Thymic-deficient hosts rely primarily on antigen-driven expansion to restore the peripheral T-cell compartment following T-cell depletion (TCD). The degree to which this thymic-independent pathway can restore immune competence remains poorly understood but has important implications for a number of clinical conditions including stem cell transplantation and human immunodeficiency virus (HIV) infection. A model of HY-mediated skin graft rejection by athymic, TCD mice was used to show that restoration of naive and recall responses via peripheral expansion requires transfer of only 25 × 106 lymph node (LN) cells representing approximately 10% of the T-cell repertoire. Consitutive expression of bcl-2 in the expanding inocula restored recall responses to HY at a substantially lower LN cell dose (1 × 106), which is normally insufficient to induce HY-mediated graft rejection in athymic hosts. Interestingly, bcl-2 had no effect on primary responses. Interleukin-7 (IL-7) potently enhanced thymic-independent peripheral expansion and led to HY graft rejection using an LN cell dose of 1 × 106 in both primary and recall models. The restoration of immune competence by IL-7 appeared to be mediated through a combination of programmed cell death inhibition, improved costimulation, and modulation of antigen-presenting cell (APC) function. These results show that immune competence for even stringent antigens such as HY can be restored in the absence of thymic function and identify IL-7 as a potent modulator of thymic-independent T-cell regeneration.
Introduction
Acute depletion of the T-cell compartment occurs in a number of clinical situations including stem cell transplantation, human immunodeficiency virus (HIV) infection, and after intensive cytotoxic therapy for cancer. Restoration of immune competence following T-cell depletion (TCD) requires the regeneration of a functionally intact, diverse T-cell pool that is capable of responding to foreign antigens and, potentially, altered self-antigens expressed by tumors. Previous studies have shown that there are 2 main pathways capable of substantial peripheral T-cell regeneration: thymic-dependent regeneration from bone marrow progenitors and thymic-independent peripheral expansion of mature T-cell populations.1 The relative contribution of these 2 pathways is dynamic and dependent on the degree of thymic function. Current concepts hold that the capacity of the host to restore immune competence depends primarily on the extent to which thymic pathways contribute to T-cell regeneration. However, due to disease, therapy-related toxicity, and age-related changes, thymic function is frequently limiting, resulting in a relative reliance on thymic-independent peripheral expansion in many clinical situations associated with TCD.2-8 The degree to which the peripheral expansion of mature T-cell populations can restore host immune competence following TCD remains poorly understood and is the focus of this report.
Peripheral expansion is predicted to give rise to limited immune competence for a variety of reasons. First, although dramatic expansions in cell number can occur,9-12 hosts reconstituted in this manner display chronically reduced T-cell numbers.10,13 Second, because the expansion of peripheral T cells via this process proceeds from limited numbers of cells and is heavily influenced by interactions with cognate antigen,14the regenerated repertoire is susceptible to dramatic skewing15 and shows limited T-cell receptor diversity.16-19 Third, the process of peripheral expansion leads to an accumulation of T cells that display a highly activated phenotype.13,20 Continuous or repeated exposure of these activated T cells to antigens that contribute to the process of expansion could also provide a signal for programmed cell death.21,22 This is suggested by studies in patients with TCD due to HIV infection showing increased susceptibility of T cells to apoptosis with restimulation in vitro23-27 and by direct labeling studies in vivo.28-30 Although these observations could potentially relate to direct or indirect effects of HIV, similar results have been observed in T-cell–depleted hosts after intensive chemotherapeutic regimens20 and after stem cell transplantation,31 suggesting that increased susceptibility to cell death is a common phenomenon in regenerating T-cell populations. Thus, a propensity for programmed cell death in peripherally expanding T-cell populations may also limit the capacity of peripheral expansion to restore host immune competence.
Interleukin-7 (IL-7) exerts potent effects on T- and B-cell progenitors and is absolutely required for early T-cell development.32-34 Because T-cell development can be largely restored in IL-7 knockout mice by the concurrent expression of a bcl-2 transgene,35,36 current models suggest that IL-7 acts primarily by inhibiting programmed cell death of immature thymocytes during development. Indeed, the administration of IL-7 after cyclophosphamide or bone marrow transplantation in mice leads to enhanced T-cell reconstitution, presumably via enhanced thymopoiesis.37-41
Recent studies have shown that IL-7 is also capable of rescuing mature T cells from apoptosis induced by glucocorticoids, cytokine withdrawal, and radiation.42-47 These effects are correlated with alterations in intracellular levels of bcl-2, bcl-xL, and caspase 3 activity, suggesting modulation of intracellular pathways of programmed cell death. In this report, we sought to examine the capacity of peripheral expansion to restore immune competence following TCD and to investigate the role of programmed cell death in limiting immune competence following reconstitution by peripheral expansion. Our results show that programmed cell death plays an important role in limiting immune competence in thymic-deficient hosts undergoing T-cell regeneration and, in addition, we have identified IL-7 as a cytokine that dramatically enhances immune competence in thymic-deficient hosts reconstituted via this pathway.
Materials and methods
Mice and thymectomies
C57BL/6 (B6/Thy-1.2, Ly-5.1), B6 PL Thy-1a (B6/Thy 1.1), and B6/Ly-5.2 mice were purchased from the Animal Production Unit, National Cancer Institute (NCI; Frederick, MD) and housed in a specific pathogen-free environment at the National Institutes of Health (NIH). At 4 to 6 weeks of age, mice were anesthetized and suction thymectomy was performed through a sternal incision according to approved protocol. Completeness of the thymectomies was confirmed by visual inspection at the completion of the experiments. C57BL/6-TgN(BCL2)36Wehi mice48 were originally purchased from Jackson Laboratories (Bar Harbor, ME) and bred at the National Institutes of Health. Mice were screened for human bcl-2 (hbcl-2) transgene expression at 8 weeks of age. A small aliquot of blood was obtained from the tail followed by separation of mononuclear cells over lymphocyte separation media (Biowhitaker, Walkersville, MD). The presence of hbcl-2 protein was determined by flow cytometry using intracellular labeling with a human-specific, monoclonal antibody (clone 6C8, Pharmingen, San Diego, CA) and detected with goat antihamster IgG fluorescein isothiocyanate (FITC; Southern Biotechnology Associates, Birmingham, AL). The validation of this screening technique was confirmed by polymerase chain reaction (PCR) analysis. Transgene-negative littermates were used as control lymph node donors. C57BL/6 IL-7Rα−− mice were purchased from Jackson Laboratories and bred at the NCI-Frederick.
TCD and skin grafting
Mice were depleted of T cells using rat antimouse anti-CD4 (clone GK1.5) and anti-CD8 (clone 2.43) purchased from the Biological Resources Branch, NCI (Frederick, MD). Injections were given intraperitoneally with schedules and doses determined for individual lots by in vivo experiments to achieve more than 98% depletion of CD4 and CD8 cells in spleen and lymph node (LN) at 4 days (data not shown). After 2 weeks, to allow clearance of the monoclonal antibodies, mice were injected with LN populations as indicated for the individual experiments. Skin grafting was performed using a modification of a protocol described elsewhere49 with a 0.5-cm patch of male tail skin grafted over the thorax and covered with a pressure dressing for 7 days. After 1 week, bandages were removed and a blinded observer monitored graft rejection. Complete rejection was defined as more than 80% of the graft surface area involved. Animal care was provided in accordance with procedures outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH Publication no. 86-23, 1996) and all protocols were approved by the animal care and use committee at the NCI.
