Highly malignant myeloma cells up-regulate their Fas-ligand (Fas-L) to escape immune surveillance by Fas+ cytotoxic cells. Here it is demonstrated that this abnormality is involved in the pathogenesis of the severe anemia associated with progression of multiple myeloma (MM). By measuring Fas and Fas-L in plasma cells and erythroblasts from 19 MM patients and 5 with monoclonal gammopathies of undetermined significance (MGUS), it was found that both Fas-L+ myeloma cells and Fas+ erythroid progenitors were significantly increased in patients with stage III MM whose erythroblasts, cultured in the presence of autologous plasma cells or their supernatant, underwent prompt apoptosis as evaluated by propidium iodide staining, the TUNEL assay, and detection of the APO2.7-reactive mitochondrial antigen. Flow cytometry of fresh erythroblasts revealed a considerable expression of the caspases CPP32 and FLICE in both their constitutive proenzymatic forms and in cleaved subunits. By contrast, their intracytoplasmic expression was defective in patients with inactive disease and MGUS controls. The evidence that Fas-L+ myeloma clones directly prime erythroblast apoptosis in vivo was further supported by the occurrence of fluorescein isothiocyanate–TUNEL+ erythroblasts juxtaposed to myeloma cells in bone marrow smears. These results strongly suggest that the deregulated apoptosis in myeloma clones plays an active role in the progressive destruction of the erythroid matrix by a cytotoxic mechanism based on up-regulation of Fas-L.
Introduction
Fas-ligand (Fas-L) is a type 2 glycoprotein1 expressed by activated lymphocytes and cytotoxic cells2,3 but is also detectable in a number of cell types4-6 and in tumors of different lineages.7 By cross-linking its natural membrane coreceptor, namely Fas (Apo-I/CD95),8 Fas-L induces sequential activation of caspases, and this results in the apoptotic cell death of Fas+ cells.9 The expression of Fas-L by nonlymphoid cells in a few organs such as testis,10 uterus,11 and the anterior chamber of the eye is thought to maintain a state of immune privilege by eliminating Fas+ infiltrating lymphocytes.12 A similar immune escape mechanism has also been proposed for several tumor cell types whose Fas-L counterattacks the Fas+effector cells committed to immune surveillance.
In addition to solid tumors such as melanoma,13astrocytoma,14 and colon carcinoma,15 other neoplasms of T-cell origin have been reported to overexpress Fas-L.16 Previous studies also showed that Fas-L+ plasma cell lines extinguish Fas+ T lymphoblasts in vitro17 and suggested that this mechanism operates in multiple myeloma (MM) to escape the suppressive control of cytotoxic cells. We have recently provided evidence that both overexpression and secretion of Fas-L in vitro by a number of myeloma cell clones reflect a high degree of malignancy, namely the ability to proliferate in the absence of interleukin-6 as well as the poor Fas-mediated apoptosis.18 By contrast, a variable sensitivity to cell death was present in additional myeloma cell lines, one of which showed little or undetectable production of Fas-L either on its cell membrane or in soluble form.
Clinical manifestations of MM include skeleton colonization by malignant plasma cells and a normochromic/normocytic anemia, both of which influence its progression.19 A number of different mechanisms have been postulated to account for the severity of the anemia frequent in hematologic malignancies. Chronic derangement of the cytokine network at the bone marrow level as well as a persistent defect of erythropoietin (EPO), particularly in patients with kidney failure, are believed to play a pivotal role.20-22
Recent studies have also shown that the Fas/Fas-L system is actively involved in the regulation of erythropoiesis.23 Fas is strongly up-regulated in hematopoietic progenitor cells24during chronic hematologic disorders by the increased levels of both tumor necrosis factor (TNF) and interferon (IFN)-γ, which also inhibit the erythroid colony-forming units (CFU-E) from bone marrow CD34+ cells.25 Although IFN-γ has been demonstrated to prompt the selective cleavage of dormant proenzymatic caspases CPP32 (caspase 3), FLICE (caspase 8), and ICE (caspase 1) to produce apoptosis in erythroblasts,26 a number of additional events are concurrently implicated in chronic activation of Fas on these cells and in the progressive destruction of the erythroid matrix.27,28 Fas-L+ differentiated erythroblasts have also been postulated to act as negative regulators of erythroid maturation through Fas/Fas-L interaction.23Therefore, because early erythroblasts, particularly at their prebasophilic/basophilic stage, show a high responsiveness to Fas stimulation, it is conceivable that the critical suppression of erythropoiesis observed in several hematologic malignancies of Fas-L+ cell lineage, including MM, could be promoted by these tumor cells through their own Fas-L. However, besides a Fas-L cytotoxic mechanism, a chronically enhanced apoptosis of erythroid progenitors may account for the persistent cross-linkage of Fas by nonspecific ligands, even including the excess of soluble proinflammatory cytokines.21 29
In this study, we explored the extent of apoptosis in bone marrow erythroblasts of MM patients with respect to Fas-L overexpression by the plasma cell clones. We found that the deregulated apoptosis in highly malignant plasma cell lines is powerfully detrimental to bone marrow erythroid growth and maturation and that this mechanism is engaged in the expansion of the myeloma clone within the bone marrow.
Patients, materials, and methods
Patients
Nineteen patients with MM stages I to IIIB30 were studied, along with 5 patients with monoclonal gammopathy of undetermined significance (MGUS) as controls. In 15 MM patients, bone marrow cell samples were obtained at diagnosis and before chemotherapy. None of them received blood transfusions or recombinant human (rHu) EPO during the 2 months preceding their enrollment. The study was approved by the Ethical Committee of the University of Bari, and all subjects were properly informed and gave their consent.
