The disease-specific survival (DSS) of 151 patients with chronic graft-versus-host disease (cGVHD) was studied in an attempt to stratify patients into risk groups and to form a basis for a new grading of cGVHD. The data included the outcome and 23 variables at the diagnosis of cGVHD and at the primary treatment failure (PTF). Eighty-nine patients (58%) failed primary therapy for cGVHD. Nonrelapse mortality was 44% after a median follow-up of 7.8 years. The probability of DSS at 10 years after diagnosis of cGVHD (DSS1) and after PTF (DSS2) was 51% (95% confidence interval [CI] = 39%, 60%) and 38% (95% CI = 28%, 49%), respectively. According to multivariate analysis, extensive skin involvement (ESI) more than 50% of body surface area; hazard ratio (HR) of 7.0 (95% CI = 3.6-13.4), thrombocytopenia (TP) (< 100 000/μL; HR, 3.6; 95% CI = 1.9-6.8), and progressive-type onset (PTO) (HR, 1.7; 95% CI = 0.9-3.0) significantly influenced DSS1. These 3 factors and Karnofsky Performance Score of less than 50% at PTF were significant predictors for DSS2. The DSS1 at 10 years for patients with prognostic factor score (PFS) at diagnosis of 0 (none), less than 2 (ESI only or TP and/or PTO), 2 to 3.5 (ESI plus either TP or PTO), and more than 3.5 (all 3 factors) was 82%, 68%, 34%, and 3% (P = .05, < .001, < .001), respectively. The DSS2 at 5 years for patients with PFS at PTF of 0, 2 or less, 2 to 3.5, and more than 3.5 were 91%, 71%, 22%, and 4% (P = .2, .005, and < .001), respectively. It was concluded that these prognostic models might be useful in grouping the patients with similar outcome.
Introduction
Despite the enormous efforts to decrease the incidence and severity of acute graft-versus-host disease (aGVHD), chronic graft-versus-host disease (cGVHD) has emerged as an increasingly frequent complication of allogeneic transplantation (BMT). This is possibly because of the progressive improvement in the fraction of patients surviving peritransplant complications, increased use of mismatched and unrelated donors,1 and, recently, the use of allogeneic peripheral blood as a source of hematopoietic stem cells. Between 38% and 77% of survivors transplanted with an allogeneic peripheral blood stem cell graft stimulated with granulocyte colony-stimulating factor have developed extensive stage cGVHD.2-4 Although cGVHD is usually considered to harbor the beneficial graft-versus-leukemia effect, it appears to remain as a single major determinant of long-term outcome and quality of life following BMT.
A variety of new drugs have come to the market and are currently being tested; however, only minor progress has been made in the treatment of cGVHD. Current treatment regimens fail to improve the outcome in one-third of patients with cGVHD. Paradoxically some patients are overtreated.5 For these reasons, the prediction of the outcome with standard treatment of cGVHD would be useful. This may allow the identification of patients who are likely to benefit from reduced treatment and those who are unlikely to have a sustained response to standard treatment.
The current dichotomous system of grading cGVHD based on the outcome of 20 patients 2 decades ago,6 has been widely used for assessing the clinical severity of cGVHD after BMT. However, limitations of this classification system have become apparent. Although it is highly reproducible among the transplant centers,7 the current grading/classification system is of limited utility because it does not stratify patients for outcome. It basically divides patients into those needing treatment (extensive cGVHD) and those who do not (limited cGVHD). Unfortunately, a majority of patients experience extensive stage cGVHD, an extremely heterogeneous population. We believe that the usefulness of a grading/classification system is dependent not only on its reproducibility but also on its ability to segregate the patients into meaningful prognostic categories. Therefore, a new clinical grading/classification system is needed to classify patients based on their prognosis so patients with similar features can be grouped for study and clinical management purposes.
There are several clinical and biologic features found to have prognostic significance in previous studies, including “extensive” cGVHD6,8 (either multiorgan or extensive cutaneous involvement), Karnofsky performance status,8thrombocytopenia (< 100 K/mm3),8-10progressive-type onset of cGVHD,10,11 lichenoid histology,11 and elevated bilirubin (> 1.2 mg/dL11 or > 2.0 mg/dL12). However, no consensus about the best grouping system in cGVHD has been reached.
We initiated a new study in an attempt to construct a better prognostic model that is reproducible as well as clinically relevant. We created a database on cGVHD to develop a model for predicting cGVHD-specific survival. In this study on 151 cGVHD patients, we report a prognostic analysis of disease-specific survival. With the use of multivariate analysis, we develop a new prognostic scoring system for clinical use and form a basis for a new clinical classification/grading schema, which will allow us to identify the diversity of outcome within extensive-stage cGVHD.
