesis and repair appear to precede the death of the hematopoietic cells in megaloblastic anemia, an altered cell cycle with a prolongation of the S phase would be expected. Although mitogen-stimulated blood lymphocytes of patients with megaloblastic anemia were found to have a decreased rate of DNA synthesis,12 another study did not find a decreased rate in bone marrow cells.13 On the other hand, 3 studies that measured simultaneously autoradiography with 3H-thymidine and total DNA content by quantitative Feulgen staining demonstrated that patients with megaloblastic anemia had a subpopulation of erythroblasts with DNA content between 2N and 4N, but these erythroblasts did not label with3H-thymidine.4,14,15 Because healthy controls did not have a similar subpopulation of erythroblasts, these results were interpreted as being consistent with either an arrested or an extremely slowed rate of DNA synthesis in megaloblastic anemia. Flow cytometry of bone marrow cells of patients with megaloblastic anemia showed an increase in the percentage of cells in the S phase.16
Bone marrow cells of megaloblastic anemia patients and healthy controls have experimental limitations due to the presence of multiple cellular lineages, a wide range of differentiation stages within each lineage, and rapid removal in vivo of apoptotic cells by phagocytosis. These limitations have been overcome in an in vitro system of erythropoiesis in which folate-deficient erythroid progenitor cells are cultured in either folate-deficient or folate-replete (control) medium. The majority of cells cultured in the control medium proliferate and differentiate into reticulocytes by the end of 2 days, whereas the majority of those cultured in folate-deficient medium undergo apoptosis during the second day.2 Before undergoing apoptosis, these cells cultured in folate-deficient medium have extremely low intracellular concentrations of all folate coenzymes, increased incorporation of uracil into DNA, and evidence of DNA damage as shown by increased levels of p53 protein.1 The small minority of cells that survive culture in folate-deficient medium give rise to macrocytic reticulocytes.1 This in vitro system of folate-deficient erythropoiesis is used here to demonstrate: (1) folate deficiency in differentiating erythroblasts causes them to accumulate in the S phase of the cell cycle; (2) the S-phase accumulation appears to be preapoptotic in that greater proportions of erythroblasts that undergo apoptosis during folate deficiency are in the S phase, compared with control erythroblasts; (3) the S-phase accumulations and increased rates of apoptosis during folate deficiency are p53 independent; and (4) reversal of the S-phase accumulation and apoptosis of folate-deficient erythroblasts by supplying nucleosides requires specific purines or purine nucleosides as well as thymidine.
Materials and methods
In vitro system of folate-deficient erythropoiesis
Weanling CD2F1 mice purchased from Harlan-Sprague Dawley (Indianapolis, IN) were used as described previously.1,2 Experiments testing the role of the tumor suppressor gene p53 used mice that were made p53null by germline disruption of the p53 locus by homologous recombination and sensitive to the Friend leukemia virus by backcross mating.17 The genotypes of the p53 null mice were confirmed by Southern blotting of DNA extracted from tail biopsies.
At 4 weeks of age, the mice were placed in 2 groups to receive either an amino acid–based, folate-free diet18 or, for controls, the same diet with 2 mg folic acid per kg of diet (Dyets, Inc, Bethlehem, PA). After 2 weeks of their respective diets, the mice in each group were infected with 103 spleen focus-forming units of the anemia-inducing strain of Friend virus by tail vein injection. After 4 weeks of each respective diet and 2 weeks of infection, the mice were killed and their spleens removed. The spleens were enlarged approximately 10-fold with erythroid progenitor cells because of the acute erythroblastosis effect of the Friend virus. CFU-E and proerythroblasts were further purified by sedimentation at unit gravity as described.2 These purified populations of erythroid progenitor cells have been demonstrated previously to be either folate-deficient cells (FDCs) or normal folate-replete cells (NCs), depending on the diet of the mice from which they were obtained.1
In each experiment, one half of the erythroid cells were cultured at 1 × 106 cells/mL at 37°C in humidified air plus 5% CO2 in folate-free Iscove's modified Dulbecco's medium (GIBCO-BRL, Grand Island, NY) containing 30% heat-inactivated fetal bovine serum (FBS, Hyclone, Logan, UT), 1% deionized bovine albumin, 100 μmol/L α-thioglycerol, and 2 U/mL recombinant human EPO (Ortho Pharmaceuticals, Raritan, NJ). This folate-deficient medium (FDM) contained only 20 nmol/L folate as contributed by the FBS. The remaining one half of the erythroid cells were cultured under the same conditions in the control medium (CM), which was produced by adding folic acid just before culture to yield 6.4 mmol/L as is normally present in Iscove's medium. The cultured erythroblasts were classified into 4 groups, depending on the diet of the mice from which they were purified and the folate concentration of the culture medium. These 4 groups were folate-deficient cells in control medium (FDC-CM), folate-deficient cells in folate-deficient medium (FDC-FDM), normal control cells in control medium (NC-CM), and normal control cells in folate-deficient medium (NC-FDM).
In experiments that tested nucleoside rescue of folate-deficient erythroid cells, the heat-inactivated FBS was dialyzed for 24 hours at 4°C against Dulbecco's phosphate-buffered saline (PBS). The dialysis membrane had a 6000-8000 mol wt cutoff and the 8-fold excess volume of dialysate was changed 3 times. At the initiation of cultures using the medium made with dialyzed FBS, the control cultures received folic acid to yield 6.4 nmol/L as described previously and the experimental cultures received various concentrations of ribonucleosides and deoxyribonucleosides as described.
