Abstract
There is a growing interest in using antigen-specific T cells for the treatment of human malignancy. For example, adoptive transfer of Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes (CTLs) has been effective prophylaxis and treatment of EBV-associated lymphoproliferative disease in immunocompromised patients. For all immunotherapies, however, there has been a hypothetical concern that mutations in tumor-specific antigens may lead to tumor escape. We now demonstrate that such events may indeed occur, with lethal outcome. A patient who developed lymphoma after marrow transplantation received donor-derived, EBV-specific CTLs but died with progressive disease. The tumor cells proved substantially less sensitive to cytolysis than the EBV-transformed B-cell line used for CTL generation. The major cytolytic activity of the donor CTL was directed against 2 HLA-A11–restricted epitopes in the viral EBNA-3B antigen. Sequence analysis of this gene in the tumor virus revealed a 245–base pair deletion, which removed these 2 CTL epitopes. Hence, the viral antigen in the tumor had mutated in a way that allowed escape from CTLs. Analysis of EBV polymorphisms demonstrated that before CTL infusion, more than one virus was present, including a virus with wild-type EBNA-3B. After CTL infusion, only the virus with the EBNA-3B deletion could be detected, suggesting that the infused CTLs had selected a resistant strain in vivo. Such an occurrence, even when polyclonal CTL lines are used against genetically stable virus antigens, suggests that escape mutants may be a serious problem when CTL therapy is directed against more unstable tumor cell–derived targets.
Introduction
There has been much recent interest in the use of immunotherapeutic approaches to treat cancer. Among the most promising of these is in vivo or ex vivo generation of cytotoxic T lymphocytes (CTLs) with specificity directed against tumor-specific antigens.1 2 All immunotherapeutic approaches are, however, potentially limited by the capacity of the tumor cells to mutate the target antigen chosen and thereby evade the immune response. We have studied this problem in Epstein-Barr virus–associated lymphoproliferative disease (EBV-LPD) in a bone marrow recipient.
EBV is an oncogenic herpesvirus that is associated with malignancies of T and B lymphocytes, epithelium, and muscle.3-6 Under normal circumstances, EBV is controlled by immune T cells. If these are absent, for example, in an immunosuppressed host, unrestrained outgrowth of EBV-transformed B cells may occur to produce lymphoproliferative disease.4 The ex vivo correlate of this phenomenon is the immortalized B lymphoblastoid cell line (LCL) that grows out in culture in the absence of T cells.7Recipients of T-cell–depleted stem cells from HLA-mismatched or unrelated donors have an incidence of EBV-LPD between 3% and 25%.1,8-11 Treatment of post-transplantation lymphoproliferative disorders after stem cell transplantation has been a major problem. Interferon-α and B-cell–specific monoclonal antibodies to the B-cell markers, CD21 and CD23, have had limited success.11,12 Unmanipulated donor T cells have also produced complete tumor remissions but are associated with severe graft-versus-host disease (GVHD) and disease progression, presumably because of the low frequency of EBV-specific CTL precursors within the infused T cells.10,13 More recently, a B-cell–specific humanized antibody to CD20 (rituximab; Genentech, South San Francisco, CA; and IDEC Pharmaceuticals, San Diego, CA) has become available for the treatment of follicular lymphoma. This antibody has been used with a high success rate (69%) for the treatment of EBV-LPDs after solid organ or stem cell transplantation.14 15 Treatment failures have been reported in the case of CD20− tumors, tumors that recur having down-regulated CD20, and in patients with advanced disease. Further, this antibody results in abrogation of the B-cell compartment for about 6 months. Unfortunately, rituximab was not available when the patient described here presented with her LPD.
The EBV-LPDs that arise in allogeneic stem cell recipients are usually classified as immunoblastic lymphomas, and the tumor cells are phenotypically identical to LCLs derived in vitro.16 Both tumor cells and LCLs express 9 EBV-encoded proteins: the nuclear proteins EBNA-1, EBNA-2, EBNA-3A, EBNA-3B, EBNA-3C, and EBNA-LP; the latent membrane proteins LMP1 and LMP2; and BARFO (the product of the BamHI A open reading frame).17In EBV-LPDs and in LCLs, these viral proteins are expressed in the context of costimulatory molecules such as HLA class I and II, CD80, CD86, and CD40. They are therefore excellent antigen-presenting cells and should be amenable to destruction by EBV-specific CTLs. Indeed, infusions of donor T lymphocytes, which contain a small proportion of EBV-reactive T cells, have proved effective at treating a proportion of stem cell recipients with established disease.8,18,19However, there remains a high incidence of treatment failure due to GVHD (because of alloreactive T cells contained in the infused population) and hypersensitivity reactions. Moreover, many patients fail to respond to the infusions and die with progressive disease.10 By contrast, donor-derived, EBV-specific CTLs have been effective as prophylaxis and treatment of EBV-LPD.20-22 In our study, none of 52 patients who received CTLs as prophylaxis developed EBV-LPD, compared with 11.5% of controls.20 Furthermore, infused CTLs were nontoxic, persisted for up to 68 months, rapidly restored immune responses to EBV, and were regularly able to control high EBV genome loads that existed prior to their administration. Two patients with established lymphoma were also successfully treated by this approach.20
One important caveat to this immunotherapeutic approach remains. The EBV latency proteins display a hierarchy of immunodominance that is dependent on HLA allotype.17 In most individuals, the EBNA-3 proteins are the most immunogenic, with the remaining proteins being poorly or nonimmunogenic.23-26 Hence, although LCLs present at least 9 viral antigens to autologous T cells during the in vitro activation of EBV-specific CTLs, most CTL lines display specificity for only 2 or 3 epitopes from 1 or 2 viral proteins. Further, each line is restricted by only 1 or 2 HLA class I alleles, and (at least in Caucasians) the immune response to EBV appears dominated by HLA-A3, -A11, -B7, -B8, and -B44.17,24 27-32Therefore, efforts to treat EBV-LPD may fail if the tumor mutates an immunodominant viral target antigen and evades the immune response. We now show the occurrence of this phenomenon in a patient with EBV-LPD and document a mechanism by which therapeutic escape may occur with lethal results.
Materials and methods
Cell lines
Generation of EBV-specific CTL lines from bone marrow donors for the prophylaxis and treatment of EBV-LPD in allogeneic bone marrow transplantation (BMT) patients has been described extensively elsewhere.33 Briefly, donor mononuclear cells were stimulated with B95-8 virus–transformed autologous LCLs and expanded in interleukin 2 (IL-2) from day 14. Analysis of the T-cell receptor Vβ usage of the bone marrow donor CTL line described here showed that it was indeed polyclonal and revealed no aberrant T-cell receptor usage (data not shown). When sufficient numbers of EBV-CTLs were obtained, they were tested for cytotoxic activity, immunophenotype, identity, and sterility and cryopreserved until safety tests were cleared and the patient became eligible for CTL infusion. The CTLs were then thawed, and 2 × 107 cells/m2 were infused. Peripheral blood was collected from patient UPN 426 prior to CTL infusion and at 1, 2, and 3 weeks postinjection for analysis. Culture of mononuclear cells both before and 1 week after CTL infusion resulted in the rapid spontaneous outgrowth of EBV-transformed B-cell lines. These lines could have been derived from virus-infected B cells unrelated to the tumor or by release of infectious virus in the culture and reinfection of normal B cells. However, because both of these spontaneous B-cell lines carried the same immunoglobulin rearrangements and EBNA-3B deletion as DNA obtained from tumor biopsy cells, they were assumed to have derived from the tumor (Figures1 and 5A). Therefore, they were termed the “pre-CTL tumor line” and the “post-CTL tumor line.” A dermal fibroblast line (CR-fibs) and B95-8–transformed B-cell line (CR-LCL) were prepared from a normal donor who shared HLA-A2, -A11, and -B7 with the marrow donor. OKT3 blasts were prepared from this donor and donor CA, who shared HLA-B60, by stimulation of peripheral blood mononuclear cells with 1 μg/mL OKT3 (Ortho Diagnostics, Raritan, NJ) and expansion with 100 U/mL IL-2 from day 7. Partially HLA class I–matched LCLs were taken from our bank of HLA-typed LCLs. Aliquots of the donor CTL line were thawed and tested for activity against the pre- and post-CTL tumor lines and for their peptide specificity. The CTL line was highly cytotoxic to the donor LCL and initially had significant nonspecific cytotoxic activity against the lymphokine-activated killer (LAK) cell target, HSB-2, and against HLA-mismatched LCLs (MM-LCLs) (Figure2A). The nonspecific activity declined with time in culture, while the specific activity remained high.
