Abstract
Tissue factor (TF), a transmembrane glycoprotein, initiates the extrinsic coagulation cascade. TF is known to play a major role in mediating thrombosis and thrombotic episodes associated with the progression of atherosclerosis. Macrophages at inflammatory sites, such as atherosclerotic lesions, release numerous cytokines that are capable of modulating TF expression. This study examined the role of oncostatin M (OSM), a macrophage/ T-lymphocyte–restricted cytokine, in the expression of TF in vascular smooth muscle cells (SMCs). It is reported here that OSM stimulated a biphasic and sustained pattern of TF messenger RNA (mRNA). The effect of OSM on TF mRNA expression was regulated at the transcriptional level as determined by nuclear run-offs and transient transfection of a TF promoter-reporter gene construct. OSM-induced TF expression was regulated primarily by the transcription factor NF-κB. Activation of NF-κB by OSM did not require IκB-α degradation. Inhibition of MEK activity by U0126 prevented OSM-induced TF expression by suppressing NF-κB DNA binding activity as determined by gel-shift analysis. Further, inhibition of Erk-1/2 protein by antisense treatment resulted in suppression of TF mRNA expression, indicating a role for Erk-1/2 in modulating NF-κB DNA binding activity. These studies suggest that the induced expression of TF by OSM is primarily through the activation of NF-κB and that activation of NF-κB is regulated in part by the MEK/Erk-1/2 signal transduction pathway. This study indicates that OSM may play a key role in promoting TF expression in SMCs within atherosclerotic lesions.
Introduction
Thrombosis plays an integral role in the development and progression of atherosclerosis.1 It is also believed to contribute to neointimal development following acute arterial injury.2 The thrombus contains growth factors and cytokines that have been implicated in smooth muscle cell (SMC) proliferation and migration.3 Data from several studies have suggested that, in acute vascular injury and in atherosclerosis, tissue factor (TF) plays a major role in initiating thrombosis.4-7 TF initiates the clotting cascade by serving as a cofactor for plasma factor VIIa.8,9 In normal arteries, TF is found predominantly in the adventitia. In experimental animal models, TF is rapidly induced in medial SMCs following balloon arterial injury and has been shown to accumulate in the neointima.2,5,10 In human atherosclerotic plaques, TF is found associated with SMCs, endothelial cells, macrophages, and extracellular matrix.10,11 With the use of a functional clotting assay of human coronary atherectomy specimens, it was shown that the TF present in atherosclerotic plaques was active.7 Numerous studies have demonstrated that inflammatory cytokines such as interleukin (IL)-1 and tumor necrosis factor-α (TNF-α) can induce TF expression in endothelial cells.12,13 However, in acute arterial injury and in atherosclerosis, SMCs and macrophages are the most likely source for TF.14 Recently, SMCs stimulated with platelet-derived growth factor (PDGF) and thrombin were shown to express TF protein on their surface as well as stored intracellularly as a latent form.14 Given that macrophages and T lymphocytes are known components of atherosclerotic plaques,15,16 cytokines released by these cells may contribute to the induction of TF within the lesion. In the present study, we investigated whether oncostatin M (OSM) is capable of regulating TF expression. OSM is a macrophage/T-lymphocyte–restricted cytokine and is related to the family of cytokines that include leukemia inhibitory factor, IL-6, IL-11, and ciliary neurotrophic factor.17,18These cytokines share a common signal transducer receptor component, gp 130, and have overlapping biological activities.19 OSM has been shown to stimulate growth of rabbit aortic SMCs and acquired immune deficiency syndrome–Kaposi sarcoma cells.20-22 In bovine and human endothelial cells, OSM promotes the expression of urokinase plasminogen activator, basic fibroblast growth factor, granulocyte colony-stimulating factor, and granulocyte-macrophage colony stimulating factor.23-25 In human fibroblasts, OSM modulates not only matrix metalloproteinases but also tissue inhibitors of matrix metalloproteinases.26,27 Further, OSM was demonstrated to stimulate angiogenesis in vivo and was shown to be present in human aortic aneurysms.28 29 Because OSM is produced by activated monocytes/macrophages and T lymphocytes, cell types found frequently at sites of inflammatory wound repair and atherosclerotic lesions, we examined the potential role and mechanism of OSM in promoting TF expression in SMCs. We report that OSM promoted a biphasic pattern of TF expression. This induction is dependent on activation of NF-κB that is regulated by the MEK/Erk-1/2 signaling pathway.
Materials and methods
Materials
Human TF and G3PDH complementary DNAs (cDNAs) were obtained from American Type Culture Collection (Rockville, MD); S2765 from Chromogenix (West Chester, OH); Factor VIIa and X from Enzyme Research Laboratories (South Bend, IN); human recombinant OSM from R&D Systems (Minneapolis, MN); luciferase assay system, pGL2, pSV-β-galactosidase control vectors, U0126 (ERK-1/2 inhibitor), and Transfast transfection reagent system from Promega (Madison, WI); NF-κB inhibitor peptide and NF-κB control inhibitor peptide from Biomol (Plymouth Meeting, PA); NF-κB and PanErk-1/2 monoclonal antibodies from Signal Transduction (Lexington, KY); phospho-Erk-1/2 monoclonal antibody from Biolabs (Beverly, MA); antihuman OSM and IκB-α polyclonal antibodies from Santa Cruz (Santa Cruz, CA); and polyclonal antihuman TF antibody from American Diagnostica (Greenwich, CT).
Cell culture
Human SMCs were obtained from Clonetics (San Diego, CA). Canine SMCs were obtained by enzymatic digestion. Briefly, medial tissue was minced and digested overnight at 37°C in Dulbecco modified Eagle (DME)/F12 medium supplemented with 150 U/mL collagenase, 1 U/mL elastase, 0.25 U/mL DNase I, 2 mM Glutamax-I, and 2% fetal bovine serum (FBS) for 24 hours. SMCs were cultured in DME/F12 medium supplemented with 10% FBS, 2 mM Glutamax-I, and 50 μg/mL gentamicin. Cells were characterized as SMCs by morphological criteria and by staining with α-smooth muscle actin and smooth muscle myosin heavy chain (Sigma, St Louis, MO). Canine SMCs from passage 1 to 5 and human SMCs from passage 6 to 8 were used for experiments. Throughout the experiments, the canine and human cells were incubated in a defined medium, consisting of DME/F12 with 0.4% Redu-Ser II, 2 mM Glutamax-1, and 50 μM gentamicin for at least 24 hours. All experiments were repeated at least once in human SMCs to confirm observations made in canine SMCs.
