Engagement of the plasma membrane receptor Fas can induce apoptosis of leukemic cells. Signaling through Fas requires the formation of a death-inducing signaling complex (DISC) that involves the cytoplasmic domain of Fas, the adaptor molecule FADD/MORT-1, and procaspase-8. The present study investigated whether another caspase, known as procaspase-2L, played a role in Fas-mediated cell death. A series of human leukemic variant cells was derived by stable transfection with aCASP2L antisense construct (CASP2L/AS).Specific down-regulation of procaspase-2L decreased the sensitivity of these cells to apoptosis induced by an agonistic anti-Fas antibody (Ab, clone CH11), as determined by studying DNA fragmentation, chromatin condensation, and externalization of phosphatidylserine on the plasma membrane. In leukemic cells transfected with an empty vector, anti-Fas Ab treatment activated caspase-8, decreased the expression of the BH3 domain-only protein Bid, triggered the release of cytochrome c from the mitochondria to the cytosol, and activated caspase-3. All these events could not be observed when CASP2L/AS cells were similarly treated with anti-Fas Abs. CASP2L/AStransfection did not inhibit the formation of the DISC and no direct interaction between procaspase-2L and either Fas or FADD or procaspase-8 was identified. Down-regulation of procaspase-2L inhibited anti-Fas Ab–mediated cleavage of c-FLIP (FLICE-inhibitory protein), a protein that interferes with the formation of a functional DISC. These results suggest that the long isoform of caspase-2 plays a role in the Fas-mediated pathway to cell death by contributing to caspase-8 activation at the DISC level.
Introduction
Apoptosis can be induced in many cell types by engagement of the Fas (CD95/APO-1) receptor by its natural ligand (FasL) or agonistic antibodies (anti-Fas Ab).1,2Fas-mediated cell death was proposed to play a role in T-cell3 and erythroid cell4 homeostasis. Fas receptor was also suggested to be involved in the early phase of cytotoxic drug-mediated leukemic cell apoptosis.5,6Signaling through Fas requires its aggregation and the formation of an intracellular protein complex referred to as the death-inducing signaling complex (DISC).7,8 This complex consists of the cytoplasmic domain of Fas, the adaptor molecule FADD/MORT-1, and the cysteine aspartic acid–specific protease Mch5/Flice/Mach/procaspase-8.9-13 This latter protein belongs to a family of structurally related cysteine proteases that currently includes 14 enzymes in mammals.14 All are synthesized as inactive proenzymes that have to be cleaved at key aspartate residues to be activated.15 Caspases can be divided into 2 groups according to the length of their prodomain, those with a short prodomain (procaspase-3, -6, and -7) and those with a long prodomain (the remainder). These enzymes were shown to function as a proteolytic cascade in which caspases with a long prodomain act upstream of those with a short prodomain.16 In the Fas-mediated cell death pathway, caspase-3 was suggested to play an important role in the cascade, downstream of caspase-8.17
The binding of procaspase-8 to FADD/MORT-1 is mediated through tandem death effector domains (DED). The FLICE-inhibitory protein, c-FLIP (Casper/I-Flice/Flame-1/Cash/Clarp/Mrit/Usurpin) that contains similar DED but an inactive caspase domain, interferes with the formation of a functional DISC.18-25 On recruitment to the DISC, procaspase-8 is processed into its active subunits, which are released from the complex to cleave intracellular substrates and to signal apoptosis.26 The amount of active caspase-8 generated at the DISC and the level of expression of procaspase-3 could determine whether downstream pathways involve the mitochondria or not.27 Type I cells in which caspase-8 directly activates downstream caspases to induce the cell demise were distinguished from type II cells in which caspases have to cleave the proapoptotic BH3 domain-only protein of the Bcl-2 family known as Bid to trigger cell death.28,29 In this latter pathway, cleaved/activated Bid migrates from the cytosol to the mitochondria and induces the opening of the permeability transition pores, the loss of inner mitochondrial transmembrane potential, and the release of apoptogenic factors such as cytochrome c and apoptosis-inducing factor (AIF).30-33 In the cytosol, cytochrome c triggers oligomerization of the adaptor molecule APAF-1 in the presence of adenosine triphosphate (ATP). Oligomerized APAF-1 recruits and activates caspase-9 that, in turn, activates caspase-3 and other downstream caspases in a proteolytic cascade.34-36
The specific contribution of other caspases to Fas-mediated cell death remains poorly understood. One of these is caspase-2, initially described as Nedd2/Ich1, whose gene was highly expressed in mouse embryonic brain and down-regulated in the adult.37,38 Mice deficient in caspase-2 have shown that this enzyme acts both as a positive and a negative regulator of cell death.39Alternative splicing of the CASP2 gene37generates at least 2 messengers that encode a long isoform, caspase-2L, whose overexpression induces cell death, and a short isoform, caspase-2S, that antagonizes cell death. Caspase-2L is the dominant isoform that is expressed in most tissues. Its contribution to apoptosis is attested to by several lines of evidence. Procaspase-2L can be activated by cleavage early in the process of apoptosis, its overexpression induces mammalian cell death, antisense-mediated decrease of procaspase-2L expression delays cell death induced by trophic factor deprivation in various cell lines, and oocytes from caspase-2–deficient mice are resistant to chemotherapeutic drug-induced apoptosis.39-42 The prodomain of procaspase-2 interacts with the TNFR1 receptor via the adaptor molecules TRADD, RIP, and RAIDD/CRADD, suggesting its upstream involvement in the proteolytic cascade that mediates cell death induced by tumor necrosis factor-α (TNF-α).43,44 Procaspase-2 has also been shown to be a target of caspase-3, suggesting its downstream involvement in the caspase cascade activated by other stimuli.45
To analyze the role of caspase-2L in Fas-mediated apoptosis, we have derived a series of variant human leukemic cell populations by transfection with a CASP2L antisense construct(CASP2L/AS). Our results indicate that the presence of caspase-2L contributes to caspase-8 activation and downstream events, including Bid cleavage, cytochrome c release, caspase-3 activation, and DNA fragmentation in leukemic cells exposed to anti-Fas Abs. Furthermore, c-FLIP cleavage was prevented by down-regulation of caspase-2L, suggesting a potential mechanism for caspase-2–mediated sensitization to Fas agonists.
Materials and methods
Chemicals and reagents
The antihuman Fas monoclonal Abs (IgM, clone CH-11) were obtained from Biovalley Medical and Biological Laboratories (Conches, France); the recombinant soluble FasL was collected from the supernatant of FasL-transfected Neuro2A cells (a kind gift from Dr Fontana, Lausanne, Switzerland). Soluble human TRAIL (Alexis Biochemicals, San Diego, CA; 100-200 ng/mL) was cross-linked with 2 μg/mL enhancer. [2-14C]-Thymidine (59 mCi/mmol) was obtained from Amersham (Les Ulis, France). Geneticin (G418), Hoechst 33342, and cycloheximide (CHX) were obtained from Sigma Chemical (St-Quentin-Fallavier, France). The fluorogenic peptide derivatives Ac-Asp-Glu-Val-Asp-amino-4-methylcoumarin (Ac-DEVD-AMC) and benzyloxy-carbonyl-Ile-Glu-Thr-Asp-7-amido-4-trifluoromethylcoumarin (Z-IETD-AFC) as well as the caspase-2 inhibitor benzyloxy-carbonyl-Val-Asp(OMe)-Val-Ala-Asp(Ome)-fluoromethylketone (Z-VDVAD-fmk) were bought from Calbiochem (Meudon, France). All other chemicals were of reagent grade and purchased from Sigma, ICN Pharmaceuticals (Orsay, France) or other local sources.
Cell line and culture conditions
The U937, Jurkat, and CEM human leukemic cell lines were obtained from the American Type Culture Collection (Manassas, VA) and grown in suspension culture at 37°C in the presence of 5% CO2 in RPMI-1640 medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cell culture products were purchased from Biowhittaker (Fontenay-sous-bois, France). To ensure an exponential growth, cells were resuspended in fresh medium 24 hours before each treatment. Cell viability was checked at regular intervals by using a trypan blue exclusion assay.