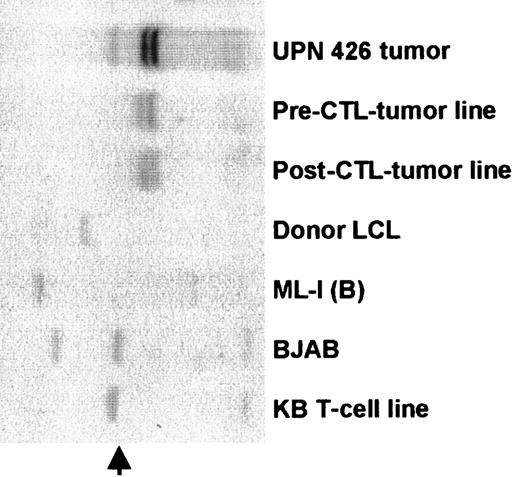
The tumor lines have the same immunoglobulin VH rearrangement as tumor biopsy cells.
DNA was prepared from lung biopsy cells and from the spontaneous lines that grew from patient peripheral blood before and after CTL infusion. Controls were ML-1 and BJAB, which are B-cell lymphoma lines; the donor LCL; and a T-cell line KB (germline). DNAs were digested withBglII, separated on a 0.8% agarose gel, transferred to a nylon membrane, and probed with a JH probe. Identical bands in the pre- and post-CTL LCLs and the tumor biopsy cells shows that all 3 are derived from the same cell. The additional band in the tumor biopsy cells probably represents the germline immunoglobulin DNA (arrow) in non-B cells in the tumor tissue.
The tumor lines have the same immunoglobulin VH rearrangement as tumor biopsy cells.
DNA was prepared from lung biopsy cells and from the spontaneous lines that grew from patient peripheral blood before and after CTL infusion. Controls were ML-1 and BJAB, which are B-cell lymphoma lines; the donor LCL; and a T-cell line KB (germline). DNAs were digested withBglII, separated on a 0.8% agarose gel, transferred to a nylon membrane, and probed with a JH probe. Identical bands in the pre- and post-CTL LCLs and the tumor biopsy cells shows that all 3 are derived from the same cell. The additional band in the tumor biopsy cells probably represents the germline immunoglobulin DNA (arrow) in non-B cells in the tumor tissue.
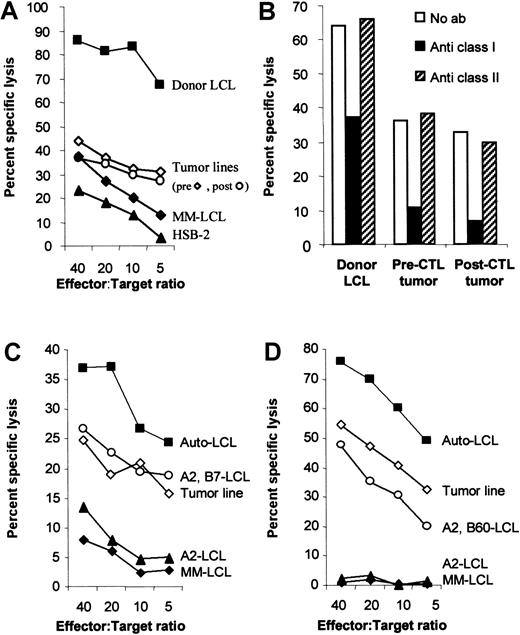
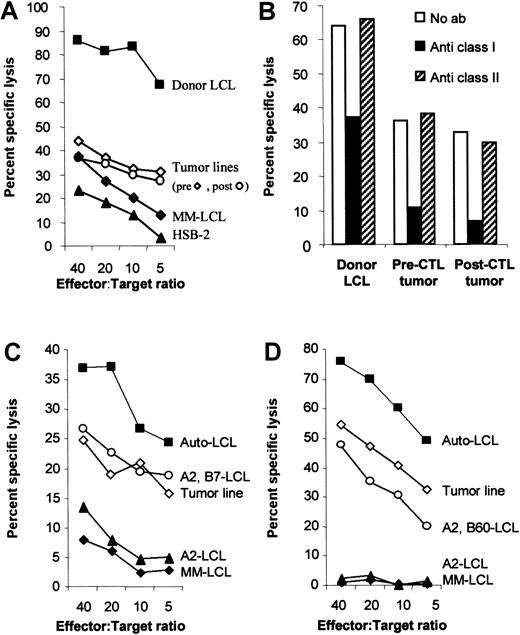
The tumor lines are less sensitive to killing by HLA class I–restricted CTLs, even though they have no defect in antigen presentation.
(A) The tumor lines are less sensitive to killing than the donor LCLs. To compare the sensitivity of donor-derived B95-8 virus–transformed B-cell line and the recipient-derived tumor cell lines to killing by the donor CTL line, donor CTLs were incubated with51Cr-labeled target cells for 4 hours in a standard chromium release assay at the effector:target ratios indicated. The target cells were the B95-8 virus–transformed donor LCLs, the patient-derived tumor lines that grew spontaneously from patient blood before and 7 days after CTL infusion, an HLA mismatched LCL (MM-LCL), and the LAK cell–sensitive target, HSB-2. (B) Antitumor activity was HLA-restricted. To determine whether the antitumor activity of the CTL line was due to CTL or LAK cell activity, target cells were incubated with standard blocking antibodies to HLA class I (W6/32) and class II (CR3/43). After 30 minutes of incubation, effector cells were added at an effector:target ratio of 10:1. After 4 hours, the plates were harvested and the percent chromium release calculated. Killing of both tumor lines was HLA class I–restricted. (C) The tumor line is killed through HLA-B7. CHJ is HLA-matched at HLA loci A2, B7 with the donor. In a standard cytotoxicity assay, CHJ CTLs kill the pre-CTL tumor line with similar efficiency as they kill normal LCLs that share only A2 and B7. The killing is likely specific for B7 because LCLs that only match at A2 are not killed. (D) The tumor line is killed through HLA-B60. RF is HLA-matched at HLA loci A2, B60 with the donor. In a standard cytotoxicity assay, RF-CTLs kill the pre-CTL tumor line with similar efficiency as they kill normal LCLs that share A2 and B60. The killing is likely specific for B60 because LCLs who only match A2 are not killed.
The tumor lines are less sensitive to killing by HLA class I–restricted CTLs, even though they have no defect in antigen presentation.
(A) The tumor lines are less sensitive to killing than the donor LCLs. To compare the sensitivity of donor-derived B95-8 virus–transformed B-cell line and the recipient-derived tumor cell lines to killing by the donor CTL line, donor CTLs were incubated with51Cr-labeled target cells for 4 hours in a standard chromium release assay at the effector:target ratios indicated. The target cells were the B95-8 virus–transformed donor LCLs, the patient-derived tumor lines that grew spontaneously from patient blood before and 7 days after CTL infusion, an HLA mismatched LCL (MM-LCL), and the LAK cell–sensitive target, HSB-2. (B) Antitumor activity was HLA-restricted. To determine whether the antitumor activity of the CTL line was due to CTL or LAK cell activity, target cells were incubated with standard blocking antibodies to HLA class I (W6/32) and class II (CR3/43). After 30 minutes of incubation, effector cells were added at an effector:target ratio of 10:1. After 4 hours, the plates were harvested and the percent chromium release calculated. Killing of both tumor lines was HLA class I–restricted. (C) The tumor line is killed through HLA-B7. CHJ is HLA-matched at HLA loci A2, B7 with the donor. In a standard cytotoxicity assay, CHJ CTLs kill the pre-CTL tumor line with similar efficiency as they kill normal LCLs that share only A2 and B7. The killing is likely specific for B7 because LCLs that only match at A2 are not killed. (D) The tumor line is killed through HLA-B60. RF is HLA-matched at HLA loci A2, B60 with the donor. In a standard cytotoxicity assay, RF-CTLs kill the pre-CTL tumor line with similar efficiency as they kill normal LCLs that share A2 and B60. The killing is likely specific for B60 because LCLs who only match A2 are not killed.