Bone marrow cell phenotyping and cultures
Cells were obtained by bone marrow needle aspiration and freshly typed by double fluorescence analysis in a FACScan (Becton Dickinson, Mountain View, CA). The expression of both Fas and Fas-L molecules on plasma cells and erythroblasts was explored by phenotypic differentiation with CD38 or glycophorin A (GpA) detection,31 respectively. Phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (MoAbs) to Fas from clone UB2 (Immunotech, Marseille, France), a biotinylated anti–Fas-L immunoglobulin G1κ (IgG1κ) from clone NOK-1 alternatively treated with FITC- or PE-coupled streptavidin (Pharmingen, San Diego, CA), a PE-conjugated anti-CD38 MoAb (Becton Dickinson, San Jose, CA) and a FITC-conjugated anti-GpA MoAb (Immunotech) were used for this purpose.
Aliquots of bone marrow samples were also processed to prepare cultures of both plasma cell and erythroid lineages. Briefly, myeloma cells were enriched by passing the mononuclear cells previously obtained by Ficoll-Hypaque sedimentation over a discontinuous density gradient of a Percoll solution at 90% to 30%. Plasma cells collected at both 60% and 45% gradient concentration were enriched to more than 80%. Then, 1 × 106 cells were cultured in complete medium (RPMI 1640 plus 10% fetal calf serum and 2 mM glutamine) in the presence of 500 U/mL interleukin-6 (Genzyme, Cambridge, MA). Cultures were maintained in a CO2-humidified incubator up to 3 weeks to establish continuous proliferation. Enrichment in malignant plasma cells was monitored weekly by CD38+ bright cytofluorimetric detection.
Erythroblast proliferation was also stimulated in parallel aliquots of bone marrow samples by preparing an Iscove's modified Dulbecco's medium supplemented with 20% fetal calf serum, 500 U/mL ampicillin (Sigma, St Louis, MO), 100 U/mL rHu-EPO (Janssen-Cilag, Schaffausen, Switzerland), 10 μg/mL insulin, and 5% sterile human AB serum, as described.28 Cultures were maintained up to 9 days and used for coculturing experiments.
Cocultures of erythroblasts with plasma cells
Mixed cultures of erythroblasts and plasma cells were established to investigate the potential cytotoxic effect of Fas-L+ cells within each culture. Briefly, 2 × 105 to 5 × 105 of 8-day cultured erythroblasts were incubated overnight in 24-well plates at 37°C in the presence of plasma cells at a 1:1 ratio. In most instances erythroblasts were cultured in the presence of enriched autologous myeloma cells, whereas parallel experiments included treatment of erythroblasts for an equivalent time with the conditioned supernatant (SN) from the relative plasma cell culture.
A number of control experiments included inhibition of the interaction between Fas and Fas-L in culture to assess the specificity of erythroblast suppression. Separate erythroblast preparations from selected patients were preincubated with increasing amounts of nonagonist Fas-reactive IgG1-UB2 (Immunotech) MoAb (0.01, 0.1, 1.0, 10, and 100 μg/mL) to saturate the receptor binding. These cells were cocultured with myeloma cells, and the extent of their apoptosis was measured. In parallel, alternative experiments included the pretreatment of myeloma cells with similar levels of the recombinant Fas-Fc chimeric protein (Pharmingen) to block their Fas-L linkage sites. These cell preparations were then used to measure erythroblast apoptosis. In additional experiments we prepared enriched erythroblast populations from the bone marrow of a few patients by magnetic cell-sorting procedures (Miltenyi Biotec, Bergisch Gladbach, Germany). These methods used the positive selection of erythroblasts by magnetic GpA-coupled microbeads and provided adequate amounts of purified erythroblasts according to the manufacturer's instructions. We used these cells for further control experiments measuring their spontaneous apoptosis in the absence of either cocultured myeloma cells or their conditioned SN.
Measurement of apoptosis
Three different approaches were adopted to detect apoptosis in cocultured erythroblasts in comparison with control unstimulated cells from each patient. We first used propidium iodide (PI) cell staining, which included overnight treatment of cells with 100 μg PI following their preparation with 70% ethanol at 4°C. Measurement of the subdiploid DNA-containing erythroblast population in each coculture was then completed by flow cytometry on the relative GpA+ subset.
Erythroblast apoptosis was also assessed by the TUNEL assay (Boehringer-Mannheim, Mannheim, Germany), which measures the extent of nuclear DNA fragmentation by labeling the fluorescent nucleotides at the 3′ ends with terminal deoxynucleotidyl transferase (TdT). We used this method both in a cytofluorimetric assay and in fresh cells from bone marrow smears to evaluate the occurrence of erythroblast apoptosis in vivo. Cells were treated with 4% paraformaldehyde for 20 minutes prior to their incubation with an anti-GpA MoAb in parallel with anti-κ or anti-λ light chain antisera. The test was completed with the fluorescent nucleotide mixture in the presence of TdT, and the slides were examined under a fluorescence microscope.
Lastly, an additional attempt was devoted to exploring the expression of a 38-kd mitochondrial antigen recognized by the APO2.7 MoAb (Immunotech) and considered as an early marker of apoptosis.32 Its amplitude was also estimated by flow cytometry in double fluorescence experiments.
