Stromal cell–derived factor-1 (SDF-1), the ligand for the CXCR4 receptor, is a highly efficacious chemoattractant for CD34+hematopoietic progenitor cells. However, the SDF-1/CXCR4 signaling pathways that regulate hematopoiesis are still not well defined. This study reports that SDF-1α can stimulate the tyrosine phosphorylation of Janus kinase 2 (JAK2) and other members of the JAK/signal transduction and activation of transcription (STAT) family, including JAK1, tyrosine kinase 2, STAT2, and STAT4 in the human progenitor cell line, CTS. SDF-1α stimulation of these cells also enhanced the association of JAK2 with phosphatidylinositol 3 (PI3)-kinase. This enhanced association was abolished by pretreatment of cells with AG490, a specific JAK2 inhibitor. Furthermore, pretreatment of CTS cells with AG490 significantly inhibited SDF-1α–induced PI3-kinase activity, and inhibition of JAK2 with AG490 ablated the SDF-1α–induced tyrosine phosphorylation of multiple focal adhesion proteins (including focal adhesion kinase, related adhesion focal tyrosine kinase, paxillin, CrkII, CrkL, and p130Cas). Chemotaxis assays showed that inhibition of JAK2 diminished SDF-1α–induced migration in both CTS cells and CD34+ human bone marrow progenitor cells. Hence, these results suggest that JAK2 is required for CXCR4 receptor-mediated signaling that regulates cytoskeletal proteins and cell migration through PI3-kinase pathways in hematopoietic progenitor cells.
Introduction
Blood cells are generated from hematopoietic stem cells within the bone marrow microenvironment. During fetal development, stem cells migrate to the bone marrow from the fetal liver.1 In bone marrow transplantation, hematopoietic stem cells home to the extravascular compartment of the bone marrow and durably repopulate transplanted recipients with both myeloid and lymphoid blood cells.2,3 Chemoattractants appear to play essential roles in directing the migration of hematopoietic stem cells to the bone marrow, although how they act on a molecular level is not yet fully understood. Stromal cell–derived factor-1 (SDF-1), also called pre-B-cell growth-stimulating factor, is an α-chemokine that binds to the CXCR4 receptor.4-6 There are 2 isoforms of SDF-1, α and β, that are generated by differential splicing from a single gene. CXCR4 has been shown to be expressed on many cell types, including hematopoietic stem cells and progenitor cells.6-8 In vitro, SDF-1 attracts CD34+progenitor cells,9-11 cobblestone area-forming cells,10 and long-term culture-initiating cells.12 Mice that lack SDF-1 or that do not express CXCR4 exhibit many defects, including the absence of both lymphoid and myeloid hematopoiesis in the fetal bone marrow.13 More recently, it was shown that SDF-1α/CXCR4–mediated migration is essential for the homing and engraftment of human stem cells into nonobese diabetic/severe combined immunodeficient mice.14These results suggest that homing and long-term maintenance of hematopoietic cells within the bone marrow are modulated by the SDF-1α/CXCR4 signaling pathway.
Although our understanding of the functional role of SDF-1α/CXCR4 in regulating hematopoiesis has increased substantially in recent years, relatively little is known about the signaling pathways that mediate these effects. CXCR4 is a member of the CXC chemokine receptor family. Like other α-chemokine receptors, CXCR4 is a 7-transmembrane surface structure linked to G proteins.15-17 Studies, including ours, have demonstrated that SDF-1α, after binding to CXCR4, causes mobilization of calcium and the activation of multiple signaling pathways, including phosphatidylinositol 3 (PI3)-kinase, PLC-γ/protein kinase c, and extracellular signal-related kinase (ERK)1/2 mitogen-activated protein kinase (MAPK).18-21SDF-1α stimulation also enhances the tyrosine phosphorylation of multiple focal adhesion components, including related adhesion focal tyrosine kinase (RAFTK), focal adhesion kinase (FAK), p130Cas, paxillin, CrkII, and CrkL.22 23 PI3-kinase signaling appears to be required for the SDF-1α–induced phosphorylation of these focal adhesion components as well as for cell migration in hematopoietic progenitor cells.
More recently, it has been proposed that Janus kinase (JAK)/signal transduction and activation of transcription (STAT) pathways may also be involved in CXCR4 receptor signaling.24 In a model T-cell line, MOLT4, SDF-1α stimulated the tyrosine phosphorylation of JAK2 and JAK3 and induced their association with the CXCR4 receptor. With this background, we sought to characterize the functional role of JAK kinases in CXCR4 receptor-mediated signaling and migration in hematopoietic progenitor cells. We therefore studied bone marrow CD34+ progenitor cells and a model human hematopoietic progenitor cell line, CTS. We observed that SDF-1α induced the tyrosine phosphorylation of JAK2 and other members of the JAK/STAT family, including JAK1, tyrosine kinase 2 (TYK2), STAT2, and STAT4. JAK2 was shown to be required for the SDF-1α–induced activation of PI3-kinase and for the tyrosine phosphorylation of multiple focal adhesion proteins. Inhibition of JAK2 decreased SDF-1α–induced cell migration in both CTS and primary bone marrow CD34+progenitor cells. These data provide new insights into the role of JAK kinases in the regulation of CXCR4 receptor-mediated signaling and migration in hematopoietic progenitor cells.