HLA types of cell lines
Only HLA class I typing is shown because there was no killing through HLA class II (Figure 2B). The donor, the donor LCL, and the pre- and post-CTL tumor lines were HLA-A2, -A11, -B7, and -B60. The recipient was HLA-A11, -B7, and -B60. The donor (CR) of the partially HLA-matched fibroblasts and OKT3 blasts was HLA-A2, -A11, -B7, and -B8. The CA donor OKT3 blasts were HLA-A2, -A32, -B60, and -B62. The LCLs matched at one HLA class I locus were BJ–LCL-A1 -A2, -B8, and -B62; JR–LCL-A11, -B18, and -B35; BW–LCL-A24, -A25, -B7, and -B18; BP–LCL-A1, -A31, -B44, and -B60. The HLA MM-LCL was A3, A24, B35, and B27 for Figure 2A; A26, A31, B28, and B44 for Figure 2C; and A24, A26, B18, and B65 for Figure 2C. The CHJ-CTL line (A2, A3, B7, B51) matched the tumor line at A2 and B7 and the RF-CTL line (A2, A31, B8, B60) at A2 and B60. The LCLs that matched at 1 or 2 HLA class I loci for CHJ-CTL were BS–LCL-A2, -A29, -B7, and -B44 and BG–LCL-A2, -A31, -B57, and -B62 and, for RF-CTL, were MK–LCL-A2, -A32, -B51, and -B60 and SG–LCL-A2, -A3, -B35, and -B57.
Patient samples
Ten to 20 mL of peripheral blood was drawn from the patient prior to BMT, prior to CTL infusion, and 7, 14, and 21 days after CTL infusion. Small tumor biopsies were obtained from a neck node on day 17 after CTL infusion and from a lung nodule at autopsy. These samples were used for culture, phenotype, Western immunoblotting, and DNA analysis.
Cytotoxicity assays
Cytotoxicity of the donor CTL line against various target cells was measured in a standard 4-hour chromium release assay using effector:target ratios of 40:1, 20:1, 10:1, 5:1, 2.5:1, and 1.25:1. To determine whether killing was HLA-restricted, target cells were preincubated for 30 minutes with 10 μL (175 μg/mL) of the standard blocking antibodies W6/32, a monoclonal antibody that recognizes a monomorphic HLA class I determinant, and CR3/43, which is anti-DR, -DP, and -DQ (Dako, Carpinteria, CA). Target cells included autologous LCLs, HLA MM-LCLs, HSB-2, an EBV− LAK cell–sensitive T-cell lymphoma, and dermal fibroblasts. To analyze HLA class I restriction, LCLs that shared only one of each HLA class I antigen with the patient were used. To analyze antigen specificity, CR fibroblasts that shared HLA-A2, -11, and -B7 with the donor were infected with vaccinia recombinants expressing each of the EBV latency antigens individually (gifts of Elliott Kieff, Boston, MA) and were used as targets. The fibroblast line was exposed to 100 U/mL interferon-γ 24 hours prior to infection with the vaccinia virus recombinants containing EBNA-1, -2, -3A, -3B, -3C, -LP, LMP1, and LMP2A or containing the control gene β-galactosidase at a multiplicity of infection of 5:1 for 1 hour. Target cells were labeled with chromium following infection. For peptide sensitization experiments, mononuclear cells were stimulated with 1 μg/mL OKT3 and then restimulated weekly with 100 U/mL IL-2. From day 7 the blasts were used as targets. They were labeled with51Cr-labeled sodium chromate, washed, and then incubated for 1 hour with the appropriate amount of peptide before the addition of donor CTLs.
Genomic Southern blot
DNA was extracted from cell lines and from a tumor biopsy taken 17 days after CTL infusion, using a tissue DNA extraction kit (Qiagen, Chatsworth, CA). For analysis of immunoglobulin heavy chain rearrangements, DNA was digested with BglII, separated on a 0.8% agarose gel, blotted onto a nylon membrane (MSI Inc), and probed with a VH probe (gift of Geoffrey Kitchingman, St Jude Children's Research Hospital, Memphis, TN).
PCR and sequencing
Genomic DNA was made using Qiagen Blood DNA kits and quantified on a DNA calculator (Pharmacia Biotech, NJ). EBV strain typing was performed by polymerase chain reaction (PCR) using EBNA-2–specific primers that distinguished between type 1 and type 2 EBV as previously described.2,34 Three different primer sets were used to detect the 5 epitopes that are associated with HLA-A11 in EBNA-3B. Primers were designed using Primer Designer (Sci-Ed Software, NC). Primers 5′-GTGCCTCTTACCAACATGG-3′ and 5′-GCAAGAGAGGAAGAGGAACC-3′ spanned the first A11 epitope, and 5′-CGAACTGCTAAGTTGAGACG-3′ and 5′-ATCCAACAGTATACGGCAGG-3′ spanned the remaining 4 epitopes. Primers 5′-ATAATTGTTGAGGATGACG-3′ and 5′-CTCTGCTCCATGACTTCA-3′ were described by de Campos-Lima et al35 and spanned the second and third A11 epitopes. Primers internal to the tumor virus deletion were 5′-CTGCCGTACAATCCAACAGT-3′ and 5′-CGTGGCTCTTGGCTGCTCTG-3′. Polymorphisms in the LMP1 gene were detected by primers 5′-TCCACCGGAACCAGAAGAAC-3′ and 5′-CTCCACAATTGACGGAAGAG-3′ (the LMP1 30–base pair deletion) and 5′-AACCTCTTCCGTCAATTGTG-3′ and 5′-GACACCACCTGCTCGTGAGT-3′ (the LMP1 repeat). Briefly, 1 μg DNA was added to a reaction tube containing 1 pM primers, 200 μM of each deoxynucleotide, 5 units Taq polymerase, 10 × buffer, and 2 mM MgCl2 (Promega Corp, WI). The final volume was adjusted to 100 μL with water. Purified DNA for sequencing was obtained by eluting the PCR product from the gel using a Qiaquick kit (Qiagen). DNA sequencing was performed by the Center for Biotechnology (St Jude Children's Research Hospital) on an ABI Prism DNA Sequencer (Perkin-Elmer, CT). For the PCR reaction, samples were heated to 94°C for 40 seconds, 62°C for 2 minutes, and 72°C for 2 minutes, for 35 cycles, followed by 72°C for 10 minutes. The amplified products were run on an agarose gel and stained with ethidium bromide. To confirm the identity of the amplified products, the gels were Southern blotted and probed with radiolabeled fragments of EBNA-3B (EBV 95305 to 97026) that was excised with EcoRI and SacII from pSG5–EBNA-3B or an LMP1 complementary DNA probe.
Peptides for target sensitization
Peptides that interact with the patient's HLA class I molecules have been described in the literature.17 These peptides (Table 1) were prepared either by the Center for Biotechnology, St Jude Children's Research Hospital, or by the American Peptide Company (Sunnyvale, CA). Additional control peptides restricted by HLA-B44 were VEITPYKPTW and EENLLDFVRF from EBNAs 3C and 3B, respectively.17 30
Results
Case history
The patient, UPN 426, was a 17-year-old female with high-risk acute lymphoblastic leukemia—high white blood count and t(4;11). She received an unrelated donor BMT from a 5/6 HLA-matched unrelated donor after conditioning with cyclophosphamide, ara-C, anti-thymocyte globulin (ATG), and total body irradiation (TBI). GVHD prophylaxis was in vitro depletion of donor marrow with monoclonal antibodies to CD6 and CD8 and complement and, also, post-transplant cyclosporin A.36 Her initial post-transplant course was uncomplicated apart from grade I skin GVHD and an episode of herpes zoster. On day 45 after BMT, she was admitted with fever, and a computed tomography scan showed an enlarged right paratracheal lymph node. The following day she developed pharyngitis, which progressed to oropharyngeal edema. EBV DNA levels were more than 100 000 copies per 106 mononuclear cells, an indicator of LPD.2 A repeat computed tomography scan showed rapidly progressive pulmonary infiltrates. On day 56, the patient was given 2 × 107/m2 of her donor's EBV-specific CTLs on an Institutional Internal Review Board and FDA-approved protocol.37
Following CTL infusion, the patient's pharyngitis and oropharyngeal and facial edema progressed, and on day 61 she developed an oxygen requirement. Chest x-ray revealed worsening interstitial infiltrates. Because of concern that these effects represented an inflammatory response induced by infiltrating EBV-specific CTLs,20 she was started on methylprednisolone at 3 mg/kg per dose. Her oxygen requirement increased, and she was transferred to the intensive care unit and electively intubated. On day 73, a right neck node was biopsied. This showed large areas of necrosis but with more than 90% residual population of B lymphoblasts. Three days later, bronchoscopy showed exophytic bronchial wall lesions, and biopsy of one of these lesions showed lymphoma. EBV DNA levels obtained 7 and 21 days after CTL infusion remained extremely high—indicative of progressive LPD. She therefore received one dose of 10 mg/kg cyclophosphamide and then her second dose of CTLs as per protocol. However, her renal, cardiac, and respiratory function deteriorated, and she died on day 80 post-transplant, 24 days after the first CTL infusion.