Cellinjections
Axillary and inguinal LNs were harvested from syngeneic female mice and placed in iced complete media (RPMI, with 10% heat-inactivated fetal bovine serum, penicillin, streptomycin,l-glutamine, Hepes buffer, nonessential amino acids, sodium pyruvate (all from Gibco Life Technologies, Gaithersburg, MD), and β-mercaptoethanol (Sigma, St. Louis, MO). LNs were teased apart with fine forceps, gently minced with the plunger of a syringe, and passed through a nylon mesh. The cells were washed twice in iced complete media, viable cell number was determined with trypan blue exclusion, and cells were resuspended at the appropriate concentration for injection via tail vein in RPMI without fetal calf serum at the doses described.
Male dendritic cells were purified from splenocytes following plate adherence and incubation with IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF). Briefly, male spleens were minced with the plunger of a syringe in iced complete media and passed through a nylon mesh. Red blood cells (RBCs) were lysed with ammonium hydroxide lysing buffer, washed twice in complete media, resuspended in iced Dulbecco phosphate-buffered saline (DPBS; Gibco Life Technologies) and separated over a 50% Percoll gradient by centrifugation at 1800g for 12 minutes. The cell layer was removed, washed twice in complete media, resuspended, and counted using trypan blue exclusion. Viable cells (100 × 106) were loaded onto a 150 × 25-mm tissue culture dish (Falcon 3025, Becton Dickinson, Lincoln Park, NJ), incubated at room temperature for 20 minutes followed by incubation at 37°C/5% CO2 for 2 hours. Nonadherent cells were removed by washing with 50 mL warm complete media, the media was replaced with complete media containing 1 ng/mL GM-CSF and 0.1 μg/mL IL-4 (Peprotech, Rocky Hill, NJ), and the plate was incubated overnight at 37°C/5% CO2. On day 2, the nonadherent fraction was removed by gentle pipetting, washed twice in complete media, resuspended, and counted. A fraction of the cell suspension was analyzed with flow cytometry and the remainder was injected in RPMI intraperitoneally at a dose of 1 × 105cells per mouse.
In vitro studies
Single-cell suspensions of RBC-depleted splenocytes were made as described followed by passage over a negative selection T-cell enrichment column (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The enriched fraction was washed, counted, and resuspended in complete media. The 24-well plates were coated for 18 hours with 0.1 μg/mL rat antimouse CD3 (clone 145-2C11) in DPBS or with DPBS alone. This concentration of 2C11 is insufficient to induce maximal T-cell proliferation (data not shown). T-cell–enriched splenocytes were plated at 4 × 106cells/well in 2 mL complete media. Recombinant human IL-7 (Peprotech) was added at a concentration of 10 ng/mL. At the time points indicated, wells were washed, pooled, and analyzed with flow cytometry. For proliferation studies, T-cell–enriched splenocytes were labeled with CFSE (5-[and 6]-carboxyfluorescein diacetate succinimidyl ester; Molecular Probes, Eugene, OR) according to the manufacturer's instructions. Labeled cells were incubated on a 96-well plate coated with anti-CD3 as described above.
Flow cytometric analysis
Cell suspensions were prepared in staining buffer (Hanks balanced salt solution without phenol red with 0.2% human serum albumin [Sigma] and 0.1% sodium azide [Sigma]). For intracellular labeling, cell suspensions were washed in staining buffer containing 0.03% saponin (Sigma). Prior to antibody labeling, FCγIII/II receptors were blocked with monoclonal antibody (clone 2.4G2). The monoclonal antibodies used were CD4 phycoerythrin (PE) (clone CT-CD4) and CD8a PE (clone CT-CD8a) (Caltag Laboratories, Burlingame, CA), TCR β chain PE (clone H57-597), CD45R/B220PE (clone RA3-6B2), CD11b PE (clone M1/70), CD11c FITC (clone HL3), CD45.1/Ly5.2 FITC (clone A20), CD45.2/Ly5.1 FITC (clone 104), CD54/ICAM-1 FITC (clone 3E2), CD80/B7-1 FITC (clone 1G10), CD86/B7-2 FITC (clone GL-1), CD90.1/Thy 1.1 FITC (clone HIS51), and CD90.2/Thy1.2 FITC (clone OX-7) (Pharmingen). Intracellular murine bcl-2 was detected with purified hamster antimouse bcl-2 (clone 3F11; Pharmingen) and detected with goat antihamster IgG FITC (Southern Biotechnology Associates). Appropriate isotype controls were used for all experiments. Annexin V labeling was performed in phosphate-buffered saline (PBS) containing 10 mM NaOH, 140 mM NaCl, and 2.5 mM CaCl2. Cells were then analyzed in buffer containing 2 μg/mL propidium iodide (Sigma).
Flow cytometric analysis was performed on a single laser FACScan or dual laser FACSCalibur (Becton Dickinson). Fluorescence data were collected and analyzed using CELLQuest software. Viable lymphocyte populations were gated based on forward-scatter and side-scatter characteristics.
Statisticalanalysis
Survival analysis was performed on the HY skin graft rejection experiments considering the time of complete rejection in days as the end-point. A dichotomous variable of 1 was assigned if a rejection occurred during the period of observation (100 days) and value of 0 was assigned if rejection did not occur and the event was censored. For each comparison, a log-rank test was performed and the 2-sided Pvalue is reported.
Results
Restoration of immune competence in athymic hosts reconstituted via peripheral expansion is critically dependent on the size of the expanding inocula
To assess the capability of hosts reconstituted via peripheral expansion to respond to recall antigen, thymectomized, T-cell–depleted C57BL/6 females were grafted with male tail skin and injected intravenously with 1 × 106 primed LN cells as a source of mature T cells. In the recall model, primed cells refer to LN cells taken from syngeneic female mice within 3 weeks after complete male skin graft rejection. Thymus-bearing mice reject HY-disparate skin grafts within 8 weeks. In contrast, thymectomized, T-cell–depleted mice were unable to reject HY-disparate skin grafts despite administration of primed LN cells as a source for peripheral expansion. Importantly, these mice are capable of rejecting allogeneic skin grafts within 8 to 10 days (data not shown). Therefore, the impairment in T-cell immune competence following reconstitution via thymic-independent pathways is limited to responses to antigen with a low precursor frequency suggesting that a simple limitation in the number of cells bearing antigen-specific T-cell receptor specificities may play a central role.