Materials and methods
Reagents and antibodies
Rabbit polyclonal antibodies for JAK1, JAK2, JAK3, TYK2, STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, STAT6, and CrkL were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The mouse anti-phosphotyrosine monoclonal antibody (mAb) 4G10 was a generous gift from Dr Brian Druker (University of Oregon, Portland, OR). Rabbit anti–PI3-kinase (p85) polyclonal antibodies were purchased from Upstate Biotechnology (Lake Placid, NY). Anti-CrkII, anti-p130Cas, antipaxillin, and antiphosphotyrosine mAb (PY20) were purchased from Transduction Laboratories (Lexington, KY). Rabbit polyclonal antibodies for RAFTK or FAK were generated as described previously.24-26 Normal rabbit serum, purified normal rabbit immunoglobulin G (IgG) or mouse IgG was purchased from Organon Teknika (Westchester, PA). AG490, a JAK2 kinase inhibitor, was obtained from Calbiochem (La Jolla, CA). Electrophoresis reagents and nitrocellulose membrane were obtained from Bio-Rad Laboratories (Hercules, CA). Protein A-Sepharose CL-4B and Glutathione Sepharose 4B were obtained from Amersham Pharmacia Biotech (Piscataway, NJ). The protease inhibitors leupeptin, aprotinin, and α1 antitrypsin, as well as all other reagents were obtained from Sigma Chemical (St Louis, MO). Recombinant SDF-1α was purchased from R&D Systems (Minneapolis, MN).
Cell culture and ligand stimulation of cells
A hematopoietic progenitor cell line, CTS, was established from a patient with acute myeloblastic leukemia27 and has been previously used as a model of SDF-1α–mediated migration and signaling.22,23 The CTS cell line was grown at 37°C in 5% CO2 in RPMI-1640 medium containing 10% fetal calf serum, 50 μg/mL penicillin, and 50 μg/mL streptomycin, as described previously.22,23 27
Before stimulation, the CTS cells were starved in serum-free RPMI-1640 medium for 4 hours. During the last hour of starvation, 0.1 nM sodium vanadate was added. After starvation, cells were washed twice with serum-free RPMI-1640 medium and then resuspended at 15 × 106/mL. Cells were next stimulated in vitro with 20 nM SDF-1α for different time periods at 37°C. After stimulation, cell lysates were prepared by lysis in lysis buffer (50 mM HEPES, pH 7.0; 150 mM NaCl; 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 10 mM sodium pyrophosphate, 100 mM NaF; 10 mM dithiothreitol; 1 mM phenylmethylsulfonyl fluoride [PMSF]; 10 μg/mL each of aprotinin, leupeptin, and pepstatin; and 10 mM sodium orthovanadate). Total cell lysates were clarified by centrifugation at 10 000g for 10 minutes. Protein concentrations were determined by protein assay (Bio-Rad Laboratories). To assess the effects of the JAK2 kinase inhibitor, AG490, cells were preincubated with this compound for 2 hours before SDF-1α stimulation, as described above.
Immunoprecipitation and Western blot analysis
For the immunoprecipitation studies, identical amounts of protein from each sample were clarified by incubation with protein A-Sepharose for 1 hour at 4°C. Following the removal of protein A-Sepharose by brief centrifugation, the solution was incubated with different primary antibodies, as detailed below for each experiment, for 4 hours or overnight at 4°C. Immunoprecipitations of the antibody-antigen complexes were performed by incubation for 2 hours at 4°C with 75 μL protein A-Sepharose (10% suspension). Nonspecific bound proteins were removed by washing the Sepharose beads 2 times with HNTG buffer (50 mM HEPES, pH 7.0; 150 mM NaCl; 10% glycerol; 0.1% Triton X-100; 1 mM PMSF; 10 μg/mL each of aprotinin, leupeptin and pepstatin; and 10 mM sodium orthovanadate) and one time with phosphate-buffered saline (PBS). Bound proteins were solubilized in 40 μL of 2× Laemmli sample buffer and further analyzed by immunoblotting. Samples were separated on 8% to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat milk protein and probed with primary antibody for 2 hours at room temperature or at 4°C overnight. Immunoreactive bands were visualized by using horseradish peroxidase-conjugated secondary antibody and the enhanced chemiluminescent system (Amersham Pharmacia Biotech).
Assays of PI3-kinase activity
Unstimulated or SDF-1α–stimulated cells were lysed in ice-cold lysis buffer containing 137 mM NaCl, 20 mM Tris-HCl (pH 7.4), 1 mM MgCl2, 1 mM sodium orthovanadate, 10% glycerol, 1% NP-40, and 1 mM PMSF. Immunoprecipitation was performed by using antiphosphotyrosine antibody (PY20) or anti-p85 antibody. Immunoprecipitates were washed 3 times with lysis buffer; 3 times with buffer containing 0.1 M Tris-HCl (pH 7.4), 5 mM LiCl, and 0.1 mM sodium orthovanadate; and 2 times with Tris, NaCl, EDTA (TNE) buffer containing 10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA, and 0.1 mM sodium orthovanadate. Samples were resuspended in 20 μL TNE buffer, 20 μL phosphoinositol (10 μg; Avanti Polar Lipids, Alabaster, AL), and 10 μL adenosine triphosphate (ATP) mix (1 mM HEPES, 10 μM ATP, 1 μM MgCl2, 10 μM32P-ATP), then incubated at 37°C for 10 minutes. The reaction was stopped by adding 40 μL 3 M HCl and 160 μL chloroform:methanol (1:1 vol/vol). Lipids were separated on oxalate-impregnated silica thin layer chromatography (TLC) plates using a solvent system of chloroform:methanol:water:ammonium hydroxide (28%; 35:35:3.5:7). TLC plates were dried and subjected to autoradiography at −80°C.
Preparation of human bone marrow cells
Light-density bone marrow mononuclear cells were obtained from healthy consenting donors and depleted of adherent cells, as previously described.28
Isolation of human bone marrow CD34+progenitor cells
CD34+ cells were isolated from the bone marrow of healthy donors by using the Direct CD34+ Progenitor Cell Isolation Kit (Miltenyi Biotec GMbH, Germany), according to the manufacturer's instructions. The purity of the CD34+ cells selected by this method was found to be more than 95% by flow-activated cell sorter analysis. After isolation, cells were washed 2 times with migration medium and then resuspended in the same medium (see below).
