Most thymocytes are deleted by thymic selection. The mechanisms of cell death are far from being clear. Peroxynitrite is a powerful oxidant produced in vivo by the reaction of superoxide (O2•−) with nitric oxide (NO•) and is able to mediate apoptosis. The aim of this study was to analyze whether NO and peroxynitrite could play a role in human thymocyte apoptosis. The results indicate that 3-(4-morpholinyl)-sydnonimine (SIN-1, an O2•− and NO• donor) and chemically synthesized peroxynitrite, but not S-nitroso-N-acetyl-D,L-penicillamine (SNAP, an NO• donor), have a strong apoptotic effect on human thymocytes (annexin V staining and TUNEL reaction). This effect was inhibited by exogenous superoxide dismutase (SOD), which interacts with O2•− and inhibits the formation of peroxynitrite. Because peroxynitrite formation requires NO•, thymic stromal cells were investigated to determine if they produced NO•. Inducible NOS was synthesized in cultured thymic epithelial cells in certain conditions of cytokine stimulation, as shown by messenger RNA levels, protein analysis, and nitrite production in the supernatants. SIN-1–treated thymocytes had high levels of tyrosine nitration, abolished by the addition of exogenous SOD. Tyrosine nitration was also detected in thymus extracts and sections, suggesting the presence of peroxynitrite in situ. In thymus sections, clusters of nitrotyrosine-positive cells were found in the cortex and corticomedullary areas colocalized with cells positive in the TUNEL reaction. These data indicate an association between human thymocyte apoptosis and nitrotyrosine formation. Thus, the results support the notion of a physiologic role for peroxynitrite in human thymocyte apoptosis.
Introduction
The thymus plays a central role in T-cell differentiation and T-cell repertoire selection. Clonal deletion of immature thymocytes is an important mechanism ensuring self-tolerance. Few of the thymocytes generated in the thymus survive to become mature T cells, the remainder being deleted through thymic selection.1 The cellular and molecular mechanisms underlying the deletion of immature thymocytes are unclear, but these processes are known to be driven by interactions between developing thymocytes and thymic stromal cells.2 The thymus stroma is a complex structure. One of the main cell types is the thymic epithelial cell (TEC), which has a key role in T-cell development and in the acquisition of self-tolerance.3-5
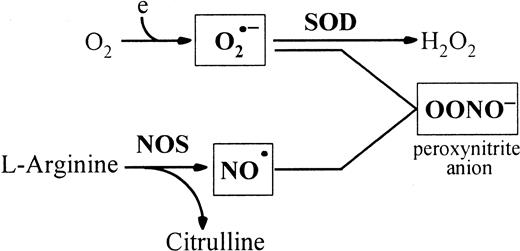
Many intracellular reactions, including respiration, reduce oxygen to superoxide (O2•−) and hydrogen peroxide. Nitric oxide (NO•) is an important messenger molecule with key roles in a wide variety of physiologic functions, including neuronal transmission, vascular relaxation, immune modulation, cytotoxicity, and inhibition of platelet aggregation. NO•synthesis is catalyzed from L-arginine by nitric oxide synthase (NOS), a hemoprotein with 3 known isoforms: the neuronal isoform (nNOS), the endothelial isoform (eNOS), and the inducible isoform (iNOS).6 Reaction of O2•− with NO• generates peroxynitrite (Figure1), which may modulate NO•signaling functions and have direct cytotoxic effects through extensive protein tyrosine nitration.7-9 Several observations suggest that reactive oxygen species (ROS) might mediate programmed cell death.10 For example, both ionizing and ultraviolet radiation generate reactive oxygen intermediates such as H2O2 and OH•, and both are capable of inducing apoptosis. Exposure to low concentrations of H2O2 induces apoptosis in a variety of cell types. NO• also induces apoptosis in macrophages, monocytes, and dendritic cells.11 The causative role of oxidative stress in T-cell apoptosis has also been demonstrated. Thiol antioxidants such as glutathione and N-acetyl cysteine can completely block major histocompatibility complex–antigen and mitogen-driven activation-induced death of myelin basic protein–specific T-cell hybridomas.12 In the murine thymus, the involvement of NO• in apoptosis has been suggested.13,14Peroxynitrite was shown to induce rat thymocyte apoptosis.15 The role of ROS in thymocyte apoptosis was also demonstrated in studies reporting that antioxidant reagents can block thymocyte apoptosis in response to glucocorticoids, calcium ionophores, or gamma radiation.16 17
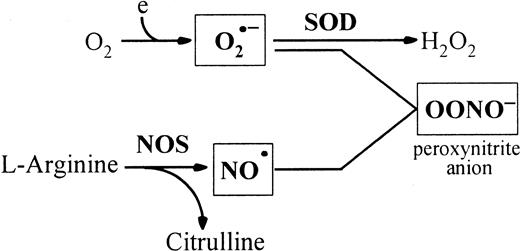
Peroxynitrite is produced by the reaction of O2•− with NO•.
NO• is produced by NOSs that utilize L-arginine and oxygen to form NO• and citrulline. SOD catalyses the dismutation of O2•− radicals. Peroxynitrite induces tyrosine nitration in vivo. Thus, the peroxynitrite concentration is mainly regulated by SOD and NOS.
Peroxynitrite is produced by the reaction of O2•− with NO•.
NO• is produced by NOSs that utilize L-arginine and oxygen to form NO• and citrulline. SOD catalyses the dismutation of O2•− radicals. Peroxynitrite induces tyrosine nitration in vivo. Thus, the peroxynitrite concentration is mainly regulated by SOD and NOS.
The possible role of oxidative stress in the human thymus has not yet been studied. We therefore examined the role of ROS in human thymocyte apoptosis. We show that peroxynitrite, but not NO•, is a powerful inducer of apoptosis of human thymocytes. This phenomenon is associated with nitrotyrosine formation both in vitro and ex vivo, suggesting a possible physiologic role of peroxynitrite in human thymocyte apoptosis.
Materials and methods
Thymic tissues
Fresh thymic fragments were obtained as discarded tissue from children younger than 5 years old undergoing corrective cardiovascular surgery at Marie Lannelongue Hospital (Le Plessis Robinson, France). Fragments of some specimens were flash-frozen in liquid nitrogen and stored at −80°C.
Thymocyte isolation and culture
Thymocytes were collected after mincing the thymus and were cultured in RPMI 1640 Glutamax I medium (Life Technologies, Cergy-Pontoise, France) supplemented with 10% fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL fungizone (Bristol Myers Squibb, Paris, France). Thymocytes (2 × 106 in 1 mL) were cultured in 24-well plates with various concentrations of diethylamine NONOate (DEA-NO; Cayman Chemical, Ann Arbor, MI), S-nitroso-N-acetyl-D,L-penicillamine (SNAP; Biomol, Plymouth Meeting, PA) or 3-(4-morpholinyl)-sydnonimine (SIN-1; Cayman Chemical, Ann Arbor, MI). Peroxynitrite was synthesized as previously described.18 At various culture times, thymocytes were harvested, counted, and tested for viability (trypan blue exclusion method). In some experiments, superoxide dismutase (SOD) from bovine erythrocytes (Sigma Chemical, Saint Quentin Fallavier, France) was added in the culture medium at concentrations varying from 100 to 1000 U/mL.
To analyze their potential production of iNOS, thymocytes were activated either by phorbol myristate acetate (PMA) (2.5 ng/mL) and ionomycin (0.25 mg/mL) for 24 hours or with concanavalin A (10 μg/mL) for 3 days. Total RNA was then extracted, and iNOS was analyzed by reverse transcriptase–polymerase chain reaction (RT-PCR).
Measurement of apoptosis
Apoptosis was analyzed by quantifying phosphatidylserine residues exposed on the external cell membrane.19 One microliter of human recombinant fluorescein isothiocyanate (FITC)-conjugated annexin V (Boehringer Mannheim, Meylan, France) and 2 μg/mL propidium iodide (PI) were added to 100 μL cell suspension in binding buffer (10 mM HEPES/NaOH, pH 7.4; 140 mM NaCl; 5 mM CaCl2). After 15 minutes of incubation in the dark, dual-color flow cytometry was performed.
Apoptosis was also quantified by measuring DNA fragmentation and by using the TdT-mediated dUTP nick end labeling (TUNEL) method with the Boehringer Mannheim kit following the manufacturer's instructions. Briefly, 106 cells were fixed in phosphate-buffered saline (PBS) containing 2% paraformaldehyde. After 2 washes with PBS, cells were permeabilized in hypotonic solution containing 0.1% Triton X-100 and 0.1% sodium citrate for 2 minutes on ice. Cells were washed twice in PBS and resuspended in TUNEL reaction mixture containing terminal deoxynucleotidyl transferase and FITC-coupled dUTP. After 2 washes, the label incorporated at damaged DNA sites was visualized on a FACScan flow cytometer (Becton Dickinson, Grenoble, France) using Cell Quest software.