Measurement of Fas-L secretion
Two mouse MoAbs to unrelated epitopes of Fas-L, namely NOK-1 and NOK-2 (Pharmingen), were used in a standardized enzyme-linked immunosorbent assay (ELISA)33,34 to measure relative soluble Fas-L (sFas-L) concentrations in SNs from plasma cell cultures. Briefly, plates were coated with 10 μg/mL NOK-2 in carbonate buffer, pH 9.6, and then supplemented with SNs for 5 hours. After washing, plates were incubated with biotinylated NOK-1, subsequently with avidin-conjugated peroxidase (Sigma), and developed withO-phenylendiamine solution. Each determination was assessed in triplicate and referred to a standard control value obtained with 2 μg/mL recombinant Fas-L (Pharmingen). The function of Fas-L secreted by cultured myeloma cells was also investigated in relation to its cytocidal property in the [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) assay. This procedure is a colorimetric method for measuring cell viability and is based on cleavage of the yellow tetrazolium salt by active mitochondria to form a dark-blue formazan product. Thus, it was used as an indirect method to measure the percent of killing of mouse lymphoblasts overexpressing human Fas, namely the WC8 cell line35 along with its Fas− parental cell line WR19L as control (kindly provided by Dr S. Nagata, Osaka, Japan). The assay included a 40-hour incubation of 1 × 105 target cells with each myeloma-conditioned SN and, in parallel, in the presence of control unconditioned medium. Therefore, 100 μL MTT solution (2 mg/mL) was added to each well. After 4 hours at 37°C, the formazan crystals were dissolved by adding 100 μL dimethyl sulfoxide (Sigma), and the 96-well plates were read in a spectrophotometer (Bio-Rad Laboratories, Irvine, CA). Dead cells were determined as reported.18
Evaluation of caspase activity
Several experiments were performed to measure caspase expression and activation in fresh erythroblasts. We used double fluorescence analysis to measure the constitutive expression of caspase 1 (ICE) and caspase 3 (CPP32) by rabbit polyclonal antisera (Alexis, Vinci, Italy), whereas caspase 8 (FLICE) was assessed by a MoAb (Biosource, Camarillo, CA). Although several of these reagents were optimized by their manufacturers for immunoblotting methods, we demonstrated their reliability in flow cytometry assay. Conversely, cleavage of the CPP32 proenzyme form, namely the classical hallmark of apoptosis activation throughout the Fas pathway, was investigated by detection of both its 12-kd cleaved subunit and the proteolytic cleavage of poly (ADP) ribose polymerase (PARP) by rabbit polyclonal reagents (Pharmingen, and New England Biolabs, Hitchin, UK, respectively). Briefly, the cells were incubated with the anti-GpA reagent and then fixed, permeabilized, and separately treated with specific anticaspase reagents to their intact proenzyme forms, the CPP32 activated subunit or PARP. Lastly, the assay was completed by a secondary FITC-conjugated antibody prior to flow cytometry. These analyses were carried out along with parallel tests using Jurkat cells as a control-established Fas+ cellular model to measure both caspase constitutive expression and cleavage following the Fas pathway activation. Therefore, Jurkat cells were evaluated by flow cytometry before and after Fas stimulation with the myeloma-conditioned SN from patient no. 16, which showed high concentration of sFas-L.
Statistical analysis
Differences between means of groups of data were calculated using Student t test, whereas the correlation of data was assessed by Spearman's Rho nonparametric method.
Results
Discrepancy in Fas and Fas-L expression in relation to the progression of MM
As shown in Table 1, the clinical stages of MM were associated with different expressions of Fas and Fas-L in bone marrow plasma cells and erythroblasts, along with an independent relation to its progression in the case of plasma cells. The mean values (Figure 1) show that stage III MM plasma cells up-regulate their Fas-L expression in the presence of a variable modulation of Fas. This pattern of weak expression of the native Fas on highly malignant plasma cells was in agreement with a previous description of their insensitivity to Fas stimulation.18 Comparison between patients with stage III MM and those with MGUS revealed a significant difference in each receptor expression (P < .02 in both instances) and indicated that advanced MM is associated with abnormalities in myeloma cell apoptosis.
Mean values (%) of both Fas and Fas-L expressed by bone marrow plasma cells and erythroblasts.
Nineteen patients with MM and 5 patients with MGUS were grouped in relation to their clinical stages. (A) A remarkable up-regulation of Fas-L was detected in plasma cells from patients with stage III MM as compared with the MGUS controls. This pattern was associated with a concurrent increase of Fas+ erythroblasts, whose defective expression of Fas-L in patients with advanced disease suggested the depletion of mature erythroblasts in the bone marrow. Note the decreasing Fas expression by myeloma cells with disease progression. The evaluation was carried out by double fluorescence analysis using CD38 bright and GpA as phenotypic markers of plasma cells and erythroblasts, respectively. (B) Correlation of individual Hb values with the relative expression of both Fas+ and Fas-L+ fresh plasma cells and erythroblasts (graphic representation of Spearman's test). The analysis revealed an inverse correlation of Hb levels with the percent expression of either Fas-L+ plasma cells or Fas+erythroblasts.
Mean values (%) of both Fas and Fas-L expressed by bone marrow plasma cells and erythroblasts.
Nineteen patients with MM and 5 patients with MGUS were grouped in relation to their clinical stages. (A) A remarkable up-regulation of Fas-L was detected in plasma cells from patients with stage III MM as compared with the MGUS controls. This pattern was associated with a concurrent increase of Fas+ erythroblasts, whose defective expression of Fas-L in patients with advanced disease suggested the depletion of mature erythroblasts in the bone marrow. Note the decreasing Fas expression by myeloma cells with disease progression. The evaluation was carried out by double fluorescence analysis using CD38 bright and GpA as phenotypic markers of plasma cells and erythroblasts, respectively. (B) Correlation of individual Hb values with the relative expression of both Fas+ and Fas-L+ fresh plasma cells and erythroblasts (graphic representation of Spearman's test). The analysis revealed an inverse correlation of Hb levels with the percent expression of either Fas-L+ plasma cells or Fas+erythroblasts.
