Polymorphonuclear neutrophils (PMNs) are the most abundant circulating blood leukocytes. They provide the first-line defense against infection and are potent effectors of inflammation. In addition, their release of soluble chemotactic factors guides the recruitment of both nonspecific and specific immune effector cells.1 Finally, since they both respond to and produce cytokines,2,3 they also modulate the balance between humoral and cell-mediated immunity by contributing to the promotion of a TH1 or TH2 response.4 In these ways, PMNs are engaged in a complex cross-talk with immune and endothelial cells that bridges innate and adaptive immunity.5
Even though many facets of their biology have been thoroughly investigated,6 PMNs still have every reason to complain of the disdain with which they are regarded by oncologists and immunologists.2 So widespread is T-cell chauvinism7 that the antitumor potential of PMNs continues to receive little attention, and researchers have not yet fully considered the possibility of exploiting their functions as effective weapons against cancer.
Natural antitumor activity
The attempts of leukocytes to respond to cancer are suggested by their systemic, regional, and intratumoral activation.8-10Infiltration of tumors by leukocytes has been associated with a favorable prognosis in some studies in humans.11-13However, for individual patients, there is no predictable relationship between leukocyte composition and the prognosis of their disease.
PMNs are usually a scarce reactive component of both human and animal tumors. In animal models, their presence may sometimes be detrimental by favoring malignant growth and progression.14Nevertheless, recent studies have suggested that they are active in immunosurveillance against several tumors.15-18 These intriguing outcomes are probably related to the result of the interplay between (1) the kind and amount of cytokines and chemotactic factors naturally released by tumor cells19 and (2) the degree of recruitment and activation of the intermingled PMNs.
Over the last decade, cytokine gene transfer strategies in animal models have provided a tool with which to dramatically increase intratumoral cytokine availability, avoid the side effects of systemic administrations, and evaluate the antineoplastic potential of locally recruited PMNs.
Cytokines at the tumor site
The natural tumor-PMN balance can be markedly altered by engineering tumors to release interleukins20 or chemokines21 in their microenvironment. Although the amount released is usually small, it may be gigantic when compared with wild-type tumors and produce dramatic effects.
Almost all the cytokines sustainedly released by engineered tumor cells, namely interleukin-1 (IL-1), IL-2, IL-3, IL-4, IL-7, IL-10, IL-12, interferon-α (IFN-α), IFN-β, IFN-γ, granulocyte-colony stimulating factor (G-CSF), and tumor necrosis factor-α (TNF-α),22,23 quickly recruit a massive local reaction that leads to the rejection of engineered tumor cells and the establishment of a significant immunity against the wild-type parental tumor. PMNs play a key role in all of these cytokine-induced tumor rejections, often in cooperation with CD8+ T lymphocytes.23 As circulating granulocytes of tumor patients have impaired cytotoxic activity,24 which is further decreased by most chemotherapeutic agents,25 the elaboration of systems capable of guiding the recruitment of PMNs and their activation within a tumor microenvironment can be put forward as a fresh therapeutic approach.
PMN recruitment into the tumor
Extravasation from the blood into a tumor is a regulated multistep process involving a series of coordinated interactions between PMNs and endothelial cells.26 Several molecular regulator families, namely selectins, integrins, and cytokines, are thought to control the steps of this process. It is well known that P-, E-, and L-selectin adhesion molecules (also known as GMP 140, ELAM-1, and LAM-1, respectively) initially tether free-flowing neutrophils to the endothelium of postcapillary venules and mediate transient interactions by causing them to roll much more slowly. Slowly progressing PMNs pick up the signals delivered by interleukins, chemokines, and other mediators released by the endothelium and become firmly attached to it via β2 integrin (MAC-1, ie, CD11b/CD18)–intercellular adhesion molecule-1 (ICAM-1) recognition.27 The endothelium is thus the most active controller of leukocyte traffic and behavior through its display of specific signals.