Chemotaxis assays
Chemotaxis assays were performed in triplicate using 5 μm-pore filters (Transwell, 24-well cell clusters; Costar, Boston, MA) as described previously.23 Briefly, the filters were rinsed with migration medium (RPMI-1640 with 0.5% bovine serum albumin [BSA] for the CTS cells; complete α-medium with 0.5% BSA for the CD34+ bone marrow cells), and the supernatant was aspirated immediately before loading the cells. CTS cells (2 × 105) or CD34+ cells (1.5 × 105), suspended in 100 μL migration medium, were loaded into each Transwell filter. Filters were then carefully transferred to another well containing 650 μL migration medium with 20 nM SDF-1α (R&D Systems). The plates were incubated at 37°C in 5% CO2 for 3.5 hours. Next, the upper chambers were carefully removed, and the cells in the bottom chambers were collected. The cells were washed and resuspended in proper volume, then quantitated for viable cells using the trypan blue exclusion method. To assess the effects of AG490, cells were preincubated with various concentrations of this inhibitor for 2 hours, and then the chemotaxic assays were done as described above. Cell migration is shown as the percentage of cell input.
Statistical analysis
The results are expressed as the means ± SD of data obtained from 3 or more experiments performed in triplicate. Statistical significance was determined using the Studentt test.
Results
SDF-1α stimulation induces the tyrosine phosphorylation of JAK2 kinase and other members of the JAK/STAT family in CTS cells
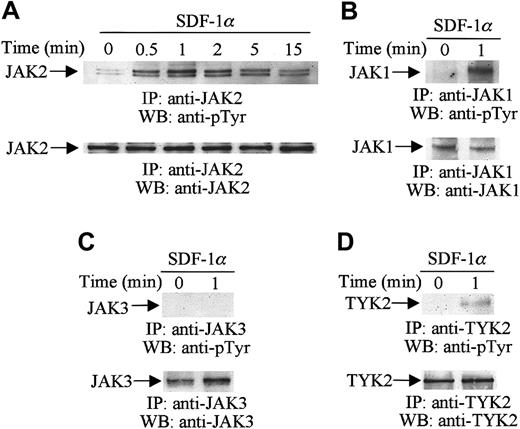
To determine whether members of the JAK family, including JAK1, JAK2, JAK3, and TYK2, are tyrosine-phosphorylated following SDF-1α stimulation, CTS cells were serum-starved and stimulated with 20 nM of SDF-1α for the indicated times (Figure1). Cell lysates were immunoprecipitated with a specific anti-JAK2 (Figure 1A), anti-JAK1 (Figure 1B), anti-JAK3 (Figure 1C), or anti-TYK2 (Figure 1D) antibody, and the immunoprecipitates were then analyzed by Western blotting with antiphosphotyrosine antibody. As shown in Figure 1A, SDF-1α stimulation induced the rapid and transient tyrosine phosphorylation of JAK2. Maximum phosphorylation of JAK2 was detected as early as 1 minute after the addition of 20 nM SDF-1α. Thereafter, the tyrosine phosphorylation declined gradually. JAK1 (Figure 1B, upper panel) and TYK2 (Figure 1D, upper panel) were also tyrosine-phosphorylated in response to SDF-1α stimulation. The time course of phosphorylation of JAK1 and TYK2 was similar to that of JAK2 (data not shown). However, the tyrosine phosphorylation of JAK3 was not altered by SDF-1α stimulation (Figure 1C). Loading of equal amounts of protein in all lanes was confirmed by stripping and reprobing with the same antibodies used for the immunoprecipitations (Figure 1A-D, lower panels).
SDF-1α induces the tyrosine phosphorylation of JAK family members.
Total cell lysates from untreated or SDF-1α-treated (20 nM) CTS cells were immunoprecipitated (IP) with anti-JAK1, anti-JAK2, anti-JAK3, or anti-TYK2 antibodies and analyzed by Western blotting (WB) with PY99 antiphosphotyrosine antibody (pTyr) (A-D, upper panels). The protein loading was verified by reprobing the membranes with the same antibodies used for the immunoprecipitations (A-D, lower panels).
SDF-1α induces the tyrosine phosphorylation of JAK family members.
Total cell lysates from untreated or SDF-1α-treated (20 nM) CTS cells were immunoprecipitated (IP) with anti-JAK1, anti-JAK2, anti-JAK3, or anti-TYK2 antibodies and analyzed by Western blotting (WB) with PY99 antiphosphotyrosine antibody (pTyr) (A-D, upper panels). The protein loading was verified by reprobing the membranes with the same antibodies used for the immunoprecipitations (A-D, lower panels).
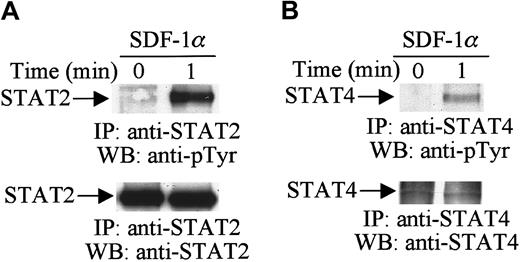
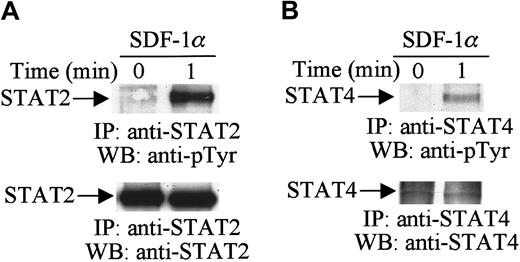
Activation of the JAK tyrosine kinases enables these kinases to mediate the tyrosine phosphorylation of specific STAT proteins.29 30 Therefore, in further analysis, we examined the effects of SDF-1α stimulation on the tyrosine phosphorylation of STAT proteins. Cell lysates from unstimulated or SDF-1α–stimulated CTS cells were immunoprecipitated with anti-STAT1 to STAT6 antibodies. Changes in the tyrosine phosphorylation of each protein were analyzed by immunoblotting with antiphosphotyrosine antibody. As shown in Figure2, SDF-1α stimulation induced the tyrosine phosphorylation of STAT2 (Figure 2A, upper panel) and STAT4 (Figure 2B, upper panel). We verified equal protein loading by reprobing blots with the same antibody used for each immunoprecipitation (Figure 2A,B, lower panels). However, SDF-1α treatment did not induce the tyrosine phosphorylation of STAT1, STAT3, STAT5, or STAT6 (data not shown). These results indicate that certain members of the JAK/STAT family, specifically JAK1, JAK2, TYK2, STAT2, and STAT4, are involved in SDF-1α–induced signaling in hematopoietic progenitor cells.