Bone marrow erythroblasts also up-regulated their Fas expression in advanced MM (P < .01 as compared with the other groups), whereas Fas-L was defectively expressed (P < .02), pointing to a prevalence of Fas-L–negative or immature erythroblasts. To validate this interpretation, we measured the percent distribution of basophilic erythroblasts on bone marrow smears as compared with other mature erythroid progenitors in each group of patients. The mean values from this analysis (Table 2) point to a progressive deficiency of both polychromatophilic and orthochromatic erythroblasts as the disease progresses. We also compared both Fas and Fas-L positivities in erythroblast and plasma cell populations, and Figure 1B plots their values against the hemoglobin (Hb) levels. Spearman's Rho revealed an inverse relationship of Hb with the CD38+ bright/Fas-L+and GpA+/Fas+ subsets (r = −0.7848 and −0.7376, respectively) and a linear correlation (r = 0.6414 and 0.8942, respectively) with the other populations.
Plasma cell and erythroblast cultures
Culture experiments were carried out to investigate the extent of defective apoptosis with respect to Fas/Fas-L deregulation in expanded plasma cell and erythroid populations and to obtain enriched cell profiles for autologous coculturing tests as an in vitro model of the mixed proliferation of myeloma cells with erythroid progenitors within the bone marrow.
We established both types of cultures from 9 patients. Aliquots of cells were harvested and analyzed by double fluorescence in the FACScan by gating the cells positive for CD38 or GpA antigens. Figure2A illustrates the distribution of both Fas-L and Fas in each culture of plasma cells and erythroblasts, respectively. A striking difference was observed in the phenotype expansions, in that the Fas-L+ subset of myeloma cells was widely expanded in plasma cell cultures from stage III patients and rose to about 90% in one (patient no. 16). A concurrent, though lower, pattern of Fas+ erythroid cell expansion was detected in their parallel erythroblast cultures. Median values of both expansions were significantly higher (P < .02 in all instances) when compared with the variable, though weak, phenotype expression in the other groups. Figure 2B shows representative patterns of this diversity between an MGUS subject and MM patient no. 16. Both CD38+bright/Fas-L+ plasma cell and GpA+/Fas+ erythroblast populations were greatly expanded in the MM patient, in keeping with the high replication rate of Fas-L+ myeloma clones observed in stage III MM.18
In vitro expansion of myeloma cells and erythroblasts separately cultured from the bone marrow of patients with MGUS or MM.
(A) A predominant expansion of Fas-L+ myeloma clones was observed in plasma cell cultures from patients with stage III MM. Similarly, a prevalent expansion, though to a lower extent, of the Fas+ erythroblast subset was recorded in parallel erythroid cultures from these patients (NE indicates not established). (B) Representative cytofluorimetric patterns of CD38+bright/Fas-L+ and GpA+ cell expansion. As compared with those from the MGUS subject, both subsets were largely expanded in the cultures from the MM patient.
In vitro expansion of myeloma cells and erythroblasts separately cultured from the bone marrow of patients with MGUS or MM.
(A) A predominant expansion of Fas-L+ myeloma clones was observed in plasma cell cultures from patients with stage III MM. Similarly, a prevalent expansion, though to a lower extent, of the Fas+ erythroblast subset was recorded in parallel erythroid cultures from these patients (NE indicates not established). (B) Representative cytofluorimetric patterns of CD38+bright/Fas-L+ and GpA+ cell expansion. As compared with those from the MGUS subject, both subsets were largely expanded in the cultures from the MM patient.
Functional sFas-L is variably secreted by myeloma cell cultures
Further studies explored the extent of Fas-L solubilization by malignant plasma cells in culture. We first adopted an optimized ELISA to measure its quantitative release in SNs and then tested their cytocidal property by a functional assay. Figure3 shows these results. The number of myeloma cultures differed because in several instances no established cell lines were obtained. A significant secretion of sFas-L was nonetheless detected in cultures from stage III patients (patients no. 12, 13, and 16-18) as compared with the controls (P < .02). This pattern was confirmed by a substantial major cytotoxic effect on Fas+ target cells in the MTT test, whose positive control included 2 μg/mL recombinant Fas-L. Therefore, at least in SNs from myeloma cultures of patients no. 16 and 17, the amount of secreted Fas-L was higher than that of the reference value. In addition, the remarkable cytocidal effect induced on WC8 cells by most SNs agreed with the ELISA measurement and suggested an apparent concordance between the 2 methods. Further support for the major release of sFas-L by cells from 6 stage III patients (patients no. 12-14 and 16-18) was obtained when the MTT test using the control Fas− WR19L cells as target failed to demonstrate the ability of those SNs to induce an evaluable extent of cell death. These results provided further evidence that highly malignant myeloma cells are up-regulated in both expression and solubilization of Fas-L in vitro and that sFas-L is functional.
Fas-L secretion by myeloma plasma cells.
Measurement by ELISA of sFas-L in SNs from cultured myeloma clones revealed its significant release by cells from stage III patients, whose levels in cultures no. 16 and 17 were higher than the reference value obtained using 2 μg/mL recombinant Fas-L as standard. The functional activity of sFas-L in SNs was assessed by the MTT assay using the WC8 cell line, a human Fas-transfected mouse lymphoma, whereas the parental cell line WR19L was used as Fas−control. OD indicates optical density.
