In attempting to develop effective anticancer immunotherapies, the relative ability of apoptotic cells to induce an immune response remains an important but controversial consideration. A novel gene-transfer approach was used by which rapid induction of pure apoptosis can be selectively achieved in a transfected tumor cell population following exposure to a semisynthetic dimerizing ligand, AP20187. Inoculation of BALB/c mice with apoptotic and viable 12B1-D1 leukemia cells, at a 12:1 ratio subcutaneously, led to early tumor growth. Heat stress up-regulated the expression of membrane heat shock proteins (HSP72 and HSP60) on apoptotic 12B1-D1 cells, and stressed apoptotic cells were capable of generating a T-cell–mediated specific antitumor response. Pulsing of stressed apoptotic leukemia cells onto syngeneic dendritic cells resulted largely in rejection of coinjected viable 12B1-D1 cells. Mice rejecting the primary 12B1-D1 inoculum were immune to the same but not to a different leukemia challenge. Our findings indicate that tumor immunogenicity is dependent on whether cells are stressed before apoptosis induction and suggest that the immune system is capable of distinguishing between stressed and nonstressed cells undergoing programmed cell death.
Introduction
The mechanism by which a cell dies determines whether an immune response will be generated.1,2 Recent studies have documented that apoptotic tumor cells have low immunogenicity in vivo, whereas those that die by necrosis can generate an antitumor response.3 This may be due in part to the high expression of inducible heat shock protein (HSP) 70 found in necrotic but not apoptotic cells.3 Because apoptotic tumor cells apparently do not increase the expression of HSPs during their demise, we hypothesized that stressing the cells before apoptosis may recreate the danger signal to which antigen-presenting cells (APCs) are primed to respond.
To test whether apoptotic tumor cells can become immunogenic after stress, we used an approach by which we selectively induced programmed cell death following heat shock. The bcr-abl–positive and Fas-negative murine leukemia cell line, 12B1, was stably transfected with complementary DNA (cDNA) encoding a recombinant protein that consists of 2 mutant FK506 binding protein (FKBP) domains and the Fas death domain.4-6 Using this novel system, we were able to trigger classic apoptotic death in stably transfected leukemia clones with the use of an FKBP-specific dimerizing drug, AP20187. The 12B1-D1 clone was selected for further study based on its sensitivity to apoptosis after incubation with AP20187. 12B1-D1 cells were found to have low basal surface expression of HSP72 and HSP60, which could be significantly increased following heat exposure. Tumor development following injection of viable 12B1-D1 cells mixed with heat-stressed and AP20187-treated leukemia cells into syngeneic BALB/c mice was significantly delayed when compared with coinjection of live 12B1-D1 cells with nonstressed apoptotic leukemia cells. The data presented herein clearly demonstrate that the immunogenicity of apoptotic leukemia cells is relatively poor but can be significantly increased by application of stress stimuli, such as induction of HSPs on the surface of apoptotic tumor cells.
Materials and methods
Bcr-abl–positive leukemia cell line
12B1 is a murine leukemia cell line derived by retroviral transformation of BALB/c bone marrow cells with the humanbcr-abl (b3a2) fusion gene and expresses the p210 bcr-abl protein.7This is an aggressive leukemia, with the 100% lethal dose (LD100) being 100 cells after tail vein injection.8 The 12B1 cell line was kindly provided by Dr Wei Chen (Cleveland Clinic, Cleveland, OH). The cell lines were tested monthly and were always found to be free of Mycoplasmacontamination.
Transfection of 12B1 cells with plasmid DNA encoding a mutant FKBP
The cDNA encoding for a recombinant protein that consists of the extracellular domain of the low-affinity nerve growth factor receptor (NGFR) fused to 2 mutant FKBP domains and the Fas death domain was kindly provided by Ariad Pharmaceuticals (Cambridge, MA). This mutant FKBP has reduced affinity for the natural ligand (FK506) and high affinity for synthetic ones.4 5 The fusion-protein gene was excised from the original cloning vehicle and ligated into the expression vector pcDNA containing a zeocin resistance gene (Clontech, Palo Alto, CA). The construct was sequenced to verify ligation fidelity and transformed into Escherichia coli, and plasmid DNA was extracted using a Qiagen Maxiprep kit (Qiagen, Valencia, CA). Plasmid DNA was then transfected into 12B1 bcr-abl–positive leukemia cells by electroporation. Transfected cells were grown in selective media to obtain stable transfectants, which were then plated in serial dilution to less than one cell per well. Multiple stable clones were obtained by selection and analyzed by flow cytometry to confirm surface expression of NGFR.
Flow cytometry
12B1 leukemia cells were washed in phosphate-buffered saline (PBS) containing 2% heat-inactivated fetal bovine serum and 0.1% sodium azide (Sigma Chemical, St Louis, MO). A total of 2 × 105 cells were placed in each well of 96-well U-bottom microtiter plates. Surface expression of specific antigens was determined by incubating with saturating amounts of monoclonal antibodies for 30 minutes at 4°C. Antibodies used included purified antimouse Fas–fluorescein isothiocyanate (FITC) conjugated (CD95) (clone Jo2, hamster IgG; Pharmingen, San Diego, CA), purified anti-HSP72 (clone C92F3A-5, mouse IgG1; StressGen, Victoria, BC, Canada), purified anti-HSP60 (clone LK-1, mouse IgG1; StressGen), and anti-NGFR tissue culture supernatant (clone 200-3-G6-4, mouse IgG1; American Type Culture Collection, Rockville, MD). The cells were then washed 3 times in PBS containing 2% heat-inactivated fetal bovine serum and 0.1% sodium azide. Secondary antibody used was phycoerythrin (PE)-conjugated affiniPure F(ab′)2 fragment goat antimouse IgG (Jackson Immunoresearch, West Grove, PA). After a 30-minute incubation, the cells were washed 3 times and fixed with PBS containing 1% paraformaldehyde (Polysciences, Warrington, PA). Ten thousand cells were analyzed using a FACScan (Becton Dickinson Immunocytometry Systems, San Jose, CA).