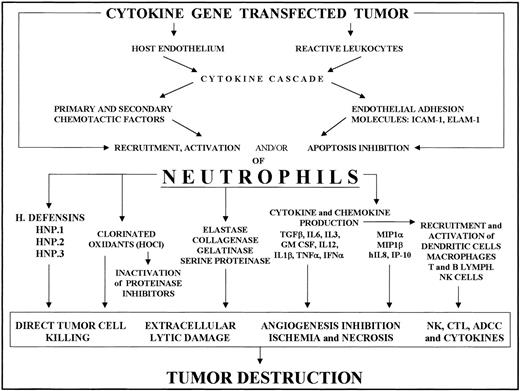
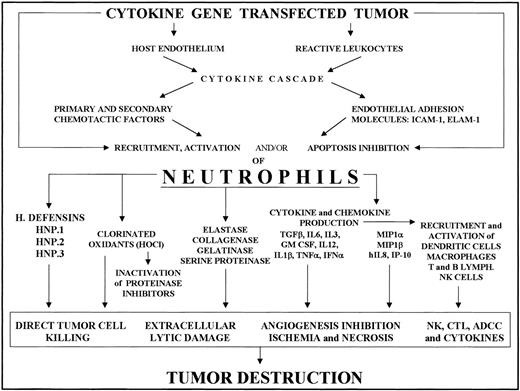
Some proinflammatory mediators or other factors directly secreted by engineered tumor cells, or elicited as downstream mediators by the released cytokine, increase the endothelial expression of several leukocyte adhesion and activation molecules. IL-1β and TNF-α induce and/or up-regulate ELAM-1, P-selectin, ICAM-1, and vascular cell adhesion molecule-1 expression in endothelial cells, whereas IFN-γ mainly promotes ICAM-1 expression.28-31 This cytokine-endothelium cross-talk is thus the first step in regulating PMN intratumoral accumulation (Figure 1).
Recruitment of neutrophils into cytokine-transfected tumor and their involvement in its destruction.
Local release of the cytokine from transfected tumor cells acts on the host endothelium and reactive leukocytes, mainly tumor-associated macrophages. The cytokine and chemokine cascade and endothelial adhesion molecule expression thus induced results in PMN recruitment and activation. Tumor destruction on the part of activated PMNs is achieved through their release of a variety of factors (cytokines, enzymes, chlorinated oxidants, etc) whose effects include direct tumor killing, extracellular lysis, inhibition of angiogenesis and activation of other reactive cells, resulting in NK cell, T cell, and antibody-dependent cytotoxicity.
Recruitment of neutrophils into cytokine-transfected tumor and their involvement in its destruction.
Local release of the cytokine from transfected tumor cells acts on the host endothelium and reactive leukocytes, mainly tumor-associated macrophages. The cytokine and chemokine cascade and endothelial adhesion molecule expression thus induced results in PMN recruitment and activation. Tumor destruction on the part of activated PMNs is achieved through their release of a variety of factors (cytokines, enzymes, chlorinated oxidants, etc) whose effects include direct tumor killing, extracellular lysis, inhibition of angiogenesis and activation of other reactive cells, resulting in NK cell, T cell, and antibody-dependent cytotoxicity.
PMN intratumoral accumulation is also attained when IL-10, a cytokine typically regarded as an anti-inflammatory mediator because it inhibits the release of other interleukins and chemokines,32-34 is present in the microenvironment. We have demonstrated that local release of high levels of IL-10 by IL-10 gene transfected mammary carcinoma cells (TSA–IL-10) in a syngeneic host results in both an anti- and a pro-inflammatory activity, through strong endothelial ELAM-1 expression in peripheral tumor microvessels.35 This expression and subsequent intratumoral accumulation of PMNs were directly attributable to IL-10, since no secondary mediators were detected.35,36 Moreover, it is likely that IL-10 attenuates the constitutive endothelial cell release of nitric oxide37 and contributes to the adhesion of PMNs to microvessels38 39 and their intratumoral accumulation.