SDF-1α induces the tyrosine phosphorylation of STAT.
Total cell lysates from untreated or SDF-1α–treated (20 nM) CTS cells were immunoprecipitated with anti-STAT2 (A) or anti-STAT4 (B) antibody and analyzed by Western blotting with PY99 antiphosphotyrosine antibody (pTyr). After stripping, the membrane was reprobed with anti-STAT2 antibody (A, lower panel) or anti-STAT4 antibody (B, lower panel).
SDF-1α induces the tyrosine phosphorylation of STAT.
Total cell lysates from untreated or SDF-1α–treated (20 nM) CTS cells were immunoprecipitated with anti-STAT2 (A) or anti-STAT4 (B) antibody and analyzed by Western blotting with PY99 antiphosphotyrosine antibody (pTyr). After stripping, the membrane was reprobed with anti-STAT2 antibody (A, lower panel) or anti-STAT4 antibody (B, lower panel).
JAK2 is involved in the SDF-1α–induced activation and phosphorylation of PI3-kinase
We have previously shown that SDF-1α stimulation activates the PI3-kinase pathway in hematopoietic cells. The PI3-kinase pathway appears to be required for SDF-1α–induced migration in CTS cells and CD34+ bone marrow progenitor cells.23 It has been reported that activation of JAK2 kinase is required for SDF-1α–induced migration in T cells.24 Thus, we sought to determine the functional role of JAK2 in the activation of PI3-kinase and their potential interrelationship.
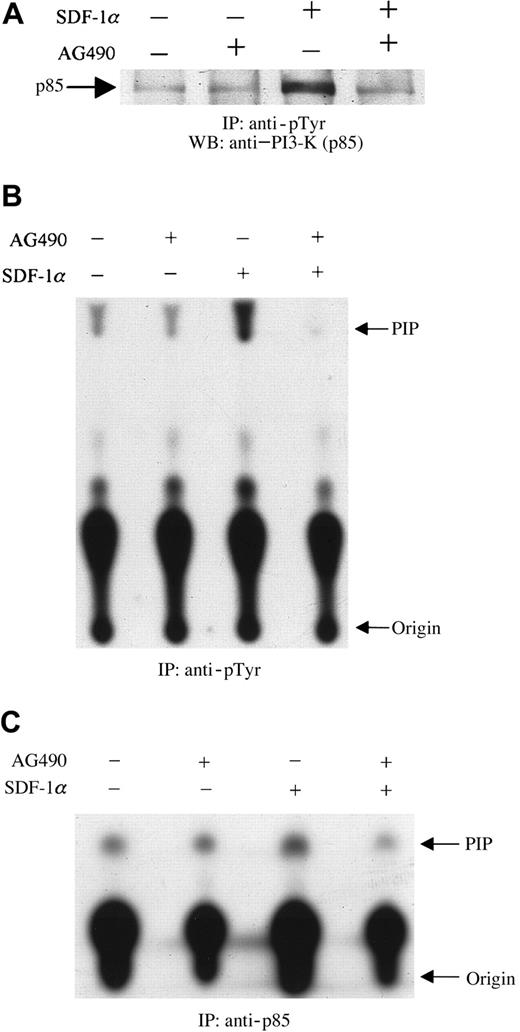
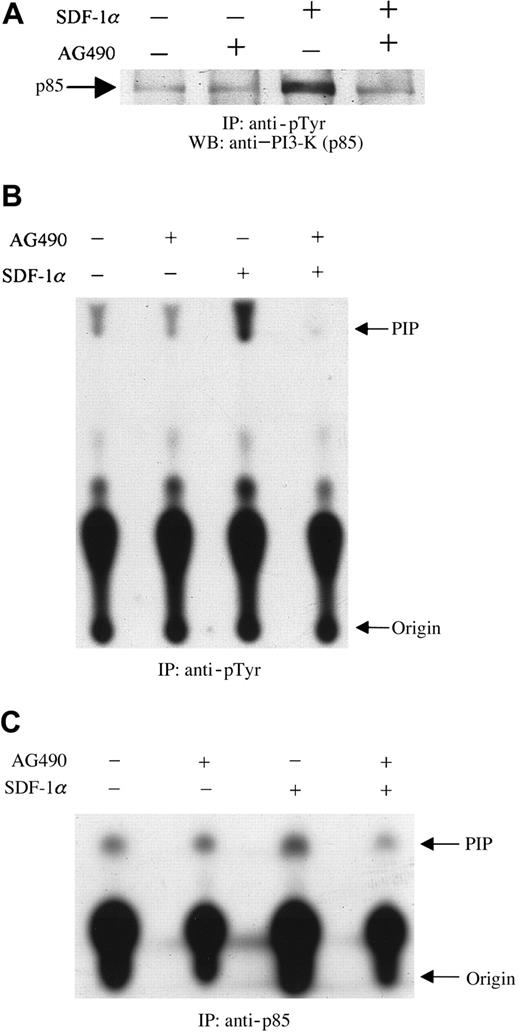
These experiments were designed to examine the effects of AG490, a specific JAK2 inhibitor,31 on the tyrosine phosphorylation of the p85 subunit of PI3-kinase. Serum-starved CTS cells at 10 × 106/mL were preincubated with 100 μM AG490 or its diluent, dimethyl sulfoxide (DMSO), for 2 hours at 37°C. The cells were then stimulated with 20 nM SDF-1α or diluent (PBS with 0.1% BSA) for 1 minute at 37°C. Cell lysates were prepared and immunoprecipitated with antiphosphotyrosine antibody (PY20). The immunoprecipitates were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and immunoblotted with anti–PI3-kinase (p85 subunit) antibody. As shown in Figure3, SDF-1α treatment induced the tyrosine phosphorylation of p85 (Figure 3A), as reported previously.23 Pretreating cells with AG490 led to a decrease in the level of tyrosine phosphorylation of p85 induced by SDF-1α stimulation (Figure 3A). Subsequently, the effect of JAK2 inhibition by AG490 on SDF-1α–induced PI3-kinase activity was measured. Cells were prepared as described above. PI3-kinase activity was measured by an in vitro PI3-kinase assay by using immunoprecipitates with antiphosphotyrosine (anti-pTyr) (Figure3B) or anti-p85 (Figure 3C) antibody, as described in “Materials and methods.” As shown, pretreatment with AG490 significantly inhibited SDF-1α–induced PI3-kinase activity.