Immunoblotting
The presence of HSP72 and HSP60 protein in the 12B1-D1 cells was confirmed by Western blotting. Following sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the gels were electroblotted to nitrocellulose using an Idea Scientific electroblotter (Minneapolis, MN). Gels were transferred in 25 mM Tris, 200 mM glycine, and 20% methanol for 1 hour at 25 V; stained with Ponceau Red to verify adequate protein transfer; and destained in TBST (50 mM Tris-Cl, 150 mM NaCl, 0.1% Tween 20, pH 7).4 Blots were blocked in 5% nonfat dried milk in TBST for 20 to 60 minutes, followed by 3 × 5-minute rinses in TBST. The protein of interest was identified using the monoclonal antibodies anti-HSP72 (clone C92F3A-5, mouse IgG1; StressGen) and purified anti-HSP60 (clone LK-1, mouse IgG1; StressGen). Primary antibody solutions were prepared in blocking solution, and blots were incubated in primary antibody for 1 hour at room temperature or 12 hours at 4°C, followed by 3 × 5-minute rinses in TBST. Alkaline phosphatase–conjugated goat antimouse secondary antibody was applied for 1 hour at room temperature or 12 hours at 4°C. Immunoreactive signals were detected by color deposition of the alkaline phosphatase substrates nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (Roche, Indianapolis, IN).
Mice
Six- to 10-week-old female BALB/c (H-2d) mice (Harlan Sprague Dawley, Indianapolis, IN) and C.B-17scid/scid mice (University of Arizona animal breeding facility) were used for the experiments. The animals were housed in a dedicated pathogen-free facility and cared for according to the University of Arizona Institutional Animal Care and Use Committee guidelines.
Generation of bone marrow–derived dendritic cells
BALB/c mouse bone marrow dendritic cells (DCs) were generated using a slightly modified protocol from that described previously.9 Bone marrow was harvested from femurs and tibiae and filtered through a Falcon 100-μg nylon cell strainer (Becton Dickinson Labware, Franklin Lakes, NJ). Red blood cells were lysed in a hypotonic buffer and the marrow was cultured in AIM V medium (therapeutic grade; Gibco BRL, Gaithersburg, MD), which contains L-glutamine, human serum albumin, 50 μg/mL streptomycin sulfate, and 10 μg/mL gentamicin sulfate. Murine granulocyte-macrophage colony-stimulating factor (10 ng/mL; kindly provided by Immunex, Seattle, WA) and interleukin (IL)-4 (10 ng/mL; Peprotech, Rocky Hill, NJ) were added to the culture. After 4 days, the nonadherent and loosely adherent cells were harvested, layered onto a metrizamide gradient (14.5% metrizamide solution; Sigma), and centrifuged. The enriched DCs were then washed and used for the in vivo experiments. Less than 10% of these DCs were contaminated by macrophages.8
Cell viability assessment using MTT assay
Parental 12B1 and transfected 12B1-D1 clones were plated in 96-well flat-bottom plates (50 000/well) in the presence of increasing concentrations of AP20187 for 24 hours. MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, stock solution 5 mg/mL (Sigma) at 10 μL per well was added for an additional 4 hours. The supernatant was aspirated and the formazan crystals were solubilized in dimethylsulfoxide, followed by determination of optical densities at 560 nm and 690 nm using a microtiter plate reader.
Frequency estimation of surviving 12B1-D1 cells after treatment with dimerizer
To estimate the sensitivity of 12B1-D1 to AP20187, we studied heat-stressed or non–heat-stressed cells. Limiting dilution was performed with 12B1-D1 cells added to 96-well plates at 30 replicates with 6 dilutions of 1000 down to 4 cells/well. Individual wells were considered negative if there was no cell growth after 2 weeks. The 95% confidence intervals of the frequencies and χ2 estimates of probability were calculated. P > .05 is statistically significant and confirms that the data follow “single hit” kinetics. The Poisson equation was used10 to calculate the frequency of surviving cells.
Confirmation of apoptotic cell death in dimerizer-treated 12B1-D1 cells
To confirm that 12B1-D1 cells were in fact dying by apoptosis, we performed Annexin V-FITC and propidium iodide (PI) staining using the Annexin-V-FLUOS staining kit (Roche) followed by flow cytometric analysis of cells. DNA fragmentation analysis was done using the apoptotic DNA ladder kit from Roche. Electron microscopy was also performed to confirm specific apoptotic morphology.
In vivo tumor growth experiments
Nonstressed 12B1-D1 cells or those incubated at 42°C in a water bath for 1 hour were treated with 40 nM AP20187 for 6 hours, then washed 3 times in PBS. In experiments with DCs, the 12B1-D1 cells were incubated with the DCs for 6 hours in the presence of AP20187. A total of 5 × 105 viable 12B1-D1 cells, determined by trypan blue exclusion, were inoculated subcutaneously (SC) into the right groin of BALB/c or SCID mice. In experiments with DCs, the ratio of viable tumor cells to DCs was 1:1. Tumor size was measured every other day with calipers once the tumors became palpable. Tumor volume was calculated using the formula: length × width2 × π/6. Differences in mean tumor volume between groups were compared using an unpaired t test. In rechallenge experiments, 2 × 104 live 12B1-D1 cells (LD100) were injected into the right groin 40 days after the first challenge, whereas 106 A20 leukemia cells (LD100)11 12 were injected into the left groin.