It has been recently reported that IL-10 up-regulates the expression of the liver-expressed chemokine (LEC), a human β chemokine also known as NCC-4, HCC-4, and LMC.40 LEC is chemotactic for human monocytes and dendritic cells, but not for neutrophils.41,42 Nevertheless, local accumulation of LEC secreted by engineered TSA cells (TSA-LEC) quickly induces their rejection in association with an impressive recruitment of antigen- presenting cells, lymphocytes, and particularly PMNs.21
PMNs intratumorally recruited by highly expressed endothelial adhesion molecules also play a key role in mounting a strong antitumor response.21
Induction of ELAM-1 and up-regulation of ICAM-1 in the blood vessels, even in the case of tumors formed by cells engineered to release G-CSF, IL-2, IL-4, and IL-12, are attributable to both the cytokine-released and downstream-induced secondary mediators, such as CXC chemokines.43,44 Experiments with tumor cells transfected to release G-CSF, IL-2, and IL-12 as well as TNF-α,45-47 have disclosed an evident production of macrophage inflammatory protein-2 (MIP-2), also known as growth-related oncogene/cytokine–induced neutrophil chemoattractant (GRO/KC).48-50 This molecule is the murine functional homologue of human IL-8, which up-regulates the binding affinity of a family of adhesion molecules called integrins on PMNs and of its counter-receptor, ICAM-1, on endothelial cells.51Integrin-mediated adhesion leads to extravasation of PMNs highly attracted to the tumor site by MIP-2, which binds to the PMNs' CXCR1 or CXCR2 counterreceptor (see review in Luster52). MIP-2, together with other factors belonging to the IL-8 granulocyte chemoattractant family, is often released by tumor-associated macrophages under the stimulus of the cytokines released by tumor cells or the secondary mediators they induce.53 We found that macrophages and endothelial cells were clearly stained by anti–MIP-2 antibody when TNF-α as well as IL-1β were present in the tumor microenvironment.44 46 In all these cases, MIP-2 expression was associated with marked recruitment of PMNs, whose accumulation was further enhanced by the further release of MIP-2 produced by PMNs themselves in response to stimulation by TNF-α in the tumor microenvironment.
PMN-induced tumor destruction
Recruited PMNs produce several cytotoxic mediators, including reactive oxygen species, proteases, membrane-perforating agents, and soluble mediators of cell killing, such as TNF-α, IL-1β, and IFNs (Figure 1).
Oxidants employ 2 mechanisms to injure tumor cells. They act synergically with protease and other agents, and inactivate plasma antiproteases to allow proteases to operate.54
Recent dissection of the cytolytic armamentarium of PMNs has suggested a primary role for hypochlorous acid (HOCl) in mediating tumor cell lysis by activated PMNs after their leukocyte function–associated antigen 1–dependent recognition of the target cell surface.55 Furthermore, a distinct adhesion pathway, mediated by CD11b/CD18 up-regulation on activated PMNs, enables these cells to adhere to the vascular endothelium and create a subjacent microenvironment, allowing accumulation of oxidants and proteolytic enzymes at local concentrations sufficient to cause endothelial damage and matrix degradation.56 In addition, PMN-released HOCl reacts with primary amines to form relatively stable chloramines with immunostimulatory properties.57
Although reactive oxygen and reactive nitrogen intermediates are toxic molecules that contribute to the control of tumors, they also mediate inhibition of T-cell proliferation by suppressing macrophage functions. This mechanism accounts, at least in part, for the immunosuppressed state seen in certain infectious diseases, malignancies, and graft-versus-host reactions (see review in Bogdan et al58).
A further mechanism of PMN-mediated tumor cell killing is antibody-dependent cell-mediated cytotoxicity (ADCC).59The role of antibody-independent recognition of tumor cells by cytotoxic T cells has been extensively researched, whereas only a few recent works have shown that in vivo tumor ADCC also exists.60-63 Granulocyte-macrophage–CSF (GM-CSF) augments the normal PMN ADCC of melanoma, neuroblastoma, and colorectal cancer cells.64-66
Recent preclinical studies of the treatment of advanced renal cell carcinoma by combining bispecific antibodies (with one specificity against the epidermal growth factor–receptor (EGF-R) overexpressed on the majority of renal cell carcinomas, and another specificity against Fc receptors on human leukocytes) with G-CSF or GM-CSF therapy have demonstrated that granulocytes are the most active effector cell population.67 Although systemic application of bispecific antibodies is suitable at present only for adjuvant treatment of minimal residual disease due to poor tumor cell accessibility, local administration, either alone or in combination with autologous effector cells, is highly effective in eradicating tumor cells.68
In our murine model, IL-10–releasing TSA cells initially grow and are then rejected by the combined action of CD8+ lymphocytes, natural killer (NK) cells, and PMNs.35 69 As already mentioned, the marked anti-TSA antibody response that follows this rejection may be responsible for PMN-mediated tumor ADCC.