TF procoagulant activity
The TF activity expressed by SMCs was measured by a chromogenic assay. Briefly, cells were seeded in 24-well plates at a density of 1 × 105 cells per well in 200 μL culture medium. SMCs were stimulated with OSM (10 ng/mL) for the indicated times. The cells were washed 3 times in Tris-buffered saline (50 mM Tris HCl, 120 mM NaCl, 2.7 mM KCl, 3 mg/mL bovine serum albumin, pH 7.4), followed by incubation for 30 minutes at 37°C with 300 μL Tris-buffered saline containing human factor VIIa and X (5 and 150 nM, respectively) and CaCl2 (5 mM). Then, 250 μL of the supernatant was added to 25 μL of a chromogenic substrate for factor Xa (S2765, 0.2 μM final concentration). The chromogenic reaction was stopped after 3 minutes by addition of 20 μL of 50% acetic acid solution and absorbance was measured at 405 nm with a spectrophotometer. The TF activity was expressed in arbitrary units (AU), using reference curves determined by rabbit brain thromboplastin. The logarithms of the procoagulant activity were linearly related to the absorbance up to 100 AU. TF clotting assay was performed as described previously.30
TF enzyme–linked immunosorbent assay
Confluent human SMC in 6-well plates were exposed to OSM (10 ng/mL) for the indicated times. SMC were lysed and analyzed for TF antigen, using enzyme-linked immunosorbent assay kits (American Diagnostica) according to the manufacturer's instructions.
Total RNA extraction and Northern blot analysis
RNA was extracted, using RNeasy kit (Qiagen, Valencia, CA). RNA samples (5 to 10 μg) were denatured with dimethyl sulfoxide/glyoxal and electrophoresed on a 1.2% agarose/10 mM sodium phosphate gel and were transferred onto nylon filters by capillary blotting. The filters were then hybridized in Quickhyb solution (Stratagene, La Jolla, CA) to random primed 32P-labeled cDNA TF probe for 1 hour at 68°C. Filters were washed at 68°C in 0.2 × standard saline citrate/0.2% sodium dodecyl sulfate (SDS) and exposed to Kodak MS films for 48 hours at −70°C. For comparison of RNA loading, filters were rehybridized with G3PDH probe.
RNA stability analysis
SMCs were stimulated for 1 and 24 hours with 10 ng/mL OSM. Actinomycin D (5 μg/mL) was then added to the cultures, and RNA was extracted at the indicated times. Northern analysis was performed, and the membranes were probed with TF and G3PDH. Autoradiographic signals were analyzed on a Macintosh 9600 computer, using the public domain NIH Image analysis program (developed at the U.S. National Institutes of Health and available on the Internet athttp://rsb.info.nih.gov/nih-image/). The TF signal density was normalized to G3PDH density. The corrected density was then plotted as a percentage of the 0-hour value (log scale) against time.
Nuclear run-off analysis
Nuclei (2 × 107) from SMCs stimulated for 1 hour with OSM (10 ng/mL) were isolated, and in vitro transcription was carried out in 100 μL of 10 mM Tris-HCl (pH 8.0) buffer, containing 5 mM MgCl2; 300 mM KCl; 0.1 mM EDTA; 1 mM dithiothreitol (DTT); 0.5 mM cytidine triphosphate, guanosine triphosphate, adenosine triphosphate (ATP); and 200 μCi α32P uridine triphosphate (NEN, Boston, MA) for 30 minutes at 30°C. The reaction was terminated by adding 1 μL proteinase K (20 mg/mL) and 10 μL 10% SDS followed by incubation at 40°C for 1 hour. Radiolabeled RNA was precipitated by LiCl after acid phenol/chloroform extraction. Linearized, denatured TF, G3PDH, and pGEM-4Z (Promega) plasmid DNA (5 μg) was vacuum transferred onto nylon membranes (Schleicher and Schuell, Keene, NH), using a slot blot apparatus. The nylon membranes were hybridized with radiolabeled RNA (3 × 107 cpm) in Quickhyb solution for 4 hours. The membranes were then washed with 0.5 × standard saline citrate/0.2% SDS at 60°C before autoradiography for 48 hours at −70°C.
Transfections and luciferase assays
The construction of pTF(−2106)LUC and p19LUC vectors have been described previously.31 The pGL2 promoter control and p19LUC vectors were used as positive and negative controls, respectively. The pSV-β-galactosidase control vector was used to allow for normalization for transfection efficiencies. SMCs were seeded at a density of 5 × 105 cells per well in 6-well plates and grown in the culture medium overnight. Transient transfections were performed according to the manufacturer's instruction. The cells were transfected with 1 μg pTF(−2106)LUC, or pGL2 or p19LUC together with 1 μg pSV-β-galactosidase control vector. After transfection, the cells were incubated in the serum-free medium for 48 hours throughout experiments. Then, cells were incubated at 37°C for a further 5 hours either in the presence or absence of 10 ng/mL OSM. β-galactosidase and luciferase activity assay was performed according to the manufacturer's instruction, and luciferase values were normalized to β-galactosidase levels.
Oligonucleotide transfection and reverse transcriptase–polymerase chain reaction analysis
The following phosphorothioated oligonucleotides were used as antisense directed against p42 and p44 Erk: 5′-GCCGCCGCCGCCGCCAT-3′. Control oligonucleotides consisted of scrambled antisense sequence, 5′-CGCGCGCTCGCGCACCC-3′ (Biomol). Oligonucleotides were transfected into SMCs in 24-well plates, using Lipofectin reagent (Gibco-BRL, Grand Island, NY) as described by the manufacturer. After 48 hours, the SMC cultures were stimulated for 1 hour with OSM (10 ng/mL). The effect of antisense treatment on Erk-1/2 protein expression was analyzed by preparing total cell protein extract, using sample buffer. RNA was extracted as described above, and 100 ng RNA was used for TF and ribosomal s17 messenger RNA (mRNA) analysis, using a one-step reverse transcriptase–polymerase chain reaction (RT-PCR) kit (Gibco-BRL). Quantitative RT-PCR was performed as previously described with minor modifications.32 Forward primers were end-labeled with32P-γATP. Preliminary experiments were performed to determine the number of PCR cycles to ensure that the PCR was done in a quantitative range. TF and ribosomal s17 primers used were as follows: TF forward, 5′-CACCTTACCTGGAGACAAACCTC-3′; TF reverse, 5′-TGGGCAACAGAGCAAGACTC-3′; s17 forward, 5′-GAAGGCGGCCCGGGTCATCA-3′; and s17 reverse, 5′-GTAGGCTGA/GGTGACCTG-3′. RT-PCR conditions used were 30 minutes at 45°C (RT step), 2 minutes at 94°C, followed by 18 cycles of 94°C for 15 seconds, 55°C for 20 seconds, and 72°C for 20 seconds. The final extension was carried out at 72°C for 10 minutes. The PCR products, TF (900 base pairs [bp]) and ribosomal s17 (350 bp) were separated on 2% TAE agarose gel. To visualize the products, gels were exposed to Kodak MS films. The products were quantified by cutting the bands and counting radioactivity in a scintillation counter.
