A careful prognostic evaluation of patients referred for high-dose therapy (HDT) is warranted to identify those who maximally benefit from HDT as well as those who clearly fail current HDT and are candidates for more innovative treatments. In a series of 110 patients with myeloma who received HDT as first-line therapy, times to event (disease progression and death) were studied through proportional hazard models, in relation to different prognostic factors, including a chromosome 13 fluorescence in situ hybridization (FISH) analysis using a D13S319 probe. Δ13 was detected in 42 patients (38%). Follow-up time among surviving patients and survival time were 48 ± 3 and 51 ± 7 months, respectively (median ± SE). In the univariate analysis, Δ13 was the most powerful adverse prognostic factor for all times to event, especially for the survival time (P < .0001) and was followed by β2-microglobulin (β2m) levels 2.5 mg/L or higher (P = .0001). The comparison of survival prognostic models including β2m 2.5 mg/L or greater and another factor favored the Δ13/β2m combination. In 22 patients (20%) with no unfavorable factor, the median survival time was not reached at 111 months. In contrast, among 55 patients (50%) with one unfavorable factor and 33 patients (30%) with 2 unfavorable factors, median survival times were 47.3 ± 4.6 months and 25.3 ± 3.2 months, respectively (P < .0001). We conclude that Δ13, adequately detected by FISH analysis, is a very strong factor related to poor survival, especially when associated with a β2m level of 2.5 mg/L or higher. Routine FISH Δ13 assessment is strongly recommended for patients considered for HDT.
Introduction
Currently, an increasing number of patients with myeloma are referred for high-dose therapy (HDT), usually 1 or 2 courses of HDT with an autologous peripheral blood stem cell (PBSC) support.1 A careful prognostic evaluation of these patients is warranted to identify patients who maximally benefit from HDT as well as those who fail current HDT and are candidates for more innovative treatments. Up to now the best single and routinely performed prognostic factor remains serum β2microglobulin (β2m). Its powerful prognostic significance has been recognized in many studies over the last 15 years2-6 and confirmed in patients receiving HDT.7-11 Recently, abnormalities of chromosome 13 (monosomy and/or deletions, Δ13) have been associated with a short response duration and survival.12-15 In 2 studies, Δ13 had been detected using conventional cytogenetics (CC), which is hampered by the low mitotic activity of myelomatous plasma cells.12,13 Strong evidence now indicates that CC fails to recognize about one half of Δ13 as compared to fluorescence in situ hybridization (FISH), with incidences of 15% to 20% and almost 40%, respectively.12-17 Recently, 2 studies have used FISH to detect Δ13 and have demonstrated its adverse prognostic value, but these patients had received conventional chemotherapy.14 15 This study was designed to reevaluate the prognostic value of Δ13 detected by the FISH technique and to attempt to define a new prognostic staging for patients referred for HDT.
Patients, materials, and methods
Patients
The study included 110 patients with multiple myeloma diagnosed in the centers of Lille (85 patients) and Nantes (25 patients) between May 1990 and September 1998. All patients received an intensive treatment according to IFM protocols (mainly IFM 94 and 95), following 4 to 5 cycles of VAD chemotherapy (vincristine, Adriamycin, dexamethasone). The median time from diagnosis to HDT was 5.2 months. Total body irradiation was used in the conditioning regimen in 57 patients. The types of stem cells were bone marrow in 26 patients, PBSCs in 74 patients (2 patients with CD34 selection), PBSCs and bone marrow in 5 patients, and no stem cell support in 5 patients (high-dose melphalan 140 mg/m2 alone). All patients were evaluable for response. The patient and treatment characteristics are given in Table1.
Cytogenetic studies
Conventional cytogenetics and FISH were performed as previously described.16,18 19 For FISH, we used a D13S319-specific probe, purchased from Vysis (Voisin-le-Bretonneux, France) according to the manufacturer's instructions. This probe maps to the 13q14 region, in the common deleted region observed in B-cell chronic lymphocytic leukemia (B-CLL). For 44 patients, the analysis was performed on highly purified plasma cells from fresh bone marrow collected at diagnosis. For 66 patients, the analysis was performed on frozen cytospin slides of bone marrow collected at diagnosis. All samples were collected before treatment and 100 cells were analyzed in both techniques.