Patients and methods
Patients
Between June 1979 and December 1998, cGVHD was diagnosed in 151 patients who underwent BMT at Johns Hopkins Oncology Center. Patients with equivocal diagnosis of cGVHD (n = 5), an incidental preterminal diagnosis of cGVHD (n = 2), incomplete clinical information (n = 2), and no follow-up (n = 2) were not included in this study. Patients were diagnosed and treated by the GVHD team of our center, and the diagnosis of cGVHD was confirmed histologically. Some of the patients (first 44) have been previously reported.11
Demographics, clinical, and laboratory variables analyzed
The following 23 variables documented at diagnosis and at the time of primary treatment failure (PTF) were analyzed as potential prognostic factors, including age; sex; donor/recipient sex matching; pretransplant disease status; preparative regimen; type and date of BMT; GVHD prophylaxis; prior occurrence of aGVHD (classified as none [ = 0], skin only [ = 1], or systemic involvement [ = 2]); positive cytomegalovirus status (donor and/or recipient); mode of presentation of cGVHD; histology of cGVHD; alkaline phosphatase; total bilirubin; platelet count; absolute serum immunoglobulin G level; and blood eosinophil percentage. The extent and severity of cGVHD in the most frequently involved sites such as skin, liver, mouth, and eye8 13 were assessed. Other surrogate parameters indicating disease severity, such as weight loss after the BMT, performance status measured by Karnofsky Performance Score (KPS), and the presence of infectious complications, were also evaluated. The main rationale for the evaluation at PTF was to see whether the same outcome predictors identified at diagnosis would have prognostic significance for the patients who failed primary therapy.
Coding system of the clinical variables analyzed
The most common sites of involvement were coded on a scale of 1 to 3, indicating the extent and severity of cGVHD as shown below. These codes were entered into the database for analyses.
Skin and fascia.
The extent of skin and/or fascial involvement was assessed and scaled, based on the extent of skin involved with rash and/or sclerodermatous changes described in clinic notes (code 1, no skin involvement; code 2, skin cGVHD involving 50% or less of body surface area [Rule of 9s]; code 3, skin cGVHD involving more than 50% of body surface area).
Performance status.
The KPS index was used (code 1, KPS 100% to 80% [able to carry on normal activity, no special care needed]; code 2, KPS 70% to 50% [unable to work, able to live at home, and care for most personal needs, varying amount of assistance needed]; code 3, KPS less than 50% [unable to care for self, requires institutional or hospital care or equivalent, disease may be rapidly progressing]).14
Mouth/eye involvement.
Mouth involvement was defined as clinical (if disease histologically documented elsewhere) and/or histologic documentation of oral GVHD with or without ulcers associated with symptoms and functional abnormalities. Eye involvement was defined as dry eyes (abnormal tear production on Schirmer test and/or positive Rose bengal staining).15 Code 1 was defined as no clinical evidence cGVHD (positive Schirmer test, > 5 mm/wetting). Patients with no clinically evident oral cGVHD who were diagnosed by screening biopsies were also included here. Code 2 was defined as clinically evident oral (severe dry mouth, food sensitivity, lichenoid mucosal changes and/or erythema, and/or ulcer/blisters) or ocular cGVHD (dry eyes with negative Schirmer test, ≤ 5 mm/wetting, or conjunctivitis without keratitis). Code 3 was defined as severe oral and/or ocular cGVHD, causing functional impairment or loss such as difficulty in swallowing, requirement of pain medication and/or feeding tube placement secondary to severe oral cGVHD, or decrease in visual acuity secondary to keratoconjunctivitis.
Infection (within 1 month prior to or after diagnosis of cGVHD or PTF).
Code 1 was defined as no evidence of infection requiring treatment, code 2 as nonsystemic (localized) infections, and code 3 as systemic or disseminated infections, including viral, fungal, and pneumocystis carinii pneumonias (PCP).
Weight loss.
The proportion of weight loss at the diagnosis of cGVHD and at the PTF compared to the baseline weight obtained just before BMT (code 1, no weight loss; code 2, weight loss of 10% or less of baseline; code 3, weight loss more than 10% of baseline at BMT).
Treatment of cGVHD
The treatment decision was managed by the GVHD team. Therefore, all patients were treated in a consistent manner, depending on the time of diagnosis and on the treatment for initial or recurrent disease. Only 2 patients with localized cGVHD did not receive any therapy. Patients were treated with corticosteroid-based immunosuppressive regimens according to the extent of cGVHD involvement. Seventy-four patients (49%) were treated with cyclosporine and corticosteroid, 37 (25%) were treated with corticosteroid and azathioprine, 44 (29%) received thalidomide in addition to the regimens above. Twenty-two patients (15%) received psoralen ultraviolet-A (PUVA). Topical measures were applied as indicated. All patients were given standard institutional antimicrobial prophylaxis, including oral trimethoprim-sulfamethoxazole or dapsone for PCP, penicillin for gram-positive bacteria, and nystatin or fluconazole for fungal infections during immunosuppressive therapy for cGVHD.