Results
Transfected 12B1 cells expressing mutant FKBP and the Fas death domain undergo apoptosis after exposure to AP20187
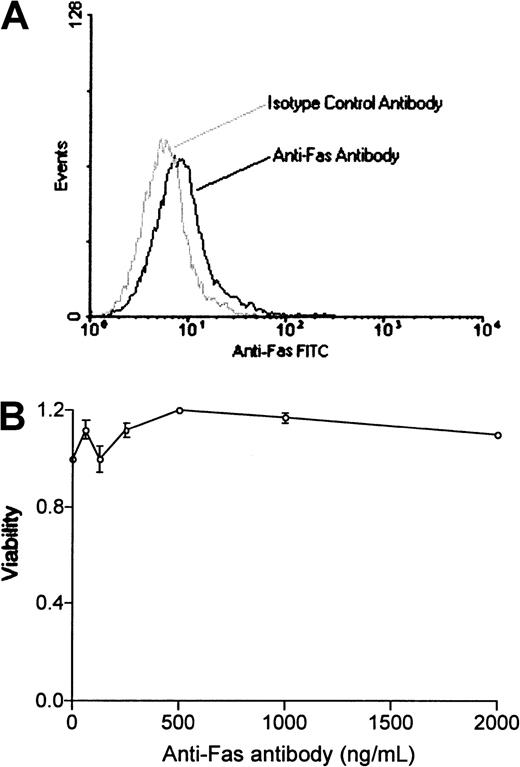
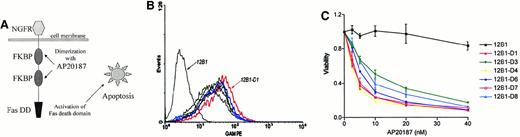
We used a bcr-abl–positive leukemia, 12B1, to investigate whether apoptotic leukemia cells can elicit an immune response if induced to express HSPs. 12B1 cells do not express endogenous Fas on their surface (Figure1A) and consequently do not undergo apoptosis after incubation with anti-Fas antibody (Figure 1B). We therefore established a system to induce selective apoptosis of 12B1. The cDNA encoding for a fusion protein consisting of the extracellular domain of the low-affinity NGFR fused to 2 mutant FKBP domains and the Fas death domain was transfected into the 12B1 leukemia line (Figure2B). Multiple stable clones were obtained by zeocin antibiotic selection and were analyzed by flow cytometry to confirm surface expression of NGFR (Figure 2B). Zeocin-resistant, NGFR-expressing clones were studied further for in vitro sensitivity to the dimerizer AP20187. Using MTT assays, we demonstrated that transfected clones (12B1-Dx) were sensitive to increasing concentrations of AP20187, whereas the parental line (12B1) was not (Figure 2C). The clone with the highest expression of NGFR, 12B1-D1, was also one of the most susceptible to AP20187. To verify that 12B1-D1 cells were dying by apoptosis, we performed flow cytometry on cells stained with FITC-conjugated Annexin V and PI (Figure3A). We also performed electron microscopy (Figure 3B) and DNA fragmentation analyses (Figure 3C). All of these assays confirmed that 12B1-D1 cells were undergoing cell death with the classic features of apoptosis. We should note that heat stress before AP20187 treatment of cells did not alter the features of apoptosis as determined by Annexin staining (data not shown) and DNA fragmentation analysis (Figure 3C). Limiting dilution analyses were done to assess the sensitivity of 12B1-D1 to AP20187. Using the Poisson equation,10 13 we calculated that the frequency of surviving cells, when AP20187 was continuously present in the assay, was approximately 1 in 400 cells (Figure4). For the in vivo experiments (described below), heat-stressed and nonheat-stressed 12B1-D1 cells were exposed to the drug for only 6 hours; limiting dilution assays indicated that this short exposure resulted in survival of 1 of 13 and 1 of 12 cells, respectively (Figure 4). These assays therefore confirm that a heat shock of 42°C for 1 hour did not modify the sensitivity of 12B1-D1 to AP20187.
12B1 cells do not express Fas or respond to anti-Fas antibody.
(A) 12B1 cells do not express Fas on their surface. 12B1 cells were incubated with purified antimouse Fas–FITC-conjugated antibody, washed, and analyzed by flow cytometry. (B) Failure of increasing concentrations of antimouse Fas antibody to induce apoptosis in 12B1 cells in vitro as evaluated by MTT assay after 24 hours.
12B1 cells do not express Fas or respond to anti-Fas antibody.
(A) 12B1 cells do not express Fas on their surface. 12B1 cells were incubated with purified antimouse Fas–FITC-conjugated antibody, washed, and analyzed by flow cytometry. (B) Failure of increasing concentrations of antimouse Fas antibody to induce apoptosis in 12B1 cells in vitro as evaluated by MTT assay after 24 hours.
System used to induce selective apoptosis of 12B1.
(A) Transmembrane fusion protein consisting of a low-affinity nerve growth factor receptor (NGFR) accessible on the cell surface, 2 mutant FK506 binding protein (FKBP) domains, and a Fas death domain (Fas DD) intracellularly. (B) Expression of surface NGFR by stably transfected 12B1 clones. The parental line 12B1 has no expression, whereas clone 12B1-D1 has high levels of NGFR on its surface. (C) Sensitivity of transfected 12B1 clones to increasing concentrations of AP20187. Parental 12B1 cells were found to be resistant to AP20187, whereas transfected clones such as 12B1-D1 were sensitive. Evaluated by MTT assay after 24 hours.
System used to induce selective apoptosis of 12B1.