Setup of conditions of flow cytometry analysis
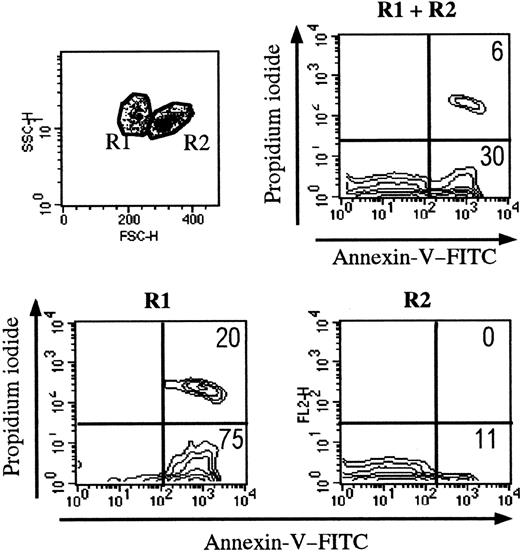
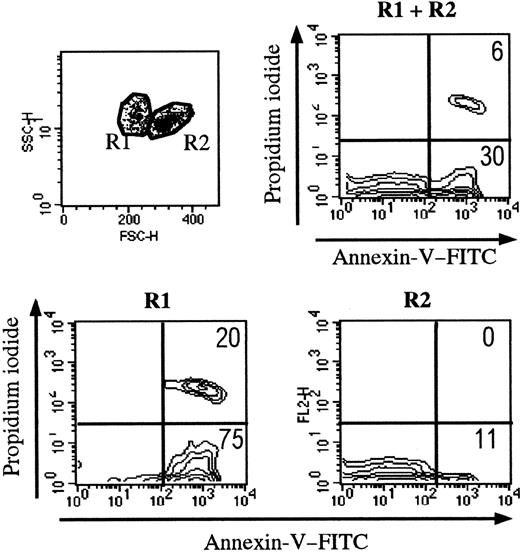
Trypan blue staining was used to determine cell viability before each experiment. When 106 thymocytes were cultured in control conditions, 0.86 × 106 ± 0.06 × 106 viable cells were recovered after 18 hours. We then measured thymocyte binding of FITC-conjugated annexin V by flow cytometry (Figure2). According to forward- and side-scatter criteria, 2 regions (R1 and R2) were identified. As shown in Figure 2, R1 cells were mainly apoptotic (annexin V+PI−) and necrotic (annexin V+ PI+) thymocytes; R2 mainly contained living cells. After 18 hours of culture, about 30% of all thymocytes were apoptotic (FITC–annexin V+ and PI−) and were essentially found in R1. Of note, freshly isolated human thymocytes contained only a few apoptotic cells, found in R1 (data not shown). Except when indicated, the flow cytometry analysis was performed in the whole population of thymocytes (R1 and R2).
Setting up the analysis of thymocytes during apoptosis.
Apoptotic cells present distinct side-scatter/forward-scatter features. The R1 gate essentially contains apoptotic cells (defined by annexin V staining). The R2 gate mainly contains living cells.
Setting up the analysis of thymocytes during apoptosis.
Apoptotic cells present distinct side-scatter/forward-scatter features. The R1 gate essentially contains apoptotic cells (defined by annexin V staining). The R2 gate mainly contains living cells.
In some experiments, thymocytes were labeled with anti-CD4–Cy5 (diluted 1:3) and anti-CD8–phycoerythrin (diluted 1:3) monoclonal antibodies (Dako, Trappes, France) before using the annexin V staining protocol.
Immunofluorescence on frozen human thymic sections
Thymic sections were from 4 different newborn individuals (1 week to 2 years). Cryostat thymic sections (5 μm) were fixed with acetone for 10 minutes. To detect nitrotyrosine, rabbit polyclonal antinitrotyrosine antibody (Upstate Biotechnology, Lake Placid, NY) was applied in a dilution of 1:100 for 60 minutes. The sections were then washed 3 times and revealed using goat antirabbit bound to tetramethylrhodamine isothiocyanate (GAR-TRITC; Immunotech, Marseille, France) at a 1:100 dilution for 30 minutes. Double labeling with monoclonal antikeratin antibodies at a 1:50 dilution (mix of LP34 and MNF116 monoclonal antibodies) (Dako) revealed by goat antimouse immunoglobulins coupled to fluorescein at a 1:100 dilution (GAM-FITC; Silenius, Eurobio, France) was performed to visualize the epithelial network in the thymus. Two types of controls were performed:(1) The antinitrotyrosine antibody (10 μL) was mixed in 1 mL PBS with free nitrotyrosine (10 M) for 1 hour at room temperature before being applied on thymic sections, and (2) the fluorescent second-layer antibodies were directly tested on thymic sections.
Three-color immunofluorescence was performed as follows: After fixation and washings, the slides were incubated with antinitrotyrosine antibody (1:100 dilution for 1 hour) revealed by GAR-TRITC (1:100 dilution for 30 minutes), then with antikeratin-mix antibodies (1:50 dilution for 30 minutes) revealed by RAM-AMCA (rabbit antimouse aminoethylcoumarin acetic acid, Dako) diluted 1:10 and, lastly, with monoclonal anti-iNOS antibody coupled to FITC (diluted 1:25) for 2 hours (Transduction Laboratories, Lexington, KY).
To assess the in situ cell death, we used a kit for detection of apoptosis based on labeling of DNA strand breaks (Boehringer Mannheim). Briefly, the slides were fixed with paraformaldehyde 2% for 30 minutes followed by 3 washes. They were then permeabilized in 0.1%Triton X-100 in PBS for 2 minutes on ice. The slides were then washed 3 times, and the TUNEL reaction mixture was added on the samples for 60 minutes at 37°C in the dark. The slides were then washed 3 times before applying the antinitrotyrosine antibody as described above. A negative control was included. It consists of incubating the sections with the label solution without terminal transferase instead of TUNEL reaction mixture.
Fluorescence was then visualized using the Leica DMRB microscope (Wetzlar, Germany) equipped with a Sony digital charge-coupled device camera for capturing images (Koehn, Germany).
Culture of thymic epithelium
Primary cultures of human TECs were established as previously described.20 Briefly, small fragments of thymic tissue were washed in RPMI and transferred to culture dishes. The culture medium consisted of RPMI 1640 Glutamax I (Life Technologies) supplemented with 20% horse serum, 0.2% Ultroser (Life Technologies), 100 U/mL penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL fungizone and was replaced twice-weekly. Cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2. After 8 to 12 days of primary culture, the confluent monolayers were treated with 0.075% trypsin (Life Technologies) and 0.16% ethylenediaminetetraacetic acid (EDTA) for 5 minutes at 37°C. To study NOS regulation, TECs were subcultured in 24-well plates (5 × 105 cells/well) and incubated overnight to allow cells to adhere. After 2 washes, 1 ng/mL recombinant human interleukin (IL)-1β (Sigma), 10 ng/mL recombinant tumor necrosis factor (TNF)-α, and 500 U/mL recombinant human interferon (IFN)-γ (Genzyme, Cergy Saint Christophe, France) were added, separately or together, in RPMI containing 5% horse serum. When indicated, 0.5 mM Nω-nitro-L-arginine methyl ester (L-NAME, Sigma) was also added. After various incubation times, TECs were treated with trypsin-EDTA solution and the cell suspension thus obtained was washed, centrifuged, and used for flow cytometry, protein extraction, or total RNA preparation. The epithelial nature of the cells was shown by means of immunofluorescence with an antikeratin antibody (clone CK1, Dako); the percentage of epithelial cells was consistently higher than 95%. Fibroblasts represented around 5% of the cultured cells, as shown by staining with an anticollagen III antibody (ICN, Orsay, France).
RNA preparation and RT-PCR
Total RNA was prepared from TEC pellets by using the acid guanidinium thiocyanate-phenol-chloroform extraction method as described by Chomczynski et al.21 The cell pellet was homogenized in 0.1 mL denaturing solution (4 M guanidinium thiocyanate; 25 mM sodium citrate, pH 7; 0.5 N lauryl sarcosyl; and 0.1 M 2-mercaptoethanol). Extracted total RNA was purified with 0.5 vol 7.5 M ammonium acetate and 2.5 vol 100% ethanol and then centrifuged at 15 000 rpm for 30 minutes at 4°C. The pellet was washed in 75% ethanol, dried under a vacuum, and stored at −80°C after dissolution in water. The total RNA concentration was determined from absorbance at 260 nm in a Gene Quant spectrophotometer (Amersham Pharmacia Biotech, Buckinghamshire, England). Purity was checked by measuring the 260 nm:280 nm optical density (OD) ratio.
The oligonucleotide primers used for RT-PCR (Genset, Paris, France) have the following sequences: iNOS primers, forward 5′-ATGTCTGGCAGGACGAGAAG-3′ and reverse 5′-CTGAATGTGCTGTTTGCCTC-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers, forward 5′-ATCACCATCTTCCAGGAG-3′ and reverse 5′-CCTGCTTCACC- ACCTTCTTG-3′.