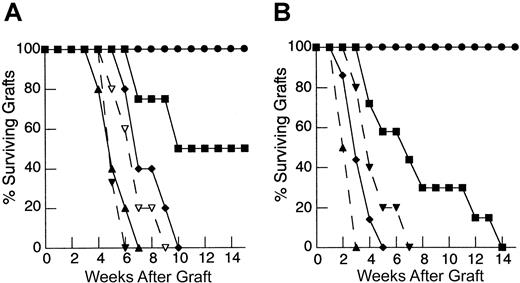
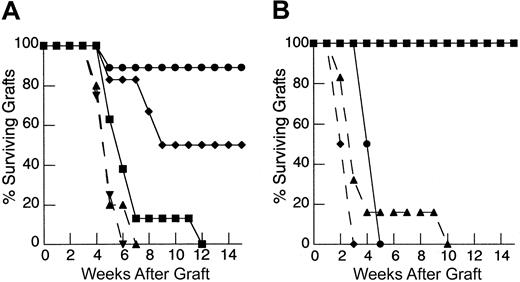
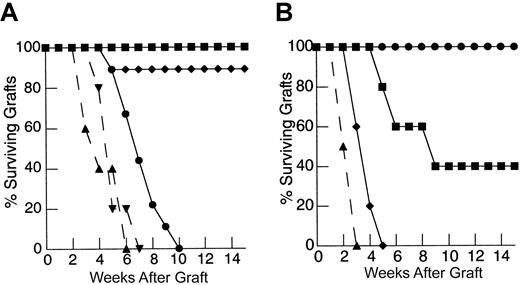
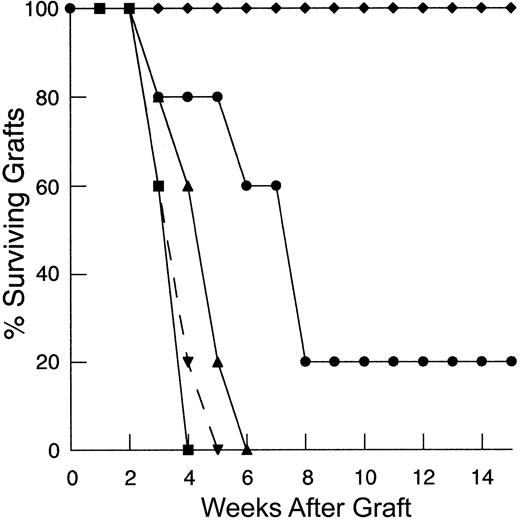
To determine the degree to which the deficiency in HY graft rejection observed following thymic-independent T-cell regeneration was related to an inadequate starting T-cell inocula, and, if so, to establish the size of the inocula required to restore responses to recall antigen, thymectomized T-cell–depleted mice were reconstituted using progressively larger primed mature LN inocula. As the dose of primed LN cells was increased, there is an increase in the rate of graft rejection (Figure 1A). Indeed, T-cell–depleted mice reconstituted from 25 × 106 primed LN cells were able to reject HY grafts at a rate analogous to that observed in control groups.
Rejection of HY-disparate skin grafts via peripherally expanded lymph node cells is critically dependent on the size of the starting inocula.
(A) Adult thymectomized, C57BL/6 females were T cell depleted and grafted with male tail skin as described in “Materials and methods.” Primed cells were collected from the draining LN of T-cell–replete syngeneic female mice 3 weeks after successful male skin graft rejection, teased into a single-cell suspension, and injected via the tail vein 24 hours after skin grafting. Unprimed cells were collected from LN of T-cell–replete females naive to male antigen. Percent surviving grafts are shown as measured by visual inspection as described in “Materials and methods.” Thymectomized, T-cell–depleted mice (TXY/TCD mice) receiving 5 × 106primed LN cells are partially able to reject HY-disparate skin grafts (▪, n = 4) when compared to mice receiving no LN inocula (●, n = 5). When the LN cell dose is increased to 10 × 106, there is rejection in all mice but the rate of graft rejection remains delayed (♦, n = 5). At a dose of 25 × 106 LN cells (▴, solid line, n = 5), graft rejection occurs at a rate analogous to thymus-bearing control animals (▾, dashed line, n = 6) also receiving primed inocula. Note the modest effect of the primed inocula when this group is compared to thymus-bearing animals receiving an unprimed inocula (▿, dashed line, n = 5). (B) To assess primary immune responses, TXY/TCD mice were given graded numbers of LN cells from syngeneic females naive to male antigen via tail vein. The mice were then sensitized by intraperitoneal injection of 1 × 105 enriched male dendritic cells from male splenocytes as described in “Materials and methods.” These cells express B7-1, B7-2, CD11c, and major histocompatibility complex (MHC) class II and represent 50% to 60% of the cells injected in all experiments. Twenty-one days after LN cell transfer and enriched male dendritic cell sensitization, the mice were grafted with male tail skin as before and observed for rejection. As with the recall responses, mice receiving 1 × 106 naive cells were unable to reject HY-disparate skin grafts (●, n = 5). Administration of 10 × 106 naive cells led to graft rejection in all of the mice but at a delayed rate (▪, n = 7). Transfer of 25 × 106 LN cells (⧫, n = 7) completely restored responses so that rejection occurred at similar rate to thymus-bearing control groups receiving enriched dendritic cells (▴, dashed line, n = 6). Thymus-bearing control groups receiving no enriched dendritic cells (▾, dashed line, n = 5) required approximately 2 weeks longer to reject HY grafts.
Rejection of HY-disparate skin grafts via peripherally expanded lymph node cells is critically dependent on the size of the starting inocula.
(A) Adult thymectomized, C57BL/6 females were T cell depleted and grafted with male tail skin as described in “Materials and methods.” Primed cells were collected from the draining LN of T-cell–replete syngeneic female mice 3 weeks after successful male skin graft rejection, teased into a single-cell suspension, and injected via the tail vein 24 hours after skin grafting. Unprimed cells were collected from LN of T-cell–replete females naive to male antigen. Percent surviving grafts are shown as measured by visual inspection as described in “Materials and methods.” Thymectomized, T-cell–depleted mice (TXY/TCD mice) receiving 5 × 106primed LN cells are partially able to reject HY-disparate skin grafts (▪, n = 4) when compared to mice receiving no LN inocula (●, n = 5). When the LN cell dose is increased to 10 × 106, there is rejection in all mice but the rate of graft rejection remains delayed (♦, n = 5). At a dose of 25 × 106 LN cells (▴, solid line, n = 5), graft rejection occurs at a rate analogous to thymus-bearing control animals (▾, dashed line, n = 6) also receiving primed inocula. Note the modest effect of the primed inocula when this group is compared to thymus-bearing animals receiving an unprimed inocula (▿, dashed line, n = 5). (B) To assess primary immune responses, TXY/TCD mice were given graded numbers of LN cells from syngeneic females naive to male antigen via tail vein. The mice were then sensitized by intraperitoneal injection of 1 × 105 enriched male dendritic cells from male splenocytes as described in “Materials and methods.” These cells express B7-1, B7-2, CD11c, and major histocompatibility complex (MHC) class II and represent 50% to 60% of the cells injected in all experiments. Twenty-one days after LN cell transfer and enriched male dendritic cell sensitization, the mice were grafted with male tail skin as before and observed for rejection. As with the recall responses, mice receiving 1 × 106 naive cells were unable to reject HY-disparate skin grafts (●, n = 5). Administration of 10 × 106 naive cells led to graft rejection in all of the mice but at a delayed rate (▪, n = 7). Transfer of 25 × 106 LN cells (⧫, n = 7) completely restored responses so that rejection occurred at similar rate to thymus-bearing control groups receiving enriched dendritic cells (▴, dashed line, n = 6). Thymus-bearing control groups receiving no enriched dendritic cells (▾, dashed line, n = 5) required approximately 2 weeks longer to reject HY grafts.
To examine the induction of primary immune responses after thymic-independent regeneration, thymectomized T-cell–depleted mice were injected with LN cells from syngeneic female mice naive to male antigen at the same time as enriched male dendritic cells as a sensitizing population. In this model sensitization must occur following transfer into T-cell–depleted hosts. In T-cell–replete mice, sensitization with enriched male dendritic cells accelerated the rate of graft rejection by approximately 2 weeks, confirming the ability of these cells to sensitize to HY antigen (Figure 1B). In contrast, as seen in the recall experiments, thymectomized T-cell–depleted mice reconstituted with 1 × 106 naive LN cells failed to reject male grafts despite sensitization with enriched male dendritic cells. Importantly, titration of the lymph node dose to 25 × 106 LN cells led to complete restoration of responses to HY antigen.