(A) Transmembrane fusion protein consisting of a low-affinity nerve growth factor receptor (NGFR) accessible on the cell surface, 2 mutant FK506 binding protein (FKBP) domains, and a Fas death domain (Fas DD) intracellularly. (B) Expression of surface NGFR by stably transfected 12B1 clones. The parental line 12B1 has no expression, whereas clone 12B1-D1 has high levels of NGFR on its surface. (C) Sensitivity of transfected 12B1 clones to increasing concentrations of AP20187. Parental 12B1 cells were found to be resistant to AP20187, whereas transfected clones such as 12B1-D1 were sensitive. Evaluated by MTT assay after 24 hours.
12B1-D1 cells exposed to AP20187 undergo apoptosis.
(A) The 12B1-D1 clone was cultured in the presence of 40 nM AP20187, and induction of apoptosis was assessed by Annexin V and PI staining at the indicated times. (B) Electron micrographs of 12B1-D1 cells exposed to AP20187 at 0, 6 hours (6000×). (C) DNA fragmentation analysis. Lane 1, 100-bp ladder; lane 2, DNA extracted from 12B1-D1; lane 3, 12B1-D1 + HS (A), cells were heat shocked at 42°C for 1 hour; lane 4, 12B1-D1 + HS (B), cells were heat shocked at 42°C for 1 hour and then incubated for 6 hours at 37°C; lane 5, 12B1-D1 + AP20187 for 6 hours; lane 6, 12B1-D1 + HS for 1 hour + AP20187 for 6 hours.
12B1-D1 cells exposed to AP20187 undergo apoptosis.
(A) The 12B1-D1 clone was cultured in the presence of 40 nM AP20187, and induction of apoptosis was assessed by Annexin V and PI staining at the indicated times. (B) Electron micrographs of 12B1-D1 cells exposed to AP20187 at 0, 6 hours (6000×). (C) DNA fragmentation analysis. Lane 1, 100-bp ladder; lane 2, DNA extracted from 12B1-D1; lane 3, 12B1-D1 + HS (A), cells were heat shocked at 42°C for 1 hour; lane 4, 12B1-D1 + HS (B), cells were heat shocked at 42°C for 1 hour and then incubated for 6 hours at 37°C; lane 5, 12B1-D1 + AP20187 for 6 hours; lane 6, 12B1-D1 + HS for 1 hour + AP20187 for 6 hours.
Frequency estimate of 12B1-D1 cells surviving treatment with AP20187.
Limiting dilution was performed. (A) 12B1-D1 cells were plated in the presence of 40 nM AP20187. (B) The cells were previously incubated for 6 hours with 40 nM AP20187, washed, and then added to 96-well plates. (C) The cells were previously heat shocked at 42°C for 1 hour, then incubated for 6 hours with 40 nM AP20187, washed, and then seeded into 96-well plates. Individual wells were considered negative if there was no cell growth after 2 weeks. The 95% confidence intervals (CIs) of the frequencies and χ2 estimates of probability were calculated. P > .05 is statistically significant and confirms that the data follow “single hit” kinetics.
Frequency estimate of 12B1-D1 cells surviving treatment with AP20187.
Limiting dilution was performed. (A) 12B1-D1 cells were plated in the presence of 40 nM AP20187. (B) The cells were previously incubated for 6 hours with 40 nM AP20187, washed, and then added to 96-well plates. (C) The cells were previously heat shocked at 42°C for 1 hour, then incubated for 6 hours with 40 nM AP20187, washed, and then seeded into 96-well plates. Individual wells were considered negative if there was no cell growth after 2 weeks. The 95% confidence intervals (CIs) of the frequencies and χ2 estimates of probability were calculated. P > .05 is statistically significant and confirms that the data follow “single hit” kinetics.
Stressed apoptotic tumor cells express HSP72 and HSP60 on their cell surface, and these cells elicit an antitumor response
In vivo experiments were performed to test the immunogenicity (or lack thereof) of apoptotic 12B1-D1 cells. SC inoculation of 5 × 105 AP20187-treated 12B1-D1 cells into the groin of BALB/c mice invariably led to early tumor growth (by day 10). This indicates that despite injection of more than 90% apoptotic cells, a host response potent enough to result in rejection of the remaining viable tumor cells (approximately 4 × 104 live cells based on Figure 4) did not take place. Silent apoptotic death induced by AP20187 therefore appeared to fail to alert the immune system to danger. We reasoned that stressing the tumor cells before triggering apoptosis might induce HSPs, hence supplying danger signals that may be critical for the generation of tumor-specific immunity. 12B1-D1 cells were consequently heat-stressed before apoptosis induction. This resulted in up-regulation of membrane HSP72 and HSP60 as determined by flow cytometry (Figure 5A) and of total cell HSP72 as assessed by Western blotting (Figure 5B). Total HSP60, which is abundantly expressed even under control conditions, did not appear to be increased.
Expression of HSPs by 12B1-D1 cells.
(A) Surface expression of HSP72 and HSP60 on 12B1-D1 cells as determined by flow cytometry. Panel 1: 12B1-D1 cells were incubated with isotype control antibody followed by PE-conjugated goat antimouse IgG and analyzed by flow cytometry. Panels 2-5: 12B1-D1 cells incubated with anti-HSP72 antibody (left) and anti-HSP60 antibody (right) followed by PE-conjugated goat antimouse IgG. Panel 2: Untreated 12B1-D1 cells. Panel 3: 12B1-D1 cells were heat shocked for 1 hour at 42°C and then incubated for 6 hours at 37°C. Panel 4: 12B1-D1 cells were incubated for 6 hours with 40 nM AP20187. Panel 5: 12B1-D1 cells were heat shocked for 1 hour at 42°C, then incubated for 6 hours with 40 nM AP20187. (B) Immunoblot of 12B1-D1 cell lysates. Lane 1, untreated 12B1-D1; lane 2, 12B1-D1 + HS (A), cells were heat shocked at 42°C for 1 hour; lane 3, 12B1-D1 + HS (B), cells were heat shocked at 42°C for 1 hour and then incubated for 6 hours at 37°C; lane 4, 12B1-D1 + AP20187 for 6 hours; lane 5, 12B1-D1 + HS for 1 hour + AP20187 for 6 hours.