A 50 μL RT reaction mixture containing 2 μg total RNA, 5 μL 10 × RT buffer (Eurobio, Les Ulis, France), 1.5 mM each deoxynucleoside triphosphate (dNTP) (Eurobio), 40 U RNasin (Promega, Charbonnières, France), 1 μM reverse primer, and 4 U avian myeloblastosis virus (AMV) reverse transcriptase (Eurobio) was incubated at 42°C for 60 minutes. PCR was carried out in a total volume of 100 μL containing 10 μL RT reaction mix, 10 μL PCR buffer (Eurobio), 1.5 mM MgCl2, 0.5 μM each primer, 0.2 mM each dNTP, and 2.5 U EurobioTaqII polymerase (Eurobio). The mixture was overlaid with mineral oil and amplified in a PHC3 thermal cycler (Techne, Cambridge, United Kingdom) as follows: denaturing step, 94°C for 1 minute; annealing step, 55°C (iNOS) or 60°C (GAPDH) for 1 minute; extension step, 72°C for 2 minutes. The final elongation step lasted 10 minutes at 72°C. PCR products were analyzed on 1.5% agarose gel containing ethidium bromide.
Western blotting
Primary cultures of human TECs were solubilized in 1% Triton X-100; 150 mM NaCl; 50 mM Tris, pH 8.0; 5 mM EDTA, pH 8.0; 0.02% NaN3; 1 mM phenylmethylsulfonyl fluoride; and 0.15 U/mL aprotinin for 20 minutes at 4°C. Insoluble material was removed by centrifugation at 4°C for 10 minutes. The protein concentration was measured with the bicinchoninic acid (BCA) protein assay reagent (Pierce, Rockford, IL).
Samples were suspended by boiling in sample buffer and were then analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis in 8% to 18% linear acrylamide gradients gels overlaid with 5% acrylamide stacking gels. Protein bands were electroblotted onto nitrocellulose filters. Blots were saturated in buffer containing 20 mM Tris, pH 7.4; 500 mM NaCl; 0.1% Tween-20; and 1% bovine serum albumin. Anti-iNOS (Transduction Laboratories) or antinitrotyrosine (Euromedex, Souffelweyersheim, France) antibodies diluted in this buffer were added and allowed to bind overnight. Bound immunoglobulins were detected indirectly by using peroxidase-conjugated antimouse immunoglobulin antibodies. Immunoreactivity was determined using the enhanced chemiluminescence reaction (Amersham). To check the specificity of the antinitrotyrosine reactivity, the antibody (10 μL) was absorbed with 90 μL free competing nitrotyrosine (10 mM) for 1 hour before being applied in the Western blot experiments as described above.
Immunohistochemical analysis of cytocentrifuged cells
Cultured TECs (5 × 104 per slide) were cytocentrifuged (Cytospin 2, Shandon, United Kingdom), air-dried, fixed in acetone, and tested with the labeled streptavidin biotin (LSAB) labeling kit (Dako). Briefly, the cells were incubated for 5 minutes with the blocking antibody (normal goat serum), then with 5 μg/mL anti-iNOS monoclonal antibody (Transduction Laboratories) for 30 minutes, and washed in PBS. Biotinylated antimouse immunoglobulins were then added for 30 minutes. After 3 washes in PBS, horseradish peroxidase–conjugated streptavidin was added for 10 minutes. After 3 washes the amino-3-ethyl-9 carbazole (AEC) substrate was added for 10 minutes, and the slides were rinsed with distilled water and mounted in glycerol/PBS.
Nitrite analysis
Nitrite production by TECs was measured as an index of NOS activity. Confluent cells in 24-well plates were washed twice with PBS and incubated with Dulbecco modified Eagle medium without phenol red, with or without cytokines, for the indicated times. The medium was then removed, and nitrite was measured by mixing 200 μL supernatant with 50 μL Griess reagent as previously described.22Absorbance was read at 550 nm, and the nitrite concentration was determined from a calibration curve constructed with sodium nitrite standards.
Results
ONOO− but not NO• induces thymocyte apoptosis
Comparative effects of NO• donors on thymocyte apoptosis.
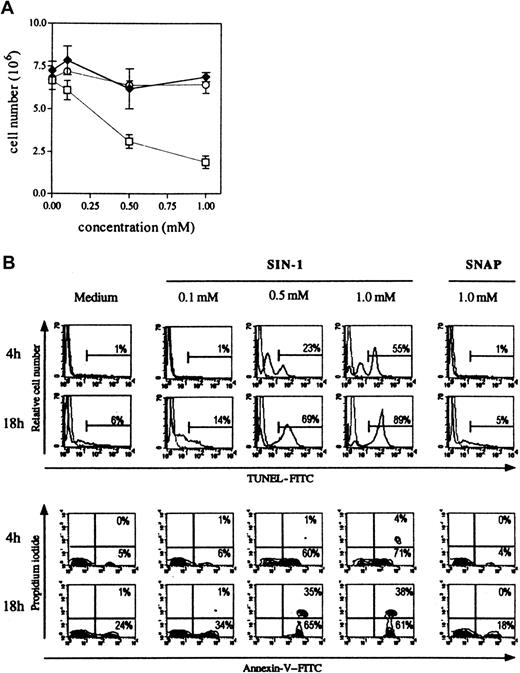
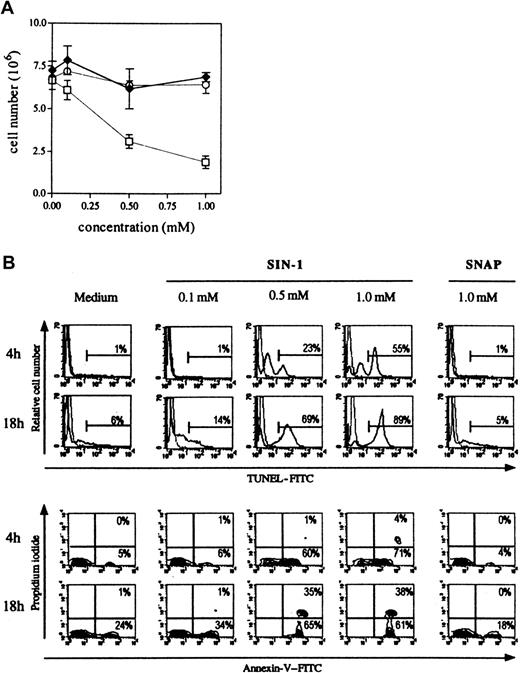
Three different NO• donors were used: SNAP and DEA-NO that produce only NO•, SIN-1 that produces NO• and O2•−, and thus, subsequently, peroxynitrite (Figure 1). As shown in Figure3A, only SIN-1 reduced the number of cells recovered after 18 hours of culture. When 8 × 106cells were cultured in the presence of the different NO•donors, 7.0 × 106 ± 0.3 × 106 cells were collected in control conditions, 6.9 × 106 ± 0.3 × 106 in the presence of 1 mM SNAP, 6.4 × 106 ± 0.5 × 106 in the presence of 1 mM DEA-NO, and 1.9 × 106 ± 0.4 × 106 in the presence of 1 mM SIN-1. SIN-1–induced human thymocyte depletion was due to apoptosis, as shown by phosphatidylserine residue exposure (annexin V binding) and DNA fragmentation (TUNEL reaction) (Figure 3B). The induction of apoptosis by SIN-1 was observed as early as 4 hours because more than half the thymocytes were TUNEL-positive (1% in control conditions) and 71% were annexin V+ and PI− (5% in control conditions) with 1 mM SIN-1. All cells were annexin V+ after 18 hours of incubation with 0.5 mM or 1 mM SIN-1, and 75% of cells were viable in control conditions. The flow cytometry analysis was performed in the whole population of thymocytes.
SIN-1, contrary to SNAP, induces human thymocyte apoptosis.
(A) The total number of cells was significantly reduced by 0.5 nM SIN-1 (■) but not by SNAP (♦) or DEA-NO (○). The results are expressed as the mean numbers of viable cells in 4 different experiments. (B) SIN-1 (0.5 mM) induced apoptosis (TUNEL reaction and annexin V staining) of a large population after as little as 4 hours. Most cells were apoptotic after 18 hours of treatment with 0.5 mM SIN-1. SNAP had no effect after 18 hours of culture. The analysis was performed on the whole population. The data are representative of 4 individual experiments. The percentages in each panel indicate the percentage of TUNEL-positive cells in the upper panel, and percentage of Annexin V–positive cells in the lower panel.
SIN-1, contrary to SNAP, induces human thymocyte apoptosis.
(A) The total number of cells was significantly reduced by 0.5 nM SIN-1 (■) but not by SNAP (♦) or DEA-NO (○). The results are expressed as the mean numbers of viable cells in 4 different experiments. (B) SIN-1 (0.5 mM) induced apoptosis (TUNEL reaction and annexin V staining) of a large population after as little as 4 hours. Most cells were apoptotic after 18 hours of treatment with 0.5 mM SIN-1. SNAP had no effect after 18 hours of culture. The analysis was performed on the whole population. The data are representative of 4 individual experiments. The percentages in each panel indicate the percentage of TUNEL-positive cells in the upper panel, and percentage of Annexin V–positive cells in the lower panel.
Effect of synthesized ONOO−.
