Studies with neutralizing antibodies have indicated roles for platelet-endothelial cell adhesion molecule-1 (PECAM-1) in leukocyte migration through the endothelium and the perivascular basement membrane. Because some of these findings have been contentious, this study aimed to explore the role of PECAM-1 in leukocyte migration by analyzing leukocyte responses in interleukin 1β (IL-1β)– and tumor necrosis factor-α (TNFα)–activated cremasteric venules of PECAM-1–deficient mice using intravital and electron microscopy. Although no differences in levels of leukocyte rolling flux or firm adhesion were observed, a delay in leukocyte transmigration in response to IL-1β, but not TNFα, was detected in PECAM-1–deficient mice. Electron microscopy indicated that this delay occurred at the level of perivascular basement membrane. To address the cytokine specificity of PECAM-1 dependence, in vitro experiments demonstrated that TNFα, but not IL-1β, could induce rapid adhesion of murine neutrophils to protein-coated surfaces, suggesting that TNFα elicited leukocyte transmigration in wild-type mice via direct stimulation of leukocytes. In summary, the results suggest a regulatory role for PECAM-1 in leukocyte migration through the perivascular basement membrane, a role that appears to be cytokine-specific and associated with the ability of the cytokine to stimulate rapid neutrophil adhesion.
Introduction
The process of leukocyte recruitment involves a series of cooperative interactions between circulating leukocytes and the endothelial cells over which they pass.1 Adhesive interactions between glycoproteins expressed on the surface of leukocytes flowing freely within blood vessels and their specific counterreceptors on endothelial cells permit leukocytes to slow and subsequently arrest their motion, despite the relatively high shear forces found within the vascular lumen. This process can be subdivided into a number of distinct but overlapping steps, involving different classes of cell adhesion molecules acting cooperatively with endothelial-cell–associated stimulating factors, such as platelet activating factor and certain chemokines. Although our understanding of the molecular events that regulate the first 2 stages, rolling and firm adhesion, has increased greatly in recent years, details of the mechanisms that allow firmly adherent leukocytes to pass through the endothelium and its associated perivascular basement membrane have yet to be fully determined.2 This process, termed leukocyte transmigration, has already been shown to involve representatives from different families of adhesion molecules3,4 including members of the β2 integrin family, such as leukocyte-function–associated antigen 1 (αLβ2, CD11a/CD18) and Mac-1 (αMβ2, CD11b/CD18),5-8 and members of the immunoglobulin superfamily, such as intercellular adhesion molecules 1 and 2 (ICAM-1, ICAM-2),9,10platelet-endothelial cell adhesion molecule-1 (PECAM-1, CD31),11,12 and junctional adhesion molecule.13
PECAM-1 is a 130-kd glycoprotein composed of 6 C2 immunoglobulin domains, a transmembrane portion, and a short cytoplasmic tail. It is expressed by platelets, by most subsets of leukocytes, and by endothelial cells, concentrated at interendothelial junctions. PECAM-1 has been shown to support cell-cell adhesion by homophilic interactions in a calcium-dependent manner, a process that appears to be mediated by domains 1 and 2 of the molecule.14,15 A number of putative heterotypic ligands for PECAM-1 have also been identified, including the integrin αvβ316,17 and adenosine diphosphate–ribosyl cyclase (CD38),18 though the in vivo relevance of such in vitro observations has yet to be determined and, in the case of αvβ3, is in contention.19
Early studies suggested that the role of PECAM-1 was predominantly as an adhesive structure because it shared sequence homology with other adhesion molecules, it was capable of mediating cell-cell adhesion, and anti–PECAM-1 antibodies could disrupt the formation of confluent endothelial monolayers in vitro.14,20 There is increasing evidence, however, that in common with other cell adhesion molecules, PECAM-1 has important signaling properties. Engagement of PECAM-1 can result in up-regulation of β1, β2, and β3 integrin function,21-23 and cellular activation can result in serine or tyrosine phosphorylation of the cytoplasmic tail of PECAM-1.24-27Phosphorylation of the cytoplasmic tail can allow the formation of docking sites for other signaling molecules28,29 and may lead to alterations in expression24,25 and/or binding character-istics of PECAM-1.30 Thus, reciprocal signaling mechanisms exist that allow engagement of PECAM-1 to modulate other cellular processes, such as integrin-mediated adhesive interactions (outside-in signaling), and also allow cellular events to regulate the binding of PECAM-1 to its ligands (inside-out signaling). This combination of characteristics—in terms of distribution, binding, and signaling—renders PECAM-1 ideally suited for a role in transmigration.