Inhibition of SDF-1α–induced activation and phosphorylation of PI3-kinase by pretreatment with the JAK2 inhibitor, AG490.
CTS cells, pretreated with AG490 (100 μM) (+) or DMSO (−) for 2 hours, were stimulated with 20 nM SDF-1α for 1 minute and then lysed. Total cell lysates were immunoprecipitated with PY20 antiphosphotyrosine antibody (pTyr). The immunoprecipitates were analyzed by Western blotting with anti-p85 antibody (A). Total cell lysates were immunoprecipitated with PY20 antiphosphotyrosine antibody (pTyr) (B) or anti-p85 antibody (C). PI3-kinase activity was measured by a PI3-kinase assay, as described in “Materials and methods.” PIP indicates phosphatidylinositol 3-phosphate.
Inhibition of SDF-1α–induced activation and phosphorylation of PI3-kinase by pretreatment with the JAK2 inhibitor, AG490.
CTS cells, pretreated with AG490 (100 μM) (+) or DMSO (−) for 2 hours, were stimulated with 20 nM SDF-1α for 1 minute and then lysed. Total cell lysates were immunoprecipitated with PY20 antiphosphotyrosine antibody (pTyr). The immunoprecipitates were analyzed by Western blotting with anti-p85 antibody (A). Total cell lysates were immunoprecipitated with PY20 antiphosphotyrosine antibody (pTyr) (B) or anti-p85 antibody (C). PI3-kinase activity was measured by a PI3-kinase assay, as described in “Materials and methods.” PIP indicates phosphatidylinositol 3-phosphate.
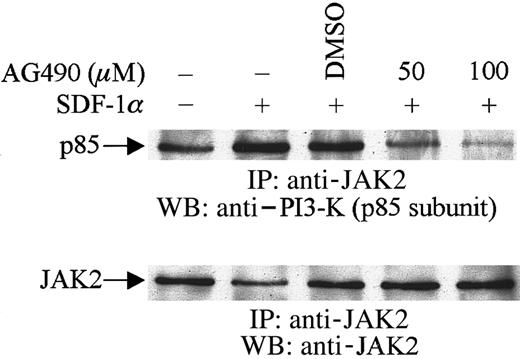
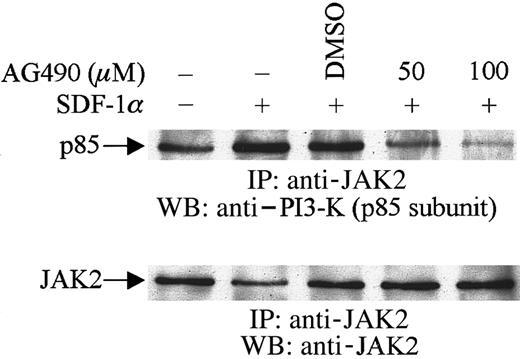
To examine the possible physical association between JAK2 and the p85 subunit of PI3-kinase, cells were pretreated with AG490 or its diluent, DMSO, followed by SDF-1α stimulation. Cell lysates were immunoprecipitated with anti-JAK2 antibody and the immunoprecipitates were then subjected to immunoblotting with anti–PI3-kinase (p85) antibody. As shown in Figure 4, SDF-1α stimulation enhanced the association of JAK2 with p85. However, this enhancement was inhibited by the pretreatment of cells with AG490. These results indicated that JAK2 is involved in SDF-1α–mediated activation and phosphorylation of PI3-kinase and that it appears to act upstream of PI3-kinase in CXCR4 signaling pathways in hematopoietic progenitor cells.
Association of JAK2 with PI3-K (p85 subunit).
CTS cells were pretreated with AG490 (+) or DMSO (−) for 2 hours and then stimulated with SDF-1α (20 nM) for 1 minute. Total cell lysates were immunoprecipitated with anti-JAK2 antibody and analyzed by Western blotting with anti-p85 antibody.
Association of JAK2 with PI3-K (p85 subunit).
CTS cells were pretreated with AG490 (+) or DMSO (−) for 2 hours and then stimulated with SDF-1α (20 nM) for 1 minute. Total cell lysates were immunoprecipitated with anti-JAK2 antibody and analyzed by Western blotting with anti-p85 antibody.
Inhibition of JAK2 with AG490 ablates the SDF-1α–induced tyrosine phosphorylation of multiple focal adhesion proteins in CTS cells
Our previous studies have shown that SDF-1α stimulates the tyrosine phosphorylation of several focal adhesion proteins, including CrkII, CrkL, p130Cas, paxillin, RAFTK, and FAK.18,22 23These focal adhesion proteins are believed to play a critical role in the formation of focal adhesions and in the regulation of cell adhesion and migration. Here, we observed that JAK2 is required for the SDF-1α–induced activation of the PI3-kinase pathway. These observations led us to study whether JAK2 is also required for the SDF-1α–induced tyrosine phosphorylation of these focal adhesion proteins.