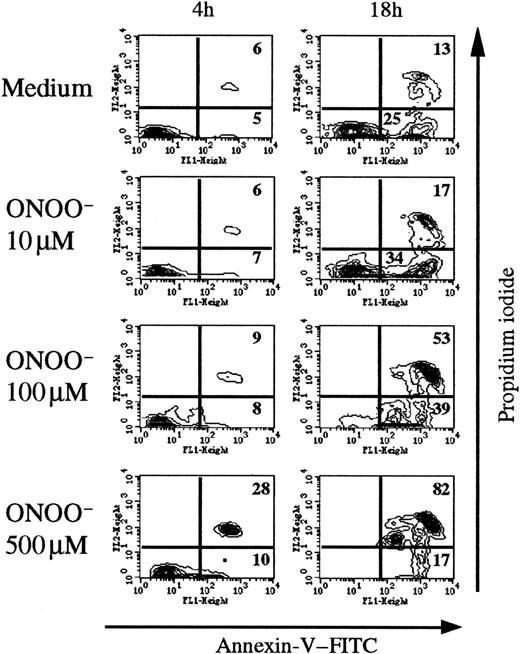
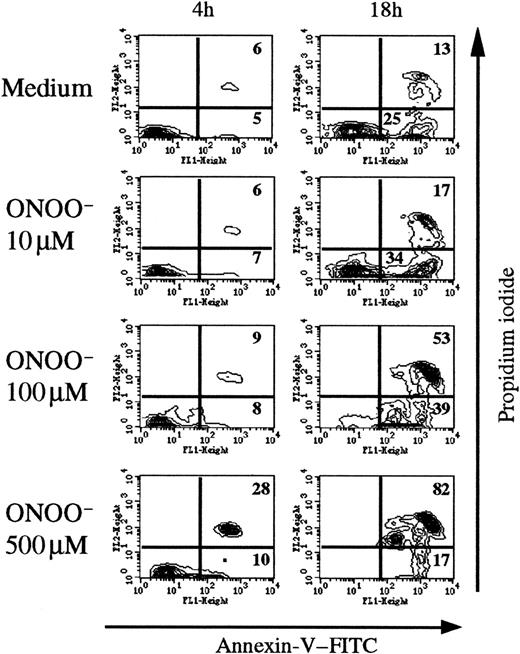
To analyze the direct effects of peroxynitrite, we added chemically synthesized peroxynitrite (10-500 μM) on human thymocytes and measured annexin V binding after various times (Figure4). A significant effect is observed among annexin V+ PI+ and annexin V+PI− cells. After 4 hours of incubation with 500 μM ONOO−, 28% of thymic cells were annexin V+PI+ (6% in control conditions). After 18 hours the proportion of annexin V+ PI+ cells increased with the ONOO− concentration, up to 82% with 500 μM ONOO− compared with 13% in control conditions.
Chemically synthesized ONOO− induces human thymocyte apoptosis.
Flow cytometric analysis of annexin V staining after 4 and 18 hours of exposure to various concentrations of ONOO−. The percentage of annexin+ cells was time- and concentration-dependent, reaching maximum values (> 80%) at 18 hours with 500 μM ONOO. The analysis was performed on the whole thymocyte population. The data are representative of 3 individual experiments.
Chemically synthesized ONOO− induces human thymocyte apoptosis.
Flow cytometric analysis of annexin V staining after 4 and 18 hours of exposure to various concentrations of ONOO−. The percentage of annexin+ cells was time- and concentration-dependent, reaching maximum values (> 80%) at 18 hours with 500 μM ONOO. The analysis was performed on the whole thymocyte population. The data are representative of 3 individual experiments.
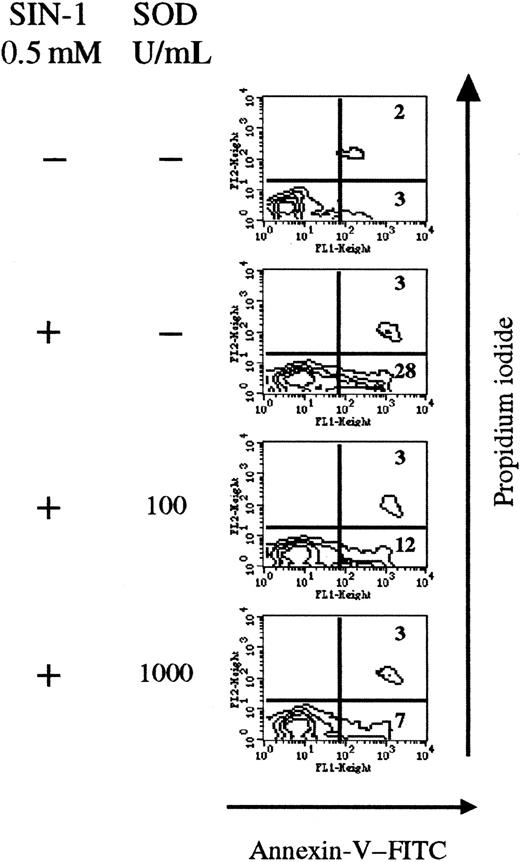
SOD inhibits the apoptotic effect of SIN-1.
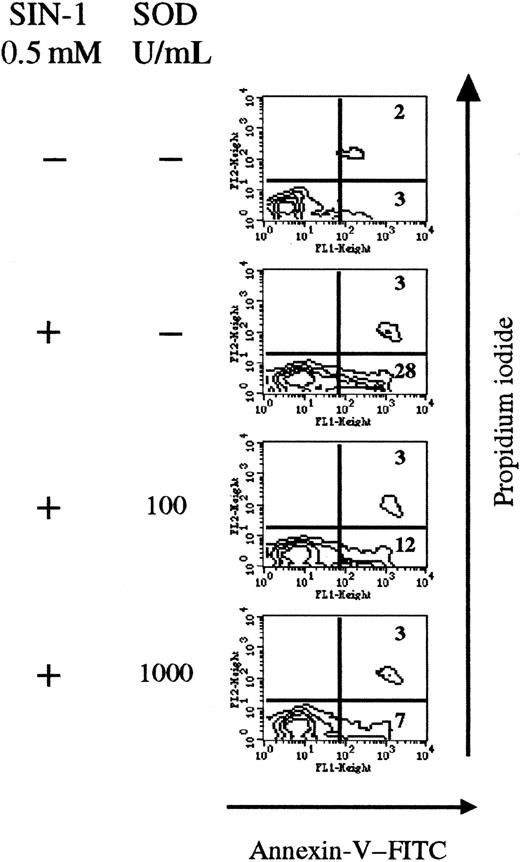
SOD competes with NO• and prevents peroxynitrite formation (Figure 1). Thus SOD was added together with SIN-1 in thymocyte cultures. After 4 hours, SIN-1 (0.5 mM) induced apoptosis of 28% of total thymic cells, compared with only 3% in control conditions. Addition of exogenous SOD significantly reduced the number of annexin V+ cells (7% of total cells at 1000 U/mL SOD) in a concentration-dependent manner (Figure5).
Exogenous SOD inhibits the apoptotic effect of SIN-1.
Cells were treated with 0.5 nM SIN-1 and various concentrations of SOD for 4 hours. SIN-1 induced annexin V staining of 31% of cells after 4 hours. Addition of SOD strikingly reduced the percentage of annexin V+ cells in a concentration-dependent manner. The analysis was performed on the whole thymocyte population. The data are representative of 3 individual experiments.
Exogenous SOD inhibits the apoptotic effect of SIN-1.
Cells were treated with 0.5 nM SIN-1 and various concentrations of SOD for 4 hours. SIN-1 induced annexin V staining of 31% of cells after 4 hours. Addition of SOD strikingly reduced the percentage of annexin V+ cells in a concentration-dependent manner. The analysis was performed on the whole thymocyte population. The data are representative of 3 individual experiments.