Cell counting, fixation, and staining
The cultured erythroid cells were collected at the initiation of culture (0 hour) and at 8, 20, 32, and 44 hours of culture. To determine viable cell numbers, aliquots of harvested cells were stained with trypan blue and cells excluding the dye were counted with a hemocytometer. To determine the presence of apoptosis and the cell cycle phase by flow cytometry, we used the dual staining method of DNA end-labeling with fluorescein isothiocyanate (FITC)-dUTP and total DNA labeling with propidium iodide (PI) described by Li and Darzynkiewicz.19 This method accurately detects apoptosis induced in the S phase in hematopoietic cells19 and has been previously used to identify the cell cycle phases in apoptotic, Friend virus–infected erythroblasts.17 20 Briefly, the harvested cells were rinsed once in ice cold PBS, fixed for 20 minutes by suspension in ice cold paraformaldehyde (1% wt/vol in PBS), rinsed again in ice cold PBS, resuspended in PBS that was then made to 70% ethanol, and were stored at −20°C. For flow cytometric analyses, the fixed cells were rehydrated in PBS and stained for double-stranded DNA ends using the terminal deoxynucleotidyl transferase (Tdt)-dUTP-nick end-labeling (TUNEL) technique with the FITC directly linked to the dUTP (Boehringer-Mannheim, Indianapolis, IN). The FITC-labeled cells were rinsed in PBS and stained for total DNA content in a solution containing 200 μg/mL RNAse A (Sigma, St Louis, MO) and 40 μg/mL of PI (Sigma).
Flow cytometry
Double-stained samples from each time of culture were analyzed by flow cytometry for: (1) extent of apoptosis in the total population, (2) cell cycle distribution for the total population, and (3) cell cycle distribution of the TUNEL-positive (apoptotic) population and the TUNEL-negative (nonapoptotic) population. FACS analysis was performed on a FACScan (Becton Dickinson, San Jose, CA) flow cytometer emitting 488 nm laser light for fluorochrome excitation. Stored as listmode files were data sets including linear forward scattered light, log side-scattered light, linear FL-2 area (DNA–cell cycle: FL-2 area and FL-2 width [for doublet discrimination]) collected at 555 to 595 nm as a single parameter or as mixed log FL-1 (dUTP-FITC at 515-535 nm) and linear (DNA, as above) dual parameter data files.
All FACS data were analyzed off-line using WinList (Verity Software House, Inc, Topsham, ME) with remote linkage to ModFit (Verity Software House) for both cell cycle modeling and dual parameter analyses. Instrument linearity was confirmed with chick erythrocyte nuclei for each DNA data set. Doublet discrimination was performed by gating on singlets in a dual parameter FL-2 area versus FL-2 width histogram. Dual parameter analysis was used to determine the percentage of the TUNEL positive (apoptotic nuclei) and 3 separate cell cycle models were constructed to determine the cell cycle components (G0/G1, S, and G2/M) for all cells, apoptotic cells, and nonapoptotic cells at 0, 8, 20, 32, and 44 hours of culture. Data from replicates were combined for statistical evaluation. Statistical significance was determined byP < .05 using Student t test.
Results
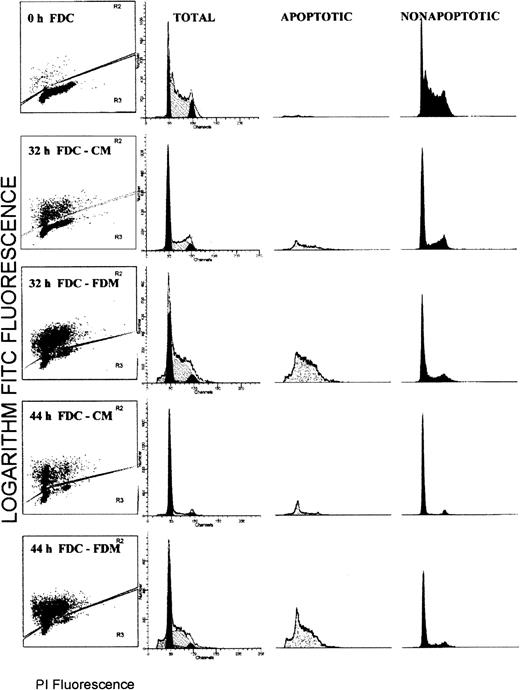
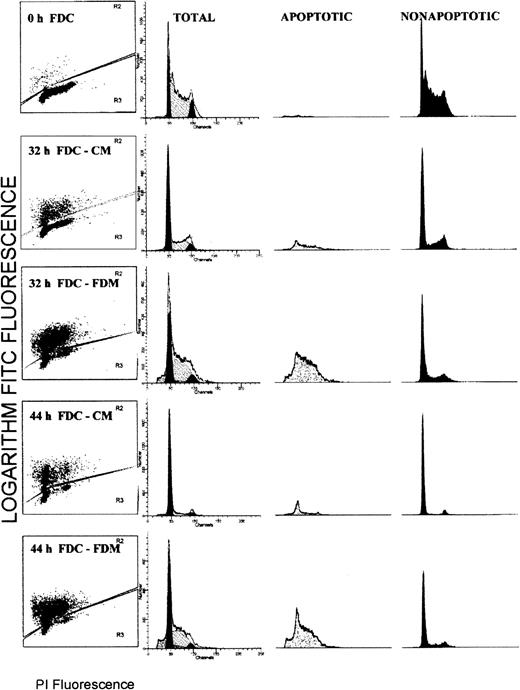
Flow cytometry analyses for simultaneous determinations of apoptosis status and the cell cycle phase were performed on the total cell populations of erythroblasts. Figure1 shows the results from a representative experiment. Each histogram is divided such that brightly FITC fluorescent (TUNEL positive) apoptotic cells are shown in the upper portion and faintly FITC fluorescent (TUNEL negative) nonapoptotic cells are shown in the lower portion. The distribution of PI fluorescence corresponding to the DNA cell cycle content for total cells is shown in the second column with modeled distributions of G0/G1, S, and G2/M phases. The PI fluorescence displays in columns 3 (apoptotic cells) and 4 (nonapoptotic cells) are proportional to those population sizes such that the apoptotic population is a minority at 0, 32 and 44 hours of culture in CM, whereas it is a majority at 32 and 44 hours of culture in FDM.
Flow cytometry distributions of total erythroblasts according to the apoptosis status and the phase of the cell cycle.