There is now considerable in vitro and in vivo experimental evidence supporting a role for PECAM-1 in leukocyte migration through the endothelium.19,31-33 More recently, certain anti–PECAM-1 antibodies have also been shown to suppress leukocyte migration through the perivascular basement membrane,34,35 a response that appears to be mediated by molecular interactions different from the mechanisms that mediate PECAM-1–dependent transendothelial cell migration.19,34,36,37 Genetically modified mice lacking PECAM-1, although exhibiting nearly normal inflammatory responses, have also suggested some abnormalities in the movement of leukocytes through the perivascular basement membrane, though at present, the dynamics and possible stimulus specificity of this observation is unclear.38 The aim of the present study was to use a combined experimental approach of intravital and electron microscopy to extend the findings of Duncan et al38 by directly quantifying leukocyte responses within cytokine-activated cremasteric venules of PECAM-1–deficient mice, focusing on responses elicited by interleukin 1β (IL-1β) and tumor necrosis factor-α (TNFα). We hypothesized that with the use of this experimental approach to observe leukocyte behavior at the cellular level, subtle defects in leukocyte transmigration in the PECAM-1–deficient mice may become more apparent. Indeed, although they provide additional evidence for a role for PECAM-1 in leukocyte migration through the perivascular basement membrane, our findings demonstrate that this PECAM-1–dependent response is transient and cytokine-specific, supporting leukocyte transmigration induced by IL-1β but not TNFα. In vitro data suggested that the difference observed in the profile of leukocyte transmigration elicited by the 2 cytokines was associated with the ability of TNFα, but not IL-1β, to stimulate murine leukocytes.
Materials and methods
Animals
Mice deficient in PECAM-1 have recently been developed by targeted gene disruption and backcrossed onto a C57BL/6 background.38 Age- and weight-matched wild-type C57BL/6 mice were purchased from Harlan-Olac (Bicester, United Kingdom).
Reagents
The following reagents were purchased: recombinant murine IL-1β and TNFα (Serotec, Oxford, United Kingdom); ketamine (Ketalar) (Parke-Davis, Eastleigh, United Kingdom); xylazine (Rompun) (Bayer, Bury St Edmunds, United Kingdom); sodium pentobarbitone (Sagatal) (Rhône Mérieux, Harlow, United Kingdom); Tyrodes balanced salts (Life Technologies, Paisley, United Kingdom); osmium (VIII) oxide (Johnson Matthey, Royston, United Kingdom); sodium cacodylate (Agar Scientific, Stanstead, United Kingdom); Biotaq DNA polymerase (Bioline, London, United Kingdom); percoll (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom). All other reagents were purchased from Sigma-Aldrich (Poole, United Kingdom).
Polymerase chain reaction
Polymerase chain reaction (PCR) was used to confirm the genotype of mice used in this study as follows: genomic material was prepared from tissue samples digested with proteinase K (10 μg/mL), and amplified by means of a reaction mixture containing appropriate murine PECAM-1 primers (1 μM), deoxynucleotide triphosphates (0.2 mM), magnesium chloride (2.5 mM), and Taq polymerase (20 U/mL) in NH4 buffer. Following amplification in a thermoblock (Biometra, Maidstone, United Kingdom), the products were separated by 1% agarose gel electrophoresis; 165–base pair (bp) fragment denoted the wild-type gene, and 1500-bp fragment denoted the recombinant, PECAM-1–deficient gene.