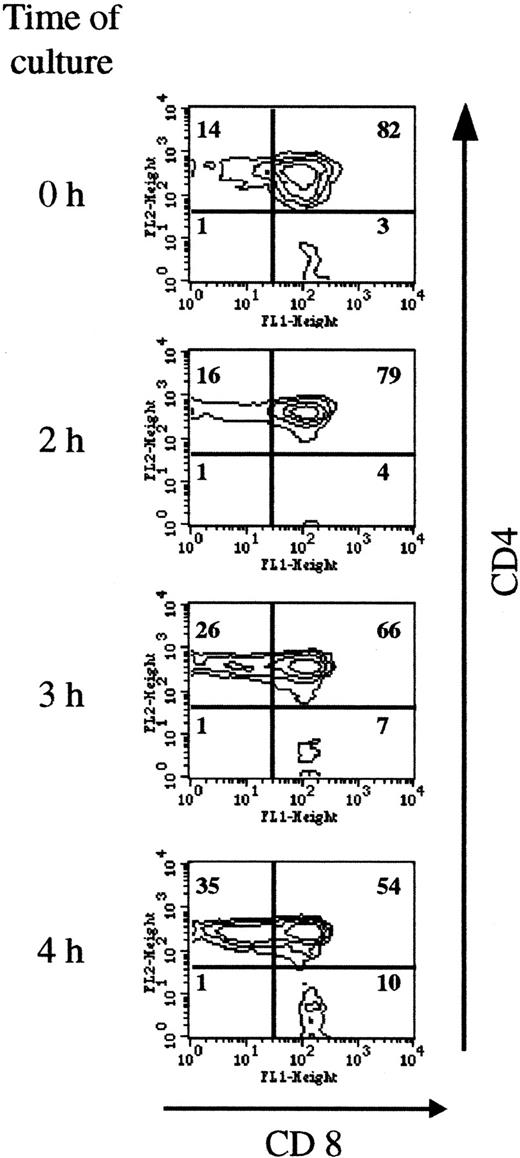
Double-positive immature thymocytes are the T-cell subpopulation most sensitive to SIN-1–induced apoptosis
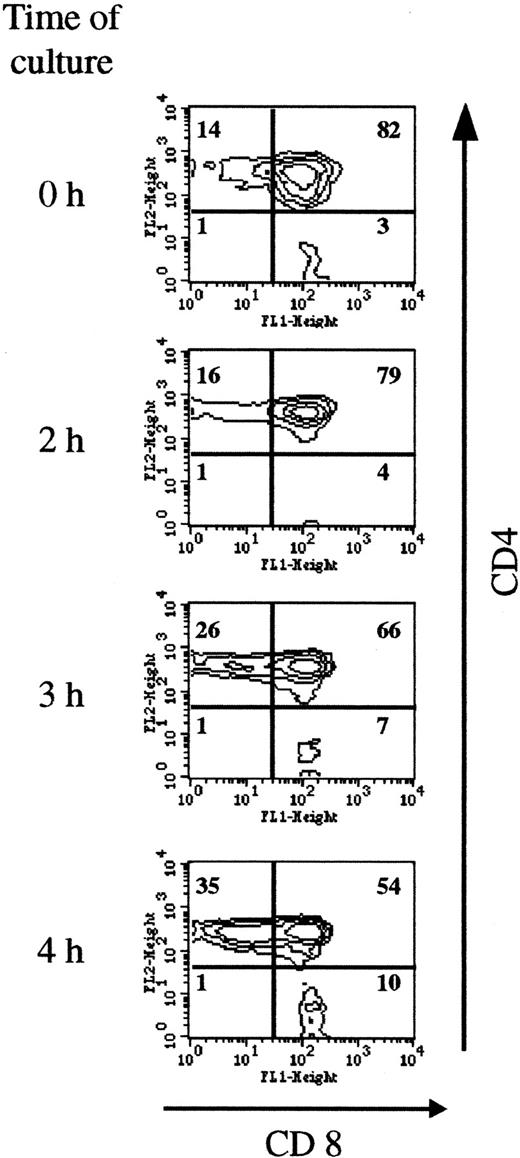
As shown in Figure 6, short incubation in the presence of SIN-1 depleted CD4+CD8+ cells (from 82% at the beginning of the culture to 54% after 4 hours of incubation with 0.5 mM SIN-1), and the percentages of CD4+ and CD8+single-positive cells increased accordingly. The fall in CD4+CD8+ cell numbers observed in the R2 gate was compensated for by an increase in cell numbers in the R1 gate (Figure 2). After 4 hours of incubation with 0.5 mM SIN-1, the percentage of annexin V+ PI− cells was higher in the CD4+CD8+ population than in the single mature cells (Table 1). This analysis was performed in the R2 gate. Taken together, these data indicated that CD4+CD8+ cells are more sensitive to SIN-1–induced apoptosis than mature cells. At a later stage (18 hours), more than 80% of the cells were apoptotic, and all the subpopulations were concerned (data not shown).
Double-positive cells are most sensitive to SIN-1–induced apoptosis.
Thymocyte CD4 and CD8 expression was analyzed on the viable cells (R2 gate). The distribution of the populations changed as early as 3 hours, with a fall in the percentage of double-positive cells (from 82% to 54% after 4 hours of treatment) and a corresponding increase in CD4+ and CD8+ cells. The data are representative of 3 individual experiments.
Double-positive cells are most sensitive to SIN-1–induced apoptosis.
Thymocyte CD4 and CD8 expression was analyzed on the viable cells (R2 gate). The distribution of the populations changed as early as 3 hours, with a fall in the percentage of double-positive cells (from 82% to 54% after 4 hours of treatment) and a corresponding increase in CD4+ and CD8+ cells. The data are representative of 3 individual experiments.
Tyrosine nitration levels increase during thymocyte apoptosis
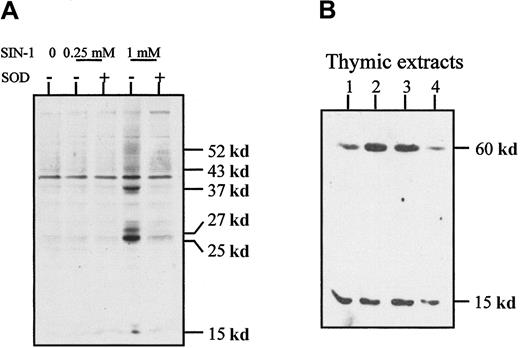
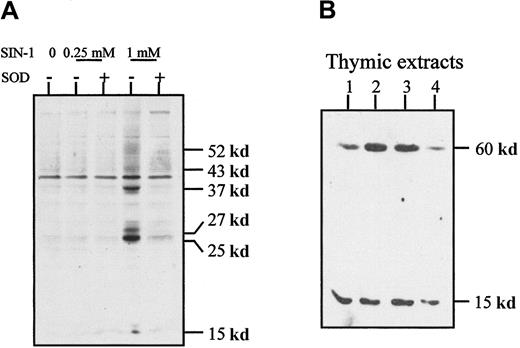
Because peroxynitrite has the functional property of inducing tyrosine nitration, we examined whether SIN-1–treated cells contain proteins with nitrated tyrosine residues. The level of tyrosine nitration was clearly increased after 4 hours of treatment with 1 mM SIN-1 and affected a few thymic proteins as shown in Western blot experiments (Figure 7A). This effect was strongly inhibited by 1000 U/mL SOD, which also reversed SIN-1–induced apoptosis of human thymocytes (Figure 5). In normal thymic extracts from children undergoing cardiac surgery, 2 major proteins with nitrated tyrosines were detected around 15 and 60 kd (Figure 7B). When the antinitrotyrosine antibody was incubated with free nitrotyrosine before its application in the Western blot experiment, the signal was strikingly reduced (data not shown).
Tyrosine nitration in thymocytes.
(A) Western blot analysis of thymocyte extracts with an antinitrotyrosine antibody showed that SIN-1 induced a high level of tyrosine nitration on several proteins, an effect partly reversed by exogenous SOD. These data are representative of 3 different experiments. (B) Western blot analysis of normal thymic extracts with an antinitrotyrosine antibody showed 2 major bands in all the samples studied. The data shown are representative of 3 different experiments and 10 distinct thymic samples.
Tyrosine nitration in thymocytes.
(A) Western blot analysis of thymocyte extracts with an antinitrotyrosine antibody showed that SIN-1 induced a high level of tyrosine nitration on several proteins, an effect partly reversed by exogenous SOD. These data are representative of 3 different experiments. (B) Western blot analysis of normal thymic extracts with an antinitrotyrosine antibody showed 2 major bands in all the samples studied. The data shown are representative of 3 different experiments and 10 distinct thymic samples.
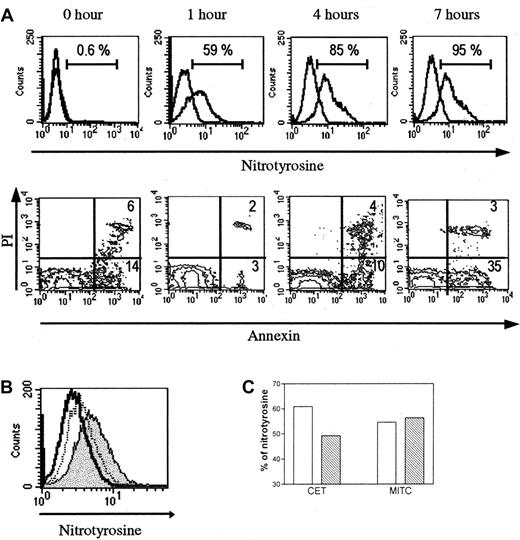
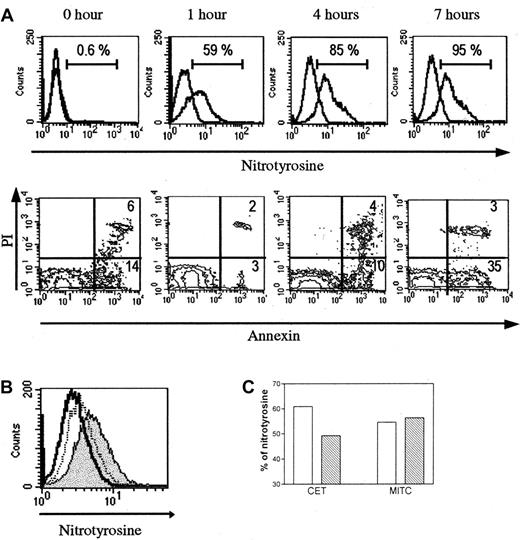
The antinitrotyrosine reactivity was also evidenced by flow cytometry after membrane permeabilization of human thymocytes. A kinetics study shown in Figure 8A indicates that nitrotyrosine-positive cell percentage increases in the first hours of the culture of thymocytes, in parallel with the increase of apoptotic cells determined by annexin V staining. Of note, the percentage of apoptotic cells is higher before culture, probably due to dying cells provoked by the mechanical dissociation. Preincubation of the antinitrotyrosine antibody with free competing nitrotyrosine reverses the immunoreactivity of the antinitrotyrosine antibody (Figure8B).