Expression of HSPs by 12B1-D1 cells.
(A) Surface expression of HSP72 and HSP60 on 12B1-D1 cells as determined by flow cytometry. Panel 1: 12B1-D1 cells were incubated with isotype control antibody followed by PE-conjugated goat antimouse IgG and analyzed by flow cytometry. Panels 2-5: 12B1-D1 cells incubated with anti-HSP72 antibody (left) and anti-HSP60 antibody (right) followed by PE-conjugated goat antimouse IgG. Panel 2: Untreated 12B1-D1 cells. Panel 3: 12B1-D1 cells were heat shocked for 1 hour at 42°C and then incubated for 6 hours at 37°C. Panel 4: 12B1-D1 cells were incubated for 6 hours with 40 nM AP20187. Panel 5: 12B1-D1 cells were heat shocked for 1 hour at 42°C, then incubated for 6 hours with 40 nM AP20187. (B) Immunoblot of 12B1-D1 cell lysates. Lane 1, untreated 12B1-D1; lane 2, 12B1-D1 + HS (A), cells were heat shocked at 42°C for 1 hour; lane 3, 12B1-D1 + HS (B), cells were heat shocked at 42°C for 1 hour and then incubated for 6 hours at 37°C; lane 4, 12B1-D1 + AP20187 for 6 hours; lane 5, 12B1-D1 + HS for 1 hour + AP20187 for 6 hours.
In vivo experiments were then performed to evaluate whether stress before apoptosis induction of 12B1-D1 cells would promote an antileukemia immune response. Non–heat-stressed and heat-stressed 12B1-D1 cells were exposed to AP20187 for 6 hours before their injection into BALB/c mice. Figure 6A demonstrates that tumors developed substantially more slowly in animals receiving viable cells mixed with heat-shocked, dimerizer-treated 12B1-D1 cells when compared with nonheat-treated cells exposed to dimerizer only (P < .01). The fact that all animals received comparable numbers of live cells (approximately 4 × 104) but tumor growth was delayed in those receiving heat-stressed cells suggests that a significant host response was induced in this group. Heat-stressed or nonstressed AP20187-exposed cells were then injected into SCID mice to assess whether this response was mediated by T cells. In contrast to what was found in immunocompetent BALB/c mice, heat stress before apoptosis induction did not retard tumor growth in immunodeficient mice (Figure 6B), indicating that the host response was, in fact, T-cell dependent.
Effects of heat shock and apoptosis induction on antitumor responses.
(A) 12B1-D1 cells (5 × 105) were incubated for 6 hours with 40 nM AP20187 or heat shocked for 1 hour at 42°C (HS), incubated with AP20187 for 6 hours, and then injected SC into BALB/c mice. Mice were followed for tumor development. Shown are pooled data from 3 experiments (n = 20 mice per group); P < .01 from day 13 onward. (B) 12B1-D1 cells were treated as in (A) and injected into SCID mice (n = 8 mice per group); P = not significant. (C) 12B1-D1 cells (2 × 104) were untreated or heat shocked for 1 hour at 42°C and immediately injected into BALB/c mice, 12B1-D1 + HS (A); or heat shocked for 1 hour at 42°C, incubated at 37°C for 6 hours, and then injected, 12B1-D1 + HS (B) (n = 8 mice per group); P = not significant. (D) Heat-stressed or nonstressed AP20187-treated 12B1-D1 cells (5 × 105) were frozen and thawed for 6 cycles to yield a necrotic lysate and coinjected with 2 × 104 live 12B1-D1 cells (n = 8 mice per group); P = not significant. Panels B, C, and D show representative data from a total of 6 experiments performed.
Effects of heat shock and apoptosis induction on antitumor responses.
(A) 12B1-D1 cells (5 × 105) were incubated for 6 hours with 40 nM AP20187 or heat shocked for 1 hour at 42°C (HS), incubated with AP20187 for 6 hours, and then injected SC into BALB/c mice. Mice were followed for tumor development. Shown are pooled data from 3 experiments (n = 20 mice per group); P < .01 from day 13 onward. (B) 12B1-D1 cells were treated as in (A) and injected into SCID mice (n = 8 mice per group); P = not significant. (C) 12B1-D1 cells (2 × 104) were untreated or heat shocked for 1 hour at 42°C and immediately injected into BALB/c mice, 12B1-D1 + HS (A); or heat shocked for 1 hour at 42°C, incubated at 37°C for 6 hours, and then injected, 12B1-D1 + HS (B) (n = 8 mice per group); P = not significant. (D) Heat-stressed or nonstressed AP20187-treated 12B1-D1 cells (5 × 105) were frozen and thawed for 6 cycles to yield a necrotic lysate and coinjected with 2 × 104 live 12B1-D1 cells (n = 8 mice per group); P = not significant. Panels B, C, and D show representative data from a total of 6 experiments performed.