Folate-deficient erythroid cells (FDCs) were cultured for 32 or 44 hours in folate-deficient medium (FDM) or control folate-replete medium (CM). The fixed cells were labeled with FITC-dUTP via the TUNEL method and stained for total DNA with propidium iodide (PI). In column 1, each histogram is divided into brightly fluorescent, TUNEL positive, apoptotic cells and faintly fluorescent, TUNEL negative, nonapoptotic cells. In columns 2, 3, and 4, the distribution of PI fluorescence is shown for the total, apoptotic, and nonapoptotic cell populations, respectively. In column 2, the distribution of the modeled cell cycle phases are shown: G0/G1phase, solid peak of least PI fluorescence; S phase, hatched peak of intermediate PI fluorescence; and G2/M phase, the solid peak of greatest PI fluorescence.
Flow cytometry distributions of total erythroblasts according to the apoptosis status and the phase of the cell cycle.
Folate-deficient erythroid cells (FDCs) were cultured for 32 or 44 hours in folate-deficient medium (FDM) or control folate-replete medium (CM). The fixed cells were labeled with FITC-dUTP via the TUNEL method and stained for total DNA with propidium iodide (PI). In column 1, each histogram is divided into brightly fluorescent, TUNEL positive, apoptotic cells and faintly fluorescent, TUNEL negative, nonapoptotic cells. In columns 2, 3, and 4, the distribution of PI fluorescence is shown for the total, apoptotic, and nonapoptotic cell populations, respectively. In column 2, the distribution of the modeled cell cycle phases are shown: G0/G1phase, solid peak of least PI fluorescence; S phase, hatched peak of intermediate PI fluorescence; and G2/M phase, the solid peak of greatest PI fluorescence.
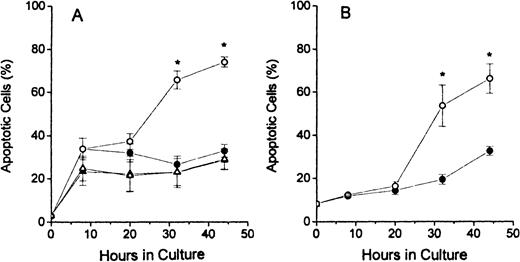
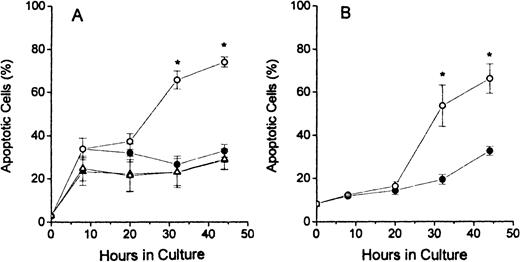
The results of TUNEL assay labeling of DNA ends are shown in Figure2. The assays of dual-stained cells were displayed as shown in column 1 of Figure 1 and the percentages of apoptotic cells determined by dividing the number of brightly FITC-stained cells (in upper portions of histograms) by the total number of cells analyzed. The FDC-FDM had marked increases in the percentage of apoptotic cells at 32 and 48 hours of culture (Figure2A). In contrast, FDC-CM, NC-FDM, and NC-CM all had no evidence of this large increase in apoptosis at these later times of culture (Figure2A). In erythroblasts from p53 null mice, in Figure 2B, this same increase in apoptotic cells at 32 and 44 hours in FDC-FDM, compared with FDC-CM, indicated that the apoptosis of erythroblasts with folate deficiency was not dependent on p53.
Percentages of apoptotic erythroblasts in culture.
Folate-deficient erythroblasts (FDCs) and normal control erythroblasts (NCs) were isolated from Friend virus–infected mice, cultured in folate-deficient medium (FDM) or control, folate-replete medium (CM), and processed for apoptosis and cell cycle phase analysis by flow cytometry as described in the legend to Figure 1. The percentage of TUNEL positive (apoptotic) erythroblasts at 0, 8, 20, 32, and 44 hours of culture are shown for FDC-CM (●), FDC-FDM (○), NC-CM (▴) and NC-FDM (▵). (A) CD2F1 mice and (B)p53 null mice. Results are ± 1 SEM for 5 separate experiments for CD2F1 FDCs, 3 separate experiments for CD2F1 NCs, and 4 separate experiments for p53 null FDCs. *Statistically significant differences in FDC-FDM.
Percentages of apoptotic erythroblasts in culture.
Folate-deficient erythroblasts (FDCs) and normal control erythroblasts (NCs) were isolated from Friend virus–infected mice, cultured in folate-deficient medium (FDM) or control, folate-replete medium (CM), and processed for apoptosis and cell cycle phase analysis by flow cytometry as described in the legend to Figure 1. The percentage of TUNEL positive (apoptotic) erythroblasts at 0, 8, 20, 32, and 44 hours of culture are shown for FDC-CM (●), FDC-FDM (○), NC-CM (▴) and NC-FDM (▵). (A) CD2F1 mice and (B)p53 null mice. Results are ± 1 SEM for 5 separate experiments for CD2F1 FDCs, 3 separate experiments for CD2F1 NCs, and 4 separate experiments for p53 null FDCs. *Statistically significant differences in FDC-FDM.