Study definitions
Diagnosis of cGVHD was made based on both clinical and histologic criteria of skin and other affected sites as previously described.6,16,17 All patients but 2 in our series had both clinical and histologic evidence of cGVHD. The histology of cGVHD was characterized as lichenoid or sclerodermatous, based on skin and oral biopsy results. In some cases, there were concomitant sclerodermatous and lichenoid histopathologic features. The histologic diagnosis was made with the use of established criteria.17 18
Date of diagnosis of cGVHD was determined as the time when an appropriate therapy was started for cGVHD. The modes of presentation were progressive (development from aGVHD without interval), quiescent (development after complete remission from aGVHD), and de novo (without preceding aGVHD) as previously described.6 The patients who had both acute and chronic GVHD features on serial biopsies were considered as having progressive-type cGVHD.
PTF was defined as the initiation of secondary treatment because of either recurrence of cGVHD that required reinstitution of systemic immunosuppressive therapy at full dose or clinically worsening cGVHD that required a second-line immunosuppressive regimen. Localized flares during therapy or tapering course with no major change in immunosuppressive regimen were not considered as PTF. Topical or systemic corticosteroid-based treatment was required for the standard primary therapy for cGVHD. Persistent disease status requiring only a modification of dose or addition of adjunctive therapy was not considered as PTF. Patients received various secondary therapies, including the original as well as other immunosuppressive regimens such as thalidomide, mycophenolic acid mofetil, and tacrolimus.
Statistical analyses
Two major statistical endpoints of this study were cGVHD-specific survival from the diagnosis of cGVHD (DSS1) and from the time of PTF (DSS2). Deaths because of the relapse of underlying hematologic disorders were censored at the time of relapse. Data were analyzed by using Cox regression models to identify prognostic variables.19 All analyses were performed with SAS software (SAS Institute, Cary, NC). The closing date was June 1, 2000. Survival distributions were estimated by using the method of Kaplan and Meier20 and compared by using the log-rank test.21
The construction of the prognostic model started with a univariate assessment of the prognostic effect of each factor to identify groups statistically, clinically, as well as biologically related. All numeric variables, including laboratory values, were initially coded as continuous variables. Subsequently, some of these variables were dichotomized. Cutoff points were chosen to make optimal use of information, with the conditions that smaller groups contain at least 20% of the patients. No dichotomized covariates were entered into the model unless the continuous analogue had a significant independent prognostic effect. This strategy was used to ensure that the selection of prognostic factors for the model would be independent of the choice of the various cutoff points. To retain sufficient statistical power, we fitted the model to the set of complete data without setting aside a validation sample.
For the multivariate analyses, all variables found to be either statistically significantly or marginally prognostic in univariate analyses were included in a multivariate proportional hazards regression analysis. Statistically nonsignificant effects were removed from the model in a stepwise fashion with re-estimation of parameters and significance levels at each step. Consequently, the final model contained only prognostically strong or statistically significant effects. Because of the multiple statistical tests performed in the process of model building, the overall type I error rate for the multivariate model is > .05.
After building a final multivariate model for survival, a composite prognostic factor score (PFS) was calculated for each individual. This score was taken to be a weighted average of prognostic factor values with weights determined by the estimated coefficients from the proportional hazards model (ie, the score is simply the patients' hazard relative to the most favorable level of all prognostic factors). Patients were then grouped according to the value of their calculated PFSs. Each prognostic category (low, intermediate, high, and very high) was retained in scoring systems if it had a significantly different survival (with P < .05 at log-rank test) compared to each other category of the scoring system. Survival curves for the resulting groups were then plotted.
Although the use of weighted averages is statistically more accurate, it is not so practical for patient usage. Therefore, the patients were also grouped based on the total number of prognostic factors, ranging from 0 to 3 to simplify the prognostic model for clinical use.
The same analyses were repeated in a subgroup of patients who had PTF (a second baseline population).
Results
Patient characteristics
Demographic features and BMT information were summarized in Table1. There were 89 male and 62 female patients, with a median age of 33 years (range, 4-62). One hundred forty patients (93%) received BMT from an HLA-identical sibling. Cyclosporine alone was given to 141 patients (93%) as GVHD prophylaxis. Thirty-three patients (22%) received an elutriated T-cell–depleted BMT. Onset of cGVHD was a median of 4.3 months (range, 0.9-34.2) after BMT.