Cytoplasmic and nuclear extract
Cytoplasmic and nuclear extracts from SMCs were prepared by scraping SMCs into cold phosphate-buffered saline, washed once, and resuspended in 200 μL hypotonic lysis buffer (10 mM HEPES, pH 8, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT and protease inhibitors) at 4°C for 15 minutes. NP-40 was added to a final concentration of 0.5%, vortexed for 10 seconds, and centrifuged at 7000g for 5 minutes. The supernatant (cytoplasmic) was stored at −70°C. The nuclei were then solubilized with sample buffer for Western blotting.
Electrophoretic mobility shift assay
For electrophoretic mobility shift assay (EMSA) studies, nuclei were extracted with 100 μL cold extraction buffer (20 mM HEPES, pH 8, 20% glycerol, 0.5 M NaCl, 1.5 mM MgCl2, 0.1 mM EDTA, 0.5 mM DTT and protease inhibitors) for 30 minutes on ice. After centrifugation, supernatant was frozen at −70°C. Protein concentrations in both nuclear and cytoplasmic extracts were determined. Nuclear extract (5 μg) was used for the detection of NF-κBp65 and AP-1 transcription factor, using EMSA kits (Geneka Biotechnology, Montreal, QC, Canada). The following oligonucleotides were used: AP-1 site, 5′-CGCTTGATGAGTCAGCCGGAA-3′; mutant AP-1 site, 5′-CGCTTGATGACCCAGCCGGAA-3′; NF-κB site, 5′-AGCTTGGGGTATTTCCAGCCG-3′; and mutant NF-κB site, 5′-AGCTTGGCATAGGTCCAGCCG-3′. The italicized nucleotides are the consensus binding sequence for the respective transcription factors. The bold underlined and italicized nucleotides represent the mutation sites. Binding reactions were performed according to the manufacturer's protocol.
Western blotting
Protein concentrations were determined, and equal amounts of protein were separated on a 4% to 12% Bis-tris polyacrylamide gel. After transferring to nylon filters, the membranes were blocked with 20 mM Tris-HCl (pH 7.5) containing 0.15 M NaCl, 3% gelatin, and 0.5% Tween-20 for 1 hour at room temperature. Membranes were then probed with a polyclonal antibody to IκB-α or with a monoclonal antibody to NF-κB in 20 mM Tris-HCl (pH 7.5) containing 1% gelatin, 0.15 M NaCl, and 0.05% Tween-20 for 1 hour at room temperature. Blots were then washed with 20 mM Tris-HCl (pH 7.5) containing 0.15 M NaCl and incubated with a peroxidase-linked goat antimouse antibody for 30 minutes. Following washing, bands were developed, using Super Signal chemiluminescent reagent (Pierce, Rockford, IL).
Statistical analysis
Statistical analysis was performed by using StatView 4.51 (Abacus Concepts, Berkeley, CA). Factorial analysis of variance with the Fisher exact test was used as appropriate. All values were expressed as mean ± SEM and P values < .05 were considered statistically significant.
Results
OSM induction of TF expression in SMCs
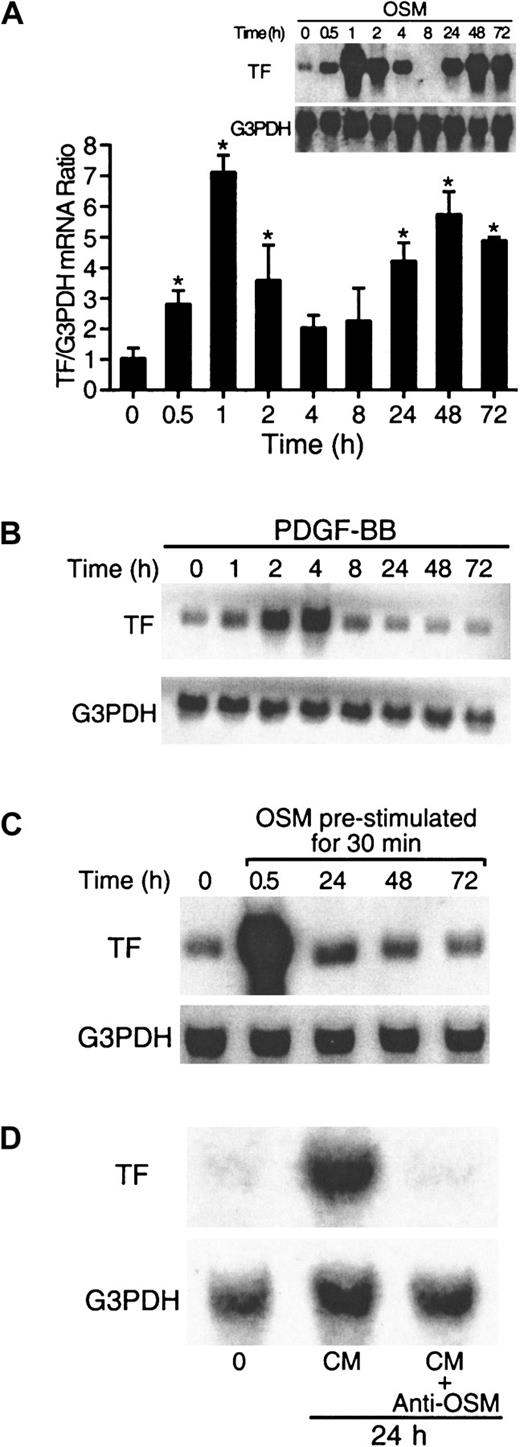
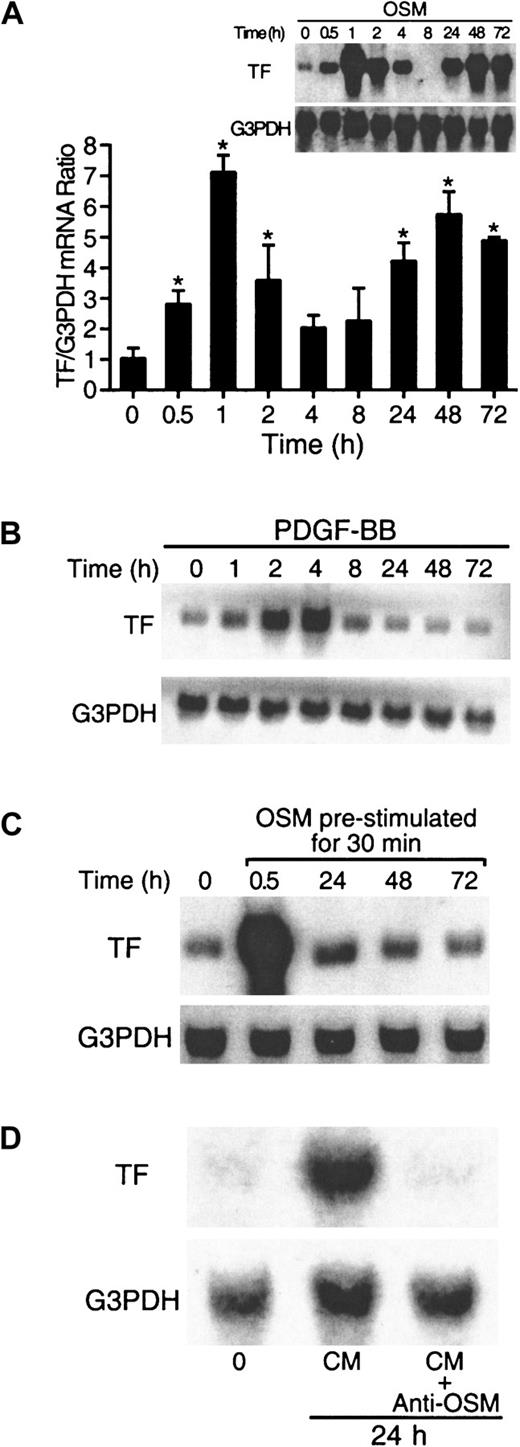
To determine if OSM could increase the expression of TF mRNA in cultured SMCs, cells were treated with 10 ng/mL OSM for the times indicated. Low levels of TF mRNA were detected in unstimulated cells (Figure 1A). OSM induced a biphasic increase in TF mRNA expression (Figure 1A). In the first phase, increased levels of TF mRNA expression were observed within 30 minutes, and maximal levels were observed at 1 hour. In the second phase, TF mRNA levels peaked at 48 hours and remained elevated up to 72 hours. Boiled OSM was used to rule out the effect of lipopolysaccharide (LPS) on TF mRNA induction (data not shown). In contrast, PDGF-BB, a known activator of TF,14 induced only a monophasic pattern of TF expression, peaking at 4 hours and returning to control levels by 24 hours (Figure 1B). Other factors tested (thrombin and FBS) also produced only a monophasic pattern (data not shown).