Intravital microscopy
Leukocyte-endothelial cell interactions were induced by intrascrotal (IS) administration of TNFα or IL-1β, with control mice receiving saline (IS). After the desired interval, most commonly 4 hours, the mice were anesthetized with ketamine (100 mg/kg intraperitoneally [IP]) and xylazine (10 mg/kg IP) and maintained at 37°C on a custom-built heated microscope stage. The cremaster muscle was exteriorized and prepared for intravital microscopy as previously described.39 Briefly, following incision of the scrotum, one testis was gently withdrawn to allow the cremaster muscle to be incised and pinned out flat over the window in the microscope stage. The cremaster muscle was kept warm and moist by continuous application of warmed Tyrodes balanced salt solution.
Leukocyte-endothelial cell interactions were observed on an upright fixed-stage microscope (Axioskop FS) (Carl-Zeiss, Welwyn Garden City, United Kingdom) fitted with water-immersion objectives. Video recordings were made with a color video camera (Model C5810-01) (Hamamatsu Photonics, Enfield, United Kingdom) and videocassette recorder (Model AG-MD830E) (Panasonic, Bracknell, United Kingdom). In most series of experiments, the carotid artery and jugular vein were exposed and cannulated to allow for blood sampling, blood pressure measurement, and administration of further anesthetic (sodium pentobarbitone, 3 mg/kg per hour). Total leukocyte counts were performed on blood samples by means of Kimura stain.40Differential cell analysis was determined in smears prepared in a cytocentrifuge (Cytospin-3) (Shandon, Runcorn, United Kingdom) and stained with May-Grünwald/Giemsa stains. Blood pressure was measured by means of an electronic pressure transducer (Harvard Apparatus, Edenbridge, United Kingdom).
Postcapillary venules, 20 to 40 μm in diameter, were identified for study. Rolling leukocytes were defined as those moving more slowly than the associated blood flow, and rolling flux was quantified as the number of rolling cells moving past a fixed point on the venular wall per minute, averaged over 5 minutes. Firmly adherent cells were those remaining stationary for 30 seconds or longer within a given 100-μm segment of venule. Extravasated leukocytes were those in the perivenular tissue within 50 μm of the 100-μm vessel segment under observation. Several vessel segments (range, 3-5) from multiple vessels (range, 3-5) were studied for each animal. Additionally, in selected experiments, erythrocyte centerline velocity was determined by means of an Optical Doppler Velocimeter (Microcirculation Research Institute, College Station, TX), which allowed Newtonian shear rates to be calculated as previously described.41
Electron microscopy
In selected experiments, following the dynamic quantification of leukocyte responses, the cremaster muscle was removed and fixed in a solution containing 2.5% glutaraldehyde (2.5%), sodium cacodylate (50 mM), hydrochloric acid (4 mM), and calcium chloride (0.18 mM). Samples were then postfixed in osmium VIII oxide (1%) and, following dehydration in methanol, were embedded in araldite resin prior to sectioning. Vessels were located within sections (1 μm) stained with toluidine blue. Ultrathin sections (0.1 μm) of the target area were mounted on copper mesh grids and stained with uranyl acetate and lead citrate. A transmission electron microscope (Hitatchi 7000) (Hitatchi, Hayes, United Kingdom) was used to assess the position of migrating leukocytes relative to the endothelium and the perivascular basement membrane, as we have previously described.35 For each vessel, the number of leukocytes in each of the following positions was noted: A, within lumen of venule; B, crossing endothelium; C, between endothelium and perivascular basement membrane; D, crossing basement membrane; E, outside venule, but within 50 μm of it. For each venule, the fraction of leukocytes that had crossed the endothelium but were still inside the basement membrane was calculated according to the following equation: C/(C + D + E). In each series of experiments, tissue samples from at least 4 animals were analyzed, and at least 3 vessels from each animal were studied in detail.