Fas-L secretion by myeloma plasma cells.
Measurement by ELISA of sFas-L in SNs from cultured myeloma clones revealed its significant release by cells from stage III patients, whose levels in cultures no. 16 and 17 were higher than the reference value obtained using 2 μg/mL recombinant Fas-L as standard. The functional activity of sFas-L in SNs was assessed by the MTT assay using the WC8 cell line, a human Fas-transfected mouse lymphoma, whereas the parental cell line WR19L was used as Fas−control. OD indicates optical density.
Myeloma cells prime erythroblast apoptosis in cocultures
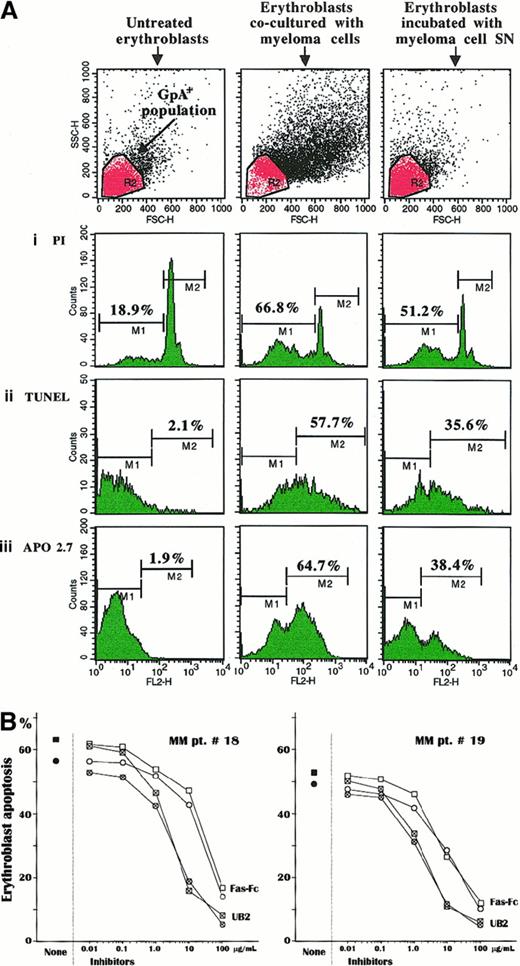
Coculturing experiments of erythroblasts with autologous myeloma cells were carried out to resemble in vivo conditions within the bone marrow and assess the susceptibility of Fas+ cultured erythroblasts to apoptosis in response to Fas-L presentation either by myeloma cells or in its secreted form in culture medium. Therefore, the analysis investigated erythroblast apoptosis by PI staining, TUNEL, and APO2.7-reactive marker detection in 6 cultures from patients no. 2, 8, 12, and 16-18 and in that from MGUS in patient no. 2. The results for patient no. 16 are presented in Figure4A. Despite the low levels of spontaneous apoptosis in control unstimulated erythroblast cultures, as also confirmed in cells sorted by magnetic GpA microbeads, a dramatic variation of those parameters was recorded in the erythroblast population incubated with either the autologous myeloma cells or the relative SN. The extent of subdiploid cells (M1) was greatly enhanced up to more than 3 times, and there was a parallel, though lower, increase in the erythroblast suspension treated with the conditioned SN. Similarly, the experiments measuring the extent of erythroblast apoptosis by TUNEL detection (M2) as well as the expression of the mitochondrial marker (M2) showed a variable increment of both parameters in cocultured erythroblasts (Figure 4Aii,iii). This activation pattern of cell death was detected in most experiments, though with slight variations. The highest apoptogen effect (TUNEL M2 > 45%) occurred in erythroblast populations whose autologous myeloma cells were major producers of Fas-L (patients no. 16 and 17). In contrast, cultured erythroblasts from the control MGUS subject no. 2 were not clearly affected by the autologous plasma cell–conditioned SN, because no evident variations in the extent of apoptosis were detected (data not shown). A few inhibitory experiments were also completed on cell preparations from patients no. 18 and 19 and from the MGUS patient no. 3 to prove involvement of the Fas/Fas-L pathway. Both erythroblast and plasma cell populations were separately preincubated with UB2 anti-Fas and Fas-Fc construct, respectively, to prevent their Fas/Fas-L interaction. Figure 4B shows the dose effect of these inhibiting tests. We observed a progressive decline of erythroblast apoptosis by both procedures in relation to their inhibitor accretion. In both MM patients, Fas erythroblast saturation with UB2 MoAb almost completely prevented the apoptogen potential of the plasma cells by more than 1 μg/mL of antibody, whereas Fas-Fc had to be added at a nearly 10-fold higher concentration to obtain a comparable effect. As expected, very little inhibition was observed in the MGUS patient no. 3 by incubating erythroblasts with more than 10 μg/mL of UB2 (data not shown). These inhibition tests provided a clear-cut validation that apoptosis was directly activated in erythroblasts by their immediate interaction with Fas-L of myeloma cells.
Apoptotic effect exerted by Fas-L+ myeloma cells on erythroblasts.