A markedly high titer of anti-TSA antibodies is also elicited during the early phases of LEC-releasing TSA tumor cell rejection. Here, too, antibodies may provide further guidance for the PMN-dependent tumor rejection.21
A family of antimicrobicidal peptides called defensins has been described in humans.70 Defensins are the most abundant component of the azurophil granules of PMNs and are highly toxic against several types of tumor cells.71
These granules also contain elastase and cathepsin G, 2 proteases particularly injurious to endothelial cells.72 It is still uncertain whether adhesion of PMNs to tumor cells is required to cause injury. However, the ultrastructural studies performed during the growth and rejection phases of several tumors engineered to release cytokines show PMNs in close contact with injured tumor cells.23 47 An absolute need for adhesion cannot, of course, be inferred from observations of this kind.
Histological and ultrastructural investigation of tumor growth area morphology shows that the damage produced by PMNs takes 2 forms: predominantly colliquative necrosis when their cytotoxicity against tumor cells prevails23 and predominantly ischemic and/or hemorrhagic necrosis when their main target is the vascular endothelium.20,23,35,73 74
Modulation of PMN behavior by cytokines
Serial immunohistological examination and polymerase chain reaction analysis after a subcutaneous challenge with TSA–IL-2, TSA–IL-4, TSA–IL-10, TSA–IL-12, and TSA–TNF-α cells have shown that the local release of all these cytokines elicits a quick and effective PMN-mediated antitumor activity.20 44The kinetics, however, are not the same, and the boosted PMN functions are often different.
Presence of pro-inflammatory cytokines
Paracrine release of IL-2, IL-4, IL-12, and TNF-α induced prompt tumor rejection (Figure 2A) marked by the formation of areas of colliquative necrosis typically associated with a massive presence of degranulated PMNs with exocyted granules in close contact with tumor cells displaying ultrastructural signs of irreversible damage (Figure 2B). This indication of direct killing by PMNs was particularly evident in the presence of paracrine IL-2 and TNF-α.
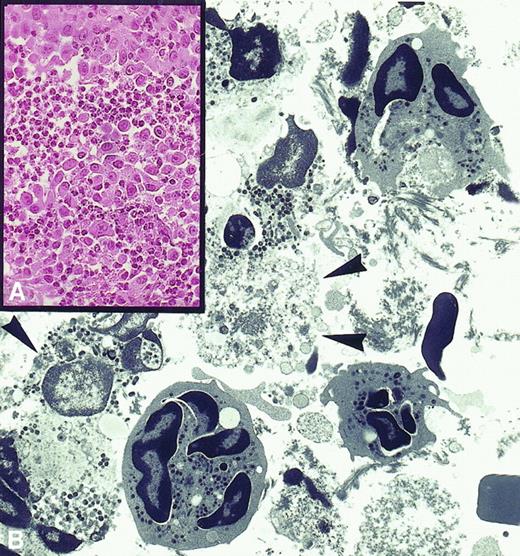
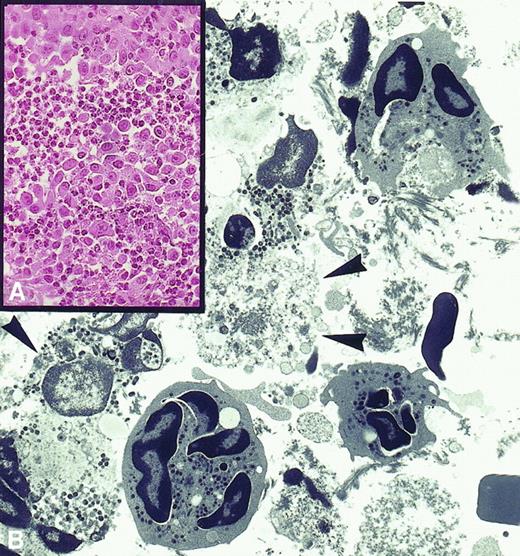
PMN-induced tumor destruction.