For the 44 patients with purified plasma cells from fresh bone marrow, the cells were fixed, after thawing, in methanol/acetic acid (3:1, v/v) for 30 minutes and air dried. After hybridization with the D13S319 probe and wash, cells were counterstained with DAPI in antifade, and examined using an epifluorescence microscope (Axioplan 2, Zeiss, Iena, Germany) equipped with a charged couple device camera and appropriate filters. According to previous studies, Δ13 was defined by the presence of only one fluorescent signal in at least 6% of plasma cells.16 However, in this series, the lowest percentage of plasma cells exhibiting only one signal was 72%.
For the 66 patients with unselected cells, but with frozen cytospin slides, we used the D13S319-specific probe in a FISH method corresponding to interphase FISH coupled to immunologic revelation of the plasma cells. Briefly, after fixation in methanol/acetic acid (v/v), slides were incubated with fluorescein isothiocyanate (FITC)–labeled polyclonal antihuman light chains (final dilution, 1:100), as previously described.19 Afterward, the probe was layered on the slides (overnight hybridization). After washes, slides were counterstained with DAPI and were examined under a fluorescence microscope (DMRXA Leica, Wetzlar, Germany) using specific filters. Bone marrow samples from 8 healthy donors (bone marrow donors) were used as controls and were studied in the same conditions as those applied for the multiple myeloma patients (with the exception of immunostaining). The number of cells demonstrating one spot never exceeded 8% and, using mean plus 2 SDs, monosomy was ascertained when over 15% cells exhibited one red signal. However, in this study, all patients had at least 30% plasma cells with one red signal.
Statistical analysis
The following initial parameters were examined for their prognostic value on survival and progression: age, sex, stage (Durie and Salmon), substage, bone marrow plasmacytosis, M-component isotype, type of light chain, creatinine, β2m, lactate dehydrogenase (LDH), C-reactive protein (CRP), hemoglobin, leukocytes, platelets, calcium, albumin serum level, CC, and Δ13 FISH analysis. The distributions of each variable were compared between patients with Δ13 and those without through chi-square method or Wilcoxon test.20 Curves for progression-free survival (PFS), proportion of patients still in remission, survival after progression, and overall survival (OS) were calculated from diagnosis as time to event (death without progression or progression, progression, death after progression, death, respectively) using the Kaplan-Meier method21 and compared using the log-rank test.22 The estimate of the relative risk (RR) of event and its 95% confidence interval (95% CI) were estimated through the proportional hazard model.23 In these prognostic analyses, continuous variables were categorized in the following way. Each variable was first divided into 3 categories at approximately the 33rd and 67th percentiles. If the relative event rates24(observed number of events divided by the expected number of events in a category, assuming no variation of event rate across categories) and the median times to event in 2 adjacent categories were not substantially different, these categories were grouped together.25 If no clear patterns were observed, the median and usual limits (as 2.5, 3, 4, and 6 mg/L for β2m) were taken as cut points. As a consequence, 2 to 3 categories were used for each continuous variable. Then, the ability of each parameter to increase predictive ability of the β2m in overall survival was tested through backward stepwise proportional hazard model23 including as prognostic factors β2m, the other tested parameter, and their interaction. Quality of the subsequent statistically significant models were classified according to the decrease in −2 log (likelihood) when compared to the model with no covariates, a decrease which is approximately distributed as a chi-square in which degrees of freedom correspond to the number of factors present in the model,26 the greater corresponding to the best fit for a fixed number of degrees of freedom. The same procedure was used to improve the prediction of the model including β2m and Δ13. No further improvements were looked for according to the number of deaths in our sample. The best prognostic models derived with 2 or 3 parameters were simplified by using the same procedure as the one described for univariate analysis leading to final models with 3 or 4 categories, only according to the number of unfavorable factors. The comparison of these final models with previously published models was performed by using Kaplan-Meier estimates of survival curves and quantified through the decrease in −2 log (likelihood), calculated on the same sample as explained above. All analyses were performed with SPSS software.27
Results
After a median follow-up time of 48 ± 3 months among surviving patients, 79 progressions and 53 deaths, all after progression except one, were observed. The median PFS time, time to progression, survival after progression, and OS time for the whole series were 25 ± 2, 25 ± 2, 12 ± 3, and 51 ± 7 months, respectively. Response to treatment was no response (< 20% decrease of the M-component) in 22 patients (20%), minor response (20%-49% decrease of the M-component) in 10 patients (9%), partial response (50%-90% decrease of the M-component) in 22 patients (20%), very good partial response (> 90% decrease of the M-component) in 33 patients (30%), and complete response (absence of the paraprotein on immunofixation and ≤ 5% plasma cells in the bone marrow) in 23 patients (21%).