Complementary DNA construct and cell transfection
CASP2L complementary DNA (cDNA) was generated by reverse transcriptase– polymerase chain reaction (RT-PCR) performed on RNA extracted from U937 cells,46 and subcloned in an antisense orientation(CASP2L/AS) into pTARGET vector (Promega, Charbonniëres, France). About 1.0 × 107 cells were electroporated at 270 kV with 10 μg pTARGET-CASP2L/ASor empty vector. The cells were cultured for 3 weeks in medium containing 1.0 mg/mL G418 to generate mixed cell populations. In addition, 48 hours after electroporation, some transfected cells were plated in 96-well plates at different cell densities and cultured during 4 weeks in medium containing 1.0 mg/mL G418. Clones were selected for subsequent analyses and maintained in 1.0 mg/mL G418 containing medium.
Antibodies
Monoclonal Abs used in this study include antihuman procaspase-3 (clone 19), procaspase-7 (clone 51), and FADD (clone 1) purchased from Transduction Laboratories (Lexington, KY); antihuman procaspase-2 (clone G310-1248), procaspase-8 (clone B9-2), and cytochrome c (clone 7H8.2C12) obtained from Pharmingen (San Diego, CA); and antihuman procaspase-8 from Biosource International (Camarillo, CA). Affinity purified polyclonal Abs used included antihuman caspase-2 and antihuman HSC-70 from Santa Cruz Biotechnology (Santa Cruz, CA), antihuman Bid from R&D Systems (Oxon, United Kingdom), antihuman PARP from Boerhinger Mannheim (Meyla, France), antihuman caspase-10 and antihuman c-FLIP from Pharmingen, and another antihuman c-FLIP from Upstate Biotechnology (Souffelweyersheim, France). The rabbit polyclonal anticaspase-3 serum, which recognizes the proenzyme (p32) and its p19 and p17 subunits, was kindly provided by Dr D. Nicholson (Merck, Toronto, ONT, Canada). Goat horseradish peroxidase-conjugated antimouse or antirabbit Abs were obtained from Jackson Immunoresearch Laboratories (West Grove, PA). Enhanced chemiluminescence (ECL) reagents were from Amersham.
Western blot, coimmunoprecipitation, and fractionation analysis
For Western blot analysis, cells were washed twice in ice-cold phosphate-buffered saline (PBS), lysed in RIPA buffer (150 mM NaCl, 50 mM Tris-HCl, pH 8.0, 0.1% Na-sodium dodecyl sulfate [SDS], 0.5% Na-desoxycholate) in the presence of protease inhibitors (0.1 mM PMSF, 2.5 μg/mL pepstatin, 10 μg/mL aprotinin, 2.5 μg/mL trypsin inhibitor, 5 μg/mL leupeptin) for 30 minutes on ice, then centrifuged at 15 000g for 20 minutes at 4°C. Supernatants were collected and 50 μg proteins were incubated in loading buffer (125 mM Tris-HCl, pH 6.8, 10% β-mercaptoethanol, 4.6% SDS, 20% glycerol, and 0.003% bromophenol blue) prior to SDS–polyacrylamide gel electrophoresis (SDS-PAGE) on a 12% to 15% SDS polyacrylamide gel. Following electrophoresis, proteins were electroblotted to polyvinylidene difluoride (PVDF). After blocking nonspecific binding sites overnight with 5% nonfat milk in TPBS (PBS, 0.1% Tween 20), membranes were incubated for 2 to 3 hours at room temperature with the primary Abs. After 2 washes in TPBS, membranes were incubated with the corresponding peroxidase-conjugated secondary Abs for 30 minutes at room temperature. Immunoblots were revealed by ECL and autoradiography. For coimmunoprecipitation studies, cells were collected by centrifugation, washed twice with ice-cold PBS, and then homogenized in a lysis buffer (50 mM Tris, pH 7.4; 150 mM NaCl; 1 mM NaVO4; 1% bovine serum albumin [BSA]; 1% NP-40; 2 mM phenylmethylsulfonyl fluoride [PMSF]; 1.0 mg/mL aprotinin) at 4°C for 30 minutes. The extracts were then centrifuged and supernatants were collected. The antihuman caspase-8 (Pharmingen), caspase-2 (Santa Cruz), and Fas (clone APO1-3, Alexis Biochemicals, San Diego, CA) were added overnight, which was followed by incubation with protein A/G agarose for 1 hour and centrifugation. Immunoprecipitates were washed in lysis buffer without NP-40 and analyzed by Western blotting. For subcellular fractionation, cells were resuspended in ice-cold buffer A (250 mM sucrose, 20 mM HEPES pH 7.4, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DDT, 17 μg/mL PMSF, 8 μg/mL aprotinin, 2 μg/mL leupeptine) and dounced briefly on ice. Unlysed cells and nuclei (750g for 10 minutes) and mitochondria (10 000g for 25 minutes) were pelleted by sequential differential centrifugation. Supernatants were further clarified by centrifugation at 100 000g for 60 minutes (S100 fraction).
Immunocytofluorometry and confocal microscopy analysis
Relative level of expression of Fas in control and transfected variant lines was determined by cytofluorometry and confocal microscopy analysis. Nonpermeable cells were incubated in the absence and presence of antihuman Fas (clone DX2 from Pharmingen) as previously described,5 and the fluorescence intensity distribution was studied by using a FACScan flow cytometer (Becton Dickinson, Le Pont de Claix, France). Cells were also fixed for confocal microscopy analysis as previously described.47
Identification of apoptosis
Staining of nuclear chromatin with Hoechst 33342 for 30 minutes at 37°C was used for identification of morphologic changes by fluorescence microscopy (each percentage was established by counting 400 cells). DNA fragmentation was measured using a previously described DNA filter elution assay48 and expressed as percentage of fragmented relative to total DNA. Exposure of phosphatidylserine on the outer plasma membrane leaflet was identified by annexin V–fluorescein isothiocyanate (FITC) staining in the presence of propidium iodide (Boehringer Ingelheim, Heidelberg, Germany). Briefly, cells were resuspended in 10 mM HEPES, pH7.4, 140 mM NaCl, 2.5 mM CaCl2, in the presence of annexin V–FITC (1.0 mg/mL) and propidium iodide (1.0 mg/mL) for 10 minutes in the dark and analyzed using a FACScan flow cytometer (Becton Dickinson).
Caspase activity
Cytosolic extracts were prepared by resuspending the cells at indicated times in a lysis buffer containing 100 mM HEPES (pH 7.5), 5 mM EDTA, 5 mM DTT, 20% glycerol, and 0.3% NP-40. Samples were centrifuged at 10 000g for 15 minutes at 4°C and supernatants were collected as cytosolic extracts. Caspase activities were measured as described17 by monitoring fluorescence continuously in a dual luminescence fluorimeter (LS 50B PerkinElmer, Courtaboeuf, France) using specific excitation and emission wavelengths for Ac-DEVD-AMC and z-IETD-AFC peptide derivative substrates, respectively. Reactions were carried out at 37°C using a water-jacketed sample compartment. The assay mixture contained 100 mM HEPES (pH7.5), 20% glycerol, 5 mM DTT, 5 mM EDTA, 200 μM fluorogenic peptides, and 200 μg proteins per assay. Enzyme activities were determined as initial velocities (intensity/min/mg) and related to results obtained in untreated cells.