A limited autopsy restricted to lungs was performed and showed bilateral nodules 2 mm to 2 cm in size. She also had hilar adenopathy and pulmonary congestion and consolidation. Histologic studies showed persisting lymphoma with a monomorphic population of lymphocytes expressing B cell markers.
Increased resistance of tumor cells to donor CTL lysis
Because our previous patients with EBV lymphoma had responded to CTL treatment,20 we investigated the possibility that this patient's tumor cells were resistant to CTL lysis. EBV-transformed B-cell lines grew spontaneously and rapidly from the patient's peripheral blood in the absence of cyclosporin A, immediately before and 7 days after CTL infusion. Comparison of the immunoglobulin VH rearrangements in the tumor biopsy cells and the spontaneous lines by Southern analysis demonstrated that the lines and the tumor were derived from the same progenitor cell (Figure 1). Thus, these lines were termed the pre-CTL tumor line and the post-CTL tumor line, respectively, and were assumed to derive from the tumor. The sensitivity of these tumor lines to killing by the donor CTL line was compared with that of the B95-8–transformed donor LCL (the stimulating cell line). Figure 2A shows that both tumor lines are killed poorly by comparison with the donor LCL. Because the CTL line also had some cytotoxic activity against an HLA MM-LCL and HSB-2, a T-cell lymphoma that is sensitive to LAK cells, we determined whether the limited tumor cell killing was by LAK cells or by bona fide major histocompatibility antigen (MHC)-restricted CTLs. Blocking antibodies to HLA class I and II were added to the tumor cells in a cytotoxicity assay. Figure 2B shows that tumor cell killing was partially blocked by anti-HLA class I antibodies. Thus, the donor CTL line had limited antitumor activity. By contrast, in the case of a previous patient whose tumors had responded to CTLs, killing of the spontaneous LCL (79% at an effector:target ratio of 20:1) was almost identical to killing of the donor LCL (81% at 20:1) (not shown). The poor susceptibility to killing that we observed in the current case might be explained by differences in the donor and recipient HLA type, strain differences between the stimulating B95-8 virus and the tumor virus, or by abnormalities in virus gene expression. To exclude an antigen-processing defect of the tumor line, which would result in underrepresentation of HLA-restricted EBV epitopes, cytotoxicity assays were performed with partially matched CTL lines. Two EBV-specific CTL lines that killed through B7 (Figure3C) or B60 (Figure 3D) were used. The tumor line was killed to the same extent as other partially matched LCL lines excluding an antigen-processing or other defect that would result in tumor resistance to CTL lysis.
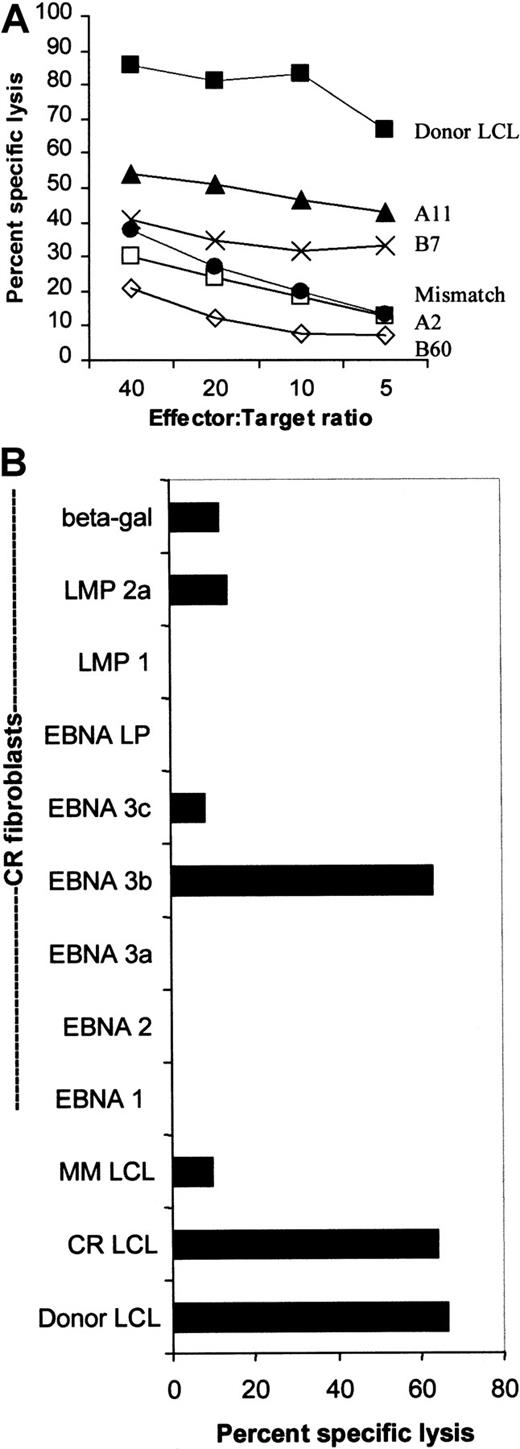
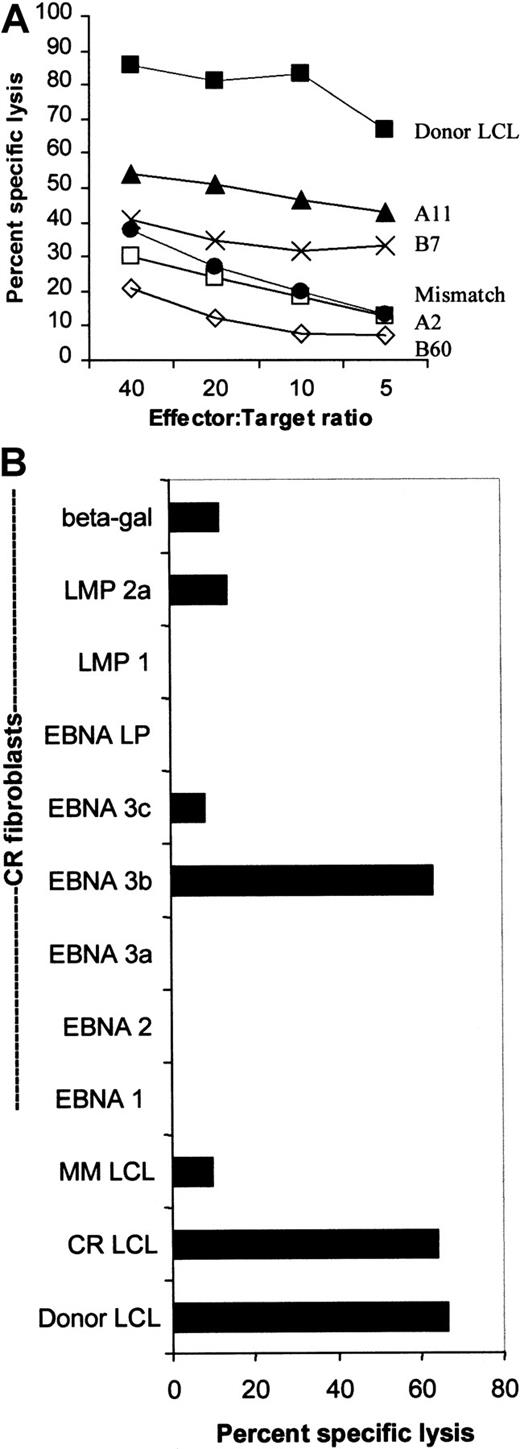
The donor CTL line was restricted through HLA-A11 and specific for EBNA-3B.
(A) The donor CTL line was tested for its ability to kill B95-8–derived LCLs from individuals who shared only one of each of its HLA class I antigens. Killing is shown across a range of effector:target ratios and was restricted mostly through HLA-A11. Killing through A2 and B60 was less than killing of an HLA class I MM-LCL. (B) The donor CTL line was EBNA-3B–specific. The donor, CR, shared HLA-A2, -A11, and -B7 with the CTL donor. This chromium release assay shows that the CR-LCLs and the donor LCLs were equally sensitive to killing by the donor CTL line. CR-derived dermal fibroblasts were infected with vaccinia virus recombinants expressing EBNAs 1, 2, 3A, 3B, 3C and LP as well as LMP1, LMP2, and the Escherichia coli–derived β-galactosidase gene. Only killing of EBNA-3B–expressing fibroblasts was greater than background killing of β-galactosidase–expressing fibroblasts.
The donor CTL line was restricted through HLA-A11 and specific for EBNA-3B.