Baseline clinical and laboratory characteristics at the time of diagnosis (before institution of treatment) are given in Table2. Thirty-one patients (20%) had de novo and 53 (35%) had progressive mode of presentation. Forty-four percent of patients initially presented with extensive skin involvement (skin code 3). Twenty-one percent of the study population had no skin involvement (skin code 1), and 34% had skin involvement of 50% or less of body surface area (skin code 2).
Survival data
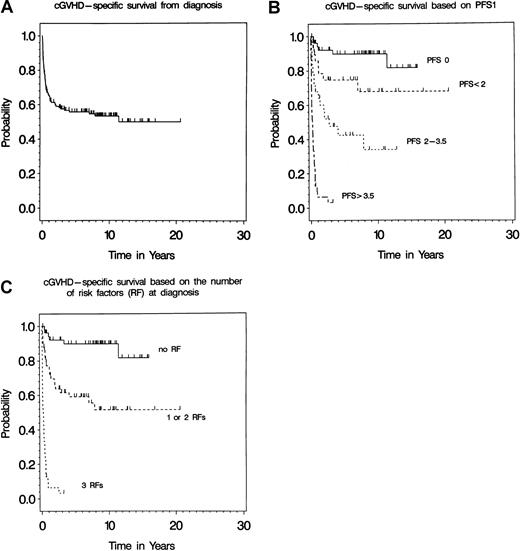
With a median follow-up of 7.8 years, the median disease (cGVHD)-specific survival (DSS) of the 151 patients was 11.4 years. As of June 1, 2000, 68 (45%) of the 151 patients were still alive at 0.2 to 20.6 years from the diagnosis. The probability of DSS at 10 years after the diagnosis of cGVHD (DSS1) was 51% (95% confidence interval [CI] = 39%, 60%) (Figure 1A). At the time of analysis, 67 patients (44%) had died due to causes other than relapse of underlying hematologic malignancies. Fifty-three (79%) of them had failed the primary therapy for cGVHD. Of these 67 deaths, 52 (78%) occurred within the first year after the diagnosis of cGVHD, 58 (87%) died from various infectious complications during the course of cGVHD, including culture-positive sepsis (n = 20), culture-negative sepsis syndrome (n = 24), aspergillosis (n = 6), cytomegalovirus pneumonia (n = 2), PCP (n = 1), and other infectious causes (n = 5). Five patients died of organ failures associated with cGVHD including lung failure (n = 4). The cause of death remained unknown in 4 patients. The latter deaths were presumed to be related to the cGVHD and, therefore, considered as an event in survival analysis. Sixteen (11%) of 151 patients died from the relapse of underlying malignancies for which the patient was transplanted. This was 19% of all deaths. None of these 16 patients had any evidence of clinically active cGVHD at the time of relapse. Thirteen (81%) patients who died of relapse had advanced hematologic disease at the time of BMT, and only 3 (19%) patients had clinically extensive features of cGVHD at the diagnosis.
Chronic GVHD-specific survival.
Probability of survival in years after the diagnosis of cGVHD in 151 allogeneic BMT patients. (A) Median follow-up of the 68 patients surviving was 7.8 years (range, 0.2-20.6) after diagnosis of cGVHD. (B) Probability of survival in years after diagnosis of cGVHD of patients grouped by their calculated PFS1. Patients were grouped as low, intermediate, high, and very high risk if their PFS1 was 0 (n = 54), less than 2 (n = 30), 2 to 3.5 (n = 28), and more than 3.5 (n = 39) at the diagnosis of cGVHD (P values = .05, < .001, and < .001), respectively. (C) Probability of survival in years after diagnosis of cGVHD of patients grouped by the total number of risk factors. Patients were grouped as low, intermediate, and high risk if they had none (n = 54), 1 or 2 (n = 58), and 3 risk factors (n = 39) present at the diagnosis of cGVHD, respectively.
Chronic GVHD-specific survival.
Probability of survival in years after the diagnosis of cGVHD in 151 allogeneic BMT patients. (A) Median follow-up of the 68 patients surviving was 7.8 years (range, 0.2-20.6) after diagnosis of cGVHD. (B) Probability of survival in years after diagnosis of cGVHD of patients grouped by their calculated PFS1. Patients were grouped as low, intermediate, high, and very high risk if their PFS1 was 0 (n = 54), less than 2 (n = 30), 2 to 3.5 (n = 28), and more than 3.5 (n = 39) at the diagnosis of cGVHD (P values = .05, < .001, and < .001), respectively. (C) Probability of survival in years after diagnosis of cGVHD of patients grouped by the total number of risk factors. Patients were grouped as low, intermediate, and high risk if they had none (n = 54), 1 or 2 (n = 58), and 3 risk factors (n = 39) present at the diagnosis of cGVHD, respectively.