Results
Stable transfection of CASP2L/ASin U937 human leukemic cells
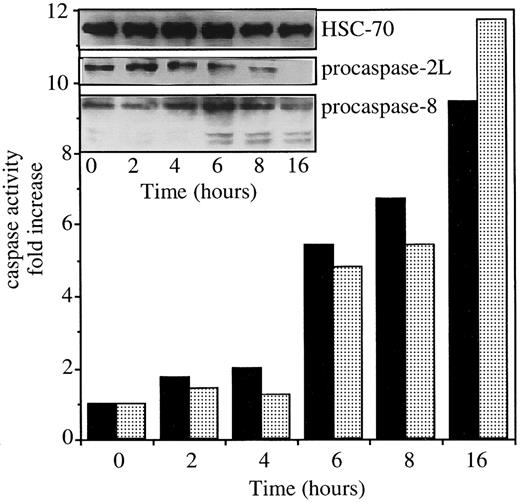
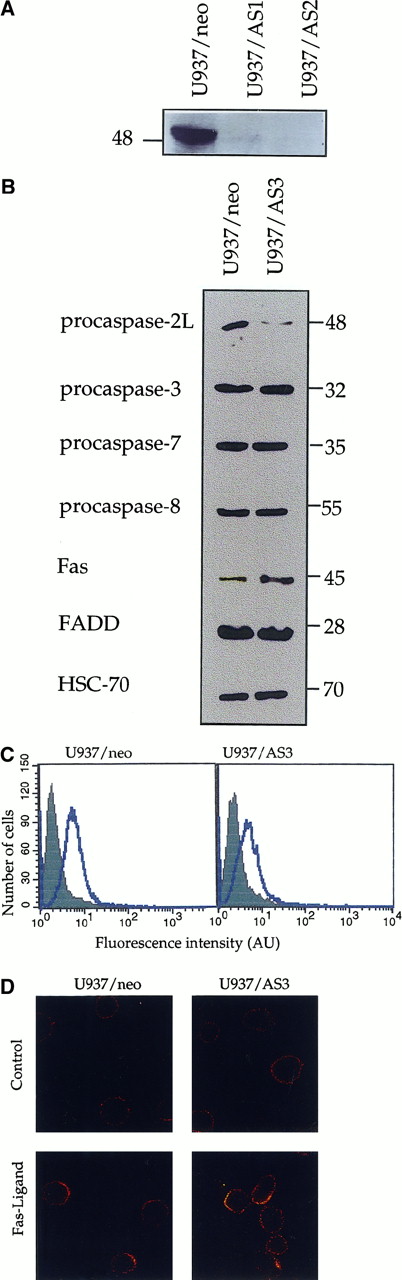
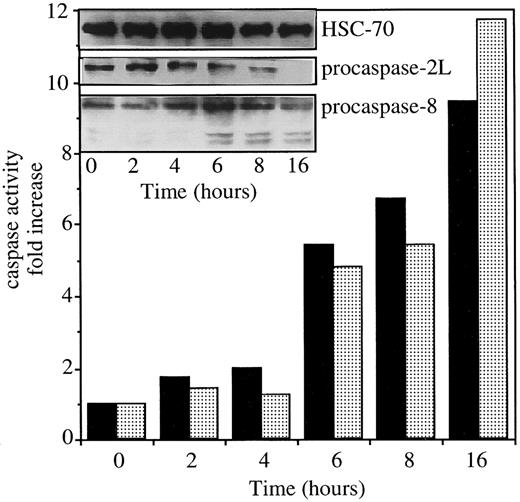
Exposure of U937 cells to 50 ng/mL CH11 anti-Fas Abs in the presence of 0.8 μg/mL CHX induces simultaneous activation of caspases 2L and 8, as demonstrated by the decreased expression of procaspase-2L, the appearance of caspase-8 active fragments (Figure1, insert), and the increased ability of cell extracts to cleave VDVAD-AFC and IETD-AFC substrates (Figure 1). To evaluate the contribution of caspase-2L to Fas-mediated cell death, the full-length CASP2L cDNA was subcloned into an antisense orientation in pTARGET plasmid vector that contains a neomycin-resistance gene. U937 cells were stably transfected by electroporation with the NEO or CASP2L/ASvectors and stable clones and mixed populations were derived under G418 selection. Western blot analysis of transfected cells using an antihuman caspase-2 Ab revealed the presence of a 48-kd protein in cells transfected with the empty vector (Figure2A; U937/neo). The expression of the 48-kd long isoform of procaspase-2 was decreased in the 2 mixed cell populations (Figure 2A; U937/AS1 and U937/AS2) as well as in a selected clone (Figure 2B; U937/AS3). The antisense constructs did not significantly modify the level of expression of Fas, FADD, and various procaspases including procaspase-3, -7, and -8 (Figure 2B). The lack of influence of CASP2L/AS construct on the plasma membrane expression of Fas was demonstrated by flow cytometry (Figure 2C). Confocal microscopy analysis showed that the clustering of Fas on stimulation with recombinant soluble Fas-L was similar in U937/neo and U937/AS3 cells (Figure 2D).
Exposure of U937 cells to CH11 anti-Fas Abs activates both caspase-2L and caspase-8.
The U937 cells were exposed to 50 ng/mL CH11 Ab (+ 0.8 μg/mL CHX) for indicated times. The ability of cell extracts to cleave VDVAD-AFC (▪) and IETD-AFC (░) substrates was measured and the expressions of procaspase-2L, procaspase-8, and caspase-8 active fragments were studied by Western blot. An antihuman HSC70 Ab was used for loading control.
Exposure of U937 cells to CH11 anti-Fas Abs activates both caspase-2L and caspase-8.
The U937 cells were exposed to 50 ng/mL CH11 Ab (+ 0.8 μg/mL CHX) for indicated times. The ability of cell extracts to cleave VDVAD-AFC (▪) and IETD-AFC (░) substrates was measured and the expressions of procaspase-2L, procaspase-8, and caspase-8 active fragments were studied by Western blot. An antihuman HSC70 Ab was used for loading control.
Characterization of U937 cells stably transfected with CASP2L/AS.
U937/AS1 and U937/AS2 are 2 mixed cell populations transfected with CASP2L/AS. U937/neo and U937/AS3 are 2 clones selected after transfection with empty pTARGET and CASP2L/AS vectors, respectively. (A) Western blot analysis of procaspase-2L expression in U937/AS1 and U937/AS2 cells. (B) Western blot analysis of procaspase-2L, -3, -7, -8, FADD, and Fas expression in U937/neo and U937/AS3 cells. HSC-70 expression level was used for loading control. (C) Flow cytometry analysis of Fas expression (open area) on plasma membrane of U937/neo and U937/AS3 cells. An isotype-matched nonrelevant Ab was used as negative control (filled area). (D) Confocal microscopy analysis of Fas expression in control and FasL (5 U/mL; 3 hours) treated U937/neo and U937/AS3 cells (magnification × 40).
Characterization of U937 cells stably transfected with CASP2L/AS.
U937/AS1 and U937/AS2 are 2 mixed cell populations transfected with CASP2L/AS. U937/neo and U937/AS3 are 2 clones selected after transfection with empty pTARGET and CASP2L/AS vectors, respectively. (A) Western blot analysis of procaspase-2L expression in U937/AS1 and U937/AS2 cells. (B) Western blot analysis of procaspase-2L, -3, -7, -8, FADD, and Fas expression in U937/neo and U937/AS3 cells. HSC-70 expression level was used for loading control. (C) Flow cytometry analysis of Fas expression (open area) on plasma membrane of U937/neo and U937/AS3 cells. An isotype-matched nonrelevant Ab was used as negative control (filled area). (D) Confocal microscopy analysis of Fas expression in control and FasL (5 U/mL; 3 hours) treated U937/neo and U937/AS3 cells (magnification × 40).