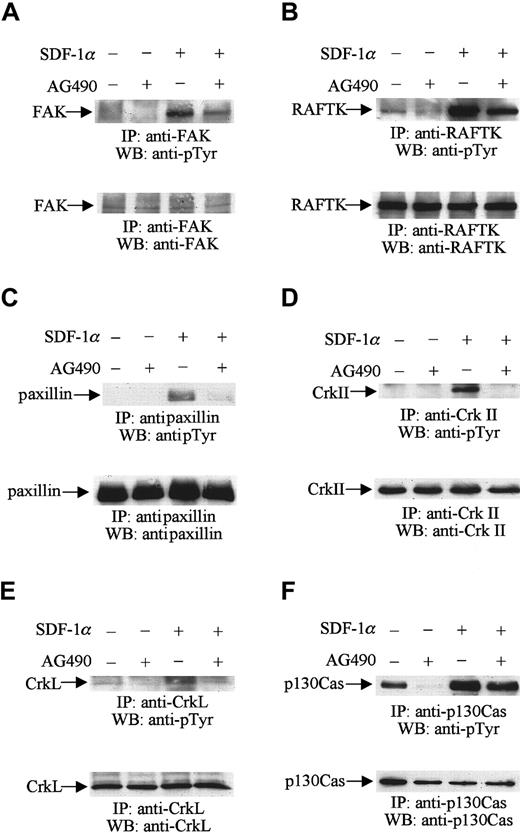
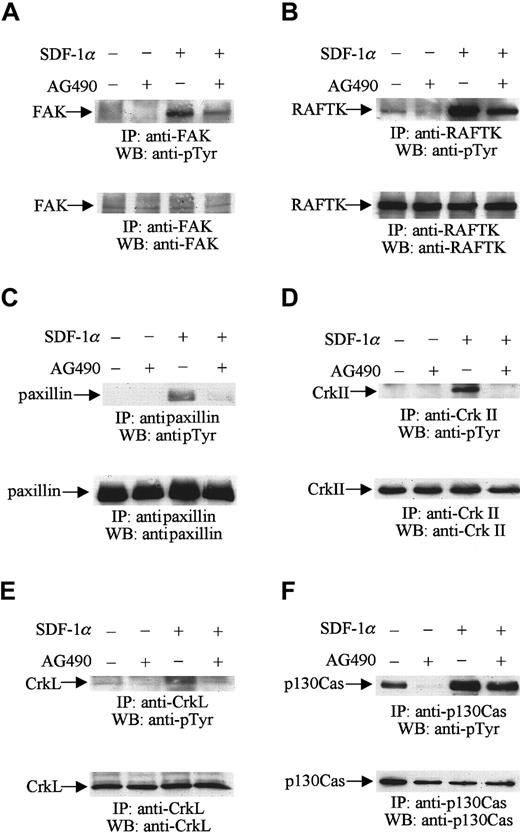
Serum-starved CTS cells were first treated with AG490 or its diluent, DMSO, as described above. The cells were then stimulated with 20 nM SDF-1α for 1 minute. Cell lysates were prepared and immunoprecipitated with anti-FAK, RAFTK, paxillin, CrkII, CrkL, and p130Cas antibodies, respectively. The immunoprecipitates were subjected to Western blot analysis using antiphosphotyrosine antibody. As we described previously,23 SDF-1α treatment increased the level of tyrosine phosphorylation of FAK (Figure5A, upper panel), RAFTK (Figure 5B, upper panel), paxillin (Figure 5C, upper panel), CrkII (Figure 5D, upper panel), CrkL (Figure 5E, upper panel), and p130Cas (Figure 5F, upper panel). Pretreatment of CTS cells with AG490 reduced the increases in tyrosine phosphorylation of these focal adhesion proteins in response to SDF-1α stimulation (Figure 5A-F, upper panels). Protein loading was examined in each immunoblot by reprobing with the same antibodies used for the immunoprecipitations. We verified that equal amounts of protein were loaded in each lane of these immunoblots (Figure 5A-F, lower panels). The results suggest that JAK2 kinase may be required for the SDF-1α–induced tyrosine phosphorylation of these focal adhesion proteins in hematopoietic progenitor cells.
JAK2 is required for the SDF-1α–induced phosphorylation of the focal adhesion components FAK, RAFTK, paxillin, CrkII, CrkL, and p130Cas.
CTS cells, pretreated with AG490 (100 μM) (+) or DMSO (−) for 2 hours, were stimulated with 20 nM SDF-1α for 1 minute and lysed. Total cell lysates were immunoprecipitated with anti-FAK (A), anti-RAFTK (B), antipaxillin (C), anti-CrkII (D), anti-CrkL (E), and anti-p130Cas (F) antibodies, and the immunoprecipitates were analyzed by Western blotting with PY99 antibody (pTyr) (upper panels). After stripping, the membranes were reprobed to assure equal protein loading (A-F, lower panels).
JAK2 is required for the SDF-1α–induced phosphorylation of the focal adhesion components FAK, RAFTK, paxillin, CrkII, CrkL, and p130Cas.
CTS cells, pretreated with AG490 (100 μM) (+) or DMSO (−) for 2 hours, were stimulated with 20 nM SDF-1α for 1 minute and lysed. Total cell lysates were immunoprecipitated with anti-FAK (A), anti-RAFTK (B), antipaxillin (C), anti-CrkII (D), anti-CrkL (E), and anti-p130Cas (F) antibodies, and the immunoprecipitates were analyzed by Western blotting with PY99 antibody (pTyr) (upper panels). After stripping, the membranes were reprobed to assure equal protein loading (A-F, lower panels).