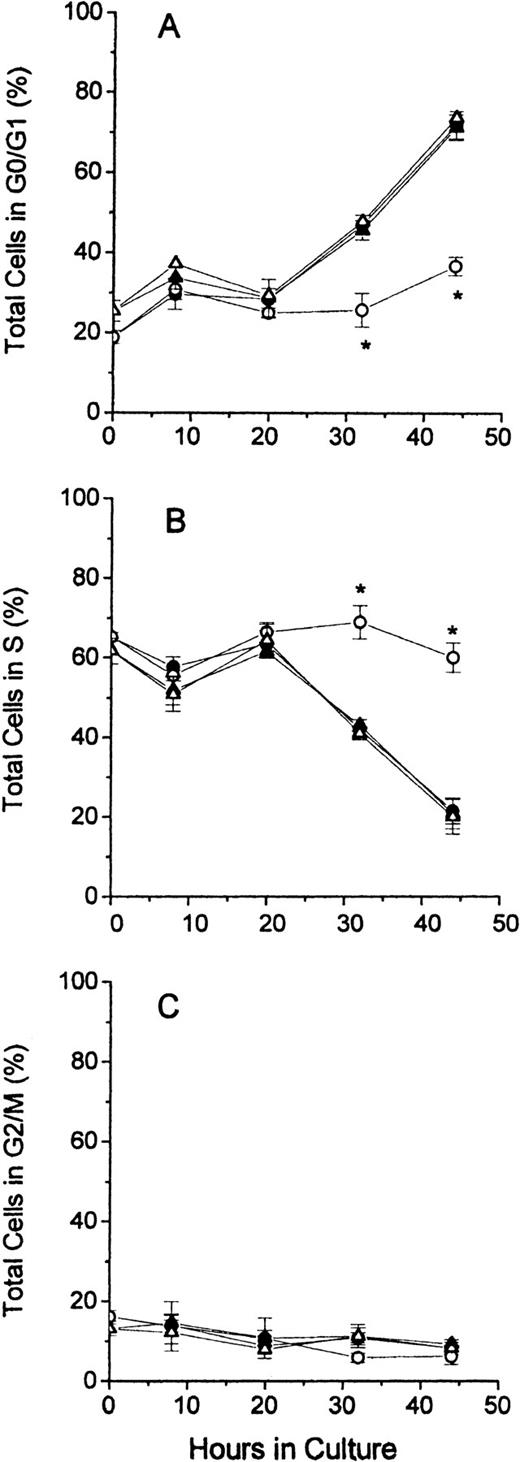
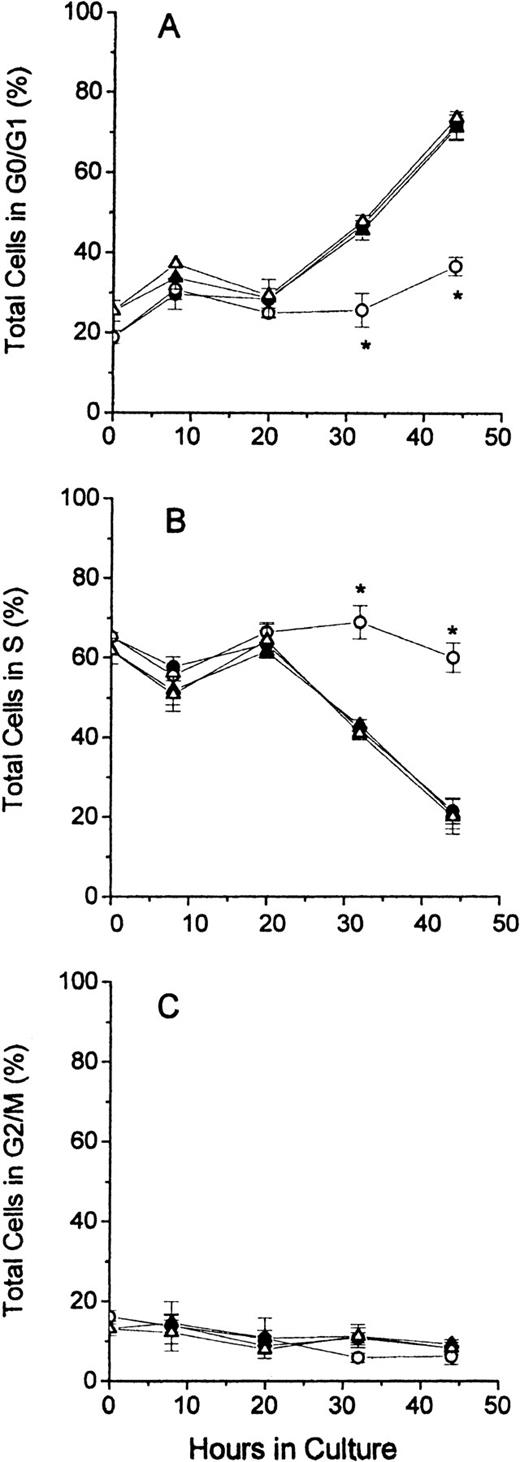
In Figure 3, the distribution of cell cycle phases for total cells is shown for all 4 groups of cultured erythroblasts. The results demonstrate that FDC-FDM differed markedly at 32 and 44 hours of culture from FDC-CM, NC-FDM, and NC-CM. The percentage of FDC-FDM that were in the G0/G1 phase remained low throughout the culture period, whereas that of FDC-CM, NC-FDM, and NC-CM had progressive increases in the percentages of cells in G0/G1 at 32 and 44 hours (Figure 3A). Conversely, the major percentage of FDC-FDM remained in the S phase throughout the culture period, whereas the percentages of FDC-CM, NC-FDM, NC-CM in the S phase declined progressively at 32 and 44 hours (Figure 3B). The percentage of FDC-FDM in the G2/M phase of the cell cycle was slightly decreased at 32 and 44 hours compared with the other 3 groups of cultured erythroblasts (Figure 3C), but at these time points, the erythroblasts in the G2/M phase represented 10% or less of the total cells.
Cell cycle phases of total erythroblasts in culture.
Folate-deficient and control erythroblasts from CD2F1 mice were cultured, collected, fixed, stained, and analyzed for flow cytometry as described in the legend to Figure 1. FDC-CM, ●; FDC-FDM, ○; NC-CM, ▴; NC-FDM, ▵. (A) Total erythroblasts in the G0/G1 phase. (B) Total erythroblasts in the S phase. (C) Total erythroblasts in the G2/M phase. Data are ± 1 SEM of 5 separate experiments for FDCs and 3 separate experiments for NCs. *Statistically significant differences in FDC-FDM.
Cell cycle phases of total erythroblasts in culture.
Folate-deficient and control erythroblasts from CD2F1 mice were cultured, collected, fixed, stained, and analyzed for flow cytometry as described in the legend to Figure 1. FDC-CM, ●; FDC-FDM, ○; NC-CM, ▴; NC-FDM, ▵. (A) Total erythroblasts in the G0/G1 phase. (B) Total erythroblasts in the S phase. (C) Total erythroblasts in the G2/M phase. Data are ± 1 SEM of 5 separate experiments for FDCs and 3 separate experiments for NCs. *Statistically significant differences in FDC-FDM.