(A) Cultured erythroblasts were measured in their spontaneous apoptosis (left panels) and after incubation with autologous myeloma cells at a 1:1 ratio (central panels) or with the relative SN (right panels). The cytofluorimetric pattern is related to the erythroblast culture of patient no. 16 and indicates a clear-cut increase of the subdiploid DNA erythroblast population (i) as well as an enlargement of both the TUNEL+ subset (ii) and the cell population expressing the mitochondrial APO2.7-reactive antigen, an early marker of apoptosis (iii). A similar, though slightly lower, effect was obtained with the myeloma cell–conditioned SN. The upper section includes the erythroblast populations gated to the cytofluorimetric analysis. (B) Inhibition experiments by PI staining (■) and TUNEL (○) measurements of the apoptogen potential of myeloma cells by preincubation of either erythroblasts or plasma cells with a nonagonist MoAb to Fas (UB2) or with the Fas-Fc construct, respectively. These inhibiting tests of erythroblast apoptosis were completed in 2 patients with advanced MM. Pretreatment with both reagents induced a progressive saturation of the binding sites of Fas on erythroblasts and, alternatively, of Fas-L on plasma cells, leading to exhaustion of the apoptosis induction through Fas/Fas-L pathway. This effect was dose-dependent and promptly induced by blocking Fas on erythroblasts, with amounts of UB2 higher than 1 μg/mL in culture medium. By contrast, saturation of Fas-L on plasma cells required a nearly 10-fold higher concentration of Fas-Fc to produce a similar effect in cultured erythroblasts from both patients.
Apoptotic effect exerted by Fas-L+ myeloma cells on erythroblasts.
(A) Cultured erythroblasts were measured in their spontaneous apoptosis (left panels) and after incubation with autologous myeloma cells at a 1:1 ratio (central panels) or with the relative SN (right panels). The cytofluorimetric pattern is related to the erythroblast culture of patient no. 16 and indicates a clear-cut increase of the subdiploid DNA erythroblast population (i) as well as an enlargement of both the TUNEL+ subset (ii) and the cell population expressing the mitochondrial APO2.7-reactive antigen, an early marker of apoptosis (iii). A similar, though slightly lower, effect was obtained with the myeloma cell–conditioned SN. The upper section includes the erythroblast populations gated to the cytofluorimetric analysis. (B) Inhibition experiments by PI staining (■) and TUNEL (○) measurements of the apoptogen potential of myeloma cells by preincubation of either erythroblasts or plasma cells with a nonagonist MoAb to Fas (UB2) or with the Fas-Fc construct, respectively. These inhibiting tests of erythroblast apoptosis were completed in 2 patients with advanced MM. Pretreatment with both reagents induced a progressive saturation of the binding sites of Fas on erythroblasts and, alternatively, of Fas-L on plasma cells, leading to exhaustion of the apoptosis induction through Fas/Fas-L pathway. This effect was dose-dependent and promptly induced by blocking Fas on erythroblasts, with amounts of UB2 higher than 1 μg/mL in culture medium. By contrast, saturation of Fas-L on plasma cells required a nearly 10-fold higher concentration of Fas-Fc to produce a similar effect in cultured erythroblasts from both patients.
Erythroblast apoptosis is activated in vivo in MM patients
A number of experiments were devoted to exploring whether erythroblasts undergo apoptosis in vivo following their putative stimulation by Fas-L+ myeloma cells. Two approaches were adopted. In 2 MM patients with severe anemia (patients no. 16 and 17) and 1 with MGUS (patient no. 5), the constitutive caspases on bone marrow fresh erythroblasts were measured by double fluorescence analysis (Figure 5A). There was a remarkable expression of CPP32 and FLICE in both MM patients. Erythroblasts from patient no. 16 showed the most significant values: 96% and 44.2% of positive cells, respectively, versus 29.1% and 20.8% for the MGUS subject (P < .02 in both analyses). However, values related to ICE in fresh erythroblasts were only moderately enhanced (19.8% vs 13.2%) whereas, as expected, the native expression of 3 caspases in control Jurkat cells was also considerably represented (ICE: 30.8%; CPP32: 80.8%; and FLICE: 67.5% of positive cells). This result pointed to a prevalent up-regulation of both constitutive proenzyme forms of CPP32 and FLICE in fresh erythroblasts from MM patients with severe anemia.
Evaluation of the apoptosis activation in bone marrow fresh erythroblasts.
(A) The cytofluorimetric analysis of the GpA+ population revealed a consistent presence of the proenzyme constitutive CPP32 and FLICE (caspases 3 and 8) in fresh erythroblasts from both MM patients with aggressive disease (no. 16 and 17) as compared with control MGUS patient no. 5, whereas ICE was only slightly represented. The measurement of these native proteases in Jurkat cells used as the control Fas+ cellular model confirmed their considerable expression in these cells. (B) Differential cytofluorimetric detection of CPP32-cleaved subunits in fresh erythroblasts from patients no. 16 and 5. The increment of both 12-kd and 89-kd (PARP) products in cells from patient no. 16 supported the previous activation of erythroblast apoptosis in vivo. A similar effect was detectable in Jurkat cells after their overnight incubation with the conditioned SN from myeloma cells of patient no. 16 containing high levels of Fas-L, as compared with untreated control cells (red). (C) Fluorescent pattern of erythroid apoptosis in bone marrow smears from patient no. 16. An erythroblast (top: PE positivity of glycophorin A) is juxtaposed (bottom) to a myeloma cell FITC+ for κ chains and is positive to the TUNEL assay (bottom) for the fluorescent condensation of the chromatin.
Evaluation of the apoptosis activation in bone marrow fresh erythroblasts.