Therefore, in both the primary response and the recall model, limitations in the capacity of athymic hosts to respond to nominal antigen could be overcome by supplying increased numbers of T cells for peripheral expansion. These results confirm that the impairment in T-cell responses observed in thymic-deficient hosts is not absolute and can be induced in this setting if sufficient T-cell numbers are provided.
Inhibition of apoptosis during reconstitution from a limited T-cell inocula restores responses to recall antigen but not to naive antigen
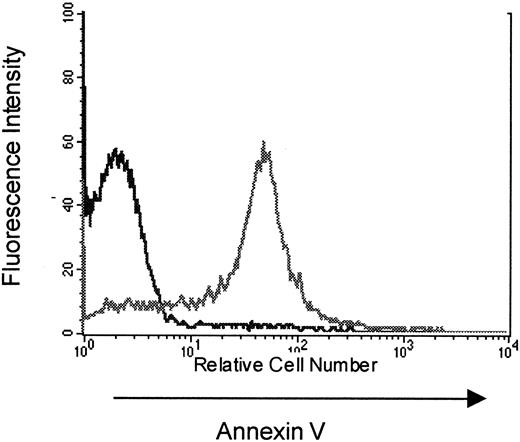
To study the mechanisms that contribute to the limitation in T-cell responses following immune reconstitution via peripheral expansion, subsequent experiments attempted to identify factors limiting peripheral expansion in hosts reconstituted from suboptimal numbers of T cells. Based on observations made in clinical settings associated with TCD,20 31 we hypothesized that increased apoptosis of peripherally expanding T cells could limit immune competence in this setting. To determine whether expanding populations are susceptible to programmed cell death in our model, T cells from thymectomized, T-cell–depleted C57BL/6 (Thy-1.2) mice reconstituted from 10 × 106 congenic B6 PL Thy-1a(Thy-1.1) LN cells were analyzed for apoptosis at various time points after transfer. When analyzed on the day of collection there was no evidence for increased T-cell apoptosis in peripherally expanding cells when compared to normal, nonexpanding populations. However, as shown in Figure2, peripherally expanding congenic T cells from thymectomized, T-cell–depleted animals collected on day 15 after transfer and restimulated with concanavalin A for 18 hours show increased annexin V staining when compared to nonexpanding controls. This provides evidence that T cells undergoing peripheral expansion in thymectomized T-cell–depleted hosts are susceptible to apoptotic cell death on restimulation and suggested that a loss of T cells to apoptosis during peripheral expansion from an insufficient inocula could potentially play a role in limiting T-cell responses in these hosts.
Increased annexin V staining of peripherally expanding T cells.
TXY/TCD C57BL/6 (Thy 1.2) mice were injected with 10 × 106 congenic LN cells from B6 PL Thy-1a females (Thy 1.1). At 5, 11, and 21 days, mice were killed for flow cytometric analysis of apoptosis in Thy 1.1+ T cells. This figure is a representative histogram showing annexin V staining on Thy 1.1+ propidium iodide–negative splenocytes (gray line) at 11 days after an 18 hours incubation with concanavalin A compared to Thy 1.2+ splenocytes from thymus-bearing, T-cell–replete controls (black line) also incubated with con A for 18 hours. In a separate experiment there was no increased annexin V staining on Thy 1.2+ splenocytes from thymectomized T-cell–replete females when compared to sham thymectomized animals treated in the same manner (data not shown).
Increased annexin V staining of peripherally expanding T cells.
TXY/TCD C57BL/6 (Thy 1.2) mice were injected with 10 × 106 congenic LN cells from B6 PL Thy-1a females (Thy 1.1). At 5, 11, and 21 days, mice were killed for flow cytometric analysis of apoptosis in Thy 1.1+ T cells. This figure is a representative histogram showing annexin V staining on Thy 1.1+ propidium iodide–negative splenocytes (gray line) at 11 days after an 18 hours incubation with concanavalin A compared to Thy 1.2+ splenocytes from thymus-bearing, T-cell–replete controls (black line) also incubated with con A for 18 hours. In a separate experiment there was no increased annexin V staining on Thy 1.2+ splenocytes from thymectomized T-cell–replete females when compared to sham thymectomized animals treated in the same manner (data not shown).
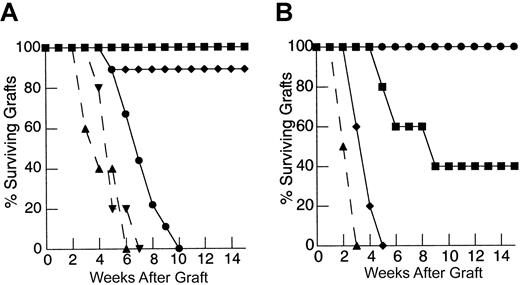
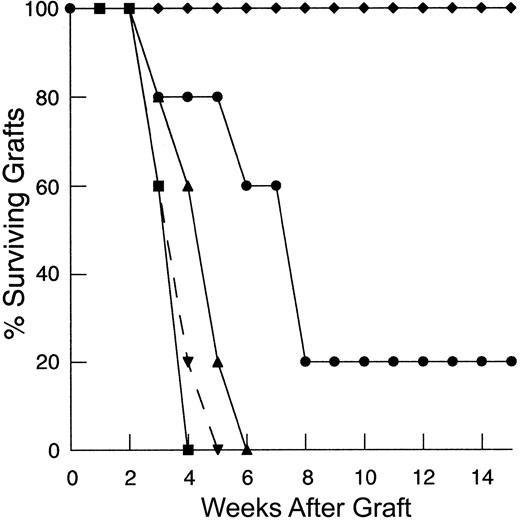
Although the finding of increased annexin V staining of restimulated, peripherally expanding T cells, along with the findings of other investigators,20,31 suggests that these cells are prone to apoptosis, it is unclear whether this process contributes to the limitation in immune responses observed in hosts reconstituted in this manner. If the loss of peripherally expanding T cells to apoptosis in our skin graft rejection model contributes to the inability to induce responses to HY antigen in animals reconstituted from a suboptimal T-cell number, we hypothesized that inhibition of apoptosis in vivo could restore HY-mediated graft rejection. Thymectomized T-cell–depleted mice were injected with 1 × 106 primed LN cells from female mice expressing an hbcl-2 transgene in lymphocytes. T cells from these animals are resistant to apoptotic cell death induced by a number of mechanisms including radiation and cytokine withdrawal.50 51 As shown in Figure3A, mice reconstituted via peripheral expansion of 1 × 106 primed, hbcl-2 transgenic LN cells were able to rapidly reject HY-disparate skin grafts, whereas mice reconstituted from the same size inocula of LN cells from nontransgenic littermate controls were unable to reject these grafts by 100 days. Therefore, inhibition of apoptosis via constitutive bcl-2 expression during peripheral expansion is sufficient to restore recall responses to nominal antigen, thereby confirming the role of apoptosis in limiting immune responses in this setting. Interestingly, no significant increase in the rate of graft rejection was observed in T-cell–replete hosts suggesting that programmed cell death is uniquely limiting in the setting of TCD.
Inhibition of programmed cell death by constitutive expression of hbcl-2 in peripherally expanding T cells restores recall responses to HY-disparate skin grafts at suboptimal inocula but fails to restore primary responses.