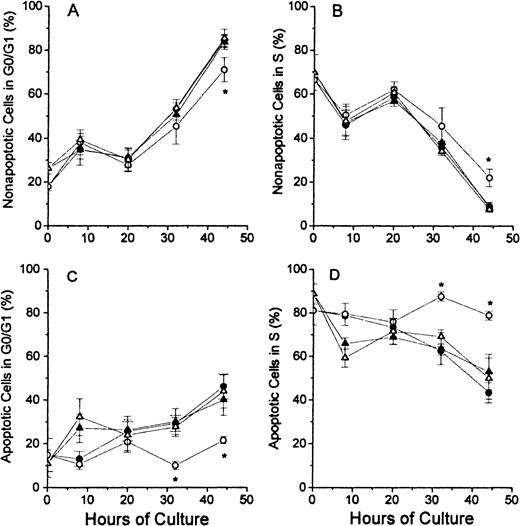
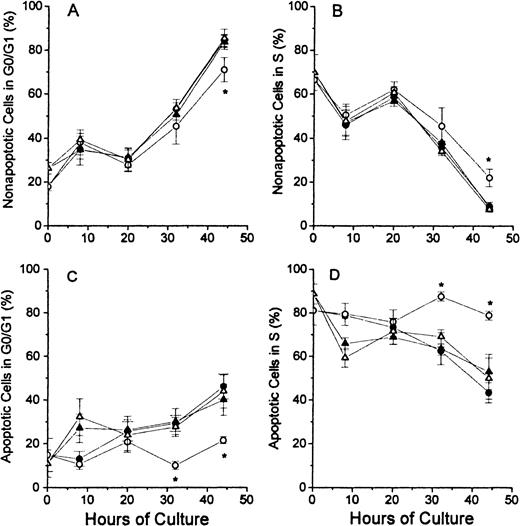
The analyses of the cell cycle phase with respect to the nonapoptotic (TUNEL negative) and apoptotic (TUNEL positive) populations at each time point of culture are shown for the G0/G1 phase and the S phase in Figure 4. In Figure 4A, the nonapoptotic cells from each of the 4 groups of cultured erythroblasts showed similar patterns, except that the FDC-FDM showed a slight decrease in the percentage of cells in G0/G1 phase at 32 and 44 hours. The difference at 44 hours of FDC-FDM was statistically significantly less than the FDC-CM, NC-FDM, and NC-CM. The analyses for cells in the S phase in Figure 4B show an opposite pattern with decreasing percentages of cells in the S phase at the later times of culture. FDC-FDM had greater percentages of cells in S phase at 32 and 44 hours (Figure 4B). At 44 hours, the FDC-FDM had more than twice the percentage of nonapoptotic cells in the S phase, compared with FDC-CM, NC-FDM, and NC-CM. In Figure 4C, the apoptotic cells in the 32- and 44-hour cultures of FDC-FDM showed a significantly less percentage of cells in the G0/G1 phase of the cell cycle. Compared with the apoptotic cells in cultures of FDC-CM, NC-FDM, and NC-CM, the FDC-FDM had less than half the percentage of cells in the G0/G1 phase (Figure 4C). In Figure 4D, the apoptotic cells from the 4 groups of cultured erythroblasts showed that the FDC-FDM had a significantly higher percentage of cells in the S phase (approximately 80%) throughout the culture period, whereas the FDC-CM, NC-CM, and NC-FDM all had progressive declines to an average of 50% or less in the S phase at 44 hours of culture.
Percentages of nonapoptotic and apoptotic erythroblasts in the G1/G0 and S phases of the cell cycle.
Folate-deficient (FDCs) or normal control (NCs) erythroblasts from CD2F1 mice were isolated, cultured, processed and analyzed by flow cytometry as described in Figure 1 legend. The percentages of nonapoptotic (TUNEL negative) cells in the G0/G1 phase are shown in panel A and those in the S phase are shown in panel B. The percentages of apoptotic erythroblasts (TUNEL positive) erythroblasts in the G0/G1 phase are shown in panel C and those in the S phase are shown in panel D. FDC-CM, ●; FDC-FDM, ○; NC-CM, ▴; NC-FDM, ▵. Data are ± 1 SEM from 5 separate experiments with FDCs and 3 separate experiments with NCs. *, statistically significant differences in FDC-FDM.
Percentages of nonapoptotic and apoptotic erythroblasts in the G1/G0 and S phases of the cell cycle.
Folate-deficient (FDCs) or normal control (NCs) erythroblasts from CD2F1 mice were isolated, cultured, processed and analyzed by flow cytometry as described in Figure 1 legend. The percentages of nonapoptotic (TUNEL negative) cells in the G0/G1 phase are shown in panel A and those in the S phase are shown in panel B. The percentages of apoptotic erythroblasts (TUNEL positive) erythroblasts in the G0/G1 phase are shown in panel C and those in the S phase are shown in panel D. FDC-CM, ●; FDC-FDM, ○; NC-CM, ▴; NC-FDM, ▵. Data are ± 1 SEM from 5 separate experiments with FDCs and 3 separate experiments with NCs. *, statistically significant differences in FDC-FDM.
To determine whether p53 was involved in the changes in the cell cycle caused by folate deficiency, we examined erythroblasts isolated from folate-deficient, Friend virus–infected, p53 null mice. The Friend virus–infected erythroblasts from these p53 null mice have been shown to have an absence of expression of known p53-inducible genes in the response to typical inducers of p53 such as γ-irradiation and actinomycin D.17 The same patterns of a lower percentage of cells in the G0/G1 phase and a persistently high percentage in the S phase at 32 and 44 hours were found in FDC-FDM, compared with FDC-CM (data not shown).
To determine whether thymidine and/or other nucleosides could reverse the alterations in the cell cycle phases and the apoptosis caused by folate deficiency, a series of experiments were performed using dialyzed FBS in the culture medium. When folic acid was added, the medium made with the dialyzed FBS permitted the complete growth and differentiation of erythroblasts as was found with undialyzed FBS. Equimolar additions to the medium of the 4 deoxyribonucleosides used for DNA synthesis (deoxyadenosine, deoxyguanosine, deoxcytidine, and thymidine) showed dose-responsive increases in viable nucleated cells and decreases in the percentages of apoptotic cells and cells in the S phase at 32 and 44 hours such that the addition of 60 μmol/L of each deoxyribonucleoside rescued folate-deficient cells as well as folic acid (data not shown). No single deoxyribonucleoside or any combination of 2 deoxyribonucleosides rescued the folate-deficient erythroblasts. Equimolar additions of adenosine, guanosine, cytidine, and uridine had no effect on the cell numbers, apoptosis, or S-phase percentages (not shown).