Analysis of nitrotyrosine in thymocytes by flow cytometry.
(A) Kinetics analysis. Untreated human thymocytes cultured for several hours show a gradual increased expression of nitrotyrosine. This increase is parallel to the number of annexin V+ cells, except before the culture where the number of annexin V+cells is higher, probably due to dying cells because of the mechanical dissociation. (B) When antinitrotyrosine antibody is absorbed with free nitrotyrosine before incubation with thymocytes, the fluorescence peak is shifted to negative values (dotted line peak). The positive peak is colored in gray, and the control peak is colorless. (C) In cocultures, thymocytes incubated with TEC in presence of L-NAME (▧) show a moderate reduction of the nitrotyrosine-positive cells, but this is not observed with the MITC line used as a control thymic cell line (■).
Analysis of nitrotyrosine in thymocytes by flow cytometry.
(A) Kinetics analysis. Untreated human thymocytes cultured for several hours show a gradual increased expression of nitrotyrosine. This increase is parallel to the number of annexin V+ cells, except before the culture where the number of annexin V+cells is higher, probably due to dying cells because of the mechanical dissociation. (B) When antinitrotyrosine antibody is absorbed with free nitrotyrosine before incubation with thymocytes, the fluorescence peak is shifted to negative values (dotted line peak). The positive peak is colored in gray, and the control peak is colorless. (C) In cocultures, thymocytes incubated with TEC in presence of L-NAME (▧) show a moderate reduction of the nitrotyrosine-positive cells, but this is not observed with the MITC line used as a control thymic cell line (■).
Tyrosine nitration is inhibited by NOS inhibitors in coculture experiments
In coculture experiments, thymocytes were incubated with TECs or with MITC (a thymic myoid cell line) used as thymic cell line control for 4 hours, and the percentage of nitrotyrosine-positive cells was determined (Figure 8C). When the coculture was performed in presence of L-NAME (0.01 mM), the percentage of nitrotyrosine-positive cells was decreased from 60.9% to 49.2% (19.2% decrease); in coculture with MITC, L-NAME (0.01 mM) had no effect because the percentage of nitrotyrosine-positive cells varies from 55% to 56%.
Detection of nitrotyrosine on thymic sections
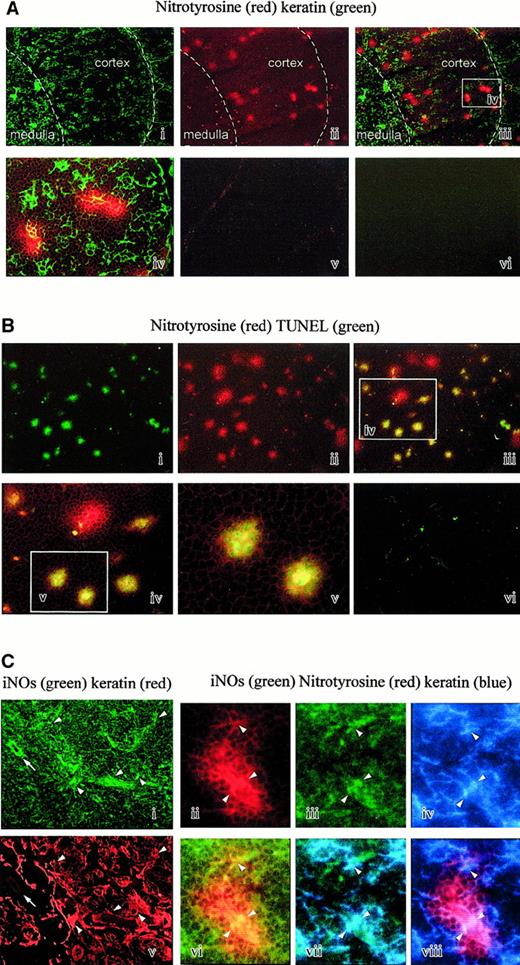
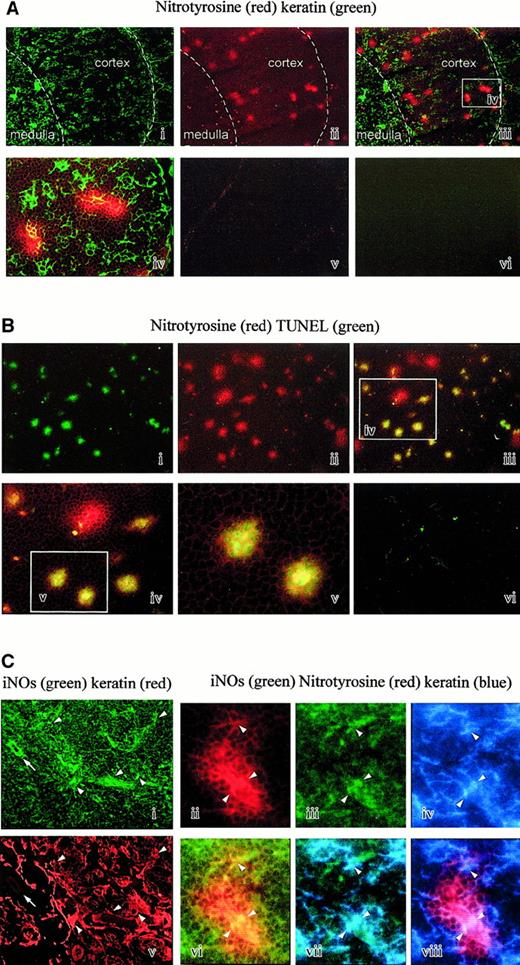
The presence of nitrated proteins in tissues is regarded as a specific footprint of peroxynitrite,8,9 although nitrating activity could also be generated by myeloperoxidase in specific conditions.23 To investigate the relevance of peroxynitrite in vivo, we analyzed the presence of tyrosine nitrations on human thymic sections by immunofluorescence using a specific polyclonal antibody. The thymic samples were from 4 newborn individuals. Small spots of positive cells were clearly detectable (Figure 9Aii). Double staining with antikeratin antibodies shows that nitrotyrosine-positive spots are located in the thymic cortex and at the corticomedullary junction, depicted as a white dotted line on the microphotographies (Figure 9Ai-iii). At a higher magnification, we could visualize TECs included in nitrotyrosine-positive spots (Figure 9Aiv). The controls performed by absorbing the antinitrotyrosine activity with free competing nitrotyrosine (Figure 9Av) or by omitting the first layers were negative (Figure 9Avi).
Analysis of nitrotyrosine on thymic sections.
(A) Nitrotyrosine and keratin double staining. Nitrotyrosine spots (red staining) are mainly observed in the cortex and at the corticomedullary junction (i). Double staining with antikeratin antibody delineates the cortex and the medulla (ii, iii). The superimposed microphotography is shown in panel Aiii (× 25). A higher magnification of the white rectangle (iv) shows that some epithelial cells (green staining) are often contained in the nitrotyrosine spots (× 100). The control performed by absorbing the antinitrotyrosine activity with competing nitrotyrosine is negative (v). The control performed by omitting the first layers is also negative (vi). (B) Nitrotyrosine and TUNEL double staining. Nitrotyrosine spots (red staining) in a cortical area (ii) are often associated with cells positive in a TUNEL reaction (green staining) (i, iii) (× 25). Double-stained cells are shown at a higher magnification of the white rectangle (iii-v). It appears that most but not all nitrotyrosine spots are associated with a TUNEL reaction (× 100). The control is negative (vi). (C) Nitrotyrosine, keratin, and iNOS triple staining. Epithelial cells (ii) are frequently iNOS+ (i), shown by the arrowhead, but keratin-negative cells are also iNOS+, shown by the arrow. Nitrotyrosine spots (iii) could include iNOS+ (iv) keratin-positive cells (v). Superimposition of antinitrotyrosine (iii) and iNOS (iv) staining is shown in panel Cvi. Superimposition of iNOS (iv) and antikeratin (v) staining is shown in panel Cvii. Superimposition of antinitrotyrosine (iii) and antikeratin (v) staining is shown in panel Cviii.
Analysis of nitrotyrosine on thymic sections.