OSM induces biphasic TF mRNA expression.
(A,B) Kinetics of TF mRNA induced by OSM and PDGF-BB. SMCs were incubated with OSM (10 ng/mL) and PDGF-BB (20 ng/mL) for the indicated times, and RNA was extracted from 5 × 106 cells at the end of each incubation and analyzed as described in “Materials and methods.” The blots were rehybridized with G3PDH to demonstrate equal RNA loading. Solid columns represent the aggregate of densitometry analysis from 4 separate experiments. *P < .05 compared to 0 hour. (C) SMCs cultures were prestimulated with OSM for 30 minutes, washed 3 times, and incubated in serum-free DME/F12 medium for the indicated times. RNA was extracted at the end of each incubation and analyzed for TF mRNA expression by Northern blotting. (D) OSM-conditioned medium from 24-hour SMC cultures were used to stimulate SMCs for a further 24 hours in the presence or absence of a neutralizing monoclonal antibody to OSM. After 24 hours, RNA was extracted and analyzed for TF expression by Northern blotting. Results are representative of 3 separate experiments.
OSM induces biphasic TF mRNA expression.
(A,B) Kinetics of TF mRNA induced by OSM and PDGF-BB. SMCs were incubated with OSM (10 ng/mL) and PDGF-BB (20 ng/mL) for the indicated times, and RNA was extracted from 5 × 106 cells at the end of each incubation and analyzed as described in “Materials and methods.” The blots were rehybridized with G3PDH to demonstrate equal RNA loading. Solid columns represent the aggregate of densitometry analysis from 4 separate experiments. *P < .05 compared to 0 hour. (C) SMCs cultures were prestimulated with OSM for 30 minutes, washed 3 times, and incubated in serum-free DME/F12 medium for the indicated times. RNA was extracted at the end of each incubation and analyzed for TF mRNA expression by Northern blotting. (D) OSM-conditioned medium from 24-hour SMC cultures were used to stimulate SMCs for a further 24 hours in the presence or absence of a neutralizing monoclonal antibody to OSM. After 24 hours, RNA was extracted and analyzed for TF expression by Northern blotting. Results are representative of 3 separate experiments.
To test whether the second phase of TF mRNA increase was due to residual OSM, we prestimulated SMC cultures with OSM for 30 minutes. The cultures were then washed and incubated for the indicated times in serum-free medium. At 30 minutes, TF mRNA levels were increased; however, TF mRNA levels were not elevated at 24, 48, and 72 hours (Figure 1C) when compared to SMC cultures incubated throughout with OSM (Figure 1A). When SMC cultures were stimulated with OSM-conditioned medium for 24 hours, an increase in TF mRNA expression was observed, which was neutralized by antibodies to OSM (Figure 1D). This observation indicates that the secondary phase of TF induction was due to residual OSM present in the medium and not through secondary growth factors induced by OSM. The time-course of TF mRNA synthesis in canine aortic SMCs was repeated in human aortic SMCs and was shown to be similar (data not shown).
Levels of TF antigen and activity were examined in the first phase of OSM-induced TF expression (0 to 8 hours). OSM transiently increased TF antigen and activity with maximal levels between 2 and 4 hours (Table1). Consistent with our observation that residual OSM promoted the late-phase TF mRNA induction (Figure 1D), TF antigen and activity levels were still elevated at 72 hours (data not shown).
OSM promotes activation of TF gene transcription
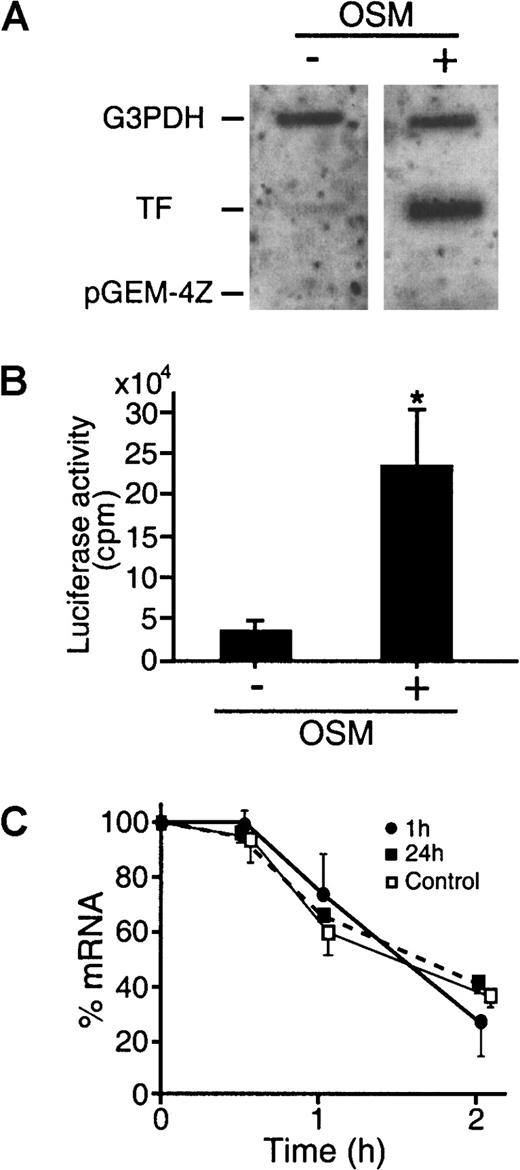
To evaluate whether gene activation, mRNA stability, or a combination of both were responsible for the increased TF mRNA observed following OSM stimulation, nuclear run-off, TF promoter studies, and mRNA stability studies were performed. Having established that both phases of TF induction were due to OSM, the remaining studies were focused primarily on the early phase of TF induction. Exposure to OSM for 1 hour significantly increased TF gene expression over unstimulated cells, whereas the transcription rate of the G3PDH gene was unaffected (Figure 2A). Thus, results from our Northern analysis studies, together with the nuclear run-off experiments, suggest that the increase in TF mRNA induced by OSM reflects specific activation of the TF promoter and enhanced TF gene transcription. To confirm that OSM induced active TF gene transcription, transfection studies were performed, using a 2106-bp fragment of the TF promoter coupled to the luciferase gene (pTF(−2106)LUC). Data obtained from transfection experiments are consistent with nuclear run-off studies (Figure 2B). In unstimulated cells, pTF(−2106)LUC promoter activity was low. There was a 9-fold increase in pTF(−2106)LUC activity when SMCs were stimulated with OSM (10 ng/mL), indicating that OSM can activate the TF promoter.