Decreased expression of procaspase-2L delays anti-Fas Ab–induced apoptosis in U937 cells
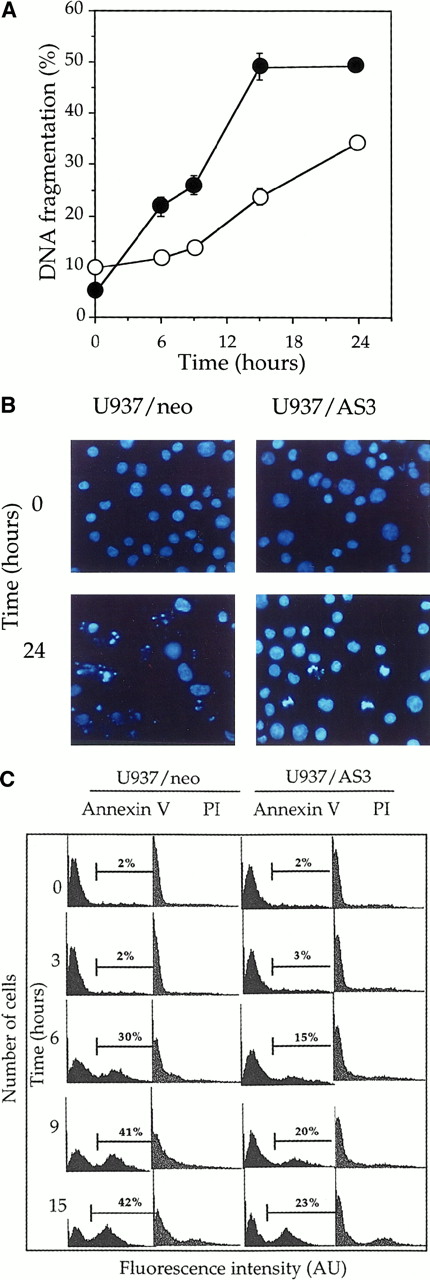
To determine the role of procaspase-2L in cell response to Fas agonists, we have exposed U937/neo and U937/AS3 clones to 50 ng/mL anti-Fas (clone CH11) Ab in the presence of 0.8 μg/mL CHX. In the tested conditions, neither anti-Fas Ab nor CHX was toxic to the cells.49 The occurrence of DNA fragmentation, monitored by a DNA filter elution assay, was delayed in U937/AS3 compared to U937/neo, with approximately 25% and 50% of fragmented DNA 15 hours after treatment, respectively (Figure3A). Trypan blue exclusion assays indicated that in most cells, plasma membranes were able to exclude the dye, even 24 hours after treatment, suggesting that the observed DNA fragmentation was related to apoptosis rather than necrosis (not shown). Apoptotic cell death was further demonstrated by Hoechst 33342 staining of nuclear chromatin in anti-Fas-Ab–treated cells (Figure 3B). The morphologic study confirmed that anti-Fas Ab–induced cell death was delayed in U937/AS3 compared to U937/neo cells. Externalization of phosphatidylserine on the outer leaflet of the plasma membrane is an early event in cells that are induced to undergo apoptosis.50 Using FITC-labeled annexin V and flow cytometry analysis, we observed that the appearance of annexin V+ cells was also delayed in U937/AS3 compared to U937/neo cells after anti-Fas Ab treatment (Figure 3C). In parallel, U937/neo and U937/AS3 cells remained unlabeled by propidium iodide, further indicating that the plasma membrane was not disrupted (Figure3C). Similar modulation in kinetics of apoptosis induction was observed in U937/AS1 and U937/AS2 cells (data not shown). Altogether, these results indicated that decreased expression of procaspase-2L in U937 cells delayed anti-Fas Ab–induced apoptosis.
Down-regulation of procaspase-2L expression delays anti-Fas Ab–induced apoptosis in U937/AS3 cells.
(A) At indicated times after anti-Fas Ab treatment (x-axis; hours), DNA fragmentation was quantified by a DNA filter elution assay in U937/neo (●) and U937/AS3 (○) cells. Data points, means of 2 independent experiments in triplicate; bars indicate SD. (B) Morphologic aspect of control and anti-Fas Ab–treated (CH11 50 ng/mL, CHX 0.8 μg/mL; 24 hours) U937/neo and U937/AS3 cells stained by Hoeschst 33342 (magnification × 40). (C) Flow cytometry profile of FITC-conjugated annexin V and propidium iodide (PI)–labeled U937/neo and U937/AS3 at various times after anti-Fas Ab treatment. Percentages of annexin V+ cells are indicated. The percentage of labeled cells with PI did not exceed 10%, 15 hours after treatment.
Down-regulation of procaspase-2L expression delays anti-Fas Ab–induced apoptosis in U937/AS3 cells.
(A) At indicated times after anti-Fas Ab treatment (x-axis; hours), DNA fragmentation was quantified by a DNA filter elution assay in U937/neo (●) and U937/AS3 (○) cells. Data points, means of 2 independent experiments in triplicate; bars indicate SD. (B) Morphologic aspect of control and anti-Fas Ab–treated (CH11 50 ng/mL, CHX 0.8 μg/mL; 24 hours) U937/neo and U937/AS3 cells stained by Hoeschst 33342 (magnification × 40). (C) Flow cytometry profile of FITC-conjugated annexin V and propidium iodide (PI)–labeled U937/neo and U937/AS3 at various times after anti-Fas Ab treatment. Percentages of annexin V+ cells are indicated. The percentage of labeled cells with PI did not exceed 10%, 15 hours after treatment.
Decreased expression of procaspase-2L prevents procaspase-3 activation and cytochrome c release in anti-Fas Ab–treated U937 cells
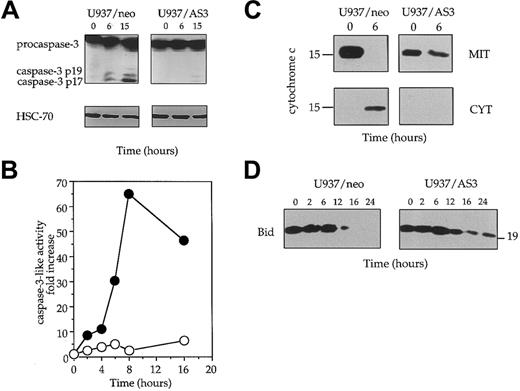
We have shown previously that Fas-induced cell death was associated with procaspase-3 cleavage/activation in U937 cells.51 Using a rabbit polyclonal Ab raised against the long subunit of caspase-3, we observed that procaspase-3 was rapidly cleaved into a p19 and a p17 fragment in Fas-treated U937/neo cells. This cleavage, which suggested caspase-3 activation, was not observed in U937/AS3 cells (Figure 4A). The cleavage of poly(ADP-ribose) polymerase, a well-known target for active caspase-3, was also delayed in U937/AS3 compared to U937/neo cells (not shown). To confirm that decreased expression of procaspase-2L prevented caspase-3 activation, we measured the ability of lysates from untreated and anti-Fas Ab–treated U937/neo and U937/AS3 cells to cleave the DEVD-AMC substrate that mimics the specific target site for some effector caspases, mainly caspase-3. This activity was strongly increased in U937/neo cells exposed to anti-Fas Abs, whereas it remained low in U937/AS3 clone (Figure 4B).
Decreased expression of procaspase-2L prevents activation of the mitochondrial pathway to cell death.
(A) The appearance of the active p19 and p17 fragments of caspase-3 was detected by Western blot analysis in U937/neo and U937/AS3 after anti-Fas Ab treatment. HSC-70 expression level is shown as protein loading control in each lane. (B) Kinetics of DEVD-AMC hydrolysis in U937/neo (●) and U937/AS3 (○) cells. Enzyme activities were measured as initial velocities and expressed relative to control untreated cells. One representative of 2 independent experiments is shown. (C) Subcellular localization of cyctochrome c was performed from enriched mitochondrial (MIT) and cytosolic (CYT) extracts prepared from control and anti-Fas Ab–treated (6 hours) U937/neo and U937/AS3 cells. (D) Expression of Bid was monitored in U937/neo and U937/AS3 cells by Western blot analysis at indicated times after anti-Fas Ab treatment.
Decreased expression of procaspase-2L prevents activation of the mitochondrial pathway to cell death.
(A) The appearance of the active p19 and p17 fragments of caspase-3 was detected by Western blot analysis in U937/neo and U937/AS3 after anti-Fas Ab treatment. HSC-70 expression level is shown as protein loading control in each lane. (B) Kinetics of DEVD-AMC hydrolysis in U937/neo (●) and U937/AS3 (○) cells. Enzyme activities were measured as initial velocities and expressed relative to control untreated cells. One representative of 2 independent experiments is shown. (C) Subcellular localization of cyctochrome c was performed from enriched mitochondrial (MIT) and cytosolic (CYT) extracts prepared from control and anti-Fas Ab–treated (6 hours) U937/neo and U937/AS3 cells. (D) Expression of Bid was monitored in U937/neo and U937/AS3 cells by Western blot analysis at indicated times after anti-Fas Ab treatment.