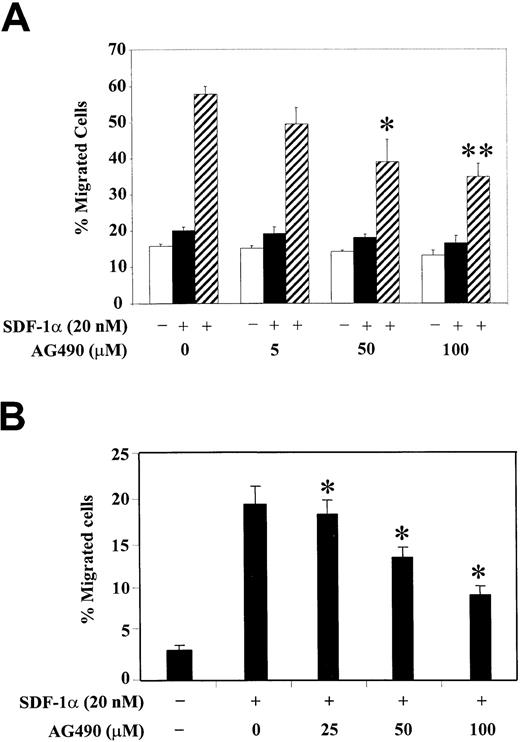
Inhibition of JAK2 kinase decreases SDF-1α–induced migration of CTS cells and CD34+ primary bone marrow progenitor cells
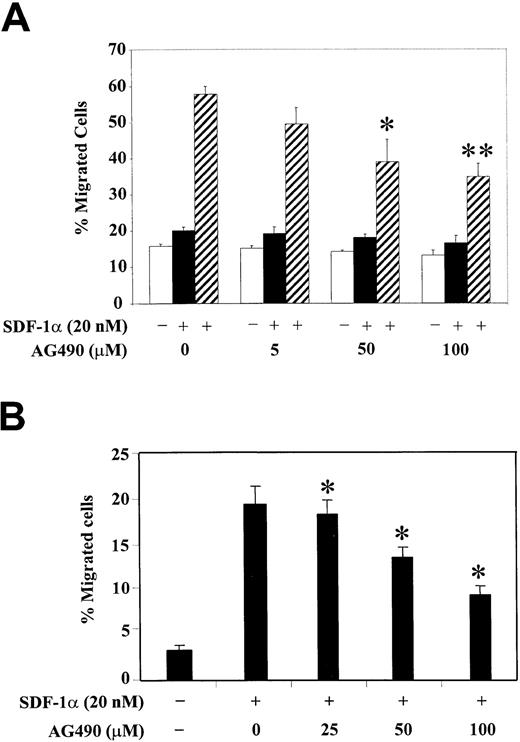
To correlate the functional role of JAK2 kinase with the observed phosphorylation changes of PI3-kinase and various focal adhesion proteins, we studied the SDF-1α–induced migration of hematopoietic progenitor cells, either CTS cells (Figure6A) or CD34+ human bone marrow cells (Figure 6B), after their pretreatment with different concentrations of AG490. Cell migration in response to SDF-1α was examined using a Transwell migration assay as described in “Materials and methods.” We observed that treatment with AG490 significantly inhibited the SDF-1α–induced migration of CTS cells in a dose-dependent manner. The AG490 treatment had no significant effect on cell migration in the absence of SDF-1α or with SDF-1α in both the upper and lower chambers (Figure 6A). Similar results were found with CD34+ primary bone marrow progenitor cells (Figure 6B). These results suggest that activation of JAK2 is required for the SDF-1α–induced migration of hematopoietic progenitor cells.
Inhibition of JAK2 with AG490 decreases SDF-1α–induced migration of CTS cells or CD34+ human bone marrow progenitors.
(A) CTS cells were pretreated with various concentrations of AG490 or control DMSO (0) for 2 hours. The effect of AG490 on cell migration was analyzed in a migration assay in the absence of SDF-1α (■, medium control), in the presence of SDF-1α in both the upper and lower chambers (▪), or with SDF-1α in the lower chamber only (▨). (B) CD34+ human bone marrow cells were pretreated with various concentrations of AG490 or control DMSO (0) for 2 hours. Cell migration in response to medium alone (−) or SDF-1α (+) was measured. Migration is shown as the percentage of cell input. The data represent the mean ± SD of 3 separate experiments performed in triplicate. *P < .05 as compared with control. **P < .01 as compared with control.
Inhibition of JAK2 with AG490 decreases SDF-1α–induced migration of CTS cells or CD34+ human bone marrow progenitors.
(A) CTS cells were pretreated with various concentrations of AG490 or control DMSO (0) for 2 hours. The effect of AG490 on cell migration was analyzed in a migration assay in the absence of SDF-1α (■, medium control), in the presence of SDF-1α in both the upper and lower chambers (▪), or with SDF-1α in the lower chamber only (▨). (B) CD34+ human bone marrow cells were pretreated with various concentrations of AG490 or control DMSO (0) for 2 hours. Cell migration in response to medium alone (−) or SDF-1α (+) was measured. Migration is shown as the percentage of cell input. The data represent the mean ± SD of 3 separate experiments performed in triplicate. *P < .05 as compared with control. **P < .01 as compared with control.
Discussion
SDF-1α plays a key role in the regulation of migration and homing of hematopoietic progenitor cells. The molecular mechanisms involved in mediating these effects are not well characterized. Cell migration is a complex process and is mediated by multiple signaling mechanisms. The PI3-kinase pathway has been reported to be involved in cytokine- or chemokine-induced migration in various cell types.32,33 Our previous work has shown that PI3-kinase and a number of focal adhesion proteins are involved in CXCR4 signaling in hematopoietic progenitor cells and that PI3-kinase is required for the SDF-1α–induced migration of hematopoietic progenitor cells.23 Here, we have extended our studies and demonstrated that the cytoplasmic tyrosine kinase, JAK2, is involved in CXCR4 receptor-mediated signaling through PI3-kinase and that JAK2 appears to be required for the SDF-1α–induced migration of hematopoietic progenitor cells.