(A) The donor CTL line was tested for its ability to kill B95-8–derived LCLs from individuals who shared only one of each of its HLA class I antigens. Killing is shown across a range of effector:target ratios and was restricted mostly through HLA-A11. Killing through A2 and B60 was less than killing of an HLA class I MM-LCL. (B) The donor CTL line was EBNA-3B–specific. The donor, CR, shared HLA-A2, -A11, and -B7 with the CTL donor. This chromium release assay shows that the CR-LCLs and the donor LCLs were equally sensitive to killing by the donor CTL line. CR-derived dermal fibroblasts were infected with vaccinia virus recombinants expressing EBNAs 1, 2, 3A, 3B, 3C and LP as well as LMP1, LMP2, and the Escherichia coli–derived β-galactosidase gene. Only killing of EBNA-3B–expressing fibroblasts was greater than background killing of β-galactosidase–expressing fibroblasts.
The tumor originated from donor lymphocytes. Most EBV lymphomas arising in BMT recipients are of donor origin, but about 10% derive from recipient B cells.3 In this case the donor-recipient pair was mismatched at HLA-A2, which was carried only by the donor. If the dominant cytotoxic activity of the CTL line was restricted by HLA-A2, and the tumor was derived from recipient cells, the CTL line would have little antitumor activity. However, HLA typing of the tumor lines that grew from the patient both before and 7 days after CTL infusion showed both to be HLA-A2+, and they were therefore of donor origin. Hence, HLA mismatching could not have accounted for tumor cell escape.
The immunizing virus and the tumor virus were both type 1 EBV. Two major strains of EBV (type 1 and type 2) have been identified, and type-specific CTL epitopes have been described.17,38 Type 1 EBV (the B95-8 strain) was used to stimulate the CTL line. If the CTLs recognized type-specific epitopes, they might not recognize a tumor that carried type 2 EBV. However, PCR analysis using primers that distinguish between type 1 and type 2 EBV showed that the virus from the tumor biopsy, and from both tumor cell lines, were all type 1 (data not shown).34
Tumor cells expressed appropriate EBV antigens. Some EBV-carrying tumor lines can down-regulate EBV antigens and thus escape CTL-mediated cytolysis. Burkitt lymphoma cells express only EBNA-1 and BARFO (type 1 latency), while Hodgkin lymphoma cells express EBNA-1, BARFO, LMP1, and LMP2 (type 2 latency). Although the LPDs that arise post-BMT usually express all the latency-associated proteins expressed on LCLs (type 3 latency), type 3-specific antigens may be down-regulated.39 However, immunofluorescence and Western blot analysis of tumor biopsy cells, lung autopsy cells, and the tumor lines using monoclonal antibodies to EBNA-2 and LMP1 showed that both proteins were expressed and indicated that the pattern of type 3 latency was maintained (data not shown).
Antigen specificity and HLA restriction of donor CTL line
Decreased susceptibility of the tumor cells to cytolysis might also be explained by mutations in the tumor virus that affect immunodominant CTL epitopes. To determine the epitope specificity of the donor CTL line, we examined both the EBV antigens recognized and the restricting HLA class I determinants. HLA restriction was tested using LCL targets that shared only one of each of the donor HLA class I antigens. Figure 3A shows that most of the killing is directed through HLA-A11, with some killing restricted by HLA-B7. There was no measurable killing restricted by HLA-A2 and -B60, because killing of target cells expressing these antigens was less than killing of an HLA MM-LCL.
The antigen specificity of the CTL line was tested against HLA class I–matched fibroblasts infected with vaccinia constructs expressing each of the EBV latency-associated proteins. The fibroblast donor, CR, shared HLA-A2, -A11, and -B7 with the donor CTL line, and the LCL from this donor (CR-LCL) was killed at the same level as the donor LCL. Significant killing of CR fibroblasts occurred only when they were expressing EBNA-3B (with low-level killing of LMP2A-expressing fibroblasts) (Figure 3B). Thus, the dominant cytotoxic activity of the donor CTL line was HLA-A11–restricted and EBNA-3B–specific.
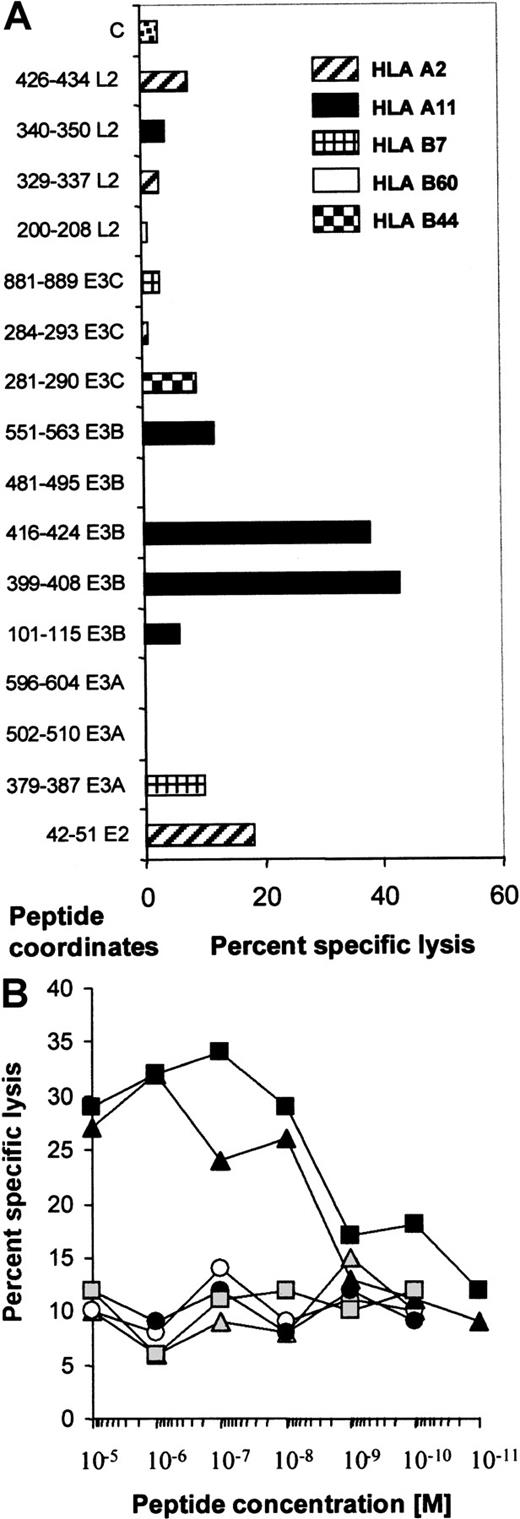
Epitope specificity of the donor CTL line
To try to identify the epitopes recognized by the donor CTL line, we identified peptides from the literature that could be predicted to sensitize target cells of the donor's HLA type (A2, A11, B7, B60). These peptides were used to pulse OKT3 blasts from the partially HLA-matched donors, CR (A2, A11, B7, B8) and CA (A2, A32, B60, B62). HLA-B44–restricted peptides were used as negative controls. An initial screen was performed using 10−5 mM peptide (Figure4A), and peptides inducing significant lysis were titrated to reveal their efficiency. Figure 4B shows that only 2 peptides from EBNA-3B, AVFDRKSDAK and IVTDFSVIK, retained their ability to sensitize targets at 10−8 mM, while the other peptides, including the EBNA-2 peptide, had only background activity. Three HLA-B7–restricted peptides were unable to elicit killing.
The donor CTL line demonstrated specificity only for 2 epitopes in EBNA-3B.
(A) This figure shows the cytotoxic activity of the donor CTL line against HLA-matched CA (HLA-B60) or CR (HLA-A2, -A11, and -B7) OKT3 blasts pulsed with 10−5 mM peptide at an effector:target ratio of 5:1. Peptide coordinates are shown. E2 represents EBNA-2, and L2 represents LMP2. C represents killing of OKT3 blasts in the absence of added peptide. Peptides that have been shown to sensitize target cells to killing through HLA-A2, -A11, -B7, and -B60 as well as control B44-restricted peptides are described in Table 1. (B) The donor CTL line was specific for peptides in EBNA-3B. The peptides that looked active in sensitizing CR OKT3 blasts at 10−5 M were used in the same assay at dilutions from 10−5 to 10−11 M. Only AVFDRKSDAK and IVTDFSVIK, the peptides deleted from EBNA-3B, titrated out to 10−8 M. indicates 42-51 (E2); ○, 379-387 (E3A); ▴, 399-408 (E3B); ▪, 416-424 (E3B); ●, 551-563 (E3B); and ░, 281-290 (E3C).
indicates 42-51 (E2); ○, 379-387 (E3A); ▴, 399-408 (E3B); ▪, 416-424 (E3B); ●, 551-563 (E3B); and ░, 281-290 (E3C).