To delineate more precisely the pathway of caspase-3 activation during anti-Fas Ab treatment, cytochrome c release from the mitochondria to the cytosol was monitored. After a 6-hour exposure to anti-Fas Abs, the cytochrome c release observed in U937/neo cells was not identified in U937/AS3 cells treated in similar conditions (Figure 4C). In addition, the time-dependent decrease of Bid expression observed in U937/neo cells was delayed in U937/AS3 cells (Figure 4D). Altogether, these results suggested that caspase-2L might interfere with Fas-mediated apoptosis pathway upstream of the mitochondrial release of proapoptotic molecules and Bid cleavage.
Decreased expression of procaspase-2L inhibits anti-Fas Ab–mediated caspase-8 activation
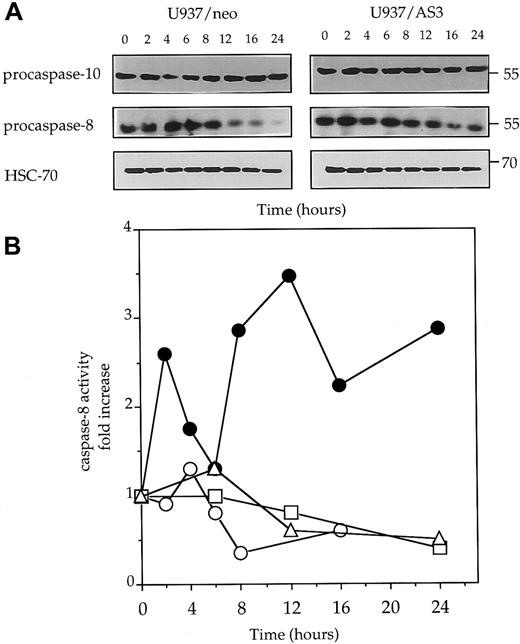
Because caspase-8 activation has been shown to play a central role in Fas-mediated cell death,9-13 52 we monitored procaspase-8 expression and caspase-8 activity in U937/neo and U937/AS3 cells exposed to anti-Fas Abs. The decrease of procaspase-8 expression, suggesting its cleavage into active fragments, was observed to be almost inhibited in U937/AS3 compared to U397/neo cells (Figure5A). As a control, procaspase-10 expression remained unchanged in anti-Fas Ab–treated U937/neo and U937/AS3 cells (Figure 5A). The hypothesis that down-regulation of caspase-2L expression could prevent caspase-8 activation in response to Fas agonists was confirmed by measuring IETDase activity in U937/neo and U937/AS cells following exposure to anti-Fas Abs. IETDase activity demonstrated a biphasic kinetics in U397/neo cells that was not observed in U937/AS3 cells (Figure 5B).
Decreased expression of procaspase-2L prevents anti-Fas Ab–mediated procaspase-8 activation.
(A) Western blot analysis of procaspase-10 and procaspase-8 expression in U937/neo and U937/AS3 cells at indicated times (x-axis; hours) after anti-Fas Ab treatment. HSC-70 expression level is used as protein loading control. One representative of 3 independent experiments is shown. (B) Kinetics of IETD-AFC hydrolysis in U937/neo (●), U937/AS3 (○), U937/AS1 (■), and U937/AS2 (▵) cells. Enzyme activities were measured as initial velocities and expressed as relative to control untreated cells.
Decreased expression of procaspase-2L prevents anti-Fas Ab–mediated procaspase-8 activation.
(A) Western blot analysis of procaspase-10 and procaspase-8 expression in U937/neo and U937/AS3 cells at indicated times (x-axis; hours) after anti-Fas Ab treatment. HSC-70 expression level is used as protein loading control. One representative of 3 independent experiments is shown. (B) Kinetics of IETD-AFC hydrolysis in U937/neo (●), U937/AS3 (○), U937/AS1 (■), and U937/AS2 (▵) cells. Enzyme activities were measured as initial velocities and expressed as relative to control untreated cells.
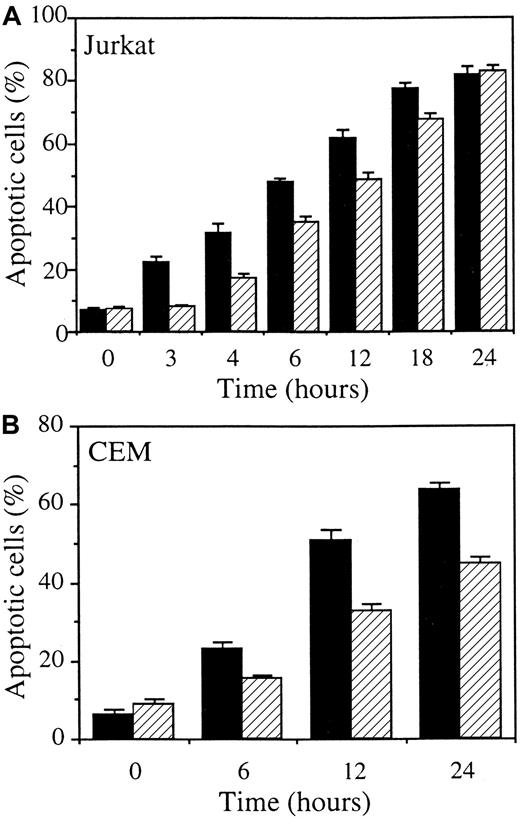
Stable transfection of CASP2L/AS also interferes with Fas-mediated cell death in Jurkat and CEM leukemic cell lines
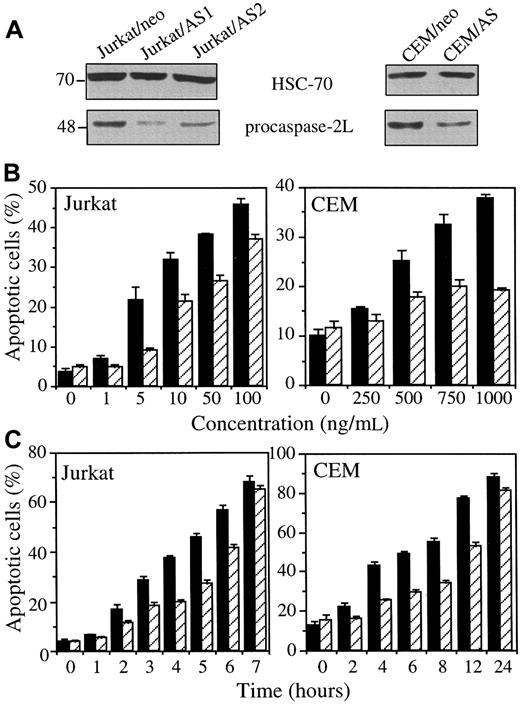
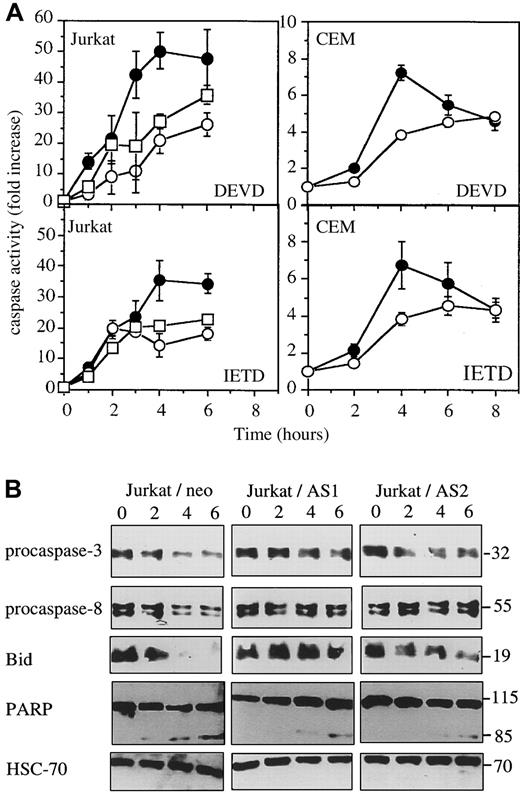
The previous experiments were repeated in mixed cell populations obtained by stably transfecting 2 other human leukemic cell lines with either the empty vector (Jurkat/neo and CEM/neo) or the CASP2L/AS-containing vector (Jurkat/AS1, Jurkat/AS2, and CEM/AS). Western blot analysis demonstrated that procaspase-2L expression was decreased in Jurkat and CEM cell populations transfected with the antisense construct (Figure6A). When exposed to CH11 anti-Fas Abs (in these cells, CHX is not required to potentiate CH11 activity), Jurkat/neo and CEM/neo cells underwent apoptosis, as demonstrated by Hoechst 33342 staining of nuclear chromatin (Figure 6B,C). Contrary to U937 cells, CHX was not required for CH11 Abs to induce apoptosis in Jurkat and CEM cells. Dose-response and time-course experiments confirmed the results obtained inCASP2L/AS- transfected U937 cells by showing that decreased expression of procaspase-2L in Jurkat/AS and CEM/AS cells significantly delayed Fas-mediated apoptosis (Figure 6B,C). In addition, by using DEVD-AMC and IETD-AFC as substrates to measure caspase activities, we observed that activation of these enzymes was delayed in cells transfected with the antisense construct (Figure7A). This was in accordance with Western blot analyses showing that the decreased expression of procaspase-3, procaspase-8 and Bid as well as the cleavage of the 116-kd PARP into a 85-kd fragment, observed in Jurkat/neo cells exposed to anti-Fas Abs, was delayed in 2 Jurkat/AS mixed populations treated under similar conditions (Figure 7B). The same observations were made by comparing CEM/neo and CEM/AS cells (data not shown).
