Konopleva et al1 describe the expression of Bcl-2 in 2 acute myeloid leukemia (AML) cell lines, HL-60 and HL-60-doxorubicin–resistant, and in fresh AML cells obtained from patients with newly diagnosed or recurrent AML. The focus of their study was to investigate the effect of liposomal Bcl-2 antisense oligonucleotides (Bcl-2-ASs) on proliferation and chemotherapy sensitivity of these AML cells. Indeed, the authors report convincingly that Bcl-2-ASs reduce Bcl-2 protein levels and increase cytosine-arabinoside cytotoxicity in both HL-60 cell lines and in 11 of 19 primary AML samples.
On the other hand, the data concerning the levels of human A1 protein are less convincing for at least 2 reasons. First, in “Materials and methods” under “Western blot analysis,” the authors cite references related to the use of polyclonal antibodies to A1 which in fact do not have any data on A1. Second, the authors show results on the protein expression for Bcl-XL, Bag-1, Mcl-1, Bax, Bad, Bak, and A1 (in Table 4), but an example of Western blot analysis for A1 protein was strikingly missing (in Figure 12).
We have been studying the role of human A1 in acute leukemia for the last 3 years.2-4 We have used mainly Northern blot analysis because of the unavailability of a human A1 antibody. We tested several batches of a polyclonal antibody marketed by Santa Cruz Biotechnology (Santa Cruz, CA) but without success. Thus it would be important for Konopleva et al to provide the proper source for the A1 antibody that they used and the Western blot evidence for their ability to detect the human A1 protein.
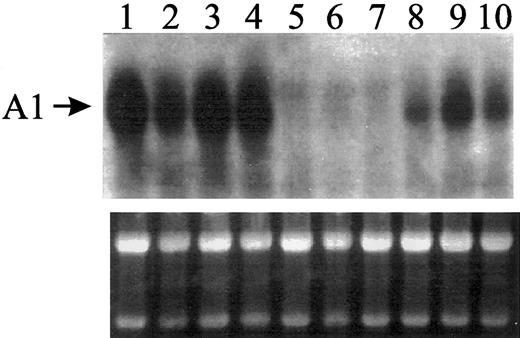
Using Northern blot analysis, we have shown that A1 mRNA expression is upregulated with myelomonocytic differentiation.2 Exposure to bleomycin at 1 μg/mL induces myeloid differentiation in the U937 leukemic cell line (increased granularity and CD11b expression)5 and upregulates A1 mRNA expression (Figure 1) in wild-type (WT) cells and in control cells transfected with a pLXSN retroviral vector (lanes 2-4 and 8-10, respectively). But in U937 cells transduced with pLXSN containing the murine Bcl-2 cDNA, the untreated and the bleomycin-treated cells lack A1 mRNA expression (lanes 5-7). These results suggest a regulatory relationship between Bcl-2overexpression and A1 downregulation.
Northern analysis for human A1 mRNA expression.
WT U937 cells or U937 cells transfected with pLSXN retroviral vector or pLXSN containing Bcl-2 in the sense orientation were exposed to 1 μg/mL bleomycin, which induces myeloid differentiation in these cells (increased granularity and CD11b expression), and assayed for the expression of A1 mRNA. Approximately 20 μg total RNA was loaded in each lane. IL-1–treated A549 lung carcinoma cell line was included (lane 1) for positive control. Other experimental groups included WT U937 cells that were either untreated or treated with bleomycin for 2 and 5 days (lanes 2, 3, and 4, respectively); U937 cells expressing Bcl-2 that were either untreated or treated with bleomycin for 2 and 5 days (lanes 5, 6, and 7, respectively); and vector-transfected U937 cells that were either untreated or treated with bleomycin for 2 and 5 days (lanes 8, 9, and 10, respectively). Northern blot was hybridized to a randomly primed32P-labeled A1 cDNA. Autoradiograph was developed after 6 days exposure to x-ray film using 2 intensifying screens at − 80°. Photograph of the corresponding ethidium bromide–stained gel is shown below the autoradiograph for loading control.
Northern analysis for human A1 mRNA expression.
WT U937 cells or U937 cells transfected with pLSXN retroviral vector or pLXSN containing Bcl-2 in the sense orientation were exposed to 1 μg/mL bleomycin, which induces myeloid differentiation in these cells (increased granularity and CD11b expression), and assayed for the expression of A1 mRNA. Approximately 20 μg total RNA was loaded in each lane. IL-1–treated A549 lung carcinoma cell line was included (lane 1) for positive control. Other experimental groups included WT U937 cells that were either untreated or treated with bleomycin for 2 and 5 days (lanes 2, 3, and 4, respectively); U937 cells expressing Bcl-2 that were either untreated or treated with bleomycin for 2 and 5 days (lanes 5, 6, and 7, respectively); and vector-transfected U937 cells that were either untreated or treated with bleomycin for 2 and 5 days (lanes 8, 9, and 10, respectively). Northern blot was hybridized to a randomly primed32P-labeled A1 cDNA. Autoradiograph was developed after 6 days exposure to x-ray film using 2 intensifying screens at − 80°. Photograph of the corresponding ethidium bromide–stained gel is shown below the autoradiograph for loading control.
In summary, although Konopleva et al show that Bcl-2 plays a central role in the proliferation and drug resistance of AML, the nature of its interaction with other antiapoptotic proteins, including A1, remains largely unknown.