(A) Rejection area of subcutaneously injected tumor cells engineered to release IL-2 is massively infiltrated by polymorphonuclear leukocytes (× 630). (B) Electron micrograph showing that these are neutrophils at various stages of disorganization and that their exocytosed granules are in close contact with severely damaged or necrotic tumor cells (× 2750).
PMN-induced tumor destruction.
(A) Rejection area of subcutaneously injected tumor cells engineered to release IL-2 is massively infiltrated by polymorphonuclear leukocytes (× 630). (B) Electron micrograph showing that these are neutrophils at various stages of disorganization and that their exocytosed granules are in close contact with severely damaged or necrotic tumor cells (× 2750).
Interestingly, the observed release of TNF-α into the circulation during infusional recombinant (r) IL-2 therapy of cancer patients is often associated with a potent activation of PMNs that interact with tumor cells and endothelial cells and cause their subsequent lysis.75,76 According to this killing activity, PMNs appear to mediate most of the therapeutic efficacy, but also the systemic toxicity of rIL-2 (ie, vascular leak syndrome).77However, these 2 opposite effects might converge in a single antitumor effect with the development of intratumoral cytokine gene transfer therapy.
In mice, direct killing by PMNs was also a hallmark of the rejection of tumor cells engineered to release G-CSF. Activated PMNs with prominent cytoplasmic projections, in fact, were observed in close contact with dead tumor cells47,78 well before any vascularization of injected tumors was possible. When recipient mice were sublethally irradiated, tumors grew and became vascularized before the rejection that occurred when PMNs and leukocytes were self-reconstituted. In this case, the tumor-associated blood vessels were the main PMN target.78
Immunohistological analysis showed that in all these situations IL-1β and TNF-α were the predominant downstream pro-inflammatory cytokines elicited in the tumor growth area. They were usually associated with MIP-2 expression in both tumor-associated macrophages and PMNs. Indeed, by thus inducing macrophages to produce PMN chemoattractants, they set up a vicious circle, since they also stimulate PMNs to produce MIP-2 and probably other factors belonging to the IL-8 granulocyte chemoattractant family (Figure 3).
Cytokine modulation of PMN intratumoral recruitment and functions.
Cytokine modulation of PMN intratumoral recruitment and functions results in tumor destruction and an antitumor immune memory. Tumor-associated macrophages are the first reactive cells ready to respond to the secondary mediators (IL-1β, TNF-α, IFN-γ, etc) induced by the cytokine systemically administered or locally released by engineered tumor cells. Depending on the cytokines present in the microenvironment, activated macrophages release ELR (glutamic acid–leucine–arginine)+ CXC chemokines, which recruit neutrophils, and/or ELR− CXC chemokines, which recruit T lymphocytes. If IL-1β and TNF-α prevail, macrophages and PMNs are induced to produce ELR+ chemokines (MIP-2/GRO/KC), which amplify accumulation of PMNs and favor their destructive functions. If IFN-γ prevails, they are induced to produce angiostatic ELR− chemokines (interferon inducible protein-10 [IP-10], monokine induced by gamma interferon [MIG]) that recruit T lymphocytes and promote the establishment of an antitumor immune memory.
Cytokine modulation of PMN intratumoral recruitment and functions.
Cytokine modulation of PMN intratumoral recruitment and functions results in tumor destruction and an antitumor immune memory. Tumor-associated macrophages are the first reactive cells ready to respond to the secondary mediators (IL-1β, TNF-α, IFN-γ, etc) induced by the cytokine systemically administered or locally released by engineered tumor cells. Depending on the cytokines present in the microenvironment, activated macrophages release ELR (glutamic acid–leucine–arginine)+ CXC chemokines, which recruit neutrophils, and/or ELR− CXC chemokines, which recruit T lymphocytes. If IL-1β and TNF-α prevail, macrophages and PMNs are induced to produce ELR+ chemokines (MIP-2/GRO/KC), which amplify accumulation of PMNs and favor their destructive functions. If IFN-γ prevails, they are induced to produce angiostatic ELR− chemokines (interferon inducible protein-10 [IP-10], monokine induced by gamma interferon [MIG]) that recruit T lymphocytes and promote the establishment of an antitumor immune memory.