(A) TXY/TCD mice were reconstituted with 1 × 106 primed LN cells constitutively expressing hbcl-2 (●, n = 9) 24 hours after placement of a male tail skin graft. This resulted in restoration of HY-disparate skin graft rejection when compared to mice receiving unprimed hbcl-2 transgenic inocula (▪, n = 6) or primed cells from nontransgenic littermate controls (♦, n = 9). Primed hbcl-2 transgenic inocula did not enhance graft rejection in thymus-bearing controls (▾, dashed line, n = 5) when compared to primed inocula from nontransgenic littermate controls (▴, dashed line, n = 5,P = .49). (B) Reconstitution of TXY/TCD mice with 1 × 106 naive hbcl-2 transgenic LN cells followed by sensitization with enriched male dendritic cells does not restore rejection of HY-disparate skin grafts placed 3 weeks after cell transfers (●, n = 5). Titration of the inocula to 10 × 106 (▪, n = 5) and 25 × 106(♦, n = 5) leads to HY-disparate graft rejection at a rate comparable to thymus-bearing controls (▴, dashed line, n = 5) analogous to nontransgenic inocula (Figure 2B).
Inhibition of programmed cell death by constitutive expression of hbcl-2 in peripherally expanding T cells restores recall responses to HY-disparate skin grafts at suboptimal inocula but fails to restore primary responses.
(A) TXY/TCD mice were reconstituted with 1 × 106 primed LN cells constitutively expressing hbcl-2 (●, n = 9) 24 hours after placement of a male tail skin graft. This resulted in restoration of HY-disparate skin graft rejection when compared to mice receiving unprimed hbcl-2 transgenic inocula (▪, n = 6) or primed cells from nontransgenic littermate controls (♦, n = 9). Primed hbcl-2 transgenic inocula did not enhance graft rejection in thymus-bearing controls (▾, dashed line, n = 5) when compared to primed inocula from nontransgenic littermate controls (▴, dashed line, n = 5,P = .49). (B) Reconstitution of TXY/TCD mice with 1 × 106 naive hbcl-2 transgenic LN cells followed by sensitization with enriched male dendritic cells does not restore rejection of HY-disparate skin grafts placed 3 weeks after cell transfers (●, n = 5). Titration of the inocula to 10 × 106 (▪, n = 5) and 25 × 106(♦, n = 5) leads to HY-disparate graft rejection at a rate comparable to thymus-bearing controls (▴, dashed line, n = 5) analogous to nontransgenic inocula (Figure 2B).
Given the similar results obtained in the LN cell titration experiments between recall and primary responses detailed above, we predicted that the constitutive expression of bcl-2 transgene in peripherally expanding T cells would also lead to restoration of primary immune responses to HY. Surprisingly, however, 1 × 106 naive hbcl-2 transgenic LN cells sensitized with enriched male dendritic cells failed to restore responses to HY antigen in athymic, T-cell–depleted hosts (Figure 3B). Indeed, the same size inocula of both transgenic and nontransgenic LN cells (25 × 106) was required to fully restore HY responses in this model. These results indicate that, unlike responses to recall antigen, constitutive bcl-2 expression in T cells undergoing thymic-independent regeneration from a limited inocula is insufficient to induce primary responses to HY antigens in thymic-deficient hosts and suggests that additional factors beyond the susceptibility to programmed cell death contribute to limitations in induction of primary T-cell responses in thymic-deficient hosts.
IL-7 has diverse effects on mature T cells in vitro, and in vivo administration leads to enhanced peripheral expansion
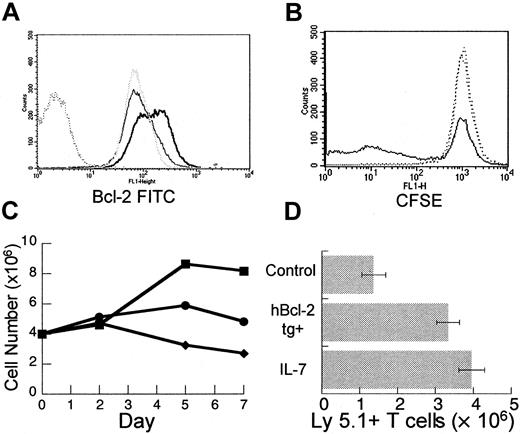
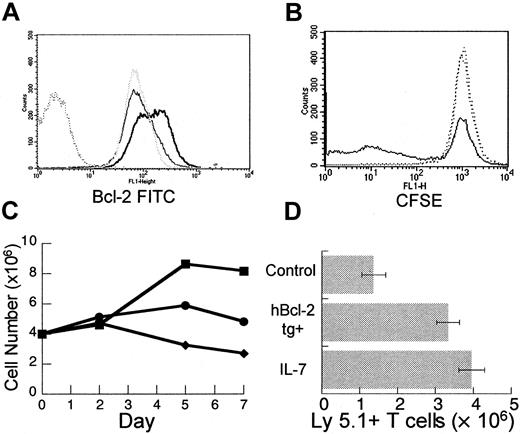
The T cells undergoing peripheral expansion display an activated phenotype regardless of activation status prior to injection into T-cell–depleted hosts.15 Therefore, modulation of T-cell activation could potentially enhance the process of peripheral expansion. To examine the effects of IL-7 on the activation of mature T cells in vitro, T-cell–enriched splenocytes were stimulated with plate-bound anti-CD3 antibody at a suboptimal concentration with and without recombinant human IL-7 (rhIL-7). By day 5, T cells incubated with rhIL-7 plus anti-CD3 displayed increased bcl-2 protein (Figure 4A) consistent with the antiapoptotic effect of this cytokine. In addition, prelabeling of T cells with CFSE indicated that rhIL-7 enhanced proliferation (Figure4B). By day 7, the number of viable T cells recovered after incubation with rhIL-7 plus anti-CD3 was significantly increased when compared to unstimulated groups or to groups incubated with anti-CD3 alone (Figure4C). Consistent with prior reports,42-46 these results indicate that IL-7 can have multiple effects on mature T cells including an antiapoptotic effect and a costimulatory effect and, therefore, may be particularly capable of modulating peripheral expansion in vivo.
IL-7 has diverse effects on mature T cells in vitro and leads to enhanced peripheral expansion in vivo.
Female C57BL/6 splenocytes were enriched for T cells using a positive selection column as described in “Materials and methods.” T-cell–enriched splenocytes were incubated in 24-well plates precoated with anti-CD3 at a suboptimal concentration of 0.1 μg/mL with or without rhIL-7 at 10 ng/mL. At days 2, 5, and 7, wells were harvested. Viable cells were counted with trypan blue exclusion and analyzed with flow cytometry. (A) By day 5 of culture, there is increased bcl-2 fluorescence intensity in cells incubated with rhIL-7 and stimulated by suboptimal anti-CD3 (heavy solid line) when compared to suboptimal αCD3 alone (gray dotted line) or media (light solid line). Isotype control shown as dark dotted line. (B) T-cell–enriched splenocytes were prelabeled with CFSE according to the manufacturer's instructions. At day 5, there is decreased CFSE intensity in a subset of T cells from wells incubated with suboptimal anti-CD3 in combination with rhIL-7 (solid line) when compared to suboptimal anti-CD3 alone or media only (dotted lines) indicating increased proliferation. Analogous results were obtained with H3 incorporation and the difference was not seen at optimal anti-CD3 stimulation (data not shown). (C) Incubation with suboptimal anti-CD3 and rhIL-7 (▪) results in increased viable T-cell number per well by days 5 and 7 compared to controls with suboptimal anti-CD3 (●) or media alone (♦). (D) TXY/TCD, C57BL/6 Ly 5.2 mice were injected intravenously with an inocula of 10 × 106 LN cells from normal or hbcl-2 transgenic donors (both Ly 5.1). One group receiving nontransgenic LN cells was treated daily with rhIL-7 at a dose of 5 μg/d intraperitoneally for 28 days. By day 28, there was a significant increase in the number of Ly 5.1+ T cells in the rhIL-7–treated group (n = 7, P = .004) or the group receiving hbcl-2 transgenic inocula (n = 7, P = .007) compared to control animals (n = 7). Data were analyzed using an unpaired t test.