To determine whether the delayed tumor development may have been the result of heat shock alone and not related to apoptosis per se, we performed additional experiments in which BALB/c mice were injected with 12B1-D1 cells that were exposed to heat but not to AP20187. Heat-stressed 12B1-D1 cells when injected into BALB/c mice grew at the same rate as nonstressed cells (data not shown), suggesting that the combination of stress and apoptosis was necessary for generation of the antitumor response. It is possible, however, that with 5 × 105 cells, any evolving immune response was overwhelmed by the large number of live tumor cells injected. Similar experiments were therefore repeated using the LD100(2 × 104 cells SC). This cell dose was determined previously by in vivo dose-titration experiments. Comparable results were obtained (Figure 6C), confirming that both apoptosis and heat stress were required for an antitumor response to take place. Two heat-treated groups were included in these experiments. In one group, 12B1-D1 + HS (A), the cells were injected immediately after the 1-hour heat shock. In the other group, 12B1-D1 + HS (B), heat shock was followed by incubation at 37°C for 6 hours. This group was included to control for the 6-hour delay in injection of heat-treated cells while they were incubating with AP20187 (Figure 6A,B). Failure to induce protection by this group argues against the possibility that simply resting the heat-stressed cells for 6 hours may have contributed to the delay in tumor growth seen in the experiments depicted in Figure6A.
We used a short time exposure of 12B1-D1 cells to AP20187 to avoid so-called secondary necrosis. Annexin V-FITC/PI staining confirmed that the majority of cells became Annexin V-FITC positive but PI negative (indicating early apoptosis) with a 6-hour exposure to AP20187 (Figure3A). To exclude the possibility that the delayed tumor development observed was the result of secondary necrotic 12B1-D1 cells from which cytoplasmic components were released, we performed the following experiments. Heat-stressed or nonstressed AP20187-treated 12B1-D1 cells were frozen and thawed for 6 cycles to yield a necrotic lysate. Coinjection of lysates with live 12B1-D1 cells did not retard tumor growth compared with mice injected with an equal number of live 12B1-D1 cells only (Figure 6D), suggesting that tumor growth delay in Figure 6A was not due to the release of cytoplasmic components.
Pulsing of DCs with stressed apoptotic tumor cells results in the generation of tumor-specific immunity
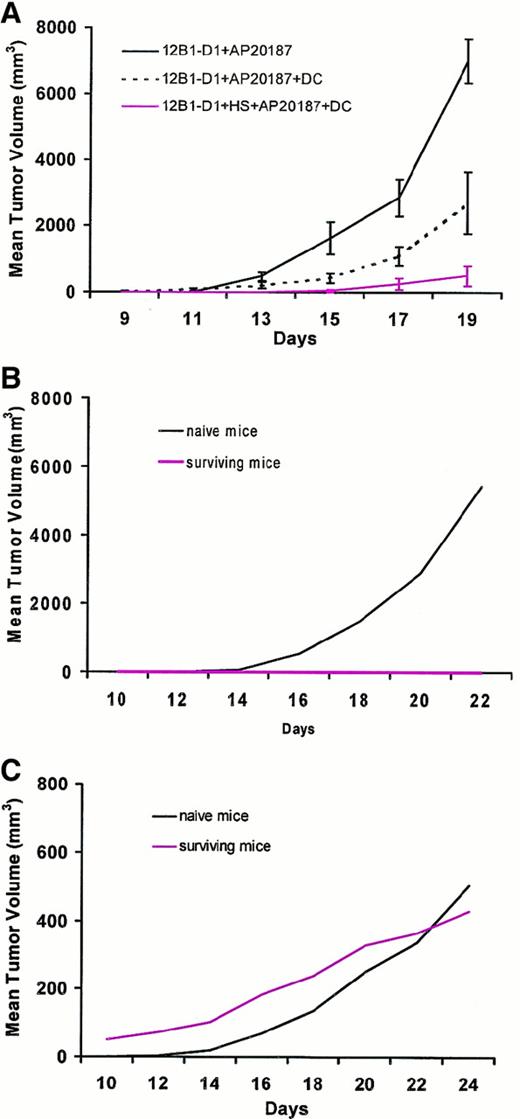
Finally, we examined whether DCs incubated ex vivo with stressed and nonstressed apoptotic cells could induce rejection of coinjected viable leukemia cells, and whether such DCs could generate long-lasting specific immunity. As in previous experiments, SC inoculation of nonstressed dimerizer-treated 12B1-D1 cells led to early tumor growth in all mice injected. DCs were incubated with nonheat-stressed 12B1-D1 cells in a 1:1 ratio for 6 hours in the presence of AP20187 before injection into BALB/c mice. AP20187 is not toxic to normal cells, allowing the coincubation with DCs. Moreover, the phenotype of DCs did not change after a 6-hour exposure to AP20187 (data not shown). The delay in tumor growth seen in this group when compared with nonstressed AP20187-treated 12B1-D1 cells (without DCs) suggests that some protection was generated by pulsing DCs with apoptotic 12B1-D1 cells (Figure 7A). Perhaps expression of HSP72 and HSP60 by about 30% of the AP20187-treated 12B1-D1 cells (Figure 5, panel 4) contributed to maturation/stimulation of DCs. Although injection of DCs incubated with nonstressed, dimerizer-treated 12B1-D1 cells delayed tumor growth, all of the mice eventually developed tumors. In contrast, injection of DCs pulsed with heat-stressed, dimerizer-treated 12B1-D1 cells, of which 90% expressed HSP72 and HSP60 (Figure 5, panel 5), led to a significant delay in tumor growth, with complete rejection of tumors in about two thirds of the challenged mice (Figure 7A). Rechallenge of surviving mice 40 days later with live 12B1-D1 cells (2 × 104; LD100) failed to generate tumors in all mice (Figure 7B). However, all of the naive mice that received the same number of 12B1-D1 cells developed SC tumors. Moreover, all of the immune and naive mice that were challenged with an LD100 (106 cells) injection of different syngeneic leukemia cells (A20) in the opposite groin developed SC tumors, which confirms the specificity of the antitumor response (Figure 7C).