The marked and rapid PMN influx and tumor necrosis observed in nude mice challenged with human tumor cells engineered to release IL-8 (the functional human equivalent of MIP-2), human MIP-1α, and murine MIP-1α79 show that both CXC chemokines, such as IL-8 and GROs, and specific CC chemokines, such as MIP-1α, regulate PMN traffic and functions, providing further evidence that recruited PMNs suppress tumor growth.
Absence of pro-inflammatory cytokines
Rejection of TSA–IL-10 cells, on the other hand, is paradigmatically different.
By suppressing the release of pro-inflammatory cytokines such as IL-1β, TNF-α, and GM-CSF, paracrine IL-10 impairs the early influx of PMNs and permits initial tumor formation by transiently paralyzing a prompt nonspecific antitumor response.35,69 However, by directly acting on endothelial cells, it induces adhesion molecules and attenuates their nitric oxide release, thus favoring leukocyte recruitment in vivo.36,80 81 Leukocytes and mainly neutrophils are responsible for the intratumoral microvasculature damage that results in multiple ischemic necrotic areas.
Local necrosis is followed by massive infiltration by PMNs and a few T and NK cells, probably recruited by mediators induced by hypoxia and necrosis.82 The late influx of reactive cells, however, is enough to lead to complete rejection of the established tumor. This does not take place in the absence of PMNs.69
The well-known immunosuppressive activity demonstrated by IL-10 in vitro has naturally impeded assessment of its potential use in the treatment of solid human tumors. Its employment has been proposed only for management of the rare myeloproliferative disorder called juvenile myelomonocytic leukemia.83 Even here, consideration has been devoted solely to its ability to inhibit the production of cytokines and growth factors by myelomonocytes in vitro.83Animal data, however, suggest that intratumoral administration of IL-10 inhibits tumor growth through a pro-inflammatory activity in which PMNs again play a fundamental role.35 69
Presence of IFN-γ
The presence in the tumor growth area of appreciable amounts of IFN-γ together with IL-1β and TNF-α was noted after injection of TSA cells engineered to release IL-2, IL-4, and IL-12.43,44 It was even more evident when local or systemic administration of rIL-12 in mice bearing a subcutaneous 7-day-old TSA tumor resulted in intratumoral expression of the messenger RNA of IL-1β, TNF-α, and IFN-γ, together with IP-10 and MIG,44 2 chemokines with well-known antiangiogenic activities. IL-1β and TNF-α probably induced the production of PMN chemoattractants by tumor-associated macrophages, as the number of tumor-infiltrating PMNs was significantly enhanced after 3 intraperitoneal administrations of rIL-12.
The presence of IFN-γ as secondary mediator, however, must also have induced both macrophages and PMNs to produce IP-10 and MIG84 85 (Figure 3). The rapid influx of PMNs with a high destructive potential and an IP-10– and MIG-mediated anti-angiogenic function resulted in vascular damage, inhibition of angiogenesis, and extensive ischemic/hemorrhagic necrosis after 3 and 8 rIL-12 administrations. Macrophages were undoubtedly essential modulators of this immune response. The crucial importance of PMNs, however, was made clear when their selective depletion abolished the rIL-12–induced antitumor effect. Whether PMNs also have a role in IL-12 therapy of human cancer has still to be determined.
IP-10 and MIG are also intense chemoattractants for monocytes and T cells.86-88 They promote T-cell adhesion to endothelial cells and are leading recruiters of the T cells, particularly CD8+, found to be indispensable, like PMNs, for complete eradication of most of our inocula and experimental tumors.
The presence of IFN-γ and its induction of a cytokine cascade are usually associated with an elevated antitumor memory reaction, since all inocula with IFN-γ in the growth area led to the rejection of a secondary challenge (Figure 3).