(A) Nitrotyrosine and keratin double staining. Nitrotyrosine spots (red staining) are mainly observed in the cortex and at the corticomedullary junction (i). Double staining with antikeratin antibody delineates the cortex and the medulla (ii, iii). The superimposed microphotography is shown in panel Aiii (× 25). A higher magnification of the white rectangle (iv) shows that some epithelial cells (green staining) are often contained in the nitrotyrosine spots (× 100). The control performed by absorbing the antinitrotyrosine activity with competing nitrotyrosine is negative (v). The control performed by omitting the first layers is also negative (vi). (B) Nitrotyrosine and TUNEL double staining. Nitrotyrosine spots (red staining) in a cortical area (ii) are often associated with cells positive in a TUNEL reaction (green staining) (i, iii) (× 25). Double-stained cells are shown at a higher magnification of the white rectangle (iii-v). It appears that most but not all nitrotyrosine spots are associated with a TUNEL reaction (× 100). The control is negative (vi). (C) Nitrotyrosine, keratin, and iNOS triple staining. Epithelial cells (ii) are frequently iNOS+ (i), shown by the arrowhead, but keratin-negative cells are also iNOS+, shown by the arrow. Nitrotyrosine spots (iii) could include iNOS+ (iv) keratin-positive cells (v). Superimposition of antinitrotyrosine (iii) and iNOS (iv) staining is shown in panel Cvi. Superimposition of iNOS (iv) and antikeratin (v) staining is shown in panel Cvii. Superimposition of antinitrotyrosine (iii) and antikeratin (v) staining is shown in panel Cviii.
We then wondered whether the spots of nitrotyrosine could be associated with an in vivo apoptosis. To this end, we performed double-staining studies on the thymic frozen sections using labeling of nitrotyrosine with the specific polyclonal antibody and of DNA strand breaks by the TUNEL reaction. TUNEL-positive cells were located in the thymic cortex and the corticomedullary junction as previously reported.24 Most spots of nitrotyrosine (Figure 9Bii) were associated with a strong TUNEL reaction (Figure 9Bi-v). The TUNEL control consisting of the same reaction mixture but without terminal transferase enzyme was negative (Figure 9Bvi).
Expression of iNOS in the human thymus
Because NO• regulates the formation of peroxynitrite, we wondered whether iNOS is produced by thymic cells.
Thymic sections.
Incubation of thymic sections with anti-iNOS antibody revealed a significant staining of several cell types. A double staining with antikeratin antibody revealed that some of them are epithelial, as shown by the arrowheads (Figure 9Ci,ii), but others are located around the vessels (arrow) and are probably endothelial cells (Figure 9Ci,ii). Triple immunofluorescence on the thymic sections with antinitrotyrosine, anti-iNOS, and antikeratin antibodies revealed that nitrotyrosine-positive spots enclosed frequently iNOS+cells (Figure 9Ciii-viii). In some spots of nitropositive cells, it was possible to identify iNOS epithelial-positive cells (Figure 9Ciii-viii).
Cultured cells.
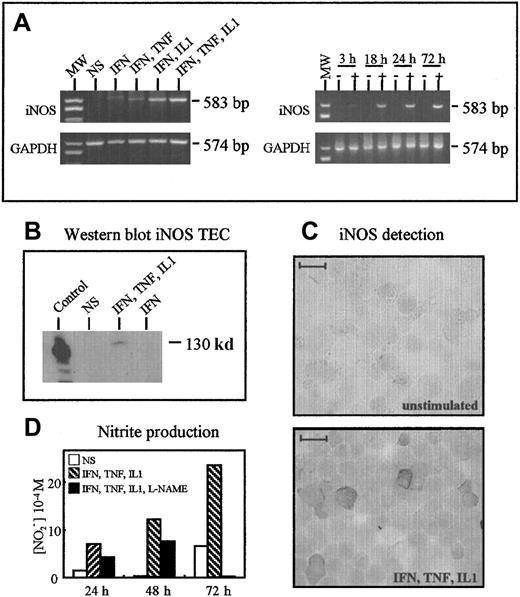
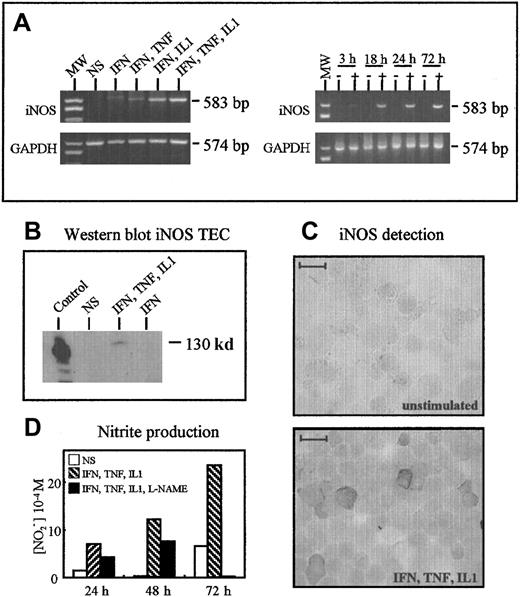
Thymocytes activated by PMA and ionomycin for 24 hours or concanavalin A for 3 days did not synthesize iNOS, as assessed by RT-PCR (data not shown). Figure 10A shows iNOS messenger RNA expression in cultured TECs after stimulation with cytokines in RT-PCR experiments. The expression was undetectable when TNF-α, IL-1β (data not shown), or IFN-γ was used alone; faint when IFN-γ and TNF-α were combined; marked when IFN-γ and IL-1β were combined; and maximal when IFN-γ, TNF-α, and IL-1β were combined (Figure 10A). A single band of the expected size (583 base pairs) was obtained in stimulated cells. GAPDH was used as a control, and similar amplification signals were obtained in the 5 conditions. The combination of IFN-γ, TNF-α, and IL-1β had a time-dependent effect, with a faint signal after 3 hours, a marked increase at 18 hours, and maximal expression after 72 hours. The iNOS was not detected in unstimulated TECs (Figure 10A).
TECs produce iNOS after activation.
(A) RT-PCR analysis of iNOS expression. TECs expressed iNOS only after stimulation by a cocktail of cytokines. Optimal expression was observed using IFN-γ, IL-1β, and TNF-α. In these optimal conditions, iNOS was barely detected after 3 hours and clearly observed after 18 hours. (B) Western blot analysis showed the presence of iNOS protein (130 kd) in TECs activated in optimal conditions but not in unstimulated cells. The positive control consisted of stimulated macrophages. (C) Immunoperoxidase analysis of iNOS expression in cultured TECs. TECs expressed significant levels of iNOS after 24 hours of optimal activation; unstimulated cells were unreactive. The scale bar represents 20 μm. (D) NO2 accumulation in TEC culture supernatants was measured by using the Griess reagent. The specificity of the reaction was checked by using L-NAME, an inhibitor of NO2 synthesis.
TECs produce iNOS after activation.
(A) RT-PCR analysis of iNOS expression. TECs expressed iNOS only after stimulation by a cocktail of cytokines. Optimal expression was observed using IFN-γ, IL-1β, and TNF-α. In these optimal conditions, iNOS was barely detected after 3 hours and clearly observed after 18 hours. (B) Western blot analysis showed the presence of iNOS protein (130 kd) in TECs activated in optimal conditions but not in unstimulated cells. The positive control consisted of stimulated macrophages. (C) Immunoperoxidase analysis of iNOS expression in cultured TECs. TECs expressed significant levels of iNOS after 24 hours of optimal activation; unstimulated cells were unreactive. The scale bar represents 20 μm. (D) NO2 accumulation in TEC culture supernatants was measured by using the Griess reagent. The specificity of the reaction was checked by using L-NAME, an inhibitor of NO2 synthesis.
After 24 hours of stimulation, TECs were analyzed for their iNOS protein content by Western blotting and immunohistochemistry. The iNOS was undetectable in unstimulated cells, but it was clearly detected after stimulation with the combination of IFN-γ, TNF-α, and IL-1β. The signal was of the expected size (130 kd) (Figure10B). In immunohistochemical studies, cultured TECs were cytocentrifuged and examined for iNOS expression with an anti-iNOS monoclonal antibody; iNOS was not detected in unstimulated cultures, but many cells expressed iNOS after cytokine stimulation (Figure 10C).
Finally, NOS activity was measured in TEC cultures using the amount of nitrites in supernatants as an index. NOlevels in the supernatants ran parallel to iNOS messenger RNA expression: They were clearly increased in stimulated cultures compared with unstimulated cultures, and they rose with time. Nitrite production was clearly decreased when L-NAME (0.5 mM), an inhibitor of iNOS activity, was added to the culture medium, showing that functional iNOS expression can be induced in activated cultured human TECs (Figure10D).
Discussion
The main results of this study are as follows: (1) Peroxynitrite is a powerful inducer of human thymocyte apoptosis; NO•is not. (2) SIN-1–induced apoptosis is reversed by adding exogenous SOD and first affects CD4+CD8+ cells. (3) SIN-1–induced apoptosis is associated with increased nitrotyrosine levels, an effect abrogated by exogenous SOD. (4) NO•, which is necessary for peroxynitrite synthesis, is produced by TECs in appropriate conditions of activation, and iNOS is expressed in cultured TECs and in thymic sections. (5) Nitrotyrosine, a marker for peroxynitrite, is found in total thymic extracts and on thymic sections colocalized with apoptotic cells detected by TUNEL reaction and, also, with iNOS+ TECs.