OSM induces an increased rate of TF gene transcription.
The transcription rate of the TF gene was assessed by nuclear run-off assays. (A) Nuclear extracts were harvested from SMCs treated with or without OSM (10 ng/mL) for 1 hour. Equal amounts of32P-labeled in vitro transcribed RNA probes were hybridized to 5 μg of denatured TF, G3PDH, and pGEM-4Z cDNA. Results are representative of 2 separate experiments. (B) Activation of TF promoter by OSM was analyzed by studying the effect of OSM on TF gene promoter fused to luciferase. pTF(−2106)LUC, which contains 2.1 kilobase (kb) of the 5′ flanking region of the TF gene promoter, and pSV–β-galactosidase control vector were transfected into SMCs. The cells were treated with OSM (10 ng/mL) for 5 hours before harvesting. Luciferase activity was determined and normalized for β-galactosidase activity. Results are expressed as mean ± SEM (n = 4 experiments). *P < .05 compared to unstimulated SMCs. (C) SMCs were exposed to vehicle (control, ■) or OSM (10 ng/mL, ▪ and ●) for 1 or 24 hours before addition of actinomycin D (5 μg/mL). Total RNA was extracted at the indicated times after addition of actinomycin D. Northern blots were performed and probed with TF and G3PDH. The signal density of each RNA sample hybridized to TF was divided by that hybridized to the G3PDH. The corrected density was then plotted as a percentage of the 0-hour value (log scale) against time. Results are representative of 3 experiments.
OSM induces an increased rate of TF gene transcription.
The transcription rate of the TF gene was assessed by nuclear run-off assays. (A) Nuclear extracts were harvested from SMCs treated with or without OSM (10 ng/mL) for 1 hour. Equal amounts of32P-labeled in vitro transcribed RNA probes were hybridized to 5 μg of denatured TF, G3PDH, and pGEM-4Z cDNA. Results are representative of 2 separate experiments. (B) Activation of TF promoter by OSM was analyzed by studying the effect of OSM on TF gene promoter fused to luciferase. pTF(−2106)LUC, which contains 2.1 kilobase (kb) of the 5′ flanking region of the TF gene promoter, and pSV–β-galactosidase control vector were transfected into SMCs. The cells were treated with OSM (10 ng/mL) for 5 hours before harvesting. Luciferase activity was determined and normalized for β-galactosidase activity. Results are expressed as mean ± SEM (n = 4 experiments). *P < .05 compared to unstimulated SMCs. (C) SMCs were exposed to vehicle (control, ■) or OSM (10 ng/mL, ▪ and ●) for 1 or 24 hours before addition of actinomycin D (5 μg/mL). Total RNA was extracted at the indicated times after addition of actinomycin D. Northern blots were performed and probed with TF and G3PDH. The signal density of each RNA sample hybridized to TF was divided by that hybridized to the G3PDH. The corrected density was then plotted as a percentage of the 0-hour value (log scale) against time. Results are representative of 3 experiments.
To investigate if mRNA stability contributed to TF mRNA accumulation, the levels of TF mRNA were measured in the presence of the transcriptional inhibitor actinomycin D (Figure 2C). The stability of TF mRNA was examined at various intervals following the addition of OSM, at the time points of maximal TF mRNA expression (1 hour and 24 hours) and was compared to unstimulated controls. SMCs were incubated with OSM (10 ng/mL) for 1 hour and 24 hours, followed by actinomycin D (5 μg/mL) to arrest transcription. TF mRNA levels were determined by Northern blot analysis at 0, 30 minutes, 1 hour, and 2 hours. TF mRNA exhibited a similar apparent half-life of 83.6 ± 11.7 minutes for controls (n = 3), 88.5 ± 17.2 minutes for 1 hour postinduction (n = 3), and 97.4 ± 6.1 minutes for 24 hours postinduction (n = 3) (Figure 2C). There were no significant differences between the values. These data indicate that the increase in TF mRNA expression in response to OSM is not dependent on mRNA stability but can be attributed to OSM-induced gene activation.
Activation of the MEK/Erk-1/2 pathway is required for OSM-induced TF gene activation
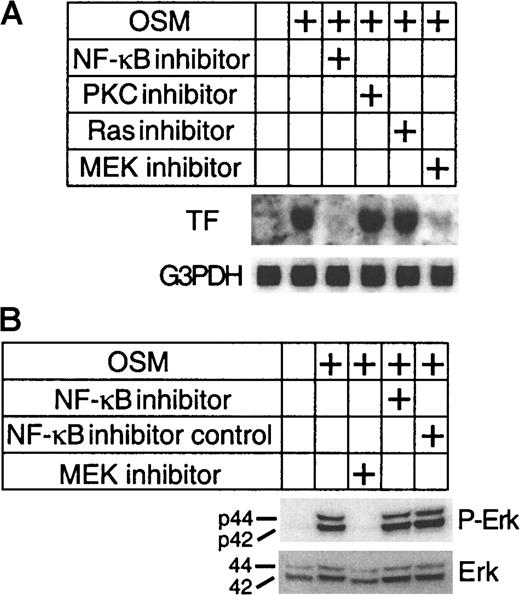
To define the signal transduction link between OSM receptor activation and TF gene induction, we investigated the effects of signaling inhibitors on TF expression. SMC cultures were preincubated for 1 hour with either 50 μM U0126 (MEK inhibitor), 50 nM NF-κB inhibitor peptide that prevents translocation of the NF-κB active complex into the nucleus,33 100 nM FTP-1 (Ras inhibitor) and 100 nM calphostin (protein kinase C [PKC] inhibitor). SMCs were then stimulated with OSM (10 ng/mL) for an additional 1 hour. Both MEK and NF-κB inhibitors suppressed TF mRNA expression, whereas PKC or Ras inhibition had no effect (Figure3A). These results indicate that the downstream signaling events induced by OSM to promote TF mRNA expression involve MEK/Erk-1/2 and NF-κB signaling pathway. Because U0126 is an inhibitor of the Erk-1/2 pathway that selectively blocks the Erk-1/2-activating enzyme MEK, we examined the effects of U0126 on OSM-induced Erk-1/2 activation. With the use of an antibody to phosphorylated Erk-1/2, we demonstrated that OSM stimulated Erk-1/2 phosphorylation (Figure 3B). This activation of Erk-1/2 by OSM was inhibited by U0126, whereas the NF-κB inhibitor had no effect. We also examined the effects of OSM on 2 other members of the Erk-1/2 family, p38 MAP kinase and stress-activated-protein kinase-1/c-Jun NH2-terminal kinase. OSM had no effect on either of these kinases (data not shown). Thus, these results suggest that activated Erk-1/2 is required for the induction of TF by OSM.