A total of 89 patients failed primary therapy. The probability of DSS at 10 years after PTF (DSS2) was 38% (95% CI = 28%, 49%) (Figure2A). Twenty-six (29%) patients became refractory, not responding to any further systemic immunosuppressive therapy. Twenty of these patients died within a median of 1 month (range, 0.25-15.5) after entering in a refractory stage. The remaining 6 patients continued to survive with limited functional performance and fluctuated symptoms. Treatment of these patients with refractory cGVHD was generally palliative, including adjunctive therapies for cGVHD such as etretinate and/or hydroxychloroquine plus prophylactic antibiotics. Of the 44 patients that have been previously reported by Wingard et al,11 18 died because of cGVHD, 4 of which were recorded after the last follow-up date (December 17, 1987).
Probability of survival after PTF.
(A) The probability of survival in years in 89 allogeneic BMT patients who failed the primary therapy for cGVHD. (B) The probability survival in years after PTF of patients grouped by the calculated PFS2. Patients were grouped as low, intermediate, high, and very high risk if their PFS2 is 0 (n = 14), 2 or less (n = 21), 2 to 3.5 (n = 26), and more than 3.5 (n = 28) at the time of PTF, respectively. The separation between the curves were statistically significant withP values of .005 and < .001, except for the difference between the first (PFS2 = 0) and the second (PFS2 ≤ 2) curve (P = .2). (C) The probability of survival in years after PTF of patients grouped by the total number of risk factors. Patients were grouped as low, intermediate, and high risk if they had none or 1 (n = 34), 2 or 3 (n = 31), and 4 risk factors (n = 24) present at the time of PTF, respectively.
Probability of survival after PTF.
(A) The probability of survival in years in 89 allogeneic BMT patients who failed the primary therapy for cGVHD. (B) The probability survival in years after PTF of patients grouped by the calculated PFS2. Patients were grouped as low, intermediate, high, and very high risk if their PFS2 is 0 (n = 14), 2 or less (n = 21), 2 to 3.5 (n = 26), and more than 3.5 (n = 28) at the time of PTF, respectively. The separation between the curves were statistically significant withP values of .005 and < .001, except for the difference between the first (PFS2 = 0) and the second (PFS2 ≤ 2) curve (P = .2). (C) The probability of survival in years after PTF of patients grouped by the total number of risk factors. Patients were grouped as low, intermediate, and high risk if they had none or 1 (n = 34), 2 or 3 (n = 31), and 4 risk factors (n = 24) present at the time of PTF, respectively.
Baseline prognostic factors for cGVHD-specific survival
Multivariate analysis demonstrated that extensive skin involvement (hazard ratio [HR], 7.0; 95% CI = 3.6-13.4], thrombocytopenia (< 100 K/μL; HR, 3.6; 95% CI = 1.9-6.8), and progressive-type onset (HR, 1.7; 95% CI = 0.9-3.0) at the diagnosis of cGVHD significantly influenced DSS1 (Table 3). The effect of the presence of any combination of factors at diagnosis and at PTF was examined by development of PFSs (PFS1 and PFS2, respectively). The PFS1 for any given patient was derived by adding together the products of the coefficient of each of the 3 significant factors at diagnosis and multiplying by the value of the factor (0 if absent, 1 if present). Extensive skin involvement had the greatest effect, and thrombocytopenia and progressive-type onset had relatively moderate effects. The probability of DSS1 at 10 years for 54 patients with none of these factors (PFS1 of 0) was 82% (95% CI = 57%, 93%). Thirty patients with PFS1 < 2 (having extensive skin involvement only or thrombocytopenia and/or progressive-type onset) and 28 with PFS1 between 2 and 3.5 (presence of extensive skin involvement plus either thrombocytopenia or progressive-type onset) had 68% (95% CI = 44%, 83%) and 34% (95% CI = 14%, 55%) probability of DSS1 at 10 years, respectively. The probability of DSS1 at 6 months for 39 patients with PFS1 more than 3.5 (presence of all 3 poor prognostic factors) was only 3% (95% CI = 0.3%, 13%).
Figure 1B shows that the prognostic scoring system predicts the median DSS1, ranging from not being reached for a PFS1 of less than 2 to 28 months for a PFS1 between 2 and 3.5. The median DSS1 was 2.5 months for a PFS1 of more than 3.5. The curves for these scores are almost equally spaced, and the difference between the curves measured by the comparison of each group to the reference group (PFS1 of 0) were all statistically significant (P = .05,P < .001, P < .001, respectively).