PMNs and the antitumor immune memory
PMNs do not seem of great importance in the elaboration of a significant immune memory against the secondary tumor cell challenge. Even so, they are certainly one of the effector arms involved in the destruction of a second inoculum that takes place once this memory is established. This was particularly evident in the case of TSA cells transfected with the IFN-γ gene.20 23 This tumor, in which the PMN infiltrate is almost absent, was rejected in only 25% of cases, though the immune memory established after the first challenge always provided complete protection against a subsequent TSA–parental cell inoculum.
Provided there is IFN-γ in the tumor growth area, the cytokine released by the engineered tumor cells plays a significant role in skewing the memory reaction toward TH1 and TH2. However, secondary rejection is not simply the work of T cells. Massive PMN recruitment, in fact, is consistently evident in both TH1- and TH2-deflected memories. Since their selective removal impairs or even abolishes rejection after establishment of the immune memory,21,69 89 it may well be that PMNs play hitherto unsuspected roles.
PMN-mediated ADCC may significantly contribute to the immune memory leading to secondary tumor cell rejection.59,64 65However, there may be a more complex interplay in which T cells release a guidance factor that directs the powerful destructive action of PMNs. Close contacts between granulocytes, lymphocytes, and tumor cells are typical of immune memory reactions, in keeping with the possibility that cytolytic activity of granulocytes is guided by factors secreted by lymphocytes.
PMNs and anticancer therapy in humans
New, interesting perspectives are opening to exploit PMN functions for anticancer strategies in patients.
During therapy with G-CSF, significantly enhanced in vitro cytotoxicity of isolated PMNs against glioblastoma, squamous cell, ovarian, and breast carcinoma cells was observed with sensitizing monoclonal antibody to the oncogene products EGF-R and HER-2/neu.90-92
Indeed, a phase I study in patients with breast and ovarian cancer showed that biological and clinical activities at well-tolerated doses of bispecific antibodies to FcRI selectively induced on neutrophils and HER-2/neu expressed on tumor target cells.91 92
In hematologic malignancies, where tumor cells are relatively accessible to antibodies and effector cells, malignant B cells displayed a particularly high susceptibility to neutrophil-mediated ADCC.93
Phenotypic and functional evidence of potent PMN activation has also been observed during rIL-2 infusion in patients with advanced malignant melanoma and renal cell carcinoma.94,95 Furthermore, patients showing disease response to treatment have a significantly greater production of PMN oxidants, such as HOCl, which is regarded as instrumental in tumor cell lysis.55 77
Evidence suggests that production of the tumoricidal long-lived oxidant HOCl, along with up-regulation of PMN surface integrins, may also contribute to the antineoplastic efficacy of infusional therapy with TNF-α.96-98
However, besides their involvement in the therapeutic efficacy of certain cytokines, PMNs may be partly responsible for the toxic side effects of high-dose systemic cytokine therapy.77
Conclusion
The poor results obtained in humans with systemic cytokine therapy have cast a shadow over this type of approach, especially since its failures were ascribable mainly to multiple side effects and enrollment in phase I trials of patients with advanced disease and a deeply compromised immune system. The extremely encouraging results obtained with local intratumoral cytokine release in animal models, on the other hand, suggest the possibility of successful antitumor management through an improvement in cytokine gene therapy biotechnology and procedures for patients with a reasonable immunological performance and low tumor load or minimal residual disease.
It is clear, in any event, that in addition to being the body's main defenders against infection and foreign invaders, PMNs could be a perfect weapon for the suppression of tumor growth and tumor rejection in T-cell memory reactions, while their ability to respond to and produce cytokines2,3 involves them in the cross-talk between tumor cells and the endothelium and between nonspecific and specific immune cells.5
A deeper insight into the biological role of immunoregulatory molecules acting as cytokines that stimulate specific PMN functions may thus lead to the elaboration of a new approach to the treatment of cancer.
Acknowledgment
We thank Dr John Iliffe for critical review of the manuscript.
Supported by grants from the Italian Association for Cancer Research (AIRC); the Istituto Superiore di Sanità Special Programs of Gene Therapy and Antitumor Therapy; C.N.R. Target Project on Biotechnology; MURST 40% and cluster 03—Molecular Engineering—funds, and the US Department of the Army grant DAMD 17-98-1-8030 (G.F.); and by a fellowship from the Italian Foundation for Cancer Research (E.D.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Piero Musiani, G. d' Annunzio University of Chieti, Anatomia Patologica, Osp. SS. Annunziata, Via Valignani, 66100 Chieti, Italy; e-mail: musiani@unich.it.