Murine neutrophil adhesion assay
Blood was collected from donor C57BL/6 animals by cardiac puncture and anticoagulated with EDTA (10 mM). Following dextran sedimentation, the neutrophil fraction was purified by centrifugation over a 2-layer percoll gradient (80% over 64%), a procedure that yielded a leukocyte preparation with greater than 90% neutrophils. The cells were then labeled with the fluorochrome Cell Tracker Orange (3 μM) (Pharmingen, San Diego, CA), and the adhesion assays were performed in triplicate in 96-well plates precoated with bovine serum albumin (BSA) (1 μg/mL). Murine neutrophils (105 per well) were coincubated for 30 minutes with murine TNFα, murine IL-1β, or formyl-methionyl-leucine-phenylalanine (fMLP) at the concentrations indicated in “Results.” Fluorescence readings were taken from each well by means of a fluorescence plate reader (Cytofluor 2300) (Millipore, Watford, United Kingdom) (excitation at 541 nm and emission at 565 nm) before and after nonadherent neutrophils had been washed off. Neutrophil adhesion was calculated as the ratio of the 2 readings, and the data are expressed as the percentage of unstimulated adhesion.
Statistical analysis
Data are presented as the mean ± SEM. Statistical significance was assessed by means of 1- or 2-way analysis of variance, or Mann-Whitney U test as appropriate; P < .05 was considered significant. Analysis was performed with Prism 3.0 for Windows (Graphpad Software, San Diego, CA).
Results
IL-1β and TNFα induce leukocyte transmigration through murine cremasteric venules
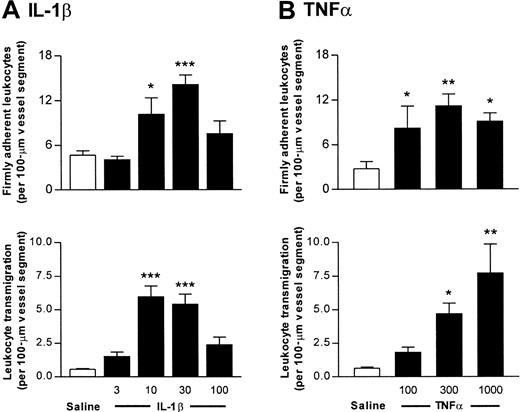
Prior to investigating leukocyte responses in PECAM-1–deficient mice, we characterized the profile of leukocyte responses in cytokine-activated cremasteric venules of wild-type mice. The cytokines under investigation, IL-1β (3 to 100 ng) and TNFα (100 to 1000 ng), were administered by intrascrotal injection, and 4 hours later, leukocyte responses were quantified as observed by intravital microscopy. Figure 1 shows the dose-response relationship of cytokine-induced leukocyte firm adhesion and transmigration. Neither IL-1β nor TNFα produced significant changes in leukocyte rolling flux (data not shown). Overall, IL-1β appeared to exhibit a greater level of potency in this model (approximately 10-fold), with doses of 30 ng IL-1β and 300 ng TNFα inducing comparable effects on leukocyte firm adhesion and transmigration. These doses were used in all subsequent experiments.
Dose-response relationship of leukocyte responses induced by IL-1β and TNFα in murine cremasteric venules.
Wild-type mice were treated either with intrascrotal saline or with IL-1β (3 to 100 ng) (A) or TNFα (100 to 1000 ng) (B) 4 hours before the cremaster muscle was prepared for intravital microscopy. Upper and lower panels show leukocyte firm adhesion and transmigration, respectively, from the same experiments. The data represent mean ± SEM (n = 4 to 5 mice per group; *P < .05, **P < .01, ***P < .001).
Dose-response relationship of leukocyte responses induced by IL-1β and TNFα in murine cremasteric venules.
Wild-type mice were treated either with intrascrotal saline or with IL-1β (3 to 100 ng) (A) or TNFα (100 to 1000 ng) (B) 4 hours before the cremaster muscle was prepared for intravital microscopy. Upper and lower panels show leukocyte firm adhesion and transmigration, respectively, from the same experiments. The data represent mean ± SEM (n = 4 to 5 mice per group; *P < .05, **P < .01, ***P < .001).