Considering only the number of prognostic risk factors (RFs) rather than the calculated prognostic scores, 3 groups with significantly different survival outcomes were identified with 82% (95% CI = 57%, 93%) and 52% (95% CI = 35%, 65%) and 0% probability of DSS1 at 10 years for low (no RF; n = 54), intermediate (1 or 2 RFs; n = 58), and high risk (all 3 RFs; n = 39), respectively (Figure 1C). The probability of DSS1 at 6 months for the high-risk group was only 3% (95% CI = 0.3%, 13%).
Prognostic factors for nonrelapse mortality at PTF
The probability of survival after PTF is shown in Figure 2A. The probability of DSS at 10 years after PTF (DSS2) was 38% (95% CI = 28%, 49%). Multivariate analysis demonstrated that 4 factors at the PTF were independent predictors for nonrelapse mortality; extensive skin involvement (HR, 5.0; 95% CI = 1.5-17.0), thrombocytopenia (HR, 2.3; 95% CI = 1.1-4.6), progressive-type onset (HR, 2.4; 95% CI = 1.3-4.6), and KPS less than 50% (HR, 3.4; 95% CI = 1.7-6.7) at PTF significantly influenced DSS2 (Table4).
The probability of DSS2 at 5 years for 14 patients with none of these factors (PFS2 of 0) was 91% (95% CI = 51%, 98%). Twenty-one patients with PFS2 of 2 or less, 26 with PFS2 between 2 and 3.5 had 71% (95% CI = 46%, 86%) and 22% (95% CI = 7%, 41%) probability of DSS2 at 5 years, respectively. The probability of DSS2 at 6 months for 28 patients with PFS2 of more than 3.5 was only 4% (95% CI = 0.3%, 16%). Figure 2B shows that the PFS2 predicts the median DSS2 ranging from not being reached (for a PFS2 of ≤ 2) to 7.5 months (for a PFS2 between 2 and 3.5) and 0.7 month (for a PFS2 of > 3.5). The curves for these scores are also spaced nicely, and the difference between these curves and the lowest risk group (PFS2 = 0) were statistically significant, except for the difference between the first (PFS2 = 0) and the second (PFS2 ≤ 2) curve with P values of .005, < .001, and .2, respectively.
On the basis of the total number of prognostic risk factors at PTF, 3 groups were identified with 81% (95% CI = 62%, 91%) and 25% (95% CI = 10%, 42%) and 0% probability of DSS2 at 5 years for low (≤ 1 RF; n = 34), intermediate (2 or 3 RFs; n = 31), and high risk (all 4 RFs; n = 24), respectively (Figure 2C). The probability of DSS2 at 6 months for the high-risk group was 4% (95% CI = 0.3%, 18%).
Discussion
Clinical grading or classification of cGVHD has long been used not only as a tool for retrospective evaluation after transplantation but also for making decisions concerning the need and type for treatment. Although treatment protocols often specify extensive stage as an indication for treatment, clinical management decisions actually entail evaluation of many other factors in addition to overall extent of disease. For example, the need for treatment is often urgent when patients evolve into severe progressive cGVHD or when manifestations of cGVHD show rapid progression from day to day. Therefore, a new classification/grading system that will allow us to identify the diversity of outcome within extensive stage cGVHD is needed.
Several prognostic parameters have been studied in cGVHD. Extensive cGVHD,6,8 Karnofsky performance status less than 70%,8 and thrombocytopenia9 have been reported in different studies to be important determinants for survival. In addition, our group previously reported that patients with progressive presentation (aGVHD evolving into cGVHD without resolution of aGVHD), lichenoid histology, and elevated bilirubin (> 1.2 mg/100 mL) had a worse outcome than patients who had none of these clinical features.11 The University of Nebraska12recently analyzed their data for the risk factors for the cGVHD onset and survival. Specific organ involvement, stage (limited or extensive), and therapeutic response did not predict survival after the onset of cGVHD. However, no consensus for a new prognostic staging/grading system has been reached. This is possibly because of the heterogeneity in these findings.
The spectrum of abnormalities in cGVHD appears to be changing as a result of earlier diagnosis and institution of immunosuppressive therapy. Ocular, esophageal, pulmonary, and serosal involvement have occurred less frequently in recent years. Weight loss and contractures have also been reduced.22 Also, the criterion for the diagnosis of cGVHD can vary from one institution to another. Patients may be overdiagnosed and overtreated, based on the clinical manifestations without requirement of histopathologic confirmation. Of 123 patients who have been referred to our center for a comprehensive evaluation of their GVHD, 9 patients (7%) had no clinical evidence of having had cGVHD, and 26 patients (21%) had no evidence of ongoing activity. Therefore, it is extremely important to establish a diagnosis and activity of cGVHD before beginning treatment.5
In the present study of 151 patients, the largest cohort to date in cGVHD, we identified 3 independent prognostic factors predictive of nonrelapse mortality in cGVHD. Univariate analysis confirmed the prognostic value of most previously reported factors, especially progressive-type onset and thrombocytopenia. The extent of skin involvement was the most important prognostic factor at diagnosis as well as at the time of PTF. To our knowledge, this is the first time that the extent of skin involvement has been shown to be highly significant for cGVHD-specific survival in multivariate analyses; extensive skin involvement (> 50% of body surface area) was associated with short survival. This finding may simply reflect the magnitude of GVHD reaction, such as the activation of more autoreactive T cells, associated with increased cytokine release that might result in more extensive skin involvement.