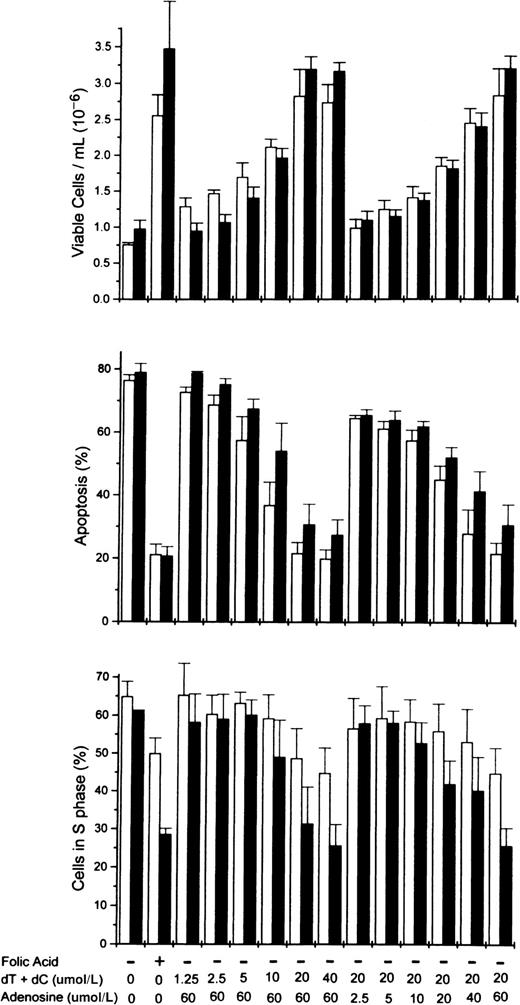
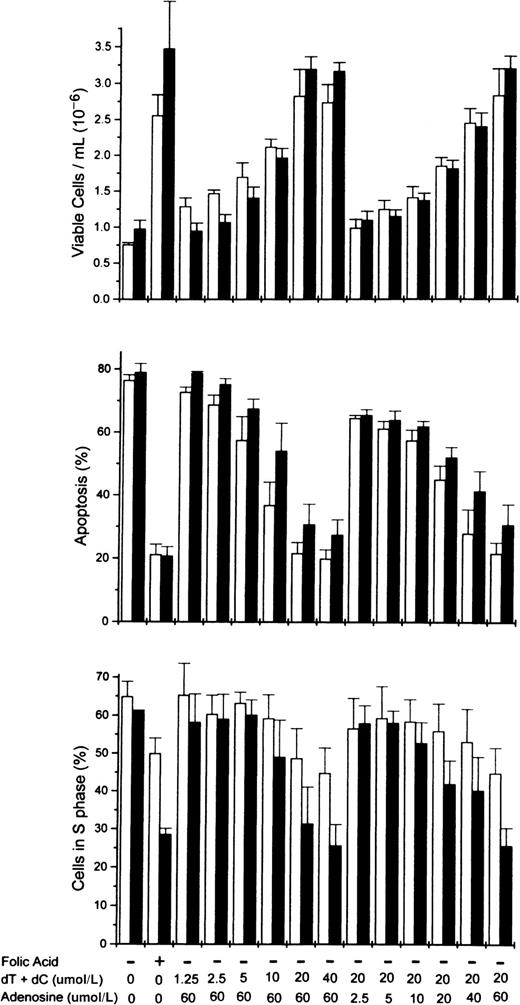
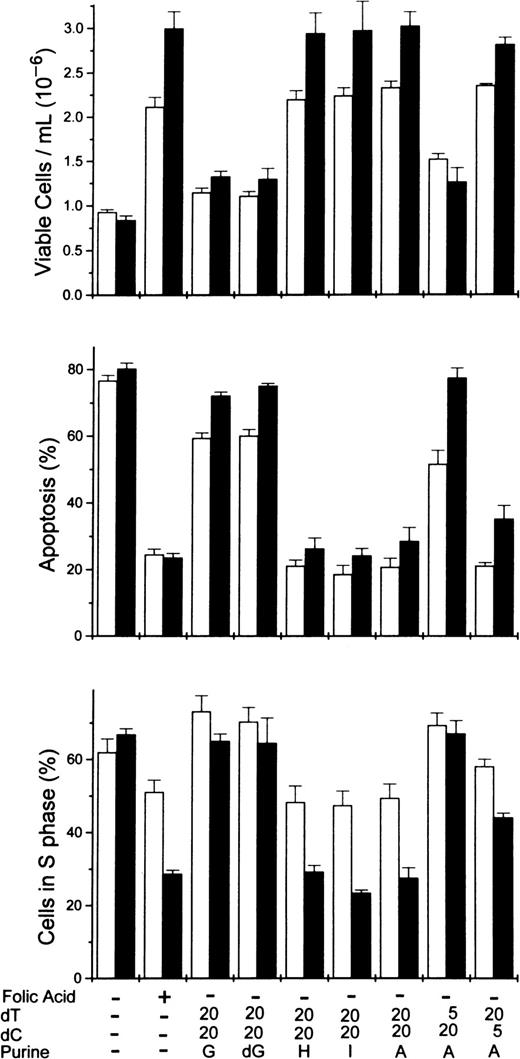
To determine more precisely the nucleoside requirements for the rescue of folate-deficient erythroblasts, 3 ribonucleosides/deoxyribonucleosides were added at varying concentrations to the folate deficient/dialyzed FBS cultures. The results showed that both deoxycytidine and thymidine were required as well as hypoxanthine or one of several purine nucleosides. In Figure5, the dose-responsive plateau for full erythroblast rescue by equimolar amounts of deoxycytidine and thymidine was achieved by 20 μmol/L, whereas the full rescue by adenosine was achieved by 60 μmol/L. Full rescue of the folate-deficient erythroblasts was also seen at 60 μmol/L hypoxanthine, inosine, or deoxadenosine when substituted for adenosine (Figure6). However, guanosine and deoxyguanosine failed to rescue the folate-deficient erythroblasts when substituted for adenosine (Figure 6). Further analysis of the thymidine and deoxycytidine requirements showed that thymidine could not be reduced below 20 μmol/L, whereas the deoxycytidine could be reduced to 5 μmol/L with the full rescue of the erythroblasts (Figure6).