Peroxynitrite induces human thymocyte apoptosis
Peroxynitrite is a potent inducer of human thymocyte apoptosis in vitro. Interestingly, this mechanism does not occur in all thymic types of cells, because SIN-1 treatment of TECs does not induce apoptosis of TECs (data not shown). Apoptosis was defined by 3 criteria: annexin V staining (an early event), the TUNEL reaction (a late event), and the absolute number of viable cells (a very late event). As regards the role of peroxynitrite in thymic apoptosis in vivo, our results show that nitrotyrosine, a marker for peroxynitrite, is found in the thymic extracts and on thymic sections colocalized with apoptotic cells assessed by TUNEL reaction. Thus peroxynitrite induces apoptosis of human thymocytes in vitro and is associated with thymocyte apoptosis in vivo.
As regards the activity of NO• in the thymus, NOS systems have been detected in multiple cellular sites in rat thymus by using the nicotinamide adenine dinucleotide phosphate reduced form (NADPH) diaphorase reaction.25 Comparing iNOS expression in autoimmune-prone Lewis rats with other resistant strains showed a lower expression in Lewis rats compared with resistant strains, suggesting a iNOS-dependent mechanism for the suppression of potentially autoreactive T-cell clones.26 In the murine thymus, very few iNOS+ cells were detected in unstimulated animals, but a major increase in the number of iNOS cells in both fetal and adult thymuses was observed after stimulation with an anti-CD3 antibody. Expression of iNOS was detected in the corticomedullary junction and medulla as well as in the stroma fraction.14In the human thymus, we found iNOS+ cells in the cortex and the medulla and around the vessels, and some of them were epithelial. This result was not surprising because we showed that cultured TECs produced iNOS when activated by IFN-γ, TNF-α, and IL-1β, 3 cytokines potentially produced by the thymic microenvironment.27 28 Conversely, human thymocytes activated either by a mitogen (concanavalin A) or with PMA and ionomycin did not produce detectable iNOS (data not shown), suggesting that the death of thymocytes observed in the tissue is induced after cell-cell contacts and interactions.
Because apoptosis of human thymocytes in vitro was induced by peroxynitrite, one could hypothesize that in vivo NO has reacted with the O2•− anion that is necessary for peroxynitrite formation. The origin of the O2•− anion could be the thymocyte that is known to generate O2•− anion during apoptosis induced by glucocorticoid29 or the TECs, or another tripartite cell, such as macrophages or eosinophils. These latter have been recently described in the murine thymus and release high levels of O2•−anion.30
Although tyrosine nitration and DNA fragmentation are associated ex vivo, it is not clear which event is the cause. In our flow cytometry analyses, nitrotyrosine appeared in thymocytes, as early as 1 hour and before annexin V staining, indicating that tyrosine nitration was an early event in the apoptosis process. However, we cannot exclude that O2•− anion produced in thymocytes at a late stage of apoptosis induced by another mechanism (such as glucocorticoids) will cause synthesis and subsequent nitrotyrosine formation. In this case, nitrotyrosine formation could be a late event in the apoptotic process.
Several reports point to a role of ROS in murine thymocyte apoptosis. A study documented the oxygen dependence of the initial steps of thymocyte death induced by glucocorticoids or gamma irradiation, and it showed that the antioxidant N-acetyl cysteine inhibited the induction of thymocyte apoptosis by these reagents.17 Thymocyte apoptosis induced by CD45 cross-linking was also inhibited by the addition of ROS scavengers.31 ROS can also regulate signals involved in caspase activation and apoptosis and contribute to peripheral activation-induced cell death.32,33 N-acetyl cysteine completely blocks activation-induced cell death in T-cell hybridomas and in myelin basic protein–-specific T-cell lines.12,34 Recently, it was shown that peroxynitrite primes normal human T lymphocytes to undergo peroxynitrite-driven apoptotic death following activation in vitro.35 The mechanism involved is the inhibition of activation-induced protein tyrosine phosphorylation through nitration of tyrosine residues by peroxynitrite. Thus, peroxynitrite could play a physiologic role as a modulator of activation and death in the immune central and peripheral systems.
Our coculture experiments indicate that tyrosine nitration of thymocytes in contact with TECs is partially inhibited in the presence of iNOS inhibitors. In addition, TECs were found to express iNOS on thymic sections. These results bring additional arguments in favor of a physiologic role of peroxynitrite in tyrosine nitration of thymocytes and apoptosis. Insofar as spots of nitrotyrosine-positive cells are mainly found in the cortex, that there is no need for a stimulation by anti-CD3 to observe tyrosine nitration, and that CD4+CD8+ cells are more sensitive to peroxynitrite-induced apoptosis than are single-positive cells, the death analyzed in this study essentially concerns death during the positive selection processes, ie, death by neglect. Whether the same events could occur during negative selection of human thymocytes deserves further investigation. In mice, it was shown that T-cell receptor–stimulated murine thymocytes are much more sensitive to NO• donor-induced apoptosis than are unstimulated thymocytes, suggesting a role of NO• in anti-CD3–induced apoptosis.13 Notably, in contrast to murine thymocytes, NO• does not induce apoptosis of human thymocytes, although several NO• donors induce apoptosis of mice thymocytes in a concentration-dependent manner.14
Significance of tyrosine nitration in the thymus
Peroxynitrite might lead to cell death by a mechanism involving nitration of critical tyrosine residues.9 Tyrosine nitration may interfere with intracellular signal transduction. Nitration of tyrosine residues is able to inhibit protein tyrosine phosphorylation in purified activated lymphocytes,35 and it inactivates protein tyrosine phosphatases in vitro.36Nitration of surfactant protein A by peroxynitrite has been linked to decreased protein function.37 Similarly, tyrosine nitration inactivates prostacyclin synthase, which favors atherosclerotic processes.38 Nitration of structural proteins, including neurofilaments and actin, can disrupt filament assembly and have severe pathological consequences.9
The presence of nitrotyrosine in the normal newborn human thymus suggests the presence of peroxynitrite ex vivo, although it was recently described that myeloperoxidase could also be a nitrating agent.23 The presence of nitrotyrosine was also obtained in mice, but its localization was different. In the human thymus, we found nitrotyrosine spots in the cortex and the corticomedullary junction; in mouse thymus nitrotyrosine was detected in the corticomedullary junction and medulla.39 In mice deficient in iNOS, expression of nitrotyrosine in the thymus was less than in normal mice,39 suggesting a role of iNOS in the expression of nitrated tyrosine residues.
In the human thymus, the localization of nitrotyrosine spots in the cortex and the corticomedullary junction is compatible with an association with apoptotic mechanisms. Indeed, most human apoptotic thymocytes in situ were detected at the corticomedullary junction and scattered throughout the cortex.24 In our study, using TUNEL reaction we found that most nitrotyrosine-positive cells were colocalized with TUNEL-positive cells. Using antinitrotyrosine antibody in a Western blot analysis, we showed that 2 main proteins were detectable. Their molecular weights were around 60 kd and 15 kd. Tyrosine nitration is generally associated with protein dysfunction, suggesting that biochemical modifications of these proteins could be crucial for thymic survival. Because some spots of nitrotyrosine were negative in TUNEL reaction, we could speculate that nitrotyrosine formation is an event occurring earlier than DNA fragmentation assessed by the TUNEL reaction. Alternatively, nitrotyrosine-positive cells that are negative in TUNEL reaction could be associated with necrosis induced by the peroxynitrite. TECs frequently observed in nitrotyrosine spots are potentially peroxynitrite producer cells because they are reactive with an anti-iNOS antibody. However, more direct arguments are needed to characterize the nitrated proteins and to evaluate their role on thymic apoptosis.
Protein nitration is increased in several pathological models. For example, nitrotyrosine residues have been detected with a specific monoclonal antibody in the brains of multiple sclerosis patients.40 Patients with adult respiratory distress syndrome have elevated nitrotyrosine levels.41Nitrotyrosine has also been detected in vacuolated muscle fibers from patients with myositis inclusion body but not in normal muscles.42 Our data show that nitrotyrosine could also be present at a basal level in normal situations.
Taken together, our results demonstrate that peroxynitrite is a powerful inducer of apoptosis of human thymocytes in vitro. The evidence of iNOS reactivity in TECs supports their role in NO synthesis. The presence of nitrotyrosine in the human thymus supports the presence of peroxynitrite in the thymus gland. Finally, the colocalization of thymic apoptotic cells with nitrated tyrosine residues suggests a significant role of peroxynitrite in human thymic apoptosis in vivo.
We are grateful to Claire Ducrocq (Institut de Chimie des Substances Naturelles, CNRS, Gif/Yvette, France) for chemical preparation of peroxynitrite and to C. Bruand for preparing the figures.
Supported by grants from Association Française contre les Myopathies, Centre National de la Recherche Scientifique, and Caisse Nationale d'Assurance Maladie des Travailleurs Salariés. N.M. is supported by a postdoctoral grant from Fondation Singer Polignac.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sonia Berrih-Aknin, Laboratoire de Physiologie Thymique, UPS CNRS ESA-8078, Hôpital Marie Lannelongue, 133, avenue de la Résistance, 92350 Le Plessis Robinson, France; e-mail: sonia.berrih@ccml.u-psud.fr.