The donor CTL line demonstrated specificity only for 2 epitopes in EBNA-3B.
(A) This figure shows the cytotoxic activity of the donor CTL line against HLA-matched CA (HLA-B60) or CR (HLA-A2, -A11, and -B7) OKT3 blasts pulsed with 10−5 mM peptide at an effector:target ratio of 5:1. Peptide coordinates are shown. E2 represents EBNA-2, and L2 represents LMP2. C represents killing of OKT3 blasts in the absence of added peptide. Peptides that have been shown to sensitize target cells to killing through HLA-A2, -A11, -B7, and -B60 as well as control B44-restricted peptides are described in Table 1. (B) The donor CTL line was specific for peptides in EBNA-3B. The peptides that looked active in sensitizing CR OKT3 blasts at 10−5 M were used in the same assay at dilutions from 10−5 to 10−11 M. Only AVFDRKSDAK and IVTDFSVIK, the peptides deleted from EBNA-3B, titrated out to 10−8 M. indicates 42-51 (E2); ○, 379-387 (E3A); ▴, 399-408 (E3B); ▪, 416-424 (E3B); ●, 551-563 (E3B); and ░, 281-290 (E3C).
indicates 42-51 (E2); ○, 379-387 (E3A); ▴, 399-408 (E3B); ▪, 416-424 (E3B); ●, 551-563 (E3B); and ░, 281-290 (E3C).
Deletion of HLA-A11–restricted CTL epitopes in EBNA-3B
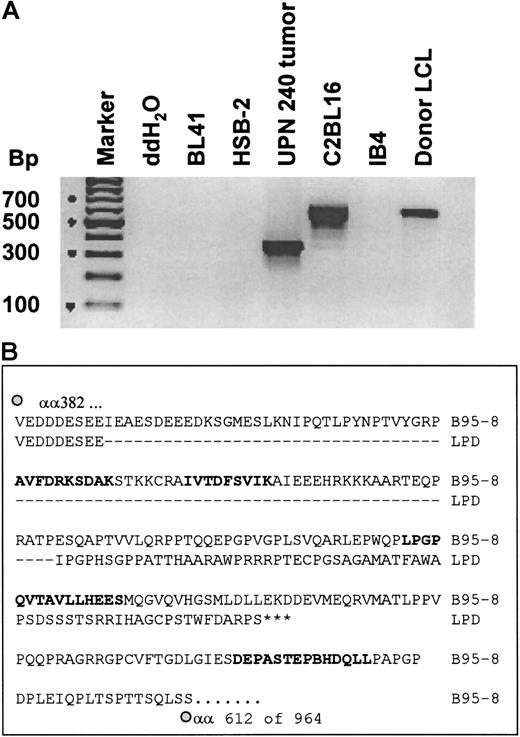
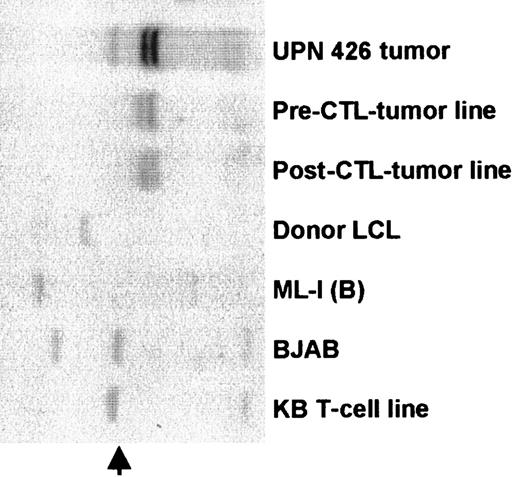
To determine whether CTL epitopes recognized by the donor CTL line were conserved in the tumor lines, we used PCR primers that spanned the A11 epitopes in EBNA-3B and allowed us to sequence all 5 A11/3B epitopes. Because the donor CTL appeared to have some killing restricted by HLA-B7 and potential recognition of EBNA-3C (Figure 3B), we also sequenced the B7 epitope in EBNA-3C. This EBNA-3C epitope, QPRAPIRPI, and the first A11 epitope, NPTQAPVIQLVHAVY, had complete sequence identity with B95-8.40 However, a second set of primers that spanned the second and third A11 epitopes revealed a deletion of 245 base pairs in the tumor biopsy cells (Figure5A) and in the pre- and post-CTL tumor lines (Figure 6A). The sequence of this fragment showed that the deletion removed the second and third A11 epitopes, AVFDRKSDAK and IVTDFSVIK, which were recognized by the donor CTLs, and disrupted the rest of the reading frame, including the remaining 2 A11 epitopes (Figure 5A). Hence, only the first 391 of the total of 947 amino acids of EBNA-3B would be expressed, together with a 62 amino acid “out-of-frame” tail. Consequently, the EBNA-3B in the tumor virus expressed only one of the 5 HLA-A11–restricted epitopes and had deleted both epitopes that sensitized target cells to killing by the donor CTL. The BL41/B95-8 cell line had a wild-type EBNA-3B fragment with complete sequence identity with the published sequence for B95-8 virus. Because most of the killing of the donor CTL line was directed against the 2 immunodominant A11-restricted epitopes in EBNA-3B, the deletion of these 2 epitopes could explain why the tumor lines were killed poorly in vitro. The observation that tumor biopsy cells (taken day 17 after CTL infusion) also carried the EBNA-3B deletion likely explains why the tumor failed to respond to the infused CTL line in vivo (Figure 5B).
The tumor biopsy cells had a deletion in the EBNA-3B gene that removed the 2 immunodominant CTL epitopes.
(A) The tumor cells have a deletion in the EBNA-3B gene; 200 ng DNA from each of the indicated cell lines and from tumor biopsy cells taken 17 days after CTL infusion was amplified using primers that spanned the second to fourth HLA-A11–restricted epitopes in EBNA-3B. These were expected to generate a fragment of 593 base pairs in wild-type EBV. The products were run on a 2% agarose gel and stained with ethidium bromide. (B) Sequence of EBNA-3B deletion in the pre-CTL tumor line; 200 ng DNA from the pre-CTL tumor line and from BL41/B95-8 was amplified and sequenced using primers described by de Campos-Lima et al.35 This figure shows a comparison of a partial sequence of the EBNA-3B protein of B95-8 EBV, from amino acids 382 to 612 (of a total of 964 amino acids), with the same region of EBNA-3B from the patient-derived tumor cell line established prior to CTL infusion. HLA-A11–restricted epitopes are in bold.
The tumor biopsy cells had a deletion in the EBNA-3B gene that removed the 2 immunodominant CTL epitopes.
(A) The tumor cells have a deletion in the EBNA-3B gene; 200 ng DNA from each of the indicated cell lines and from tumor biopsy cells taken 17 days after CTL infusion was amplified using primers that spanned the second to fourth HLA-A11–restricted epitopes in EBNA-3B. These were expected to generate a fragment of 593 base pairs in wild-type EBV. The products were run on a 2% agarose gel and stained with ethidium bromide. (B) Sequence of EBNA-3B deletion in the pre-CTL tumor line; 200 ng DNA from the pre-CTL tumor line and from BL41/B95-8 was amplified and sequenced using primers described by de Campos-Lima et al.35 This figure shows a comparison of a partial sequence of the EBNA-3B protein of B95-8 EBV, from amino acids 382 to 612 (of a total of 964 amino acids), with the same region of EBNA-3B from the patient-derived tumor cell line established prior to CTL infusion. HLA-A11–restricted epitopes are in bold.
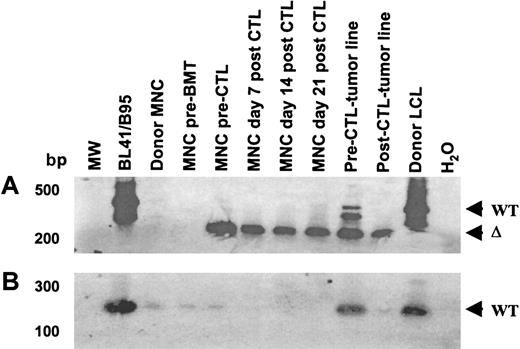
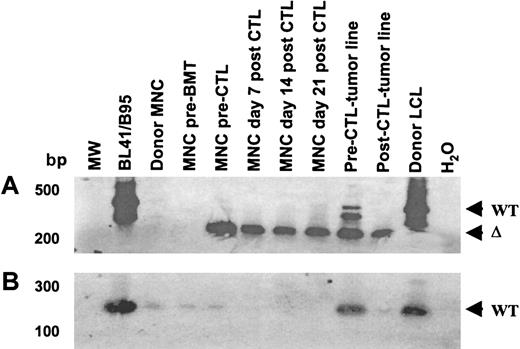
The EBNA-3B deletion variant was evident prior to CTL infusion and persisted after CTL infusion, while virus with wild-type EBNA-3B was eliminated by CTL infusion.