(A) The cytofluorimetric analysis of the GpA+ population revealed a consistent presence of the proenzyme constitutive CPP32 and FLICE (caspases 3 and 8) in fresh erythroblasts from both MM patients with aggressive disease (no. 16 and 17) as compared with control MGUS patient no. 5, whereas ICE was only slightly represented. The measurement of these native proteases in Jurkat cells used as the control Fas+ cellular model confirmed their considerable expression in these cells. (B) Differential cytofluorimetric detection of CPP32-cleaved subunits in fresh erythroblasts from patients no. 16 and 5. The increment of both 12-kd and 89-kd (PARP) products in cells from patient no. 16 supported the previous activation of erythroblast apoptosis in vivo. A similar effect was detectable in Jurkat cells after their overnight incubation with the conditioned SN from myeloma cells of patient no. 16 containing high levels of Fas-L, as compared with untreated control cells (red). (C) Fluorescent pattern of erythroid apoptosis in bone marrow smears from patient no. 16. An erythroblast (top: PE positivity of glycophorin A) is juxtaposed (bottom) to a myeloma cell FITC+ for κ chains and is positive to the TUNEL assay (bottom) for the fluorescent condensation of the chromatin.
To determine the occurrence of activated CPP32 resulting in its cleaved 12-kd subunit and the 89-kd active PARP, we used the relative rabbit reagents in further flow cytometry assays. Figure 5B shows the increment of those caspase subunits (M2) in fresh erythroblasts from patient no. 16: the cleaved form was detectable in about 70% of GpA+ erythroblasts, and more than 50% of these cells also expressed the 89-kd PARP subunit. By contrast, control fresh erythroblasts from the MGUS patient showed a remarkably lower expression of the activated caspases, whereas Jurkat cells underwent prompt cleavage of both CPP32 and PARP following incubation with the Fas-L–enriched SN from plasma cell culture of patient no. 16. Therefore, the high presence of those cleaved subunits in fresh erythroblasts from patients with severe anemia supported the hypothesis that the biochemical transduction of death signals promoted by cleavage of CPP32 was operative in vivo.
The second approach included a double fluorescence TUNEL assay on bone marrow cells to demonstrate the occurrence of apoptotic erythroblasts adjacent to myeloma cells. Figure 5C illustrates the peculiar pattern occasionally detected in smears from patient no. 16. The PE-GpA+ erythroblast (Figure 5C, top) was found to be in apoptosis by the TUNEL test (Figure 5C, bottom) and adherent to a FITC-κ+ myeloma cell. Subsequent re-evaluation of samples from other stage III patients (patients no. 12, 14, and 17) revealed a frequent occurrence of TUNEL+ erythroblasts near to or in direct contact with plasma cells, in keeping with the pathogenic agonist effect of myeloma cells in enhancing erythroblast apoptosis.
Discussion
The present study focused on the overexpression of Fas-L by highly malignant plasma cells as a major pathogenetic mechanism of bone marrow invasion. Because the clinical progression of MM includes both severe anemia and skeleton infiltration by tumor masses, we postulated that Fas-L up-regulation in myeloma clones plays a role in the destruction of the erythroid matrix observed in advanced disease.
By exploring Fas/Fas-L expression in MM patients at different clinical stages, we corroborated the indications by others17 and ourselves18 of a peculiar recurrence of Fas-L+myeloma clones in patients with aggressive disease. This pattern was apparently independent of the concurrent presence of Fas, which was prevalently down-regulated in bone marrow plasma cells. Their defective expression of the membrane-bound isoform of the receptor was associated in previous molecular cloning and complementary DNA sequencing analysis18 with the presence of a splicing variant, namely Fas Exo4,6Del,36 thus explaining the relative resistance of highly malignant myeloma cells to Fas-mediated apoptosis by the prototypic agonist anti-Fas MoAb from clone CH11.18Erythroblasts from most stage III MM patients underwent prompt apoptosis when cultured in the presence of either autologous Fas-L+ myeloma cells or, to a lesser extent, their culture medium containing the soluble form of the ligand. The small differential activity of the plasma cell membrane–bound Fas-L versus the soluble form was probably associated with the presence of accessory molecules,37 including intercellular adhesion molecule-1, as recently suggested,38 although this aspect was not investigated. In addition, cytofluorimetric analysis of fresh erythroblasts showed a remarkable presence of cleaved subunits of both CPP32 and FLICE, namely the caspases enrolled in the Fas pathway, whereas only moderate activation of ICE, a predominantly TNF receptor (TNF-R)–related caspase, was detected. Further experiments aimed at exploring the occurrence of apoptosis in vivo also demonstrated fluorescent TUNEL+ erythroblasts in close contact or juxtaposed to myeloma cells in bone marrow smears. Taken together, these results emphasize that Fas-L expressed by the malignant plasma cell clones plays a primary cytotoxic role in the chronic deterioration of hematopoiesis, which leads to the severe anemia of MM.
Anemia of hematologic malignancies is commonly associated with chronic derangement of the cytokine network. Circulating levels of TNF-β are increased in MM21,39 and in other cancers,40and this suggests a direct inhibition of erythropoiesis. Animal studies exploring this effect demonstrated the suppression of CFU-E in mice,41,42 whereas recombinant TNF-β in a phase I trial in metastatic cancer patients dramatically decreased the Hb values after 4 weeks.43 Because TNF-β is related to both growth and progression of tumor mass in MM,44 45 the increased soluble titers actively concur in exhaustion of the erythroid matrix by TNF-R–mediated cell death.