Human A1 expression in acute myeloid leukemia
We thank Drs Moreb and Zucali for their comments on our report concerning the effects of Bcl-2 antisense oligonucleotides (Bcl-2-ASs) on proliferation, apoptosis, and sensitivity to Ara-C in primary acute myeloid leukemia (AML) and myeloid leukemia cell lines.1-1 We further stated that apoptosis was induced by Bcl-2-ASs although other antiapoptotic proteins were expressed, thus establishing Bcl-2-ASs as a critical target for AS strategies in AML.
While Drs Moreb and Zucali agree with these findings, they find the data on one of the antiapoptotic proteins, A1, “less convincing.” The Bcl-2 family member A1/Bfl-1 was cloned by Choi et al1-2and by Karsan et al.1-3 It was found to be expressed in bone marrow cells and is induced by cytokines and differentiation inducers in leukemic cells.1-4
Drs Moreb and Zucali state that they “used mainly Northern blot analysis because of the unavailability of a human A1 antibody.” The A1 antibody used in our study was kindly provided by Dr John C. Reed. It generated immunoblots of better quality than those obtained with the polyclonal antibody marketed by Santa Cruz. But a paper has recently been published utilizing the Santa Cruz antibody successfully.1-5 We here provide our own method of A1 detection:
Cells were washed twice with phosphate buffered saline (PBS) buffer and lysed at 4 × 104 cells/μL in cell lysis buffer (20 mM Hepes, pH 7.4, 0.25% NP-40 containing protease inhibitor cocktail; Boehringer Mannheim, Indianapolis, IN) for 10 minutes on ice. Lysates were spun at 25 000g for 10 minutes, and an equal amount of 2 × gel-loading buffer (0.25 M Tris-HCl, 2% SDS, 4% β-mercaptoethanol, 10% glycerol, 0.02% bromophenol blue) was added to the supernatants. Equal amounts of lysate (equivalent to 5 × 105 cells) were subjected to sodium-dodecyl-sulfate polyacrylamide-gel electrophoresis (SDS-PAGE) onto 12% polyacrylamide gels. Afterward, proteins were transferred to Hybond-P membranes (Amersham Pharmacia Biotech, Buckinghamshire, England). The membranes were blocked overnight at 4°C with 7% milk in PBS buffer containing 0.3% Tween-20, followed by incubation with polyclonal antibody against A1 (1:1000 dilution) (kindly provided by Dr J. C. Reed) for 1 hour at room temperature. Membranes were washed 3 times with PBS buffer containing 0.3% Tween-20, probed with a horseradish peroxydase (HRP)–conjugated secondary antibody and then reacted with enhanced chemiluminescence (ECL) reagent (Amersham Pharmacia Biotech). Signals were detected by a phosphoimager (Storm 860 Version 4.0; Molecular Dynamics, Sunnyvale, CA).
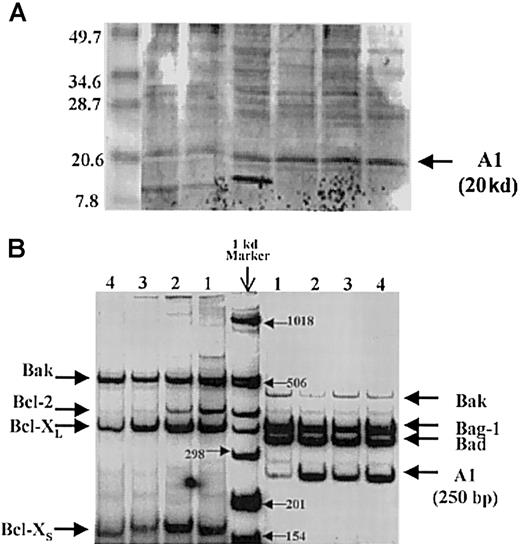
Using this method, the A1 bands are clearly discernible at the correct location, and these results are supported by the detection of A1 mRNA by reverse-transcriptase polymerase chain reaction (RT-PCR) in primary AML samples (Figure 1-1). Oligonucleotide primers (F, forward; R, reverse) used for expression analysis by RT-PCR were as follows: human A1- F 5′-CGGCATCATTAACTGGGGAAG-3′ and R 5′-GATCTTTCCTGTAACTTCTAG-3′; the expected PCR product is 250 base pairs (bp). Other pro- and antiapoptotic genes are also expressed. In a series of 135 AML analyzed for expression of A1 by semiquantitative RT-PCR, no correlation with white blood cell count or bone marrow blast count was observed.
A1 detection by Western blot analysis and reverse-transcriptase polymerase chain reaction.
(A) Detection of A1 by Western blot analysis. The A1 bands are at the correct location of 20 kd. (B) Detection of A1 mRNA by reverse-transcriptase polymerase chain reaction (RT-PCR) in primary AML samples.
A1 detection by Western blot analysis and reverse-transcriptase polymerase chain reaction.
(A) Detection of A1 by Western blot analysis. The A1 bands are at the correct location of 20 kd. (B) Detection of A1 mRNA by reverse-transcriptase polymerase chain reaction (RT-PCR) in primary AML samples.
In conclusion, we have demonstrated the presence of the antiapoptosis protein A1 in primary AML samples by RT-PCR and immunoblot analyses. We agree with Drs Moreb and Zucali that the nature of the interaction between Bcl-2 and other antiapoptotic proteins remains unknown.