(A) Primers spanning the EBNA-3B deletion were used to amplify viral DNA from patient and donor peripheral blood drawn before transplantation; from patient blood drawn prior to CTL infusion and 1, 2, and 3 weeks after CTL infusion; from the pre- and post-CTL tumor lines; and from the donor LCL. (B) Wild-type EBNA-3B was detected pre-BMT and pre-CTL but disappeared after CTL infusion. Primers from within the EBNA-3B deletion that could amplify only wild-type EBNA-3B DNA were used in an attempt to avoid competitive effects from the deletion template. The amplification products were run on a 2% agarose gel, Southern blotted, and probed with an EBNA-3B probe. The limitations of the sequence did not allow good PCR primers to be designed; nevertheless, faint wild-type EBNA-3B bands were seen in the donor LCL, donor and recipient mononuclear cells before BMT, and in recipient mononuclear cells pre-CTL infusion but not after. Wild-type EBNA-3B was also detectable in the pre-CTL tumor line and to a lesser degree in the post-CTL tumor line.
The EBNA-3B deletion variant was evident prior to CTL infusion and persisted after CTL infusion, while virus with wild-type EBNA-3B was eliminated by CTL infusion.
(A) Primers spanning the EBNA-3B deletion were used to amplify viral DNA from patient and donor peripheral blood drawn before transplantation; from patient blood drawn prior to CTL infusion and 1, 2, and 3 weeks after CTL infusion; from the pre- and post-CTL tumor lines; and from the donor LCL. (B) Wild-type EBNA-3B was detected pre-BMT and pre-CTL but disappeared after CTL infusion. Primers from within the EBNA-3B deletion that could amplify only wild-type EBNA-3B DNA were used in an attempt to avoid competitive effects from the deletion template. The amplification products were run on a 2% agarose gel, Southern blotted, and probed with an EBNA-3B probe. The limitations of the sequence did not allow good PCR primers to be designed; nevertheless, faint wild-type EBNA-3B bands were seen in the donor LCL, donor and recipient mononuclear cells before BMT, and in recipient mononuclear cells pre-CTL infusion but not after. Wild-type EBNA-3B was also detectable in the pre-CTL tumor line and to a lesser degree in the post-CTL tumor line.
Origin of the deleted virus
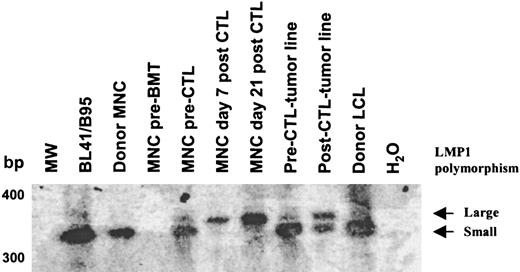
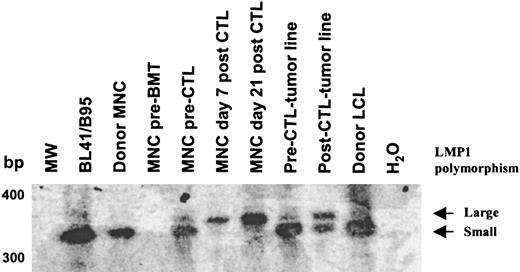
In an attempt to determine whether the deleted virus originated from the donor or recipient, we analyzed donor and recipient blood samples with primers external (Figure 6A) or internal (Figure 6B) to the deletion. Deleted virus could not be detected in donor or recipient before BMT. However, a faint band corresponding to the wild-type EBNA-3B was detected in both (Figure 6B). In contrast, 52 days after BMT and prior to CTL infusion, both wild-type and deleted EBNA-3B could be detected in the recipient, indicating that the deleted virus had emerged in the recipient after BMT. After infusion, only the deleted virus persisted, suggesting that the CTLs were able to eliminate cells containing wild-type EBV but were unable to control cells infected with the deleted virus. Consistent with this hypothesis, primers spanning polymorphic regions of LMP1 (a well-characterized 30–base pair deletion and the LMP1 repeat region) revealed 2 viruses in preinfusion peripheral blood and in the pre-CTL tumor line. By contrast in the tumor line derived after CTL infusion, the relative abundance of the virus with a smaller repeat and lacking the 30–base pair deletion was much reduced, while these polymorphisms could not be detected in the blood at all after CTL infusion (Figure7). The virus with a large LMP1 repeat was resistant to CTL infusion, with kinetics similar to that of the virus with the EBNA-3B deletion. Only the CTL-sensitive virus with the smaller repeat could be detected in donor and recipient blood prior to BMT. The relative abundance of the 2 viruses cannot be calculated from this PCR, first because the PCR was not quantitative and, second, because of competitive PCR effects between the 2 virus templates. Although it is possible that the patient harbored 2 CTL-resistant viruses, it is likely that the large repeat and the EBNA-3B deletion derive from the same virus.
Analysis of the LMP1 repeat revealed 2 viruses in the recipient before CTL infusion.
Primers spanning the LMP1 repeat region were used to amplify viral DNA. The amplification products were run on a 2% agarose gel, Southern blotted, and probed with an LMP1 complementary DNA probe. Two polymorphisms within the LMP1 repeat were detected prior to CTL infusion in the peripheral blood and the pre-CTL tumor line. After CTL infusion, the virus with the smaller repeat was not detectable in the peripheral blood, whereas the virus with the larger repeat persisted. In the post-CTL tumor line, the relative abundance of the virus with the smaller repeat was much reduced.
Analysis of the LMP1 repeat revealed 2 viruses in the recipient before CTL infusion.
Primers spanning the LMP1 repeat region were used to amplify viral DNA. The amplification products were run on a 2% agarose gel, Southern blotted, and probed with an LMP1 complementary DNA probe. Two polymorphisms within the LMP1 repeat were detected prior to CTL infusion in the peripheral blood and the pre-CTL tumor line. After CTL infusion, the virus with the smaller repeat was not detectable in the peripheral blood, whereas the virus with the larger repeat persisted. In the post-CTL tumor line, the relative abundance of the virus with the smaller repeat was much reduced.
Discussion
We have described a patient who presented with EBV-LPD 52 days after receiving a T-cell–depleted, HLA-mismatched, unrelated BMT. Despite receiving 2 doses of donor-derived, EBV-specific CTLs, she died with progressive disease 24 days after the first CTL infusion. This may have been because the CTLs were administered too late, because she had a particularly aggressive lymphoma, or because the tumor virus was not recognized by the infused CTLs. EBV-transformed B cells grew rapidly from patient peripheral blood cultured both prior to and one week after CTL infusion. Two lines of evidence suggest that these cell lines derived from the tumor. First, the tumor biopsy cells and the spontaneous lines had identical immunoglobulin rearrangements and, second, both carried the deletion in EBNA-3B. Both “tumor lines” were less sensitive to cytolysis than the donor-derived B95-8–transformed B-cell line that was used to generate the CTL line. The CTL line was found to be largely HLA-A11–restricted with specificity for 2 epitopes in EBNA-3B. Both of these epitopes were deleted in the tumor virus. We therefore propose that failure of therapy occurred because the tumor cells were not recognized by most CTL clones within the polyclonal line. This mechanism of escape may contribute to the previously documented failures of T-cell therapy in established disease.10 Down-regulation of HLA class I molecules or interference with antigen processing are other mechanisms of immune evasion.41 These were excluded in the present case because the tumor line was effectively killed by other partially HLA class I matched EBV-specific CTL lines.
In principle, CTLs generated in vitro using LCLs as antigen-presenting cells may recognize all of the virus proteins associated with type 3 latency, with the exception of EBNA-1 and BARFO.17,42 In practice, in a given donor, the CTL response is dominated by 1 to 3 epitopes from 1 or 2 proteins so that even a polyclonal EBV-specific CTL line may be oligoclonal in specificity.43,44 The viral epitopes recognized are determined by the donor HLA allotype. In most individuals the CTL response is focused on the EBNA-3 proteins, with only subdominant responses to LMP2, LMP1, EBNA-2, or EBNA-LP being found in some individuals.17 HLA-A3, -A11, -B7, -B8 and -B44 are strong restricting alleles that, if present, will dominate the CTL response to EBV. For example, CTL clones with specificity for one HLA-A11–restricted, EBNA-3B epitope, IVTDFSVIK, dominate the immune response to EBV in HLA-A11+ Caucasian individuals.28 This is thought to be because of its abundant representation as an HLA/peptide complex at the cell surface, by comparison with other peptides.45 Our donor CTL line was also dominated by the IVTDFSVIK epitope with additional killing through the next strongest A11-restricted epitope in EBNA-3B, AVFDRKSDAK. The limited HLA class I–restricted killing of the pre- and post-CTL tumor lines may have been explained by the presence in the lines of cells infected with wild-type virus (Figure 6) or by killing through epitopes that we were unable to identify. The former explanation may be more likely because, with time in culture, the abundance of the wild-type EBNA-3B relative to the deleted EBNA-3B increased. By contrast in the patient, the relative abundance of the deleted EBNA-3B decreased after CTL infusion. This suggested that the wild-type virus had a competitive advantage in the absence of immune selection.