![Fig. 3. Cytokine modulation of PMN intratumoral recruitment and functions. / Cytokine modulation of PMN intratumoral recruitment and functions results in tumor destruction and an antitumor immune memory. Tumor-associated macrophages are the first reactive cells ready to respond to the secondary mediators (IL-1β, TNF-α, IFN-γ, etc) induced by the cytokine systemically administered or locally released by engineered tumor cells. Depending on the cytokines present in the microenvironment, activated macrophages release ELR (glutamic acid–leucine–arginine)+ CXC chemokines, which recruit neutrophils, and/or ELR− CXC chemokines, which recruit T lymphocytes. If IL-1β and TNF-α prevail, macrophages and PMNs are induced to produce ELR+ chemokines (MIP-2/GRO/KC), which amplify accumulation of PMNs and favor their destructive functions. If IFN-γ prevails, they are induced to produce angiostatic ELR− chemokines (interferon inducible protein-10 [IP-10], monokine induced by gamma interferon [MIG]) that recruit T lymphocytes and promote the establishment of an antitumor immune memory.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.339/5/m_h80210589003.jpeg?Expires=1770904235&Signature=V1uxAlK1RoHl3q1-g~hTKhWdijk3hY1DhElGBpPfrIcM658dH4OpdicH7hYkD1Vtm0oDB6JXLXyZvS-TxRQ-K-rXONomSb26CGQV6AVGu87oTsoBnLRb91GEptxw4suzBNTF31HxdVqEXDHuhqRwpkzQmuQLyRSqKp-81l6UU85G1vbgBLToY-My3-Nz84WY8hCVJjswoLlSOeq73pTltIb-98nxcuL2oXmVfSCG-w4jK26iXoq25Ct2jhL0Lrj57sPt60v4VfjwgZQOBadqdZsYKfLpo~VbnAX9-byfttz3Bct2-Tb8XpgxX~ZI-4BrNv8UvT3oavih3xOowGjDJA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 3. Cytokine modulation of PMN intratumoral recruitment and functions. / Cytokine modulation of PMN intratumoral recruitment and functions results in tumor destruction and an antitumor immune memory. Tumor-associated macrophages are the first reactive cells ready to respond to the secondary mediators (IL-1β, TNF-α, IFN-γ, etc) induced by the cytokine systemically administered or locally released by engineered tumor cells. Depending on the cytokines present in the microenvironment, activated macrophages release ELR (glutamic acid–leucine–arginine)+ CXC chemokines, which recruit neutrophils, and/or ELR− CXC chemokines, which recruit T lymphocytes. If IL-1β and TNF-α prevail, macrophages and PMNs are induced to produce ELR+ chemokines (MIP-2/GRO/KC), which amplify accumulation of PMNs and favor their destructive functions. If IFN-γ prevails, they are induced to produce angiostatic ELR− chemokines (interferon inducible protein-10 [IP-10], monokine induced by gamma interferon [MIG]) that recruit T lymphocytes and promote the establishment of an antitumor immune memory.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.339/5/m_h80210589003.jpeg?Expires=1770969430&Signature=zslFr77Wp13gvAChWspwm95bnaklCGKPf~EW2gIIuoY1VH7hmC7RXZvKK74OHaqDm8Poe2wNR-NzfKD-guXezMpmZeJ8LEQjHnhaJ9xcPHzjnAN-OlPoUnjS-opZRmXA3xA2QHceT-5SX2Z5NcGVCzy6yhzjdcevzpf2Y~S3hqFGFy283pGKLIEqajfjH0iX-6PI5SGK9g1tL5JR73xAP-DXDYbTOjmdYRIHSYpE3HIDSiMTCyhew5C5y0MNcwZNEwOBMHiOqYmW-yVNWEJOsk~3YxEwVhauqOslqLt9SCvPgzbOJKDJI8syX57Y9~6GVJWPZYAZX8K3SMY3S8EcdQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)