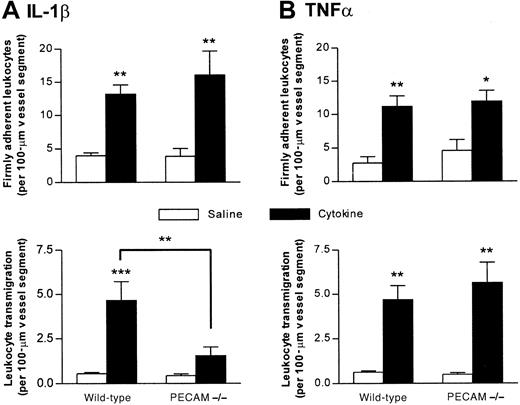
Leukocyte transmigration in response to IL-1β, but not TNFα, is diminished in PECAM-1–deficient mice
The aim of the present experiments was to investigate leukocyte responses in cytokine-stimulated cremasteric venules of PECAM-1–deficient mice compared with wild types. For this purpose, responses were quantified 4 hours postadministration of IL-1β (30 ng, IS) or TNFα (300 ng, IS). Prior to the in vivo studies, PCR analysis of tail biopsies was used to confirm the genotype of the PECAM-1–deficient mice, as detailed in “Materials and methods” (results not shown).
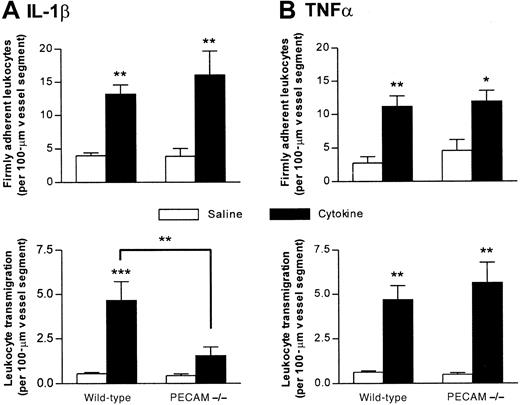
In vivo, both cytokines elicited significant and comparable increases in leukocyte firm adhesion in wild-type and PECAM-1–deficient mice, but leukocyte transmigration induced by IL-1β, but not TNFα, was significantly lower in the PECAM-1–deficient animals (Figure2). This suppression of leukocyte transmigration in the genetically modified mice represented a 67% reduction (P < .01) in the response when compared with that detected in IL-1β–stimulated venules of wild-type animals. No differences in leukocyte rolling flux, venular wall shear rates, or blood pressure were observed between the 2 strains of mice (data not shown). Furthermore, the reduction in leukocyte transmigration could not be explained by differences in systemic leukocyte counts as no significant differences in total counts were observed, though a greater number of neutrophils were detected in PECAM-1–deficient (2.99 ± 0.4 × 106/mL) compared with wild-type mice (1.78 ± 0.2 × 106/mL) (mean ± SEM; n = 5 for both groups; P < .05).
Leukocyte responses induced by IL-1β and TNFα in cremasteric venules of PECAM-1–deficient mice.
PECAM-1–deficient and wild-type mice were treated either with intrascrotal saline or with IL-1β (30 ng) (A) or TNFα (300 ng) (B) 4 hours before the cremaster muscle was prepared for intravital microscopy. Upper and lower panels show leukocyte firm adhesion and transmigration, respectively, from the same experiments. The data represent mean ± SEM (n = 5 mice per group; *P < .05, **P < .01, ***P < .001).
Leukocyte responses induced by IL-1β and TNFα in cremasteric venules of PECAM-1–deficient mice.
PECAM-1–deficient and wild-type mice were treated either with intrascrotal saline or with IL-1β (30 ng) (A) or TNFα (300 ng) (B) 4 hours before the cremaster muscle was prepared for intravital microscopy. Upper and lower panels show leukocyte firm adhesion and transmigration, respectively, from the same experiments. The data represent mean ± SEM (n = 5 mice per group; *P < .05, **P < .01, ***P < .001).