EBV is a genetically stable virus, and its immunodominant epitopes are remarkably conserved. Even when geographically diverse populations are studied, point mutations that destroy immunodominant A11-restricted epitopes in EBNA-3B have occurred only in certain isolated populations.17,28,35 Major deletions in EBNA-3B such as we describe have not previously been described in any naturally occurring EBV variants.17,28 We analyzed additional wild-type isolates from 24 normal individuals in the United States and did not find deletions in any (data not shown). The pattern of epitope dominance in an individual is also stable over time. Both of these facts suggest that CTL escape mutants such as we describe; are unsuccessful in vivo, at least in immunocompetent individuals; and that EBNA-3B, while nonessential for virus transformation, has an important virus survival function in normal individuals.44,46 In normal individuals, the immune response may coevolve with EBV so that if mutations occur in immunodominant epitopes, CTLs specific for subdominant epitopes will expand and prevent the outgrowth of B cells transformed with mutant virus. In an immunosuppressed host, the immune response may be less able to adapt to mutant viruses, which may therefore have a selective advantage. Although our patient received T-cell–depleted marrow, not all T cells are depleted and ideally a patient would receive about 5 × 105 T cells per kilogram. Such T cells may be important in the control of EBV after BMT in the immediate post-transplant period, although they cannot be detected in limiting dilution analysis.1 However, a small number of donor CTLs with a fixed repertoire may have exerted some selection against donor virus.
We were unable to determine the origin of the deleted virus. Following BMT, marrow recipients are usually repopulated by donor-derived EBV carried by B cells in the marrow infusion.47,48 Our patient was treated with acyclovir from the time of transplant until engraftment and thereafter was treated with ganciclovir. Thus, it was unlikely that free recipient virus was available to infect donor B cells. However, because the EBNA-3B–deleted virus could be detected in neither donor nor recipient prior to BMT, its origin remains obscure. The deleted virus was not detected until the patient presented with EBV lymphoma, prior to CTL infusion. To try to determine whether the deleted virus represented a mutation in a single pre-existing donor or recipient virus strain, we analyzed 2 well-characterized polymorphic regions in the EBV LMP1 gene.49 A shared polymorphism would suggest a common origin from the donor or recipient, respectively. This analysis confirmed that more than one virus was present in the recipient prior to CTL infusion. A virus carrying the 30–base pair LMP1 deletion and a large repeat region was present in patient peripheral blood mononuclear cells before the CTL infusion and persisted after treatment, coincident with the presence and persistence of the EBNA-3B deletion mutant. A virus with this set of polymorphisms could not be detected in the donor or recipient prior to transplant. A second set of polymorphisms was found in the recipient pre-CTL infusion. The virus with these polymorphisms appeared sensitive to the CTLs because it disappeared after CTL infusion. This second set of polymorphisms was also found in the donor. The preferential outgrowth of the deleted virus after CTL infusion likely occurred because the virus had lost the A11-restricted epitopes of EBNA-3B that were the predominant targets of the infused CTL line. Cells infected with the variant virus would survive, while those carrying the virus encoding wild-type EBNA-3B would be destroyed.
What is the frequency of EBV escape mutants in post-transplant lymphomas? They occurred in 1 of 4 patients with EBV-LPD that we treated and may explain some of the treatment failures observed in which 9 of 13 patients developed disease progression after receiving immune donor T cells.10 It would be of interest to determine whether some of those tumors were also caused by escape mutants. Whatever the frequency of escape mutants in EBV lymphoma, its occurrence is likely to be a frequent dilemma in immunotherapy, particularly when using in vitro–cultured CTL lines that have a fixed repertoire and no capacity for adaptation. Several strategies may overcome this problem. One is to use polyclonal CTLs, which are less likely to be successfully evaded by escape mutants. Although this was our intent and the donor CTLs were polyclonal in Vβ usage (data not shown), they were oligoclonal in specificity. A second strategy is to use CTLs specific for targets that are essential for transformation and therefore cannot be mutated. Finally, CTLs may best be used as prophylaxis or for minimal residual disease, because with fewer tumor cells there is less chance of mutation or deletion. Our results with EBV lymphoma provide an example of the need for these approaches.
Acknowledgments
We thank Yixin Yao, Jennifer Moore, and Micah Semmelmann for expert technical assistance, Jaqueline Williams for secretarial support, and Belinda Rossitter for editing the manuscript.
H.E.H. is a recipient of a Doris Duke distinguished investigator award.
Supported by National Institutes of Health grants RO1 CA61384 and Cancer Center Support CORE grant 21765; the Department of Pediatrics, Baylor College of Medicine; and the American Lebanese Syrian Associated Charities.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Cliona Rooney, Center for Cell and Gene Therapy, 1102 Bates St, Suite 1100.0, Houston, TX 77030; e-mail:crooney@bcm.tmc.edu.



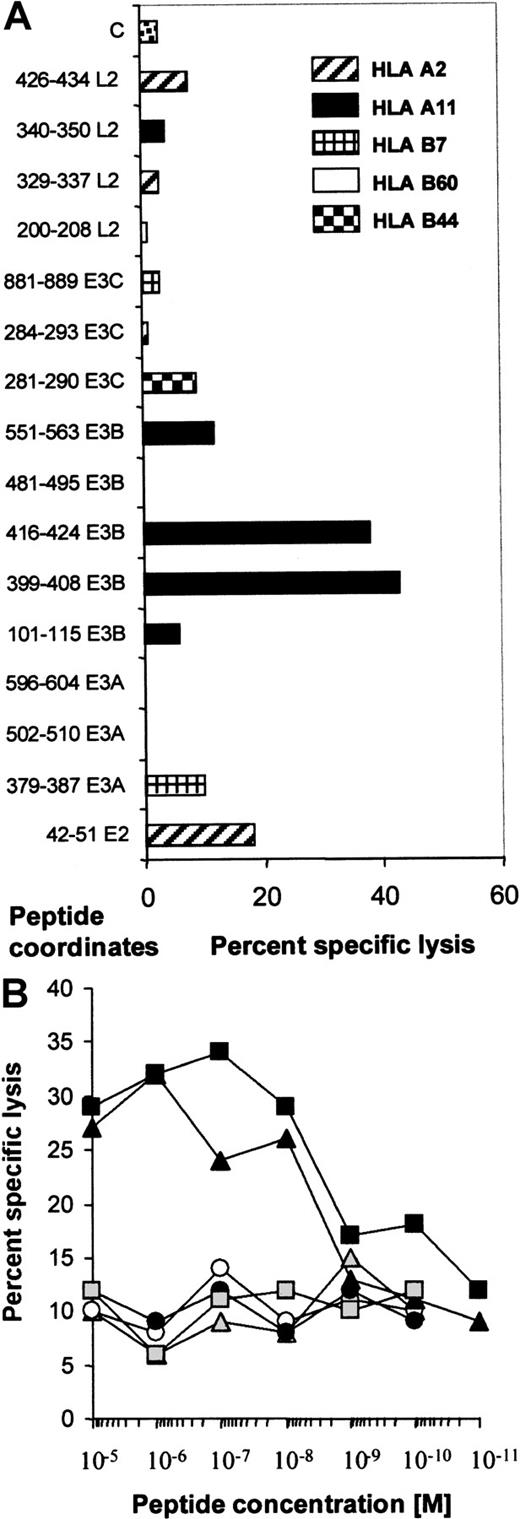
 indicates 42-51 (E2); ○, 379-387 (E3A); ▴, 399-408 (E3B); ▪, 416-424 (E3B); ●, 551-563 (E3B); and ░, 281-290 (E3C).
indicates 42-51 (E2); ○, 379-387 (E3A); ▴, 399-408 (E3B); ▪, 416-424 (E3B); ●, 551-563 (E3B); and ░, 281-290 (E3C).