Δ13 was found in 42 patients (38%). In addition to the chromosome 13 FISH analysis, 68 patients had bone marrow collected for CC. These patients consisted of the majority of patients from Lille (no CC was performed in Nantes). This cytogenetic analysis was unsuccessful in 8 patients (12%) and 60 patients had assessable metaphases. Conventional karyotype was abnormal in 29 patients and 16 of these 29 patients had Δ13 (results of CC and FISH analysis were concordant for these patients). In these latter patients, additional abnormalities besides those of chromosome 13 were investigated. The number of chromosomes was less than 46 in 7 patients, equal to 46 in 4 patients, and more than 46 in 5 patients. Structural abnormalities of chromosome 14 were frequent: add (14)(q32) to 6 patients and t(11;14)(q13;q32) to 3 patients. Deletion 6q was observed in 2 patients and deletion 16q in 2 patients (1 patient had nullosomy 16 and the other had der16 t(1;16)(p22;q11)).
A significant positive association was found between Δ13 and female sex; Δ13 was observed in 59% of female patients versus 28% of male patients (P = .008). It was also the case for Δ13 and light-chain myeloma; Δ13 was present in 64% of patients with light-chain myeloma versus 34% of patients with another isotype (P = .030). Furthermore, Δ13 myelomas tended to have a higher stage (Δ13 = 4 of 6 patients with primary plasma cell leukemia, 44% of stage III patients, and 25% of stage I/II patients;P = .054). When comparing mean levels (mean ± SE) of continuous items in patients with Δ13 versus in patients without Δ13, patients with Δ13 had a higher LDH serum level (302 ± 160 versus 255 ± 295 UI/L; P = .037), a lower hemoglobin level (10.1 ± 2.0 versus 11.3 ± 1.6 g/dL;P = .002), older age (56 ± 6 versus 52 ± 8 years;P = .030), and tended to have a higher β2m level (5.8 ± 5.1 versus 4.0 ± 2.6 mg/L; P = .092). Nevertheless, some patients had Δ13 despite low β2m; 9 (21% of patients with Δ13) and 15 patients (36%) had Δ13 and a serum β2m level below 2.5 and 3.0 mg/L, respectively.
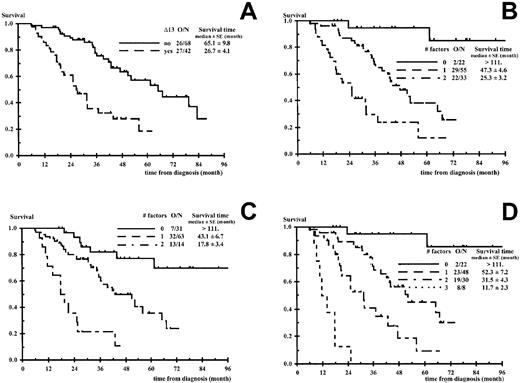
In the univariate analysis, the parameters significantly affecting PFS, survival after progression, and OS are presented in Table2. Δ13 was the most powerful adverse prognostic factor for all times to event. Median survival times for patients with and without Δ13 were 27 ± 4 and 65 ± 10 months, respectively (P < .0001). Median PFS times for patients with and without Δ13 were 17 ± 2 and 33 ± 4 months, respectively (P = .0005) (Figures1A and 2A). The response to the initial cytoreductive therapy (VAD) was not related to OS and PFS (P = .15 andP = .30, respectively) nor the type of bone marrow (fresh versus frozen) for FISH Δ13 analysis (P = .204 andP = .935, respectively). In our sample, which included 14 patients with stage I disease who received HDT according to IFM group criteria, no differences in OS and PFS could be seen between stage I and stage II patients. Concerning the 23 patients who reached a complete remission, their median complete remission duration was 28.1 ± 2.8 months (13 patients with progression). No factors reached statistical significance for complete remission duration although Δ13 (P = .094, RR = 2.5) and β2m 2.5 mg/L or greater (P = .094, RR = 3.4) had a borderline significance.
Overall survival according to the number of unfavorable factors.