Inhibition of TF expression by NF-κB and Erk-1/2 inhibitors.
(A) SMC cultures were incubated with the indicated inhibitors for 1 hour prior to stimulation with OSM (10 ng/mL) for a further hour. Total RNA was extracted at the end of each incubation and analyzed for TF mRNA expression. The blots were rehybridized with G3PDH to demonstrate equal RNA loading. (B) SMCs were preincubated for 1 hour with NF-κB and Erk-1/2 inhibitors followed by stimulation with OSM (10 ng/mL) for 10 minutes at 37°C. Extracted proteins (30 μg) were separated on a 4% to 12% Bis-tris gradient gel and transferred to a PVDF membrane. Phosphorylated and total Erk-1/2 were visualized by incubating the filters with a monoclonal antibody to p42/44 Erk-1/2 and a pan Erk-1/2 monoclonal antibody. Experiments were repeated twice.
Inhibition of TF expression by NF-κB and Erk-1/2 inhibitors.
(A) SMC cultures were incubated with the indicated inhibitors for 1 hour prior to stimulation with OSM (10 ng/mL) for a further hour. Total RNA was extracted at the end of each incubation and analyzed for TF mRNA expression. The blots were rehybridized with G3PDH to demonstrate equal RNA loading. (B) SMCs were preincubated for 1 hour with NF-κB and Erk-1/2 inhibitors followed by stimulation with OSM (10 ng/mL) for 10 minutes at 37°C. Extracted proteins (30 μg) were separated on a 4% to 12% Bis-tris gradient gel and transferred to a PVDF membrane. Phosphorylated and total Erk-1/2 were visualized by incubating the filters with a monoclonal antibody to p42/44 Erk-1/2 and a pan Erk-1/2 monoclonal antibody. Experiments were repeated twice.
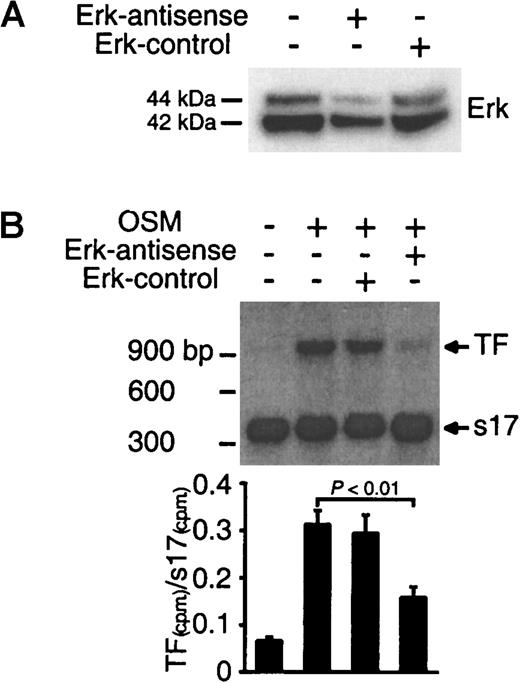
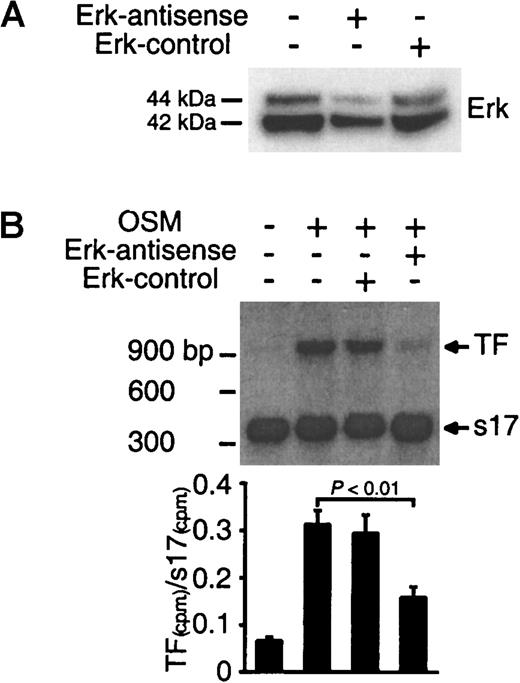
To confirm the hypothesis that Erk-1/2 mediates TF expression, experiments were performed to determine whether inhibition of Erk-1/2 by antisense treatment suppressed OSM-induced TF mRNA expression. The levels of Erk-1/2 protein in SMCs treated with Erk-1/2-antisense oligonucleotides were significantly reduced (Figure4A). Quantitative RT-PCR analysis for TF mRNA in OSM-stimulated cells demonstrated that the addition of Erk-1/2 antisense oligonucleotide suppressed TF mRNA expression (Figure 4B). These results confirm the view that signaling through the Erk-1/2 pathway is required for OSM-induced TF mRNA expression.
Inhibition of TF mRNA synthesis by Erk-1/2 antisense treatment.
(A) SMCs were transfected with antisense or sense oligonucleotides for 48 hours. Extracted proteins (30 μg) were separated on a 4% to 12% Bis-tris gradient gel and transferred to a PVDF membrane. Erk-1/2 was visualized by incubating the filters with a panErk-1/2 monoclonal antibody. (B) SMCs were transfected with antisense or sense oligonucleotides for 48 hours followed by stimulation with OSM (10 ng/mL) for 1 hour at 37°C. Total RNA was extracted, and TF mRNA expression was analyzed by quantitative RT-PCR. Solid bars represent the ratio of 32P-labeled TF and s17 PCR products isolated from 2% agarose gel. Results are representative of 2 separate experiments.
Inhibition of TF mRNA synthesis by Erk-1/2 antisense treatment.