Dose-responsive purine and pyrimidine requirements for the rescue from apoptosis of folate-deficient erythroblasts.
Folate-deficient erythroblasts were cultured in FDM made with dialyzed FBS. At 0 hour of culture, folic acid or combinations of nucleosides were added to the medium as indicated at the bottom of the figure. Thymidine (dT) and deoxycytidine (dC) were added in equimolar amounts as shown (eg, 20 indicates that 20 μmol/L dT and 20 μmol/L dC were added). Viable cell numbers, apoptosis, and phase of the cell cycle were determined at 32 (■) and 44 (▪) hours of culture. Data are ± SEM of 3 separate experiments.
Dose-responsive purine and pyrimidine requirements for the rescue from apoptosis of folate-deficient erythroblasts.
Folate-deficient erythroblasts were cultured in FDM made with dialyzed FBS. At 0 hour of culture, folic acid or combinations of nucleosides were added to the medium as indicated at the bottom of the figure. Thymidine (dT) and deoxycytidine (dC) were added in equimolar amounts as shown (eg, 20 indicates that 20 μmol/L dT and 20 μmol/L dC were added). Viable cell numbers, apoptosis, and phase of the cell cycle were determined at 32 (■) and 44 (▪) hours of culture. Data are ± SEM of 3 separate experiments.
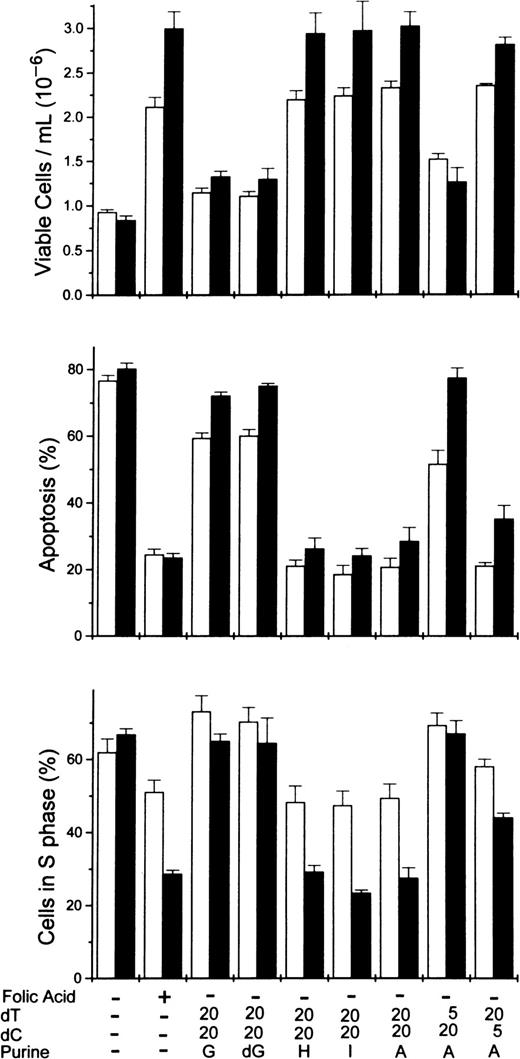
Rescue of folate-deficient erythroblasts by various purine or pyrimidine combinations.
Folate-deficient erythroblasts cultured in FDM made with dialyzed FBS received folic acid or various combinations of purines or pyrimidines at 0 hour as shown below each pair of columns. Viable cell numbers and percentages of apoptosis and cells in the S phase were determined at 32 (■) and 44 (▪) hours. Thymidine and deoxycytidine were added to give concentrations of either 5 or 20 μmol/L as indicated. All purine sources were added to give 60 μmol/L. A, adenosine; G, guanosine; dG, deoxyguanosine; H, hypoxanthine; I, inosine. All results are mean ± SEM for 3 separate experiments.
Rescue of folate-deficient erythroblasts by various purine or pyrimidine combinations.
Folate-deficient erythroblasts cultured in FDM made with dialyzed FBS received folic acid or various combinations of purines or pyrimidines at 0 hour as shown below each pair of columns. Viable cell numbers and percentages of apoptosis and cells in the S phase were determined at 32 (■) and 44 (▪) hours. Thymidine and deoxycytidine were added to give concentrations of either 5 or 20 μmol/L as indicated. All purine sources were added to give 60 μmol/L. A, adenosine; G, guanosine; dG, deoxyguanosine; H, hypoxanthine; I, inosine. All results are mean ± SEM for 3 separate experiments.
Discussion
Our results in this study demonstrate that, during terminal erythroid differentiation, folate-deficient erythroblasts accumulate in the S phase rather than progress through the cell cycle to the G0/G1 state that precedes enucleation in normal erythroblasts. Furthermore, our results here demonstrate that the accumulation in the S phase is closely associated with the increased apoptosis of folate-deficient erythroblasts1,2 and that both of these events are reversed by exogenous purines and thymidine. The rescue of cultured FDCs from S-phase accumulation and apoptosis by folic acid or nucleosides indicates that the freshly isolated FDCs are not committed to apoptosis. Like the FDCs cultured in control medium, control erythroblasts (NCs) cultured in either folate-deficient or control medium did not show the S-phase accumulation or apoptosis at later times of culture. At these late times, decreased intracellular folate coenzymes persist in FDC-FDM, compared with FDC-CM, NC-FDM, and NC-CM.1 All of these results taken together suggest that erythroblast apoptosis in folate deficiency is due to insufficient purines and thymidine for DNA synthesis secondary to decreased intracellular levels of formyl-THF and methylene-THF, respectively.