DCs pulsed with heat-stressed apoptotic cells induce a tumor-specific immune response.
(A) 12B1-D1 cells (5 × 105) were incubated for 6 hours with 40 nM AP20187 with or without an equal number of DCs, and 12B1-D1 cells were heat shocked for 1 hour at 42°C, then incubated with AP20187 and DCs for 6 hours. The cell mixtures were washed and injected SC into BALB/c mice. Mice were followed for tumor development (n = 8 mice per group). P < .01 for group 12B1-D1 + AP20187 versus 12B1-D1 + HS + AP20187 + DC from day 13 onward. (B) Naive and surviving BALB/c mice from (A) were rechallenged 40 days later with 2 × 104 12B1-D1 cells in the left groin (n = 5 surviving and 8 naive mice). (C) The same mice were challenged with 106 A20 cells in the right groin. Shown are representative data from a total of 3 experiments performed.
DCs pulsed with heat-stressed apoptotic cells induce a tumor-specific immune response.
(A) 12B1-D1 cells (5 × 105) were incubated for 6 hours with 40 nM AP20187 with or without an equal number of DCs, and 12B1-D1 cells were heat shocked for 1 hour at 42°C, then incubated with AP20187 and DCs for 6 hours. The cell mixtures were washed and injected SC into BALB/c mice. Mice were followed for tumor development (n = 8 mice per group). P < .01 for group 12B1-D1 + AP20187 versus 12B1-D1 + HS + AP20187 + DC from day 13 onward. (B) Naive and surviving BALB/c mice from (A) were rechallenged 40 days later with 2 × 104 12B1-D1 cells in the left groin (n = 5 surviving and 8 naive mice). (C) The same mice were challenged with 106 A20 cells in the right groin. Shown are representative data from a total of 3 experiments performed.
Discussion
In this study, we have demonstrated that apoptotic tumor cells can be either immunogenic or nonimmunogenic. Heat stress before apoptosis induction increases cell surface expression of HSP72 and HSP60. These up-regulated HSPs may play a role in converting nonimmunogenic apoptotic tumor cells into immunogenic ones. Apoptosis, or programmed cell death, is important in normal development and in maintaining physiologic homeostasis. In vivo, apoptotic cells are rapidly phagocytosed by APCs. Macrophages and DCs express pattern-recognition receptors, which specifically bind to apoptotic cell–associated molecular patterns and mediate the efficient phagocytosis of apoptotic bodies.14 Exogenous antigens acquired from apoptotic cells can gain access to the cytoplasm and be cross-presented on MHC class I molecules.15 The fact that professional APCs are involved in phagocytosis, processing, and presentation of apoptotic cell–derived antigens suggests that there may be immunologic consequences of apoptosis. Antibody cross-linking of scavenger receptor CD36 leads to IL-10 secretion,16 whereas αvβ3 integrin ligation promotes transforming growth factor β (TGF-β) production by macrophages.17 DCs apparently use a distinct αvβ5 receptor to phagocytose apoptotic cells.18 Recent reports have addressed the immunologic consequences following engulfment of apoptotic cells by DCs.19-21 In vitro studies have demonstrated measurable cellular responses induced by apoptotic cells. However, a potent immune response was seldom generated in vivo.22-24 When immature DCs endocytose apoptotic bodies, they are not stimulated to mature, and consequently may present their processed antigens in the absence of adequate costimulation, thereby inducing tolerance.22,24 25 This appears to be an important protective physiologic process because normal cell turnover must not induce autoimmune responses. Similarly, cancer cells undergoing apoptosis typically do not elicit immune responses against their own antigens.
We used a novel gene-transfer approach by which rapid induction of pure apoptosis downstream of the Fas/FasL apoptotic pathway can be selectively achieved in a transfected tumor cell population. This can be accomplished without any toxicity to other cells following exposure to AP20187. This system allowed us to perform both in vitro and in vivo studies. To avoid so-called secondary necrosis, we used a short 6-hour exposure of 12B1-D1 cells to AP20187. This resulted in more than a 90% apoptotic cell population with the remaining cells being clonogenic, as determined by limiting dilution assay, and viable. This cell mixture was used as a concurrent vaccine-challenge preparation. Mice injected with nonstressed apoptotic and viable 12B1-D1 cells cell mixture SC developed early growth of tumor. These tumors developed at the same rate as tumors growing following injection of an equal number of viable cells only. This indicates that a measurable immune response induced by nonstressed apoptotic cells did not take place. “Silent” apoptotic death induced by AP20187 therefore apparently failed to alert the immune system to danger.
APCs can process and present antigens acquired from apoptotic cells, but an active immune response is not induced, which may be due to a lack of danger signals.2 HSP induction following stress can provide the danger signals required for generation of a more effective immune response.26 Recent studies have indicated that tumor immunogenicity is associated with up-regulation of the highly inducible HSP70 isoform, HSP72.3,27 Inducible HSP70 can assume dual roles as a chaperone and a cytokine28 to activate monocytes and up-regulate the expression of proinflammatory cytokines. Exogenous HSP60 has also been shown to stimulate macrophages to express IL-12 and IL-15 and rapidly release tumor necrosis factor α (TNF-α).29 HSP60 appears to signal through CD14 and Toll-like receptors, thus sharing the same pathways as lipopolysaccharide.30,31 HSPs may directly mature DCs32 or act indirectly by stimulating macrophages29 and cytotoxic T lymphocytes (CTLs)33 to secrete proinflammatory cytokines. The induction of HSP expression may therefore provide the danger signals required to convert nonimmunogenic apoptotic tumor cells into immunogenic ones. Other mechanisms may also be involved, including repertoire skewing associated with antigen chaperoning by intracellular HSP.