(A) SMCs were transfected with antisense or sense oligonucleotides for 48 hours. Extracted proteins (30 μg) were separated on a 4% to 12% Bis-tris gradient gel and transferred to a PVDF membrane. Erk-1/2 was visualized by incubating the filters with a panErk-1/2 monoclonal antibody. (B) SMCs were transfected with antisense or sense oligonucleotides for 48 hours followed by stimulation with OSM (10 ng/mL) for 1 hour at 37°C. Total RNA was extracted, and TF mRNA expression was analyzed by quantitative RT-PCR. Solid bars represent the ratio of 32P-labeled TF and s17 PCR products isolated from 2% agarose gel. Results are representative of 2 separate experiments.
Regulation of NF-κB activity by the Erk-1/2 pathway
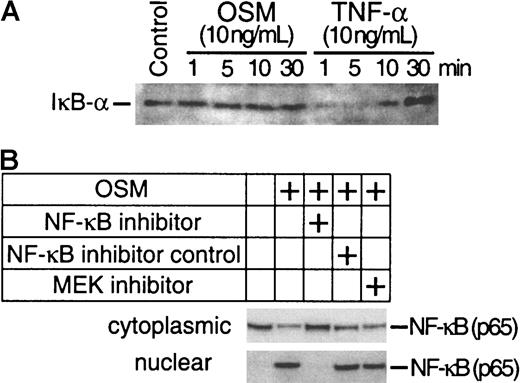
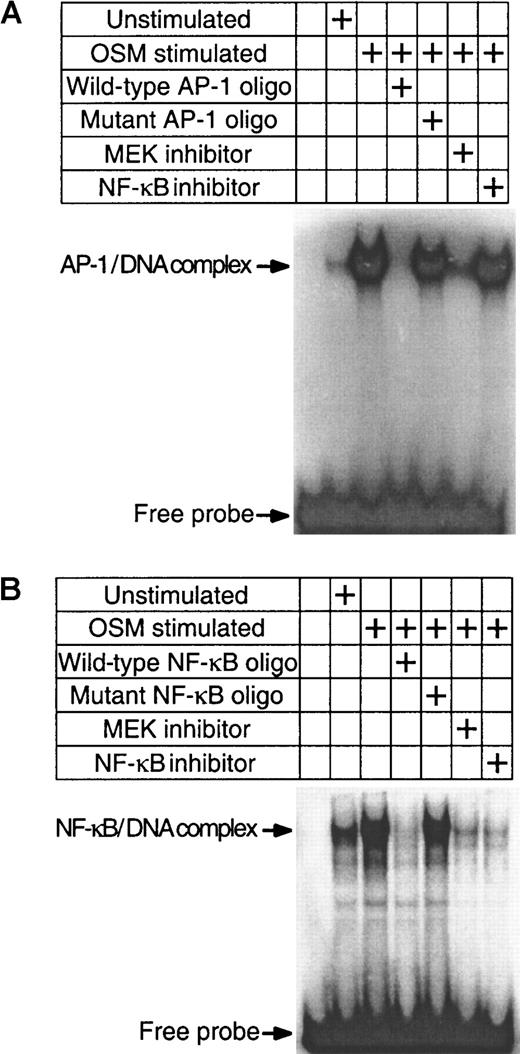
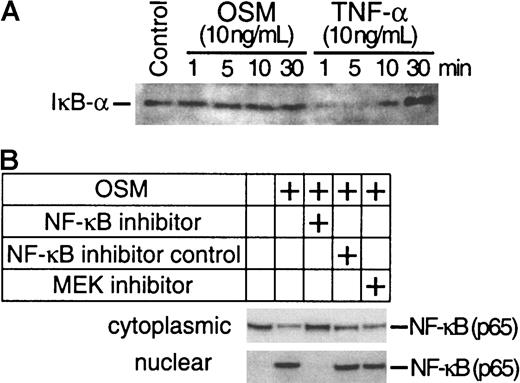
The preceding experiments have demonstrated that both Erk-1/2 and NF-κB pathways are involved in the induction of TF by OSM. To confirm whether OSM-induced activation of Erk-1/2 is involved in the activation of NF-κB, we analyzed nuclear extracts from SMC cultures exposed to OSM in the presence or absence of U0126 for NF-κB binding activity by EMSA. We also investigated whether the AP-1 transcriptional factor was involved in the induction of TF by OSM because a role for AP-1 in TF induction has been demonstrated.34 OSM markedly activated both AP-1 and NF-κB binding activity. Unlabeled homologous oligonucleotides prevented binding of 32P-labeled AP-1 (Figure 5A) and NF-κB (Figure 5B) sequences to nuclear proteins, whereas mutated oligonucleotides had no effect. U0126 inhibited both AP-1 and NF-κB binding. NF-κB–specific inhibitor peptide prevented NF-κB but not AP-1 binding. The inhibition of NF-κB binding activity by U0126 suggests that Erk-1/2 activation is linked to NF-κB activity (Figure 5B). One potential mechanism by which U0126 may be exerting its effects is to inhibit nuclear translocation of NF-κB by preventing IκB-α degradation. Western blot analysis was performed to determine whether OSM stimulated IκB-α degradation. The levels of IκB-α in OSM-treated cells were similar to the unstimulated control. In contrast, the levels of IκB-α were reduced in TNF-α–treated SMCs (Figure 6A). This finding suggests that the activation of NF-κB by OSM is independent of IκBα degradation and further rules out the possibility that U0126 inhibited NF-κB nuclear translocation by suppressing IκB-α degradation. To confirm this assumption, we examined the effects of U0126 on OSM-induced NF-κB nuclear translocation by Western blot analysis. NF-κB protein was detected in the nuclei of OSM-stimulated SMCs (Figure 6B). Incubating SMCs with the NF-κB peptide inhibitor prevented NF-κB nuclear translocation. However, similar levels of nuclear NF-κB protein were detected in OSM-stimulated SMCs treated with or without U0126. This finding, together with the observation that U0126 reduced NF-κB nuclear activity (Figure 5), suggests that the inhibitory effects of U0126 on NF-κB nuclear activity are most likely due to reduced affinity of NF-κB for its DNA binding site.
OSM induction of NF-κB and AP-1 nuclear activity.
Nuclear extracts of SMCs were prepared following OSM (10 ng/mL) treatment. EMSA was performed with the AP-1 (A) or NF-κB (B) probes. For cold and mutated competition EMSA experiments, 100-fold molar excess of unlabeled NF-κB or AP-1 probes were included in the binding reaction. Experiments were repeated twice.
OSM induction of NF-κB and AP-1 nuclear activity.
Nuclear extracts of SMCs were prepared following OSM (10 ng/mL) treatment. EMSA was performed with the AP-1 (A) or NF-κB (B) probes. For cold and mutated competition EMSA experiments, 100-fold molar excess of unlabeled NF-κB or AP-1 probes were included in the binding reaction. Experiments were repeated twice.
Assessment of IκB-α and NF-κB protein levels.