(A) Chromosome 13 deletions (Δ13). (B) Δ13, β2m ≥ 2.5 mg/L. (C) β2m ≥ 2.5 mg/L, isotype IgA associated to β2m ≥ 2.5 mg/L. (D) Δ13, β2m ≥ 2.5 mg/L, isotype IgA associated to β2m ≥ 2.5 mg/L. O/N indicates number of deaths per number of patients. P < .0001 in all panels.
Overall survival according to the number of unfavorable factors.
(A) Chromosome 13 deletions (Δ13). (B) Δ13, β2m ≥ 2.5 mg/L. (C) β2m ≥ 2.5 mg/L, isotype IgA associated to β2m ≥ 2.5 mg/L. (D) Δ13, β2m ≥ 2.5 mg/L, isotype IgA associated to β2m ≥ 2.5 mg/L. O/N indicates number of deaths per number of patients. P < .0001 in all panels.
In the multivariate analysis the comparison of OS prognostic models including β2m 2.5 mg/L or greater, another factor, and their interaction is presented in Table3. In this approach, the decrease in −2 ×logarithm (likelihood) makes the comparison possible between various prognostic models through a unique criterion distributed as a chi-square. The comparison favored the combination of β2m and Δ13 (Figure 1B) preceding the combination of β2m and IgA isotype, when associated to β2m (Figure 1C). All other combinations were clearly inferior, including the β2m/CRP combination. Different cut-off values for β2m were tested for the β2m/Δ13 model (3, 3.5, 4, and 6 mg/L) and the 2.5 mg/L level was found to be the best cut-off value (data not shown). Patients with no unfavorable factor (low-risk group), β2m less than 2.5 mg/L, absence of Δ13 represented 20% of the population (22 of 110) and had a median survival time more than 111 months (2 deaths among 22 patients). Patients with one unfavorable factor (intermediate-risk group), 50% of the population (55 of 110), had a median survival time of 47.3 ± 4.6 months (29 deaths among 55 patients; RR of death 9.8; 95% CI, 2.3-42.0). These patients consisted of 46 patients with β2m 2.5 mg/L or greater and lacking Δ13 and 9 patients with β2m less than 2.5 mg/L and Δ13. At the time of this report and taking into account the low number of patients with β2m less than 2.5 mg/L and Δ13, the OS curves of these 46 and 9 patients seem comparable and similar to that of the entire group (55 patients) (data not shown). Patients with Δ13 and β2m 2.5 mg/L or greater (high-risk group) represented 30% of the whole population and had a median survival time of 25.3 ± 3.2 months (22 deaths among 33 patients; RR of death 27.9; 95% CI, 6.3-124; P < .0001). These latter patients had a median PFS time of 15.2 ± 2.0 months. The results of the β2m/Δ13 model regarding OS and PFS are shown in Figures1B and 2B. Among the subgroup of 68 patients who had bone marrow collected for CC, we compared the use of FISH and CC. Eight patients were unclassified using CC, whereas they were evaluable using FISH; 7 patients belonged to the intermediate-risk group (6 progressions, 3 deaths); and 1 patient belonged to the high-risk group (rapid progression and death). In addition, 7 patients with an apparent normal karyotype using CC had Δ13 using FISH. Three patients (3 progressions, 2 deaths) would have been classified in the intermediate-risk group using CC, whereas they belonged to the high-risk group using FISH. The 4 other patients (3 progressions, 2 deaths) switched from the low-risk to the intermediate-risk group using FISH.
Progression-free survival according to the number of unfavorable factors.
(A) Chromosome 13 deletions (Δ13). (B) Δ13, β2m ≥ 2.5 mg/L. O/N indicates number of progressions and deaths without progression, per number of patients.P = .0005 in panel A and P < .0001 in panel B.
Progression-free survival according to the number of unfavorable factors.
(A) Chromosome 13 deletions (Δ13). (B) Δ13, β2m ≥ 2.5 mg/L. O/N indicates number of progressions and deaths without progression, per number of patients.P = .0005 in panel A and P < .0001 in panel B.