CASP2L/AS transfection delays anti-Fas Ab–mediated apoptosis in Jurkat and CEM cell lines.
(A) Western blot analysis of procaspase-2L in Jurkat (Jurkat/AS1 and Jurkat/AS2) and CEM (CEM/AS) mixed cell populations compared to those transfected with the empty vector (Jurkat/neo and CEM/neo). (B, C) Jurkat and CEM cells, transfected with either the empty vector (black bars) or the CASP2L/ASvector (hatched bars) were exposed for 3 hours to indicated concentrations of CH11 Ab (B) or to 50 ng/mL and 1 μg/mL CH11 Ab concentrations, respectively, for indicated times (C). Apoptosis was determined by Hoechst 33342 staining (mean ± SD of 3 independent experiments).
CASP2L/AS transfection delays anti-Fas Ab–mediated apoptosis in Jurkat and CEM cell lines.
(A) Western blot analysis of procaspase-2L in Jurkat (Jurkat/AS1 and Jurkat/AS2) and CEM (CEM/AS) mixed cell populations compared to those transfected with the empty vector (Jurkat/neo and CEM/neo). (B, C) Jurkat and CEM cells, transfected with either the empty vector (black bars) or the CASP2L/ASvector (hatched bars) were exposed for 3 hours to indicated concentrations of CH11 Ab (B) or to 50 ng/mL and 1 μg/mL CH11 Ab concentrations, respectively, for indicated times (C). Apoptosis was determined by Hoechst 33342 staining (mean ± SD of 3 independent experiments).
Western blot analyses in Jurkat cells exposed to anti-Fas Abs.
Jurkat and CEM cells were exposed for indicated times to 50 ng/mL and 1 μg/mL CH11 Abs, respectively. (A) DEVDase and IETDase activities were measured in Jurkat/neo (●), Jurkat/AS1 (○), Jurkat/AS2 (■), CEM/neo (●), and CEM/AS (○) as initial velocities. Results were related to those obtained in untreated cells (one representative of 2 experiments; SD, samples in triplicate). (B) Western blot analysis of indicated proteins in Jurkat/neo and Jurkat/AS cells exposed for indicated times to 50 ng/mL CH11 anti-Fas Abs.
Western blot analyses in Jurkat cells exposed to anti-Fas Abs.
Jurkat and CEM cells were exposed for indicated times to 50 ng/mL and 1 μg/mL CH11 Abs, respectively. (A) DEVDase and IETDase activities were measured in Jurkat/neo (●), Jurkat/AS1 (○), Jurkat/AS2 (■), CEM/neo (●), and CEM/AS (○) as initial velocities. Results were related to those obtained in untreated cells (one representative of 2 experiments; SD, samples in triplicate). (B) Western blot analysis of indicated proteins in Jurkat/neo and Jurkat/AS cells exposed for indicated times to 50 ng/mL CH11 anti-Fas Abs.
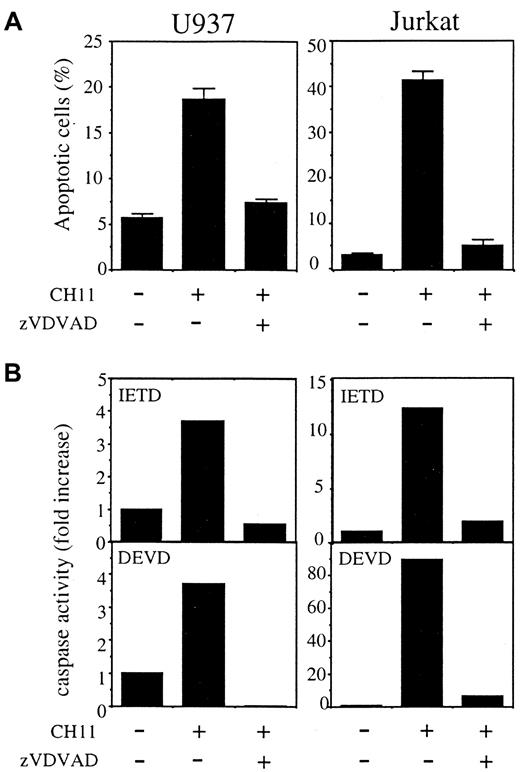
The caspase-2 inhibitor z-VDVAD prevents Fas-induced apoptosis in U937 and Jurkat cells
When added to cell culture in combination with CH11 anti-Fas Abs, the permeant peptide inhibitor Z-VDVAD-fmk, designed to inhibit caspase-2,53 54 prevented apoptosis in both U937 and Jurkat parental cell lines (Figure 8A,B). This peptide also prevented activation of the proteases that cleave DEVD-AMC and IETD-AFC substrates (Figure 8C). These results further argued for a role of caspase-2 in Fas-mediated apoptosis.
The caspase-2 permeant inhibitor Z-VDVAD-fmk prevents Fas-induced apoptosis of parental U937 and Jurkat cells.
Z-VDVAD (50 μM) was added to cell culture in combination with CH11 anti-Fas Abs (50 ng/mL), in the presence (U937) or absence (Jurkat) of CHX (0.8 μg/mL) for 3.5 hours. (A) Apoptosis was determined by Hoechst 33342 staining of nuclear chromatin (mean ± SD of 3 experiments). (B) DEVDase and IETDase activities were measured as initial velocities. Results were related to those obtained in untreated cells (one representative of 2 experiments).
The caspase-2 permeant inhibitor Z-VDVAD-fmk prevents Fas-induced apoptosis of parental U937 and Jurkat cells.
Z-VDVAD (50 μM) was added to cell culture in combination with CH11 anti-Fas Abs (50 ng/mL), in the presence (U937) or absence (Jurkat) of CHX (0.8 μg/mL) for 3.5 hours. (A) Apoptosis was determined by Hoechst 33342 staining of nuclear chromatin (mean ± SD of 3 experiments). (B) DEVDase and IETDase activities were measured as initial velocities. Results were related to those obtained in untreated cells (one representative of 2 experiments).