Although the JAK/STAT signaling pathway is conventionally considered to be a common feature of all members of the cytokine receptor superfamily,29,30 it also has been reported to participate in the signaling of the G-protein coupled receptor.34,35It has been demonstrated that β-chemokines such as monocyte chemoattractant protein-1 (MCP-1),34MIP-1α,36 and RANTES (regulated on activation, normal T cell expressed and secreted)36,37 and the α-chemokine, SDF-1α,24 stimulate the tyrosine phosphorylation of various members of the JAK and STAT protein families in monocytes and T cells. Phosphorylated JAK proteins become associated with and trigger the tyrosine phosphorylation of chemokine receptors. Activation of the JAK proteins enables the recruitment and tyrosine phosphorylation of different members of the STAT family of transcription factors. To assess the role of members of the JAK/STAT family in CXCR4 receptor-mediated signaling pathways in hematopoietic progenitor cells, we first tested whether SDF-1α treatment caused the tyrosine phosphorylation of these members in CTS cells. Similar to results reported in T cells,24 we observed that stimulation with SDF-1α induced the rapid tyrosine phosphorylation of JAK2 (Figure 1A) and its association with the CXCR4 receptor (data not shown) in CTS cells. The activation of JAK2 by SDF-1α was found to be more rapid as compared with that of most hematopoietic cytokines such as erythropoietin, thrombopoietin, interleukin 3 (IL-3), and granulocyte/macrophage colony-stimulating factor (GM-CSF).38-42 In addition to JAK family members, signaling molecules such as PI3-kinase, MAPK (ERK1/2), FAK, and RAFTK have been shown to be more rapidly activated by SDF-1 and other chemokines as compared with most cytokines.18,22-24 It has been reported that activation of JAK kinases appears to be required for phosphorylation of the chemokine receptors, CXCR4 and CCR5, and their subsequent downstream signaling.24 37 However, the molecular mechanisms that mediate the interaction between the JAK proteins and the CXCR4 receptor need to be further delineated. Besides JAK2, other members of the JAK family, JAK1 and TYK2, but not JAK3, were also tyrosine-phosphorylated on SDF-1α stimulation. Although all members of the STAT proteins were found to be expressed in CTS cells, only STAT2 and STAT4 were tyrosine-phosphorylated on SDF-1α stimulation (Figure 2).
Next, we sought to identify the possible downstream components of the JAK kinase pathway that are activated on CXCR4 receptor signaling. To date, besides the SDF-1 receptor reported recently,24 it is known that JAK2 is also associated with the erythropoietin (Epo) receptor,38 thrombopoietin (Tpo) receptor,39,40 GM-CSF receptor,41 IL-3 receptor,42 granulocyte CSF-R (G-CSF-R),43and cytokine receptors containing gp130 in their receptor chain complex (interleukin-6 receptor, ciliary neurotrophic factor receptor, leukemia inhibitory factor receptor, and oncostatin M receptor).44 Because JAK2 participates in the signaling of many hematopoietic growth factor receptors, we specifically focused on this kinase in our analyses. The major function of the JAK kinases is generally considered to be the activation of the STAT transcription factors. However, this is clearly not the only role that JAK kinases play in signaling. For example, JAK kinases are directly implicated in the activation of tyrosine kinases RAFTK,45-47 FAK,48 and PI3-kinase,49,50 and in the stimulation of the Ras-MAPK pathway.51,52 We examined the functional role of JAK2 in the activation of PI3-kinase, which has been shown to be required for CXCR4 receptor-mediated signaling and cell migration.23The increase in PI3-kinase activity is much less impressive after immunoprecipitation with anti-p85 (2.4 times, compared with unstimulated control) than after immunoprecipitation with PY20 (4.5 times, compared with unstimulated control). This finding suggests that p85 is either tyrosine-phosphorylated or associates with phosphotyrosine-containing molecules after stimulation with SDF-1α. The effects of p85 phosphorylation and PI3-kinase activity may be exaggerated after immunoprecipitation with PY20 because more p85 is recovered. Our results indicate that JAK2 acts upstream of PI3-kinase. First, we observed that JAK2 was constitutively associated with PI3-kinase and that SDF-1α stimulation enhanced this association. Second, inhibition of JAK2 with AG490 significantly abolished this enhancement.
Focal adhesions are cytoskeletal structures that form the adherent contacts of cells with the extracellular matrix. The formation of such focal adhesions is important for cell adhesion and migration. A number of proteins, including tyrosine kinases (FAK and RAFTK), adapter proteins (p130Cas, Crk, and CrkL), and cytoskeletal proteins (paxillin), are involved in the formation of such focal adhesions. The tyrosine phosphorylation of focal adhesion proteins is believed to be associated with the cell migration induced by cytokines or chemokines. We have previously shown that SDF-1α stimulation induced the tyrosine phosphorylation of these focal adhesion proteins in a PI3-kinase dependent manner. Here, we demonstrate that JAK2 appears to function as an upstream mediator in this PI3-kinase pathway. We observed that inhibition of JAK2 decreased the tyrosine phosphorylation of these focal adhesion proteins. Differences in the phosphorylation of focal adhesion components in the presence of the JAK2 inhibitor suggest that other kinases may be involved in signaling to the cytoskeletal apparatus. Extending these biochemical observations, we found that inhibition of JAK2 reduced SDF-1α-stimulated chemotaxis in both CTS cells and CD34+ primary bone marrow progenitors. Although 100 μM AG490 almost totally blocked the tyrosine phosphorylation of JAK2, this concentration just partially inhibited SDF-1α–induced migration. These data indicate that there may be other pathways besides the JAK2 pathway that are involved in the SDF-1α–induced migration of hematopoietic progenitor cells. Taken together, these results suggest that JAK2 is required for CXCR4 receptor-mediated activation of PI3-kinase, for the tyrosine phosphorylation of multiple focal adhesion proteins, and for cell migration in hematopoietic progenitor cells.
We are grateful to Janet Delahanty for editing this manuscript and to Daniel Kelley for assistance in preparing the figures. Xue-Feng Zhang is a Bertlesmann Cancer research fellow.
Supported in part by grants HL 53745-02, HL 55187-01, HL 51456-02, and HL 55445-01 from the National Institutes of Health.
The first two authors contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jerome E. Groopman, Division of Experimental Medicine, Beth Israel Deaconess Medical Center, Harvard Institutes of Medicine, 4 Blackfan Circle, Boston, MA 02115; e-mail:jgroopma@caregroup.harvard.edu.