Direct involvement of Fas in the inhibition of human erythroid colonies has been attributed to IFN-γ.46 Former studies documented its pathogenic role in aplastic anemia,47whereas its effect on either Fas up-regulation or caspase activation in erythroid progenitors has been recently suggested.26Therefore, serum elevations of this proinflammatory cytokine, which induces normochromic/normocytic anemia in cancer patients,48 also contribute to the progression of severe anemia in MM.21 Lastly, interleukin-6 is consistently up-regulated in MM. It acts as a major growth factor,49rescues malignant plasma cells from apoptosis,50 and is suspected to interfere with erythropoiesis, because it is largely secreted within the bone marrow.51
Data from the present study strongly support the contention that, in addition to these pathogenetic mechanisms, a deregulated Fas-L expression by malignant plasma cell clones plays a central role in the disturbance of erythropoiesis. Further confirmation of this pathogenetic mechanism of anemia in MM comes from recent studies describing the Fas/Fas-L–regulated apoptosis of erythroblasts in the control of erythropoiesis.23,28 In normal subjects, immature erythroblasts, particularly at the basophilic stage, lack Fas-L on their cell membrane and are driven to apoptosis by mature Fas-L+ erythroblasts23 owing to their high Fas sensitivity during erythroid differentiation. This negative regulatory feedback of erythropoiesis is physiologically active and paralleled by inadequate levels of EPO, which acts as a major growth factor for immature erythroid progenitors and regularly prevents their apoptosis.52
Therefore, because in MM immature Fas-L− erythroblasts are greatly up-regulated in their Fas expression by the high IFN-γ production, their proliferative control by apoptosis is pathologically amplified by the excess of Fas-L+ myeloma cells. This aspect was predominant in our stage III patients, in keeping with the high malignancy of these clones.17,18 The evidence that erythroblasts from these patients are primed to undergo apoptosis in vivo is supported by our demonstration that the native caspases CPP32 and FLICE were increased in fresh cells probably as an effect of their chronic stimulation within the bone marrow environment. This constitutive accumulation of caspases was also confirmed in Jurkat cells as the Fas+ cellular model, whose proenzyme forms were promptly cleaved by the Fas-L of myeloma cells. Although the intracellular increase and activation of these proteases, particularly caspase 8, are not necessarily related to induction of apoptosis as recently proved in proliferation of T cells stimulated through the CD3 pathway,53 their cleaved subunits including both the 12-kd and PARP products result from the formation of the death-inducing signaling complex (DISC) triggered by Fas.54 Conversely, ICE, which is prevalently associated with the TNF pathway,55 56 was only moderately expressed in erythroid cells in vivo. This finding also suggested that both DISC induction in vivo and erythroblast death could be primarily promoted by the excess of Fas-L+ tumor cells. The inhibition of erythroblast apoptosis by either the nonagonist anti-Fas MoAb or the Fas-Fc chimeric protein sustained this interpretation.
However, the susceptibility to apoptosis is apparently up-ruled only in immature erythroblasts, which exhibit a number of apoptogen receptors including the TNF-R type 1, both TRAIL receptors 1 and 2,57 and Fas.23 These erythroid progenitors are Fas-L−. Therefore, the feeble Fas-L expression on erythroblasts (Figure 1A) as well as the relative increment of basophilic cells (Table 2) in bone marrow of stage III MM patients reflect a deficiency of mature erythroblasts as the disease progresses. Depletion of mature erythroid cells may be dependent on the inability of the immature forms to progress in their maturation in the presence of the high Fas-L load from myeloma cells. Interestingly, detection of circulating sFas-L as a marker of invasive disease could be useful identifying patients with progressively exhausting erythropoiesis. We found serum elevations of Fas-L in 4 patients with stage III MM (patients no. 12, 14, 16, and 17) (data not shown) and are currently evaluating this parameter in a larger number of subjects.
Another interesting aspect of our study concerns the basic mechanism of myeloma clone expansion within the bone marrow. Progression of MM implies an increasing substitution of bone marrow by nests of malignant plasma cells, which contribute to both destruction of the erythroid matrix and the appearance or extension of osteolytic lesions. Interaction of myeloma clones with marrow stromal cells by a number of adhesion molecules boosts the replication of malignant plasma cells,58 though the extensive replacement of bone marrow suggests concurrent elimination of other cells. We believe that a major cytotoxic mechanism promoted by the Fas-L of myeloma clones drives such a devastating expansion to the detriment of the erythroid progenitors. Both colonization and progression of bone lesions could also account for a similar pathogenic mechanism of induction of apoptosis in osteoblasts, because these cells are sensitive to Fas stimulation.59 In preliminary experiments we found that primary normal osteoblast cultures underwent prompt apoptosis following their incubation with the myeloma culture SN from patient no.16. Although these data need to be expanded in additional research, they suggest that a similar mechanism could be involved in osteolytic bone resorption.
Insufficient erythropoiesis in MM patients has also been ascribed to defective EPO production, particularly in those with myeloma renal failure.60 A number of clinical trials have been carried out on the use of rHu-EPO to treat anemia of MM.19,61,62 However, these studies and our own experience63,64 show that only a few stage III patients respond and that this treatment seems more effective in less advanced stages. This unresponsiveness has been attributed inter alia to the effect of neutralizing autoantibodies to the recombinant hormone.65-67 Our present results indicate that Fas-L cytotoxicity on erythroid progenitors is an additional, and possibly primary, pathogenetic mechanism of MM-associated anemia.
The authors are indebted to Dr S. Nagata for providing the WR19L cell line and the transfected control.
Supported by a grant from the Ministry for the Universities and Scientific and Technological Research and by the National AIDS Research Project of the Italian Ministry of Health, Istituto Superiore di Sanità, Rome, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Franco Silvestris, DIMO, Department of Biomedical Sciences and Human Oncology, Section of Internal Medicine and Clinical Oncology, University of Bari, Piazza G. Cesare, 11—70124 Bari, Italy; e-mail: f.silvestris@dimo.uniba.it.