We reasoned that heat stressing the tumor cells before triggering apoptosis might induce HSPs, hence supplying danger signals that may be critical for the generation of tumor-specific immunity. Heat stress treatment (42°C for 1 hour) per se did not induce 12B1-D1 cells to undergo apoptosis, and heat stress before AP20187 treatment did not alter the features of apoptosis and had no effects on the sensitivities of 12B1-D1 cells to AP20187 treatment. Stress protein expression is fairly commonly associated with hematologic malignancies. We found that heat stress resulted in up-regulation of membrane HSP72 and HSP60 as determined by flow cytometry and of total cell HSP72 as assessed by Western blotting. Coinjection of mice with heat-stressed apoptotic and viable cells resulted in a substantial delay in tumor development compared with mice coinjected with nonstressed apoptotic 12B1-D1 cells and the same number of viable cells. This was due to a T-cell–mediated immune response induced by stressed apoptotic 12B1-D1 cells because this effect was not seen in SCID mice. It is not yet clear whether HSPs have to be on the surface of apoptotic cells or whether an increase of total cell levels is sufficient for this response to take place. Current studies in our laboratory are addressing this issue.
Previous studies have shown that surface expression of inducible HSP72 by tumor cells increases their susceptibility to lysis by natural killer (NK) cells.34-36 Among the cell lines tested by these investigators was the NK-sensitive, bcr-abl–positive human leukemia line K562. If NK activity were critical in our model system, one would have expected delayed growth of heat-stressed nonapoptotic 12B1-D1 cells because of increased killing in vivo by BALB/c or SCID mouse NK cells. However, this was not evident from our in vivo studies, suggesting that there may be differences between HSP72-expressing cell lines with respect to their sensitivities to NK-mediated killing.
Lysis or necrosis of cells leads to the release of intracellular danger stimuli.37 To exclude the possibility that the delayed tumor growth we observed was the result of necrotic 12B1-D1 cells from which cytoplasmic components were released, we lysed heat-stressed or nonstressed viable plus apoptotic cells. The lysates were then coinjected with 2 × 104 live 12B1-D1 cells. Interestingly, coinjection of lysates with viable tumor cells did not retard tumor growth when compared with mice injected with an equal number of viable cells only. This was consistent with our previous studies using a B-cell leukemia/lymphoma model.38 Even heat treatment before lysing the cells did not influence tumor growth. It therefore appears that the combination of efficient phagocytosis of stressed apoptotic cells coupled with increased HSP levels generated effective immunity against this tumor.
Antigens acquired by DCs from apoptotic cells can induce MHC-restricted CTLs in vitro.20 The chemokine receptor-7 (CCR-7) is up-regulated when DCs are coincubated with apoptotic tumor cells, promoting DC migration to regional lymph nodes39 and potentially generating tumor-specific immune responses.23However, whether apoptotic cells will induce potent T-cell responses in vivo may ultimately depend on the local cellular and cytokine milieu. Proinflammatory cytokines such as IL-12, interferon-γ, or TNF-α promote T-cell responses, whereas anti-inflammatory cytokines such as IL-10 and TGF-β suppress them. Recent studies have documented that macrophages ingesting apoptotic bodies inhibit proinflammatory cytokine production through an autocrine/paracrine mechanism.40 The presence of macrophages phagocytosing apoptotic cells may therefore have a negative effect on DC-mediated antitumor T-cell immunity. We examined whether DCs incubated ex vivo with stressed and nonstressed apoptotic cells could induce rejection of coinjected viable leukemia cells and generate long-lasting specific immunity. Injection of syngeneic DCs that had been pulsed with stressed apoptotic leukemia cells resulted largely in rejection of coinjected viable leukemia cells. Mice rejecting the primary 12B1-D1 inoculum were immune to the same but not to a different leukemia challenge, confirming the long-term and specific antitumor immunity.
In summary, our studies conclusively demonstrate that apoptotic tumor cells can be either immunogenic or nonimmunogenic in vivo. APCs such as macrophages and DCs can efficiently endocytose apoptotic cells, whose antigens can gain access to the cytoplasm and be cross-presented on MHC class I molecules. During physiologic processes such as normal development and tissue homeostasis, however, an active immune response is usually not induced because of the lack of danger signals. Heat stress before apoptosis induction appears to result in substantial expression of membrane HSPs on tumor cells. HSP72 and HSP60 on the surface of apoptotic cells may provide the necessary danger signals to macrophages and DCs to induce the secretion of proinflammatory cytokines, the maturation of DCs, and ultimately the generation of potent antitumor T-cell responses. Necrotic cell death therefore is not a prerequisite for the development of an immune response; apoptosis under stress conditions can also elicit T-cell responses in vivo. These findings may have implications for the development of anticancer vaccines using stressed apoptotic tumor cells.
We thank Michael Graner, Kamalesh Ramaiya, and Meghan Kreeger for their helpful comments.
Supported in part by the Leukemia and Lymphoma Society of America (E.K.), the Tee Up for Tots Fund (Y.Z), the Enid and Mel Zuckerman Fund (H.F.), and the National Cancer Institute, National Institutes of Health (CA59537) (L.W.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Emmanuel Katsanis, Department of Pediatrics, University of Arizona, 1501 N Campbell Ave, PO Box 245073, Tucson, AZ 85724-5073; e-mail: katsanis@peds.arizona.edu.