The percentages of apoptotic cells for erythroblasts cultured under each condition was higher with the sensitive TUNEL labeling in Figure 2than reported previously with morphologic evaluation.2Others have reported increased apoptosis in cell lines cultured under folate-deficient conditions.21,22 Another study found morphologic apoptosis in freshly aspirated bone marrow cells of patients with megaloblastic anemia, but internucleosomal DNA cleavage was not detected.16 Several factors may explain the absence of DNA breakdown found in these aspirated marrow cells, compared with our cultured erythroblasts: (1) rapid phagocytosis in vivo removes apoptotic cells with their cleaved DNA but apoptotic cells persist for much longer in vitro in a purified erythroblast population that lacks macrophages; (2) marrow aspirates contain cells of various lineages other than erythroblasts and these other cell types may have slower DNA cleavage rates before their being phagocytosed; and (3) the measures used to quench endogenous perioxidases in the in situ TUNEL assays of the bone marrow cells may have reduced the sensitivity of the assay. Despite these differences in detection of DNA breakdown between the aspirated human cells and murine erythroblasts in vitro, our in vitro system demonstrates morphologic apoptosis of later stage erythroblasts, the production of macrocytic reticulocytes, increased uracil misincorporation into DNA, and accumulation in the S phase of the cell cycle that have been found by others in human megaloblastic anemia.
Hematopoietic cells from patients with megaloblastic anemia or cell lines cultured under folate-deficient conditions have shown a greater percentage of cells in the S phase than normal controls.4,14-16,22 23 The relationship between the S-phase accumulations and apoptosis was not determined previously because individual cells were never examined simultaneously for the phase of cell cycle and apoptosis status. Our results here show that the S-phase accumulation is closely linked to apoptosis because at the later time of culture the FDC-FDM in the S phase have a greater percentage of apoptosis than their S-phase counterparts that are not folate-deficient (Figure 4D).
The mechanism whereby folate-deficient erythroblasts accumulate and undergo apoptosis in the S phase is not known. Our results indicating that the p53 is not required for either the S-phase accumulation or the apoptosis of the folate deficient erythroblasts are consistent with the p53 independence of accumulation of cells in the S phase after treatment with specific inhibitors of deoxyribonucleotide synthesis.24 Although p53 is not required for the S-phase accumulation or apoptosis of folate-deficient erythroblasts, a role for inhibited DNA synthesis and repair is indicated by the nucleoside rescue of folate-deficient erythroblasts. When we reported that thymidine alone could rescue folate-deficient erythroblasts,2 we were unaware that concentrations of hypoxanthine in FBS were 10 times or more than those of normal mammalian serum.25,26 Dialyzed FBS, which has less than 1 μmol/L of hypoxanthine and thymidine, permitted an analysis of the nucleosides required for the rescue of the folate-deficient erythroblasts. The specific purine nucleoside requirements for rescue are consistent with the role of formyl-THF in the de novo synthesis of purines and the known purine salvage pathways. Adenosine, deoxyadenosine, hypoxanthine, or inosine can be metabolized to supply dATP and dGTP for DNA synthesis but neither guanosine nor deoxyguanosine can supply dATP. Thus, either dATP alone or both dGTP and dATP are limiting DNA synthesis in the folate-deficient erythroblasts. Likewise, the thymidine requirement for rescue is consistent with the role of methylene-THF in the methylation of dUMP to dTMP and limiting DNA synthesis through depleted dTTP in folate-deficient erythroblasts. The requirement for deoxycytidine to prevent apoptosis when thymidine is added to the culture medium is most consistent with the feedback inhibition by dTTP on the reduction of CDP to dCDP by ribonucleotide reductase27,28 because deoxycytidine added with thymidine prevents erythroblast apoptosis2 29 (Figures 5 and 6), whereas cytidine does not (unpublished data).
Rescue with thymidine plus either adenosine or hypoxanthine of murine granulocyte colony-forming cells cultured with the methotrexate30 is consistent with our results. Specific deoxyribonucleotide depletions in spleen cells from folate-deficient rats,31 cell lines treated with antifolates,32,33 and human lymphocytes cultured in folate-deficient medium34 are also consistent with our findings. However, our results differ with a report showing increased intracellular levels of all deoxyribonucleotides in bone marrow cells from megaloblastic anemia patients.35
A mechanism for DNA damage based on decreased thymidylate synthesis has been proposed. In this scheme, the intracellular concentration of methylene-THF becomes limited and, in turn, dTMP becomes limited. The intracellular ratio of dUMP/dTMP increases with a resultant increase in the dUTP/dTTP ratio and finally increased misincorporation of uracil into DNA.9-11 Cellular attempts to correct the misincorporation through the uracil DNA glycosylase/apyrimidinic nuclease mechanism led to double-stranded DNA cleavage when uracils are misincorporated near each other in opposing strands.36 A similar mechanism for DNA damage from insufficient purine deoxyribonucleotides has not been proposed. However, insufficient dGTP and dATP as substrates for DNA polymerase can inhibit DNA synthesis and repair. Because of the generalized decrease in purine synthesis in folate deficiency, use of an alternative purine deoxyribonucleotide triphosphate substrate, analogous to the misincorporation of dUTP in place of dTTP, is not likely. Thus, in megaloblastic anemia, a halt in DNA synthesis or repair due to insufficient deoxyribonucleoside triphosphate substrates for DNA polymerases may be more likely because of impaired purine synthesis than impaired thymidylate synthesis.
Acknowledgments
We thank Dr Sarvadaman Rana, Cheng-Yao Chen, and Abudi Nashabi for technical assistance, Dr Earl Ruley for advice and support during the breeding of the Friend virus–sensitive, p53 null mice, and Dr Maurice Bondurant for review of the manuscript.
Supported by a Merit Review Award from the Department of Veterans Affairs and grant 97A159 from the American Institute for Cancer Research to M.J.K.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mark J. Koury, 547 Medical Research Bldg II, Vanderbilt University, Nashville, TN 37232-6305.