Although inferior to the β2m/Δ13 results, the results of the β2m/IgA isotype model were interesting and are shown in Figure 1C regarding OS. Furthermore, the following step of the multivariate analysis demonstrated that the OS prediction of the β2m/Δ13 model was strengthened by the addition of the IgA isotype to the model, considering the 3 following parameters: β2m 2.5 mg/L or greater, Δ13, IgA isotype associated to β2m 2.5 mg/L or greater (Figure 1D). Patients without any adverse factor had a median survival time more than 111 months (2 deaths among 22 patients). Patients with 1 or 2 factors had median survival times of 52.3 ± 7.2 and 31.5 ± 4.3 months, respectively (RR of death 8.9; 95% CI, 2.1-38.7 and 23.5; 95% CI, 5.2-106, respectively). This stratification seemed particularly useful to isolate a small subgroup of 8 patients with Δ13, β2m 2.5 mg/L or greater and IgA isotype who had a very poor prognosis; these patients had a median PFS time of 7.8 ± 0.6 months (RR of death without progression or progression 16.1; 95% CI, 6.0-43.3) and a median OS time of 11.7 ± 2.3 months (8 of 8 deaths; RR of death 156; 95% CI, 30-841) (Figure 1D). We finally compared our 2 models, Δ13/β2m and β2m/Δ13/IgA, with a previously published prognostic model28 and a proposed adaptation of this model. We considered 2 β2m/CRP models: β2m 2.5 mg/L/CRP or greater 6.0 mg/L or greater and β2m 6.0 mg/L/CRP or greater 6.0 mg/L or greater. These comparisons were done on a group of 102 patients (the whole sample except nonexcreting multiple myeloma and patients with CRP missing), and models including Δ13 appeared clearly superior. The decrease in −2 log (likelihood) was 52.695 and 35.614 for the Δ13/β2m/IgA and the Δ13/β2m models, respectively, but only 21.476 for β2m 2.5 mg/L/CRP, 6.0 mg/L or greater, and 14.391 for β2m 6.0 mg/L /CRP, 6.0 mg/L or greater.
Discussion
This study emphasizes the pejorative role of FISH Δ13 on overall survival, especially in combination with a high serum β2m level. The studied sample was composed of 44 patients diagnosed between January and September 1998, representing the patients of both centers subsequently treated by HDT, patients for whom FISH Δ13 analysis was performed on fresh bone marrow. The other 66 patients were a subsample of the patients diagnosed between May 1990 and December 1997 and then treated by HDT in the Lille center (20-30 patients per year), for whom frozen cytospin slides performed at diagnosis were still available in 1998 when the laboratory in Nantes started routine FISH Δ13 analysis. Therefore, the selection bias was very unlikely. Indeed, no differences could be demonstrated between those 2 groups of patients in overall survival (P = .204) or PFS (P = .935). No differences could be demonstrated between the survival of the 2 groups among patients with a good, intermediate, or poor prognosis (defined by zero, one, or 2 adverse prognostic factors, β2m and Δ13). Similarly, differences between the 2 subsamples were limited to the date of diagnosis, age (mean 3 years higher in patients diagnosed in 1998), bone marrow plasmacytosis, and albumin level (mean 12% and 3 g/L higher in the same group).
Chromosome 13 abnormalities were analyzed using a commercial probe mapping at 13q14, containing the D13S319 locus. This locus appears to be included in the most frequently deleted region as defined in the recent study of Shaughnessy and coworkers.29 Nevertheless, there is now evidence that marginal differences may occur with the use of different probes. In future studies it would probably be of interest to use more than one probe and carefully study, in a large group of patients, the clinical outcome with respect to different sites of deletion.
Recently Barlogie and colleagues have recognized Δ13 as a highly unfavorable prognostic factor for event-free survival, OS, and complete response duration, but the study was plagued by the use of CC and the incidence of Δ13 was only 16%.13 FISH techniques are far more efficient than CC to identify numerical chromosomal abnormalities in myeloma30-32 and indeed we observed a 38% incidence of Δ13. This incidence was comparable to that found in the recent FISH study reported by Perez-Simon and colleagues involving 63 patients who received a standard treatment (33%) and allows a more precise evaluation of Δ13 prognostic value.17 In our study the superiority of FISH over CC was established in the subgroup of 68 patients who had both analyses.