The reduction in IL-1β–induced leukocyte transmigration is due to a delay in the movement of neutrophils through the perivascular basement membrane
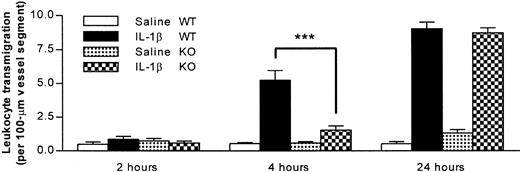
The following series of experiments aimed to determine whether the observed reduction in leukocyte transmigration through IL-1β–activated cremasteric venules of the PECAM-1–deficient mice (4 hours after stimulation) was a result of a delay or a total inhibition of leukocyte migration. For this purpose, in addition to the 4-hour in vivo test period, leukocyte transmigration was quantified at 2 additional time points, namely 2 and 24 hours postadministration of the cytokine. Figure 3 shows that in wild types, at the 2-hour time point, there was no significant difference in leukocyte transmigration in animals injected with intrascrotal IL-1β compared with saline-injected mice. As previously observed, 4 hours postinjection of the cytokine, there was a significant increase in leukocyte transmigration, a response that was further increased at the 24-hour time point. Interestingly, although once again a reduction in leukocyte transmigration was observed in the PECAM-1–deficient mice at the 4-hour time point, this effect was absent in animals treated with IL-1β for 24 hours, a time point at which the IL-1β–induced leukocyte transmigration in PECAM-1–deficient mice was identical to that observed in wild types.
Time course of neutrophil transmigration induced by IL-1β in PECAM-1–deficient and wild-type animals.
PECAM-1–deficient and wild-type mice were treated with intrascrotal saline or IL-1β (30 ng) 2, 4, and 24 hours before the cremaster muscle was prepared for intravital microscopy. The data represent mean ± SEM (n = 3 animals for 2- and 24-hour groups and n = 8 for the 4-hour group; ***P < .001).
Time course of neutrophil transmigration induced by IL-1β in PECAM-1–deficient and wild-type animals.
PECAM-1–deficient and wild-type mice were treated with intrascrotal saline or IL-1β (30 ng) 2, 4, and 24 hours before the cremaster muscle was prepared for intravital microscopy. The data represent mean ± SEM (n = 3 animals for 2- and 24-hour groups and n = 8 for the 4-hour group; ***P < .001).
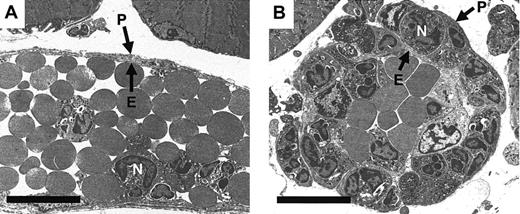
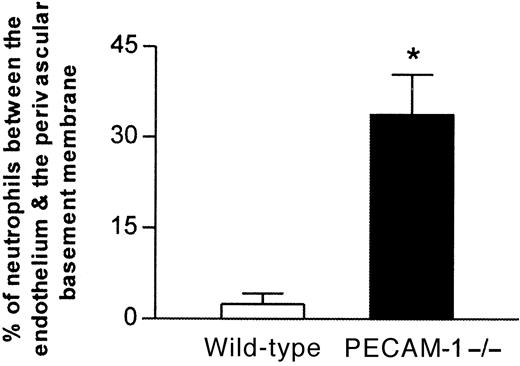
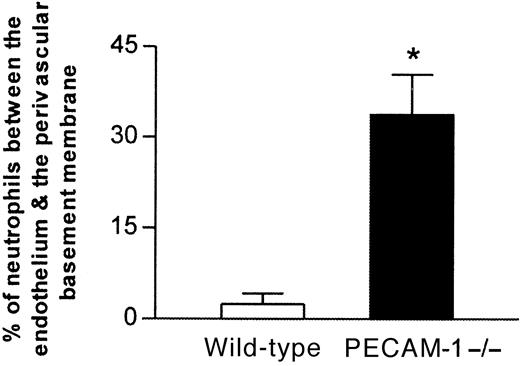
To determine the site of delay of the emigrating leukocytes in IL-1β–stimulated cremasteric venules of PECAM-1–deficient mice, cremaster muscles were further analyzed by electron microscopy, evaluations that also demonstrated that the migrating leukocytes were predominantly neutrophils (approximately 95%). Comparison of 4-hour IL-1β–stimulated cremaster venules of PECAM-1–deficient and wild-type mice demonstrated a clear and dramatic difference between their morphologies (Figure 4). In PECAM-1–deficient mice, neutrophils were frequently observed between the endothelium and the perivascular basement membrane, and in a number of vessels, this phenomenon resulted in an increase in the thickness of the venular wall by manyfold (Figure 4B). By contrast, neutrophils “trapped” in this position were seldom seen in IL-1β–stimulated