Procaspase-2L does not interact with the DISC components
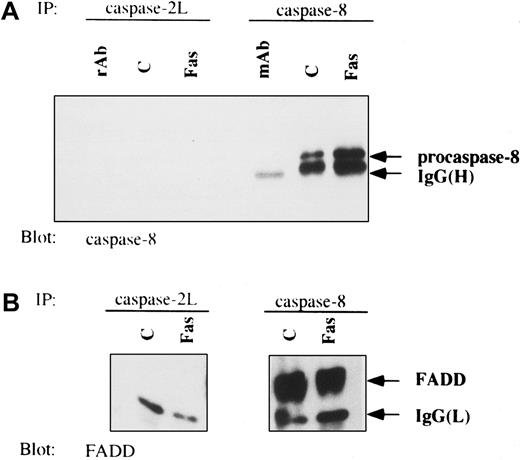
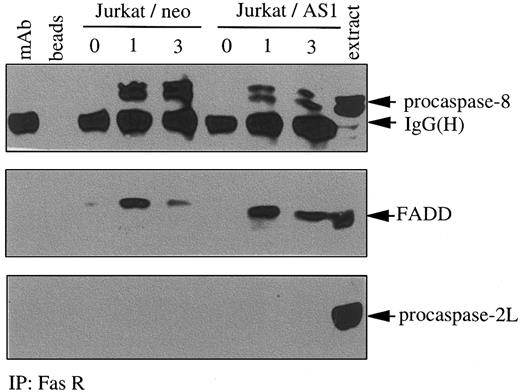
Results from the previous experiments had suggested that caspase-2L could facilitate procaspase-8 activation during Fas-induced apoptosis in U937 cells. To determine whether this effect was a consequence of a direct interaction of procaspase-2L with procaspase-8 or the adaptor protein FADD, we performed immunoprecipitation experiments in control and anti-Fas Ab–treated U937/neo cells. Even by using high concentrations of Abs, no interaction was identified between procaspase-2L and either procaspase-8 or FADD (Figure9A,B). As described previously,55 under these conditions of Ab excess, an interaction between procaspase-8 and FADD was detected in both untreated and anti-Fas–treated U937/neo cells (Figure 9B). To determine whether caspase-2L was required for the DISC formation, we realized immunoprecipitation experiments using an anti-Fas Ab in control and FasL-treated cells. This experiment, performed in Jurkat/neo and Jurkat/AS1 cell populations, demonstrated that transfection with CASP2L/AS did not prevent interaction of Fas with FADD and procaspase-8 in response to FasL. Recruitment of procaspase-8 was decreased, whereas no interaction with procaspase-2L could be detected (Figure10). Similar results were obtained by comparing U937/neo and U937/AS clones exposed to FasL in the presence of CHX (data not shown). In these conditions of immunoprecipitation, no interaction between Fas and FADD or procaspase-8 was detected in untreated cells.
Lack of interaction of procaspase-2L with FADD and procaspase-8 in anti-Fas Ab–treated U937 cells.
Coimmunoprecipitation (IP) experiments from control (c) and anti-Fas Ab–treated (Fas; 6 hours) U937/neo cells were performed with anticaspase-2L or anticaspase-8 Abs. Western blot analysis was revealed with anticaspase-8 (A) and anti-FADD (B) Abs. Abs used to perform IPs were loaded as control (rAb and mAb).
Lack of interaction of procaspase-2L with FADD and procaspase-8 in anti-Fas Ab–treated U937 cells.
Coimmunoprecipitation (IP) experiments from control (c) and anti-Fas Ab–treated (Fas; 6 hours) U937/neo cells were performed with anticaspase-2L or anticaspase-8 Abs. Western blot analysis was revealed with anticaspase-8 (A) and anti-FADD (B) Abs. Abs used to perform IPs were loaded as control (rAb and mAb).
Decreased expression of procaspase-2L does not inhibit the formation of the DISC.
Jurkat/neo and Jurkat/AS1 cells were treated for indicated times with recombinant FasL. Immunoprecipitation (IP) with an anti-Fas receptor Ab was followed by immunoblot detection of FADD, procaspase-8, and procaspase-2L. Controls include the Ab alone (mAb), beads incubated in the absence of Ab (beads), and Jurkat cell extracts (extract).
Decreased expression of procaspase-2L does not inhibit the formation of the DISC.
Jurkat/neo and Jurkat/AS1 cells were treated for indicated times with recombinant FasL. Immunoprecipitation (IP) with an anti-Fas receptor Ab was followed by immunoblot detection of FADD, procaspase-8, and procaspase-2L. Controls include the Ab alone (mAb), beads incubated in the absence of Ab (beads), and Jurkat cell extracts (extract).
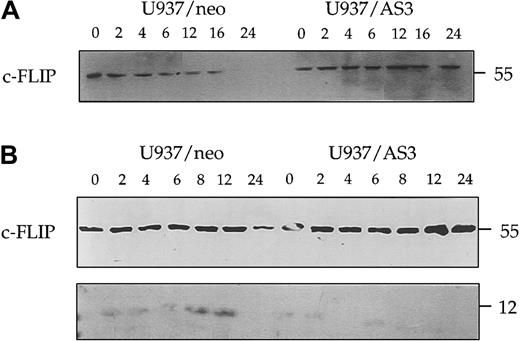
Decreased expression of procaspase-2L prevents the cleavage of c-FLIP
We also evaluated the effect of caspase-2L on c-FLIP (FLICE-inhibitory protein), a potent inhibitor of procaspase-8 activation at the DISC. Western blot experiments performed with a first polyclonal anti–c-FLIP Ab (Upstate Biotechnology) indicated that the decreased expression of c-FLIP observed in U937/neo cells treated with anti-Fas Abs was delayed in U937/AS3 cells (Figure11A). By using another polyclonal anti–c-FLIP Ab (Pharmingen), we identified a 12-kd cleavage fragment in U937/neo cells exposed to anti-Fas Abs. This cleavage fragment was not observed in U937/AS3 cells treated under the same conditions (Figure 11B). Similar results were obtained in U937/AS1 and AS2 cells (not shown).
Decreased expression of procaspase-2L prevents anti-Fas Ab–induced c-FLIP cleavage.
Kinetics of c-FLIP expression was monitored in U937/neo and U937/AS3 cells by Western blot analysis with 2 different Abs at the indicated times after anti-Fas Ab treatment. (A) Anti–c-FLIP polyclonal Ab from Upstate Biotechnology detected the 55-kd native protein. (B) Anti–c-FLIP polyclonal Ab from Pharmingen also detected a faint 12-kd band corresponding to a cleavage product.
Decreased expression of procaspase-2L prevents anti-Fas Ab–induced c-FLIP cleavage.
Kinetics of c-FLIP expression was monitored in U937/neo and U937/AS3 cells by Western blot analysis with 2 different Abs at the indicated times after anti-Fas Ab treatment. (A) Anti–c-FLIP polyclonal Ab from Upstate Biotechnology detected the 55-kd native protein. (B) Anti–c-FLIP polyclonal Ab from Pharmingen also detected a faint 12-kd band corresponding to a cleavage product.
CASP2L/AS transfection also delays TRAIL-mediated apoptosis
To determine whether caspase-2L could play a role in the apoptotic pathway triggered by engagement of another death receptor, we tested the influence of CASP2L/AS transfection on TRAIL-mediated apoptosis in Jurkat (TRAIL: 100 ng/mL) and CEM (TRAIL: 200 ng/mL) cells. In both cell lines, decreased expression of procaspase-2L was associated with a delay in TRAIL-induced apoptosis, as determined by Hoechst 33342 staining of nuclear chromatin (Figure12).
TRAIL-induced apoptosis is delayed in
CASP2L/AS-transfected cells. Apoptosis induced by 100 (Jurkat) to 200 (CEM) ng/mL recombinant TRAIL was quantified by staining nuclear chromatin with Hoechst 33342 in Jurkat/neo (▪), Jurkat/AS1 (▨), CEM/neo (▪), and CEM/AS (▨). Results are the mean ± SD of 3 independent experiments.
TRAIL-induced apoptosis is delayed in
CASP2L/AS-transfected cells. Apoptosis induced by 100 (Jurkat) to 200 (CEM) ng/mL recombinant TRAIL was quantified by staining nuclear chromatin with Hoechst 33342 in Jurkat/neo (▪), Jurkat/AS1 (▨), CEM/neo (▪), and CEM/AS (▨). Results are the mean ± SD of 3 independent experiments.