IL-7 has diverse effects on mature T cells in vitro and leads to enhanced peripheral expansion in vivo.
Female C57BL/6 splenocytes were enriched for T cells using a positive selection column as described in “Materials and methods.” T-cell–enriched splenocytes were incubated in 24-well plates precoated with anti-CD3 at a suboptimal concentration of 0.1 μg/mL with or without rhIL-7 at 10 ng/mL. At days 2, 5, and 7, wells were harvested. Viable cells were counted with trypan blue exclusion and analyzed with flow cytometry. (A) By day 5 of culture, there is increased bcl-2 fluorescence intensity in cells incubated with rhIL-7 and stimulated by suboptimal anti-CD3 (heavy solid line) when compared to suboptimal αCD3 alone (gray dotted line) or media (light solid line). Isotype control shown as dark dotted line. (B) T-cell–enriched splenocytes were prelabeled with CFSE according to the manufacturer's instructions. At day 5, there is decreased CFSE intensity in a subset of T cells from wells incubated with suboptimal anti-CD3 in combination with rhIL-7 (solid line) when compared to suboptimal anti-CD3 alone or media only (dotted lines) indicating increased proliferation. Analogous results were obtained with H3 incorporation and the difference was not seen at optimal anti-CD3 stimulation (data not shown). (C) Incubation with suboptimal anti-CD3 and rhIL-7 (▪) results in increased viable T-cell number per well by days 5 and 7 compared to controls with suboptimal anti-CD3 (●) or media alone (♦). (D) TXY/TCD, C57BL/6 Ly 5.2 mice were injected intravenously with an inocula of 10 × 106 LN cells from normal or hbcl-2 transgenic donors (both Ly 5.1). One group receiving nontransgenic LN cells was treated daily with rhIL-7 at a dose of 5 μg/d intraperitoneally for 28 days. By day 28, there was a significant increase in the number of Ly 5.1+ T cells in the rhIL-7–treated group (n = 7, P = .004) or the group receiving hbcl-2 transgenic inocula (n = 7, P = .007) compared to control animals (n = 7). Data were analyzed using an unpaired t test.
To test this hypothesis, rhIL-7 was administered at a dose of 5 μg/d intraperitoneally for 28 days to thymectomized, T-cell–depleted C57Bl/6 Ly5.2 mice during peripheral expansion of 10 × 106 Ly5.1+ LN cells. In addition, in the same experiment, the potential for peripheral expansion of hbcl-2 transgenic (Ly 5.1) inocula was determined. As seen in Figure4D, in vivo administration of rhIL-7 led to a significant increase in Ly5.1+ T-cell number. A similar result was observed in mice receiving hbcl-2 transgenic inocula, further confirming the role for apoptosis in limiting peripheral expansion. Therefore, both rhIL-7 treatment and constitutive expression of bcl-2 numerically enhance the peripheral expansion of mature T cells in athymic T-cell–depleted hosts.
IL-7 treatment restores recall and primary immune responses in athymic, T-cell–depleted hosts undergoing immune reconstitution from a limited T-cell inocula
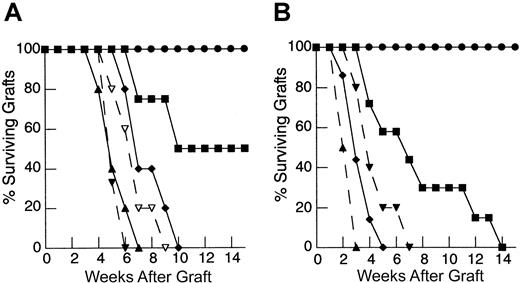
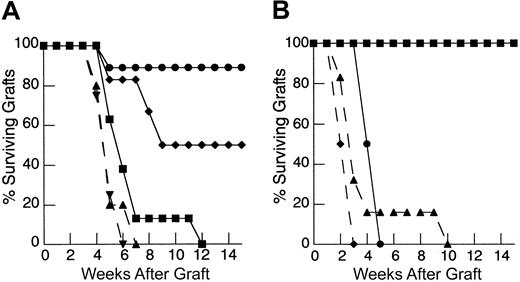
Due to the in vitro effects of IL-7 and the potent enhancement of peripheral expansion observed in vivo, we tested whether IL-7 could restore recall responses during reconstitution from suboptimal inocula. As shown in Figure5A, administration of rhIL-7 during peripheral expansion from a limited primed LN inocula (1 × 106) leads to rapid rejection of HY-disparate skin grafts. Importantly, rejection of male skin grafts in all animals required administration of rhIL-7 along with a primed LN inocula, providing evidence that the effect of IL-7 in this model was dependent on the presence of a population of mature T cells undergoing peripheral expansion. We also tested whether the ability of IL-7 to restore immune responses extended to primary responses. As with recall responses, administration of rhIL-7 also led to rapid rejection of HY-disparate skin grafts in mice reconstituted from a limited naive LN inocula and sensitized with enriched male dendritic cells (Figure 5B). The rhIL-7 appeared to have no effect on responses in T-cell–replete hosts in both recall and primary models. Therefore, IL-7 potently restores immune competence in thymic-deficient hosts undergoing T-cell reconstitution via peripheral expansion from a limited inocula. These findings contrast with the experiments using T-cell inocula constitutively expressing the bcl-2 transgene where effects were only seen with recall responses. Thus, we postulated that the ability of IL-7 to restore immune competence in this setting involves other effects on peripherally expanding T cells beyond a modulation of programmed cell death via up-regulation of bcl-2. In addition, we could not exclude the possibility that IL-7 may also enhance other cell populations critical to the generation of immune responses.
IL-7 potently restores both recall and primary responses to HY-disparate skin grafts in T-cell–depleted hosts receiving suboptimal T cell inocula.
(A) TXY/TCD mice were grafted with male tail skin and injected with 1 × 106 primed LN cells via tail vein 24 hours after grafting. Then rhIL-7 was injected intraperitoneally at a dose of 5 μg/d beginning on the day of primed cell injection and continuing for 28 days. Animals receiving rhIL-7 and 1 × 106 primed LN cells, shown in previous experiments to be a suboptimal inocula (▪, n = 8), rapidly rejected HY-disparate skin grafts at nearly the same rate as thymus-bearing animals (▴, dashed line, n = 9,P = .2) and significantly faster than animals injected with vehicle only plus primed LN cells (●, n = 9,P = .0002) or rhIL-7 without primed LN cells (♦, n = 6, P = .019). Note the lack of effect in thymus-bearing animals injected with rhIL-7 (▾, dashed line, n = 5) compared to vehicle (▴, dashed line, n = 5). (B) TXY/TCD mice were injected with 1 × 106 naive LN cells and enriched male dendritic cells. The rhIL-7 at 5 μg/d intraperitoneally was initiated on the day of cell injection and continued for 28 days. Male skin grafting was performed on day 21. Similar to recall responses, administration of rhIL-7 in the primary model led to rapid rejection of HY-disparate skin grafts in TCD mice (●, n = 8), whereas mice receiving the same inocula and injected with vehicle were completely unable to reject these grafts (▪, n = 7,P = .0002). Again, there was no difference between thymus-bearing mice injected with rhIL-7 (▴, dashed line, n = 6) or vehicle (♦, dashed line, n = 6).