In our study, thrombocytopenia was another significant predictor of poor prognosis in patients with cGVHD. Although it is subject to speculation, multiple mechanisms may have a role in the poor graft function observed in some patients with advanced GVHD. Severe infections seen during the course of cGVHD can cause bone marrow suppression and/or increased consumption of blood elements. Also, autoimmune-like thrombocytopenia and anemia have been described in patients with GVHD.23 It is possible that thrombocytopenia came out as one of the poor prognostic indicators of cGVHD as a result of marrow toxicity caused by severe infections seen during the course of severe progressive cGVHD and/or by cGVHD itself. Sullivan and colleagues previously reported that thrombocytopenia was a poor prognostic marker in patients treated with prednisone alone.9 The actuarial survival was 61% in patients without thrombocytopenia and 26% in those with thrombocytopenia because of increased infectious mortality in the latter group. It was also felt that hypomegakaryocytic thrombocytopenia was a marker for more severe cGVHD, often with progressive-type onset in patients with prior aGVHD.9 Together with steroid refractory progressive cGVHD, thrombocytopenia fewer than 100 K/μL was also reported by others as adverse outcome factors among unrelated BMT recipients with cGVHD.24 Finally, we have recently observed that thrombocytopenia post-BMT is a poor prognostic factor regardless of the GVHD status.25
The poor outcome associated with progressive-type onset is probably because most of those patients had both acute and chronic GVHD, requiring very intensive immunosuppressive therapy and developed serious infections leading to death. Development of cGVHD while on intensive immunosuppression for aGVHD indicates resistance by definition because it develops through treatment. Among patients with cGVHD, first infection and first septicemia/bacteremia were more common and had an earlier onset in individuals with progressive-type onset.26 We did not observe a significant difference in the outcome between de novo and quiescent cGVHD. This raises the question of whether or not de novo and quiescent cGVHD are separate entities.
Approximately 2 decades ago, KPS (< 70%) had been reported to be the strongest prognostic factor in cGVHD based on the observation on 52 patients treated with various combinations of immunosuppressives.8 We also observed that very poor performance status (KPS < 50%) was an independent significant factor at PTF but not at the diagnosis of cGVHD. This is most likely because there were only a few patients (6.6%) with KPS less than 50% at initial diagnosis as opposed to 41% of patients at PTF. Similarly, the presence of infection at both diagnosis and PTF was a significant factor in univariate analyses but not in multivariate analyses possibly because a relatively small number of patients presented with a systemic infection at diagnosis. However, we observed that the presence of systemic infection at diagnosis was an important clinical feature of cGVHD because it was associated with PTF.
Age, donor/recipient sex, and histology of cGVHD had no prognostic value. More interestingly, weight loss was also not found to have a prognostic significance in our study as opposed to other preliminary observations (S. Lee, personal communication, April 1999). We suspected that the possible adverse effect of weight loss on survival might have been masked by another strong poor prognostic factor such as progressive-type onset. We noted that progressive-type cGVHD was almost always associated with weight gain or lack of significant weight loss. This is possibly because the requirement of prolonged administration of corticosteroid and intravenous fluids during the course of acute and chronic GVHD. To test this hypothesis the multivariate analysis excluding the patients who had progressive cGVHD was performed. There was no interaction between weight loss and the progressive onset, ie, the effect of weight loss was not different in the 2 groups (progressive/not progressive).
As noted above, infection is a common complication and the most frequent cause of death in patients with cGVHD.9 In a study, 52% of patients with cGVHD died, the majority of infection.27 Despite that the graft-versus-leukemia effect is associated with the development of cGVHD, the relapse of underlying hematologic malignancies is another cause of death in this population. We observed that approximately 20% of all deaths in our series was due to relapse. Therefore, one of the possible explanations of somewhat different findings between the current study and the study reported previously by Wingard et al11 could be the definition of event in the statistical analysis. In that particular study on 85 patients, 10% of deaths were due to the relapse of underlying hematologic malignancies, and these were not censored in the survival analyses. Whereas we did not consider the deaths purely due to relapse as an event in the current study because of the inverse relation between the extent of cGVHD and disease relapse we observed. Therefore, we thought that cGVHD-specific survival (or nonrelapse mortality) would be a more objective and reliable parameter for assessing the outcome after cGVHD. In fact, these 16 patients who relapsed and died of underlying malignancy were more likely to have a milder form of cGVHD than those who did not relapse but died of cGVHD.