Discussion
The present study suggests that the long isoform of caspase-2 plays a role in the Fas-mediated pathway to cell death by contributing to caspase-8 activation at the DISC level. The role of caspase-2 in cell death pathways appears to depend on both the apoptotic stimulus and the cell type. Analysis of caspase-2−/− mice has implicated caspase-2 in apoptosis of oocytes triggered by the cytotoxic agent doxorubicin, whereas doxorubicin-induced cell death pathway remains efficient in B and T lymphoblasts from these caspase-2 knockout animals.39 On the other hand, B lymphoblasts from these mice were reported to be resistant to granzyme B–mediated apoptosis.39 In the present study, we used an antisense construct to down-regulate the expression of caspase-2L in the human leukemic cell line. Similar strategy has been previously used to demonstrate caspase-2 involvement in FDC-P1 murine myeloid progenitor cell death triggered by deprivation of granulocyte-macrophage colony-stimulating factor and interleukin 3.41 A potential limit to this strategy is that antisense caspase-2 could cross-react and inhibit the expression of other related caspases. However,CASP2L cDNA shares a limited homology with other caspases including those of the caspase-1– or caspase-3–related groups.14,56 In the present study, transfection ofCASP2L/AS in leukemic cells resulted in decreased expression of caspase-2L, whereas no change could be detected in procaspase-3, -7, -8, and -10 protein levels. These observations suggested that CASP2L/AS might specifically down-regulate procaspase-2L expression. Interestingly, these cells did not demonstrate any significant resistance to etoposide-induced apoptotic DNA fragmentation (data not shown), possibly due to drug-induced transcriptional activation of CASP-2 gene that would overcome the antisense effect after etoposide treatment.46 By contrast, anti-Fas Ab–mediated apoptosis, which is not associated with a transcriptional increase ofCASP-2 gene expression, was significantly delayed inCASP2L/AS transfected cells. These results suggested a role for caspase-2L in Fas-mediated leukemic cell death.
The pathway leading to Fas-mediated apoptosis depends on the clustering of Fas receptor that triggers the death signal. Decreased expression of procaspase-2L did not influence Fas receptor expression on the plasma membrane of leukemic cells. When analyzed by confocal microscopy, the clustering of Fas receptor triggered by Fas-L on the plasma membrane of leukemic cells47 was not influenced by procaspase-2L down-regulation. In these cells, Fas-mediated cell death involves the decreased expression of the native form of the BH3 domain-only protein Bid,28,29 the release of cytochrome c from the mitochondria to the cytosol, and the activation of caspase-3. Caspase-mediated cleavage of Bid28,57 was proposed to mediate, either directly or through Bax dimerization, the mitochondrial damage observed in some cells exposed to Fas agonists.29,58 Because caspase-2L down-regulation was observed to delay all these events, the enzyme was supposed to interfere with Fas-mediated cell death pathway somewhere between Fas clustering at the plasma membrane and caspase-mediated cleavage of Bid.28 29
Death signaling through Fas receptor involves the recruitment of FADD and procaspase-8 to form the DISC.8 Recruitment of procaspase-8 to the DISC leads to its proteolytic activation.12,13 Gene ablation studies in mice have shown that the presence of caspase-8 was an essential requirement for Fas-mediated cell death.52 We observed that down-regulation of procaspase-2L expression prevented Fas-mediated caspase-8 activation. One possible explanation for that observation was that procaspase-2L could be recruited to the DISC to facilitate caspase-8 activation on Fas stimulation. Procaspase-2L had been shown to associate via its amino-terminal CARD domain with RAIDD, a death adaptor molecule that is involved in death signaling through the TNF-R1 complex via association with the death domain proteins RIP and TRADD.44 Immunoprecipitation experiments failed to identify any direct interaction between procaspase-2L and either Fas or FADD or procaspase-8. These experiments also showed that decreased procaspase-2L expression did not prevent the formation of the DISC but could interfere with procaspase-8 recruitment. These observations suggest that caspase-2L could indirectly modulate the kinetics of caspase-8 recruitment and activation in the DISC, for example, through the cleavage products of a target protein. The mechanism of this modulation remains to be clarified.
Another molecule that interferes with the Fas-mediated cell death pathway is c-FLIP, a protein structurally related to procaspase-8 but lacking a catalytic active site and the residues that form the substrate binding pocket.18-25 The protein is predominantly found as a 55-kd isoform in most tissues and cell lines,19 although a minor species of 27/28 kd was also detected in some cell types.18,59 In hematopoietic cells, mainly lymphoid cells, overexpression of c-FLIP could involve a cell surface–mediated signaling pathway through a member of the immunoglobulin gene superfamily named Toso.60 The role of c-FLIP in controlling Fas susceptibility of peripheral T cells and other cell types remains controversial.59,61Overexpression of c-FLIP was proposed to specifically prevent death receptor–induced apoptosis through inhibition of procaspase-8 processing without affecting other pathways of cell death.18-21,59,62,63 However, some reports have suggested that c-FLIP could behave as a proapoptotic molecule whose effect can be inhibited by a dominant-negative caspase-8 mutant, various caspase inhibitors, and the antiapoptotic proteins of the Bcl-2 family.22-25 Another controversial issue concerns the consequences of the caspase-mediated cleavage of c-FLIP. Caspase-2 is one of the caspases that have been shown to cleave c-FLIP at Asp-341 to generate a p43 N-terminal and a p12 C-terminal fragments in vitro.21 Some authors have proposed that the cleavage of c-FLIP was required for its antiapoptotic activity,59whereas others have reported that up-regulation of the native molecule was essential for its activity.64,65 In the present study, we show that the decreased expression of c-FLIP induced by anti-Fas Ab treatment of leukemic cells is prevented by procaspase-2L down-regulation. A faint cleavage fragment of c-FLIP can be identified in anti-Fas Ab–treated control cells, whereas this fragment is not detected in anti-Fas Ab–treatedCASP2L/AS-transfected cells. Although inhibition of c-FLIP cleavage could account for the decreased sensitivity ofCASP2L/AS-transfected cells to Fas-mediated cell death, we cannot rule out the possibility that this inhibition is a nonspecific consequence of the decreased activation of caspase-8 and downstream caspases.21
To summarize, decreased expression of procaspase-2L in human leukemic cell lines prevented the activation of procaspase-8 and downstream events induced by agonistic anti-Fas Abs, including decreased expression of Bid, cytochrome c release from the mitochondria, procaspase-3 activation, apoptotic DNA fragmentation, and externalization of phosphotidylserine on the plasma membrane. These results strongly suggest that caspase-2L plays a role in caspase-8 activation at the DISC level. It remains to be determined how this caspase is activated in response to Fas agonists and whether the correlation observed between Fas-induced caspase-8 activation and c-FLIP expression is directly related to caspase-2L activity.
The authors wish to thank Francois Martin and Ali Bettaieb for helpful advice and discussion. We are grateful to Dr D. Nicholson (Merck, Toronto, Canada) and A. Fontana (Lausanne, Switzerland) for providing us the polyclonal anti–caspase-3 Ab and Fas-L Neuro2A transfected cells, respectively.
Supported by grants from the committees (Cote d'Or, Niëvre, Saone et Loire) of the Ligue Nationale Contre le Cancer, the Association pour la Recherche contre le Cancer (no. 9567) and the Conseil Régional de Bourgogne to E.S. and from the Medical Research Council of Canada (MT-15019) to R.B. N.D. obtained a studentship from the Ministëre de l'Education Nationale, de la Recherche et de la Technologie (France) and from the Sociètè Francáise d'Hématologie. R.B. is a scholar from the Medical Research Council of Canada and the Cancer Research Society (Canada).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Eric Solary, INSERM U517, Faculties of Medicine and Pharmacy, BP 87900, 21079 Dijon Cedex, France; e-mail:esolary@u-bourgogne.fr.