IL-7 potently restores both recall and primary responses to HY-disparate skin grafts in T-cell–depleted hosts receiving suboptimal T cell inocula.
(A) TXY/TCD mice were grafted with male tail skin and injected with 1 × 106 primed LN cells via tail vein 24 hours after grafting. Then rhIL-7 was injected intraperitoneally at a dose of 5 μg/d beginning on the day of primed cell injection and continuing for 28 days. Animals receiving rhIL-7 and 1 × 106 primed LN cells, shown in previous experiments to be a suboptimal inocula (▪, n = 8), rapidly rejected HY-disparate skin grafts at nearly the same rate as thymus-bearing animals (▴, dashed line, n = 9,P = .2) and significantly faster than animals injected with vehicle only plus primed LN cells (●, n = 9,P = .0002) or rhIL-7 without primed LN cells (♦, n = 6, P = .019). Note the lack of effect in thymus-bearing animals injected with rhIL-7 (▾, dashed line, n = 5) compared to vehicle (▴, dashed line, n = 5). (B) TXY/TCD mice were injected with 1 × 106 naive LN cells and enriched male dendritic cells. The rhIL-7 at 5 μg/d intraperitoneally was initiated on the day of cell injection and continued for 28 days. Male skin grafting was performed on day 21. Similar to recall responses, administration of rhIL-7 in the primary model led to rapid rejection of HY-disparate skin grafts in TCD mice (●, n = 8), whereas mice receiving the same inocula and injected with vehicle were completely unable to reject these grafts (▪, n = 7,P = .0002). Again, there was no difference between thymus-bearing mice injected with rhIL-7 (▴, dashed line, n = 6) or vehicle (♦, dashed line, n = 6).
Sensitization with IL-7Rα−/− antigen-presenting cells partially abrogates the effect of IL-7 on naive T-cell responses
To dissect possible effects of IL-7 on antigen-presenting cells (APCs) from those on expanding T cells, mice were injected with a suboptimal naive T-cell inocula as before, along with a sensitizing population of enriched dendritic cells from IL7Rα−/−males. Thus, the peripherally expanding T cells would still respond to IL-7, and the APCs used for sensitization would be unresponsive due to a lack of a functional receptor. Although the dendritic cell yield per animal was lower from the IL-7Rα−/− mice than from IL-7Rα+/+ animals, the phenotype was identical (Figure6). Further, as seen in Figure7, IL-7Rα−/− APCs are able to induce HY graft rejection in animals receiving a sufficient LN inocula (25 × 106) at essentially the same rate as thymus-bearing animals, thus establishing functionality of these APCs. However, when an insufficient LN inocula was sensitized with enriched IL-7Rα−/− dendritic cells, administration of rhIL-7 resulted in significantly delayed rejection relative to mice receiving enriched dendritic cells capable of responding to IL-7 (P = .006). Therefore, a partial loss of the effect of IL-7 on restoration of naive responses was seen, demonstrating that the effects of IL-7 in this model are not limited to peripherally expanding T cells but also include modulation of APCs.
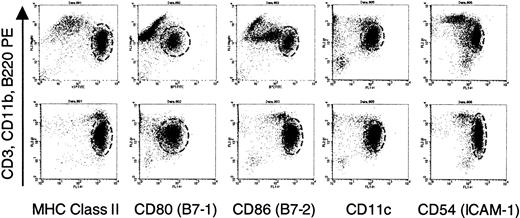
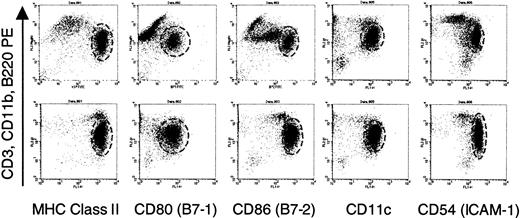
Identical phenotype of enriched dendritic cells from normal and IL-7Rα−/− males.
Dendritic cells were enriched from male splenocytes following plate adherence and incubation with IL-4 and GM-CSF as described in “Materials and methods.” Before injection, portions of the cells were analyzed by flow cytometry. Staining with FITC-conjugated antibodies expressed by dendritic cells were analyzed against a cocktail of lineage-specific antibodies conjugated to phycoerythrin (PE). The dashed circle in each graph delineates the dendritic cells. Staining for PE in the indicated cells represents intermediate labeling with anti-CD11b with no expression of B220 or CD3 (data not shown), a finding consistent with the observations of other investigators.65 The phenotype of dendritic cells from normal (top panels) and IL-7Rα−/−mice (bottom panels), as characterized by this set of markers, was identical. MHC indicates major histocompatibility complex.
Identical phenotype of enriched dendritic cells from normal and IL-7Rα−/− males.
Dendritic cells were enriched from male splenocytes following plate adherence and incubation with IL-4 and GM-CSF as described in “Materials and methods.” Before injection, portions of the cells were analyzed by flow cytometry. Staining with FITC-conjugated antibodies expressed by dendritic cells were analyzed against a cocktail of lineage-specific antibodies conjugated to phycoerythrin (PE). The dashed circle in each graph delineates the dendritic cells. Staining for PE in the indicated cells represents intermediate labeling with anti-CD11b with no expression of B220 or CD3 (data not shown), a finding consistent with the observations of other investigators.65 The phenotype of dendritic cells from normal (top panels) and IL-7Rα−/−mice (bottom panels), as characterized by this set of markers, was identical. MHC indicates major histocompatibility complex.
Lack of IL-7 signaling in APCs leads to a partial abrogation of the effect of IL-7 in restoring primary responses to HY skin grafts.
TXY/TCD mice were injected with naive LN cells and 1 × 105 enriched dendritic cells from the spleens of male IL-7Rα−/− mice or normal, IL-7Rα+/+males. The rhIL-7 at 5 μg/d or vehicle was initiated on the day of cell injections and continued for 28 days. Male skin grafting was performed on day 21. IL-7Rα−/− APCs are functionally able to induce HY graft rejection in animals receiving a sufficient T-cell inocula (25 × 106) with vehicle (▪, n = 5) at a rate analogous to thymus-bearing animals receiving enriched IL-7Rα+/+ dendritic cells (▾, n = 5). Mice receiving an insufficient LN inocula (1 × 106) with vehicle are completely unable to reject these grafts (♦, n = 6). Mice injected with a suboptimal T-cell inocula, sensitized with IL-7R−/− APCs and given rhIL-7 (●, n = 11) rejected HY-disparate skin grafts but significantly slower than mice receiving IL-7Rα+/+ dendritic cells (▴, n = 10,P = .006).
Lack of IL-7 signaling in APCs leads to a partial abrogation of the effect of IL-7 in restoring primary responses to HY skin grafts.
TXY/TCD mice were injected with naive LN cells and 1 × 105 enriched dendritic cells from the spleens of male IL-7Rα−/− mice or normal, IL-7Rα+/+males. The rhIL-7 at 5 μg/d or vehicle was initiated on the day of cell injections and continued for 28 days. Male skin grafting was performed on day 21. IL-7Rα−/− APCs are functionally able to induce HY graft rejection in animals receiving a sufficient T-cell inocula (25 × 106) with vehicle (▪, n = 5) at a rate analogous to thymus-bearing animals receiving enriched IL-7Rα+/+ dendritic cells (▾, n = 5). Mice receiving an insufficient LN inocula (1 × 106) with vehicle are completely unable to reject these grafts (♦, n = 6). Mice injected with a suboptimal T-cell inocula, sensitized with IL-7R−/− APCs and given rhIL-7 (●, n = 11) rejected HY-disparate skin grafts but significantly slower than mice receiving IL-7Rα+/+ dendritic cells (▴, n = 10,P = .006).