We have not grouped the patients according to treatment to assess this factor on the outcome because our previous report indicated that there was no survival difference between patients given different immunosuppressive regimens.11 Second, the patients also received various combinations of second- and third-line immunosuppressive therapies that make the groups more heterogeneous and the comparison more difficult. Third, there is also no evidence that any particular immunosuppressive treatment regimen is superior to another in terms of prolonging survival. Cyclosporine and prednisone are being used by many centers as the current standard treatment of extensive cGVHD based on the single phase II study28 that included the patients with adverse prognosis. This combination therapy was found to be associated with a higher response rate and improved survival when compared historically with the other high-risk patients who were treated with alternate-day prednisone without cyclosporine.9 However, a well-designed prospective randomized trial demonstrating the definite advantage of a particular treatment regimen in cGVHD is lacking possibly because of the absence of a reliable grading/classification system grouping the patients with similar outcome. As discussed by Wingard et al in a previous report,11 we believe that baseline factors are more important than the type of treatment in predicting outcome because none of the treatment arms in high-risk group patients (those with thrombocytopenia and/or extensive cGVHD) have been able to control the cGVHD.
We also evaluated the significance of the same variables at the time of PTF because these variables may have been as important determinants as the baseline prognostic factors. The majority of patients had PTF as defined in this report. As studied previously in aGVHD,29the prognostic factors at the failure of primary therapy can provide important clinical information about the patients who are referred for the management plan or for enrollment into a particular clinical study. Furthermore, a back-up prognostic model would be particularly important if the initial diagnostic information is missing for any reason.
This prognostic model/system depends on assessments of the most common sites of involvement and certain laboratory parameters. Therefore, it does not incorporate the information about the rare types of presentation of cGVHD such as bronchiolitis obliterans, neuromuscular involvement, or intestinal involvement. Nonetheless, the clinical parameters included in this model are able to cover these rare presentations of cGVHD by examining their overlapped features like performance status.
One could argue that the infrequency of patients who underwent matched-unrelated BMT, allogeneic blood cell transplant, or haploidentical BMT in this data set can affect the results. This might be true in the sense that more severe cGVHD is associated with those types of transplants, and, consequently, the course of cGVHD could be more aggressive. Although general mortality is high in unrelated donor transplant, this is probably not due to the increased risk of infections associated with cGVHD. In a previous study,30the incidence of infections was not found to be different in unrelated donor recipients with or without advanced GVHD.
Now the question is how to create a simple clinical grading system by using this extensive information. Several approaches could be developed to construct a new grading schema in cGVHD. A simplified version of the prognostic scoring system based on the total number of risk factors could be an alternative way of grouping the patients with similar prognostic features. Further testing and validation of these prognostic models in another cohort is necessary before any of these proposals can be considered for adoption as a standard. Measurements of morbidity and time to discontinuation of immunosuppressive treatment are the other potential endpoints to study.
In conclusion, our study shows that the combination of significant clinical and laboratory variables at diagnosis and at PTF (extensive skin involvement, thrombocytopenia, progressive-type onset, and KPS < 50%) provide a useful and clinically meaningful prognostic model for DSS in cGVHD. We observed that none of the morbidity parameters other than the extent of skin involvement remained significant in the multivariate models. These results indicate that those parameters correlate with progressive onset and extensive skin disease.
The optimal treatment of cGVHD is still controversial. Therefore, this new prognostic model derived from a large, broadly representative set of data could be useful to identify patients with limited life expectancy because of cGVHD, for whom new therapeutic approaches might be investigated on an objective basis. To improve comparability between publications, reports of cGVHD treatment trials should include an accurate description of the selection criteria used, based on this prognostic model. Confirmation of the validity of this prognostic model in an independent population is essential for further clinical application. This study is under way.
We are indebted to John R. Wingard, MD, for his pioneering work in this field, and for his advice, support, encouragement, and critical review of the manuscript.
Supported in part by grant CEL-151 from Roche Inc, by an unrestricted research grant from Supergen Inc, and by an unrestricted research grant from Sangstat Inc.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Görgün Akpek, Johns Hopkins Oncology Center, The Bunting and Blaustein Cancer Research Building, Floor 2M, 1650 Orleans Street, Baltimore, MD 21231; e-mail: gakpek@jhmi.edu.