In our hands, the presence of Δ13 was critically important for all times to event, especially PFS and OS; patients lacking Δ13 had a median survival of approximately 65 months compared to 27 months for patients with Δ13 (P < .0001). Nevertheless, patients with Δ13 tend to achieve complete remission more frequently, possibly reflecting more pronounced proliferative features, higher sensitivity to HDT, but rapid relapse. To a certain extent, the role of prognostic factors appeared to vary with the time to event considered. Whereas Δ13 and β2m were related to all times to event studied, the type of light chain, age, CRP, and creatinine were mainly related to survival and plasmacytosis to progression. Similarly, the IgA isotype was more related to survival and IgG to progression (Table 2). Such results could explain some variations observed in prognostic factors from one study to another, due to particularities of the corresponding sample. Only a suggestive correlation was found between Δ13 and β2m serum level, which was so far the best reported prognostic factor in myeloma.2-6 Nine of 31 patients (29%) with β2m serum level below 2.5 mg/L had Δ13. These patients had a survival time clearly inferior to that of good-risk patients with low β2m and no Δ13. They would have been wrongly classified as good-risk patients in the absence of Δ13 FISH analysis, illustrating further the strong contribution of this latter analysis to the prognostic classification.
In this study the multivariate analysis for OS prognosis was performed with the use at the first step of β2m (Table 3) and not Δ13, which was the most significant factor in the univariate analysis. We did that to determine the contribution on survival of factors in addition to the β2m level, so far the best prognostic factor. Nevertheless, when using classical stepwise approach, Δ13 was introduced at the first step and β2m at the second step, leading to the same bivariate model as the one presented in Table 3. In any case the IgA isotype was selected at the third step. Overall, whatever the method used, the 3 significant factors in the multivariate analysis were Δ13, β2m, and IgA isotype. We agree that in the latter model the highly pejorative group consisted of a small number of patients (8 patients) and this result would have to be confirmed in another sample. Nevertheless, this model with 4 prognostic categories defined by β2m, Δ13, and IgA isotype was highly significant when compared to the model with 3 prognostic categories defined by Δ13 and β2m level, and the survival time of this subgroup was actually very short with a median OS time of approximately 12 months.
Concerning the main end-point, OS, besides the effect of β2m alone, several interesting interrelationships have been published, which permitted the stratification into clinically useful categories. In particular, the stratifications according to β2m and serum albumin,33 β2m and CRP,28 and β2m and plasma cell labeling index34 have been found pertinent. In this study, the Δ13/β2m model was far superior to β2m/CRP models. The addition of serum albumin, at the cut-off value of 35 g/L, did not improve the model containing β2m (the cut-off value of 30 g/L could not be used, because only 6 patients had a lower serum level). The plasma cell labeling index was not available in the vast majority of our patients and therefore this model could not be considered. In contrast, a good stratification was achieved using β2m and IgA. The adverse prognostic value of the IgA isotype in the HDT setting has already been pointed out.9The β2m/IgA model was less powerful than the Δ13/β2m model (Table 3). Good-risk patients had a slightly inferior survival and the high-risk group consisted of only 14 patients (13%), whereas there were 33 (30%) in the Δ13/β2m model (Figure 1B,C). Nevertheless, the β2m/IgA model appeared as a valid alternative to the Δ13/β2m model for investigators lacking cytogenetics.
We conclude that Δ13, adequately detected by FISH analysis, is a very strong factor related to poor PFS and OS, especially when associated with β2m 2.5mg/L or greater. Although we have no real explanations concerning the mechanism by which Δ13 results in such an adverse prognosis, we strongly recommend routine FISH Δ13 assessment for patients considered for HDT. More needs to be done to improve HDT, especially for patients with Δ13 and high β2m who unequivocally fail current HDT, including tandem autotransplants. For these patients, our group has decided to evaluate a new tandem transplant program, using melphalan 200 mg/m2 with the first HDT cycle and, depending on age and availability of an HLA-identical sibling, melphalan 220 mg/m2 and a monoclonal anti-interleukin 6 antibody, with a second autotransplant or a nonmyeloablative allogeneic stem cell transplantation (for patients < 60 years old with an HLA-identical sibling). Thalidomide, which has recently demonstrated a strong antitumor activity in refractory myeloma,35 also deserves a complete and urgent evaluation.
We would like to thank Colette Geneix, Jacqueline Regnault, and Myriam Cambié for their excellent technical assistance.
Supported in part by the Foundation contre la Leucémie, the Comité Départemental de Loire-Atlantique de la Ligue contre le Cancer, and the CHRU Lille.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thierry Facon, Service des Maladies du Sang, Hôpital Claude Huriez, 59037 Lille Cédex, France; e-mail:t-facon@chru-lille.fr.