Discussion
Depletion of T cells is a hallmark of a number of disease processes and is an anticipated side effect of therapeutic modalities such as cancer chemotherapy and stem cell transplantation. Although much has been learned about the pathways that lead to T-cell immune reconstitution, including the substantial degree to which thymic-independent pathways can contribute to this process, it remains unclear to what extent thymic-independent pathways can restore host immune competence. In this report, we describe a model that illustrates functional deficits in immune responses to nominal antigen after thymic-independent T-cell regeneration. Using this model, we have shown that immune competence can be restored in the absence of thymic pathways by modulation of the process of peripheral expansion by increasing T-cell inocula, by inhibiting programmed cell death, and by cytokine administration.
A number of interesting findings emerge from these experiments. First, in this murine model, the number of T cells required to restore host immune competence (25 × 106 LN cells or approximately 20 × 106 T cells) is approximately equal to 10% of the total T-cell number estimated for an intact mouse.52 Although the number required would be likely to vary depending on the antigen and the precursor frequency of the antigen-specific T cells within the inocula, these results illustrate the degree of redundancy that exists within the immune system. What remains unclear is whether this number is relative and must be increased proportionally to restore immune competence in larger hosts (such as humans) or whether this represents an absolute number of T-cell receptor specificities required for response to diverse environmental antigens. Second, the finding that restoration of immune responses can occur in the absence of newly developed T-cell receptor specificities via thymic pathways emphasizes that the deficits in T-cell receptor repertoire that result from peripheral expansion are relative rather than absolute. Thus, by improving the efficiency of peripheral expansion by inhibiting cell death and, potentially, by increasing APC capacity, substantially lower numbers of antigen-specific T cells may be adequate for successful induction of immune responses. Third, the data show a clear role for programmed cell death in limiting immune responses after thymic-independent peripheral expansion. Although this is suggested by the finding of increased annexin V staining on peripherally expanded T cells manipulated ex vivo, it is more definitively shown by the increased number of T cells derived from hbcl-2 transgenic inocula and, more importantly, by restoration of immune responses to nominal antigen by constitutive bcl-2 transgene expression. Thus, peripherally expanding T cells that would otherwise be lost to apoptotic cell death are rescued and, therefore, contribute to the reconstitution of the T-cell pool. However, restoration of immune responses by inhibition of apoptosis appears to be confined to the setting of recall responses indicating important differences in the requirements for induction of responses to recall and naive antigens after thymic-independent T-cell reconstitution. This phenomenon may partially explain the restoration of recall responses and protection from opportunistic infections, which has been observed during immune reconstitution of HIV-infected patients53-56 and cannot be clearly attributed to restoration of T-cell repertoire via thymic-dependent pathways.57
The widely accepted model for T-cell death holds that there are 2 primary pathways that ultimately end in apoptosis.58,59The active process requires T-cell receptor stimulation, is mediated by Fas ligand and other tumor necrosis factor (TNF) family molecules, occurs at effective cytokine concentrations, and is inefficiently inhibited by bcl-2. The passive pathway, such as occurs with insufficient cytokine support, is strongly inhibited by bcl-2. Based on this model, our results present a potential paradox. The degree of activation of peripherally expanded T cells and the requirement for antigen would suggest that the limitations in immune responses observed in our experiments are the result of active apoptosis of peripherally expanding T cells. These models would predict that bcl-2 would be unable to restore immune responses in this setting. A potential explanation for this finding may be that there is some degree of passive cell death occurring during peripheral expansion due to competition for limiting growth factors. Alternatively, it has been reported that up-regulation of bcl-2 family members can lead to inhibition of Fas-mediated cell death in some situations.60 61 Whether inhibition of the processes involved in active cell death would have a more potent effect on immune responses in these models is currently under study.
Interleukin-7 is a cytokine with extremely diverse and potent effects on T- and B- lineage cells. It is uniquely suited to restore immune competence after TCD because of its effects on multiple pathways of T-cell regeneration. The effects of IL-7 on thymopoiesis have been well characterized. Emerging data suggest that IL-7 may also have effects on extrathymic T-cell differentiation (K. Weinberg, written communication, 2000) potentially providing a partial explanation for the restoration of responses to HY antigen observed in some animals when rhIL-7 is administered without primed T cells. In addition, IL-7 has been shown to induce proliferation of naive T cells without altering the phenotype.62 However, the effects of IL-7 on mature T-cell populations in vivo are less well characterized and the ability for this cytokine to enhance immune reconstitution via thymic-independent peripheral expansion has not been reported previously. In this report we show that IL-7 potently modulates the peripheral expansion pathway to T-cell regeneration. This action appears to be multifactorial and due to effects on expanding T cells as well as effects on APC populations. Modulation of APCs by IL-7 has not been reported and the mechanisms of this effect are currently being investigated in more detail (K. Komschlies, in preparation). Importantly, we have found IL-7 to be unique among the T-cell–active cytokines tested in inducing peripheral expansion.63 This finding raises the possibility that endogenously produced IL-7 could also play a role in regulating this process during T-cell regeneration and thus contribute to T-cell homeostasis. Indeed, it is well known that TCD leads to enhanced expansion of mature T cells and preliminary results from our laboratory (Fry et al, manuscript submitted) in patients with HIV and studies by others64 have shown elevated serum IL-7 levels in patients with TCD.
Interleukin-7 may have potential application as a biologic modifier in a number of clinical situations. First, after stem cell transplantation, prolonged lymphodepletion results in susceptibility to infection that contributes to transplant-related morbidity, especially in allogeneic transplants. Administration of IL-7 during this period would be predicted to lead to more rapid lymphocyte recovery, improved responses to foreign antigens, and a reduction in mortality from infections in the posttransplant period. Although there are clearly inherent differences between our model, which uses antibody depletion of T cells, and a stem cell transplantation model that uses cytotoxic conditioning regimens, there are many similarities including the reliance on peripheral expansion and the contribution of residual T cells to immune reconstitution. Thus, these findings are likely to be relevant to clinical transplantation. Second, although there has been success in substantially reducing viral load in patients with HIV infection, a proportion of patients fail to show complete immunologic recovery and discontinuation of antiretroviral agents results in eventual rebound in the viral load. Therefore, one of the current challenges in the treatment of HIV lies in restoring immune responses to the virus. IL-7, with its ability to restore both recall and naive responses after TCD, has the potential to be an excellent adjuvant in this setting. Whether activation of resting T cells will contribute to infection of new cells is not known. Lastly, immunotherapeutic approaches to cancer treatment will probably be most effective in the eradication of minimal residual disease remaining after standard cytotoxic therapy. However, as indicated by these findings, the TCD that results will likely provide a major barrier to the success of these approaches. Immune adjuvants such as IL-7 might vastly improve the results of cancer immunotherapy trials that are attempted in the setting of TCD.
We would like to thank Dr Scott Durum for his careful review of this manuscript.
The contents of this publication do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This project was funded in whole or part with funds from the National Cancer Institute, National Institutes of Health, under contract no. NO1-CO-56000. By acceptance of this article, the publisher or recipient acknowledges right of the U.S. Government to retain a nonexclusive, royalty-free license in and to any copyright covering the article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Terry J. Fry, Bldg 10, Rm 13N240, MSC 1928, 10 Center Dr, Bethesda, MD, 20892-1928; e-mail: tf60y@nih.gov.