(A) Total cellular proteins from SMC cultures treated for 1 to 30 minutes with OSM (10 ng/mL) and TNF-α (10 ng/mL) were separated on a 4% to 12% Bis-tris gradient gel and transferred to a PVDF membrane. IκB-α was visualized by incubating the filters with a polyclonal antibody to IκB-α. (B) SMC cultures were stimulated with OSM in the presence or absence of U0126, NF-κB inhibitor peptide, or NF-κB control peptide. Cytoplasmic and nuclear NF-κB were isolated as described in “Materials and methods.” NF-κB was visualized by incubating the filters with a monoclonal antibody to NF-κB. Experiments were repeated twice.
Assessment of IκB-α and NF-κB protein levels.
(A) Total cellular proteins from SMC cultures treated for 1 to 30 minutes with OSM (10 ng/mL) and TNF-α (10 ng/mL) were separated on a 4% to 12% Bis-tris gradient gel and transferred to a PVDF membrane. IκB-α was visualized by incubating the filters with a polyclonal antibody to IκB-α. (B) SMC cultures were stimulated with OSM in the presence or absence of U0126, NF-κB inhibitor peptide, or NF-κB control peptide. Cytoplasmic and nuclear NF-κB were isolated as described in “Materials and methods.” NF-κB was visualized by incubating the filters with a monoclonal antibody to NF-κB. Experiments were repeated twice.
Discussion
Thrombosis plays an integral role in the development and progression of atherosclerosis. Accumulation of TF within the atherosclerotic lesion is believed to play a critical role in determining its thrombogenicity.35 In the present study, we have investigated how OSM, a macrophage and T-lymphocyte–restricted cytokine, regulates TF expression in SMCs. Our results suggest that OSM expressed in atherosclerotic lesions may contribute to plaque thrombogenicity by inducing the expression of TF. Because atherosclerosis is an inflammatory disease, it is believed that macrophages within the atherosclerotic lesion secrete cytokines that are capable of promoting TF expression.36 The biphasic and sustained pattern of TF mRNA expression induced by OSM in SMCs is unique among cytokines and growth factors known to promote TF expression such as TNF-α and PDGF.14,37 After the completion of our study, an independent study showed that OSM promoted a biphasic induction of IL-6 mRNA expression in astrocytes.38 Interestingly, induction of IL-6 antigen did not show a biphasic response. Similarly, in the present study TF antigen levels remained elevated up to 72 hours (data not shown). The relative stability of TF antigen39 compared with TF mRNA expression may explain the biphasic nature of TF mRNA expression and sustained TF-antigen level. Given that SMCs express the highest number of OSM receptors,40 it is conceivable that OSM released by macrophages may promote TF expression through interaction with SMCs within the atherosclerotic lesion and contribute to thrombotic complications associated with this disease.
We have demonstrated previously that OSM can promote endothelial proliferation and migration through an indirect mechanism that involves basic fibroblast growth factor and the plasminogen activator system.41,42 Consistent with our observation, OSM was shown to promote angiogenesis in vivo and in vitro.29These studies suggest that OSM found in the atherosclerotic lesion could promote neovascularization within the plaques, a process believed to contribute to plaque instability. In this regard, OSM was also shown to stimulate expression of matrix metalloproteinases, key molecules involved in plaque rupture.27,43 Interestingly, OSM may also contribute to rupturing of aneurysms because it has been shown to be present in human aortic aneurysm specimens obtained from atherosclerotic tissue.28 Our finding that OSM promotes prolonged TF expression, together with the observations of Modur et al,28 suggests that OSM can contribute to all phases of thrombotic complications associated with atherosclerosis (ie, from plaque destabilization to plaque rupture and finally clot formation).
The TF promoter is complex and contains numerous binding sites for transcription factors involved in regulating TF gene expression.44 For instance, the induction of TF in endothelial cells and monocytic cells by agents such as TNF-α and bacterial endotoxin (LPS) appear to be regulated primarily by the AP-1, NF-κB, and Sp1 transcription factors,37,45,46 whereas the transcriptional factor Egr1 was shown to be important in promoting TF gene activation in endothelial cells under shear stress conditions.47 The present study links the Erk-1/2 and NF-κB pathways in the induction of TF expression by OSM in SMCs. Although both Erk-1/2 and NF-κB inhibition blocked TF expression (Figure 3A), EMSA studies (Figure 5) showed that, in OSM-stimulated SMCs treated with U0126 (MEK inhibitor), both AP-1 and NF-κB activity were inhibited, whereas inhibition of NF-κB translocation using a specific peptide inhibitor suppressed only NF-κB activity without affecting AP-1 activity. This finding indicates that NF-κB plays a pivotal role in promoting TF expression. However, we cannot rule out a role for AP-1 in promoting OSM-induced TF expression. In this regard, several reports have demonstrated that the interaction of AP-1 and NF-κB transcriptional factors is required for maximal induction of TF in endothelial and monocytic cells.37 45 Thus, the inhibition of TF expression by U1206 may be due to suppression of both AP-1 and NF-κB nuclear activity.
The mechanism of NF-κB activation has only been elucidated recently.48,49 NF-κB is a heterodimer composed primarily of a 50-kDa DNA binding subunit and a 65 kDa transactivator (p 65 or Rel A). NF-κB is retained in the cytoplasm associated with a family of inhibitory proteins termed IκB. This family of IκBs includes IκB-α, IκB-β, and IκB-ε. In response to inflammatory stimuli such as TNF-α and LPS, the IκBs are rapidly phosphorylated, releasing NF-κB, undergoing ubiquitination and proteolysis by the 26S proteosome. Released NF-κB then translocates to the nucleus and activates transcription of specific genes. In this study, OSM activated NF-κB through a mechanism independent of IκB-α degradation. Our observation is similar to reports that phosphorylation of IκB-α at tyrosine 42 promoted NF-κB mobilization to the nucleus via a mechanism that does not involve IκB-α degradation.50,51 Further, a recent study in human U937 monocytic cells demonstrated that treatment with IL-1β for 15 minutes caused only a 15% degradation of IκB-α yet had more than 60% DNA binding, suggesting the existence of additional mechanisms that regulate NF-κB activation.52 The finding that inhibition of Erk-1/2 by U0126 inhibited NF-κB binding activity without affecting NF-κB nuclear translocation suggests that Erk-1/2 may play a role in modulating NF-κB DNA recognition. For instance, Erk-1/2 may regulate NF-κB activity by regulating its phosphorylation status. Indeed, several studies have suggested that the binding of NF-κB to its DNA site is dependent on the phosphorylation status of the p65 subunit.53 54 Alternatively, a nuclear protein could prevent DNA recognition by either interacting with NF-κB or its DNA binding site. In summary, this study demonstrates the ability of OSM to promote prolonged expression of TF in SMCs. Our results indicate that the induction of TF expression by OSM is through the Erk-1/2 signal transduction pathway that is involved in regulating NF-κB DNA recognition.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Errol S. Wijelath, The Hope Heart Institute, Department of Molecular Biology, 528 18th Ave, Seattle, WA 98122; e-mail: ewijelath@hopeheart.org.