venules of control wild-type mice (Figure 4A). When results were assessed quantitatively rather than qualitatively, approximately 7 times as many neutrophils were trapped in IL-1β–stimulated vessels of PECAM-1–deficient mice, relative to wild-type controls (34% ± 6.7% vs 4.5% ± 1.7% respectively;P < .05) (Figure 5). No such differences were observed in TNFα–stimulated vessels (25% ± 9.7% vs 16% ± 4.2% for PECAM-1–deficient vs wild-type mice; data represent 13 to 16 randomly selected vessel segments from 4 mice per group).
Electron micrographs of IL-1β–activated venules from PECAM-1–deficient and wild-type animals.
Following in vivo quantification of leukocyte responses 4 hours after intrascrotal administration of IL-1β, cremaster muscles were removed and prepared for transmission electron microscopy. Representative electron micrographs of cremasteric venules from wild-type (A) and PECAM-1–deficient (B) mice are shown. E indicates endothelium; N, neutrophil; P, pericyte and associated perivascular basement membrane. Bar represents 10 microns.
Electron micrographs of IL-1β–activated venules from PECAM-1–deficient and wild-type animals.
Following in vivo quantification of leukocyte responses 4 hours after intrascrotal administration of IL-1β, cremaster muscles were removed and prepared for transmission electron microscopy. Representative electron micrographs of cremasteric venules from wild-type (A) and PECAM-1–deficient (B) mice are shown. E indicates endothelium; N, neutrophil; P, pericyte and associated perivascular basement membrane. Bar represents 10 microns.
Proportion of neutrophils trapped between venular endothelial cells and the perivascular basement membrane in IL-1β–treated cremasteric venules from PECAM-1–deficient and wild-type animals as determined by electron microscopy.
Following in vivo quantification of leukocyte responses 4 hours after intrascrotal administration of IL-1β, cremaster muscles of both wild-type and PECAM-1–deficient mice were removed and prepared for electron microscopy. The graph shows the number of neutrophils observed between the venular endothelium and the perivascular basement membrane, expressed as a percentage of the total number of neutrophils that had passed the endothelial cell junctions. The data represent mean ± SEM (14 randomly selected vessel segments from 4 mice per group; *P < .05).
Proportion of neutrophils trapped between venular endothelial cells and the perivascular basement membrane in IL-1β–treated cremasteric venules from PECAM-1–deficient and wild-type animals as determined by electron microscopy.
Following in vivo quantification of leukocyte responses 4 hours after intrascrotal administration of IL-1β, cremaster muscles of both wild-type and PECAM-1–deficient mice were removed and prepared for electron microscopy. The graph shows the number of neutrophils observed between the venular endothelium and the perivascular basement membrane, expressed as a percentage of the total number of neutrophils that had passed the endothelial cell junctions. The data represent mean ± SEM (14 randomly selected vessel segments from 4 mice per group; *P < .05).
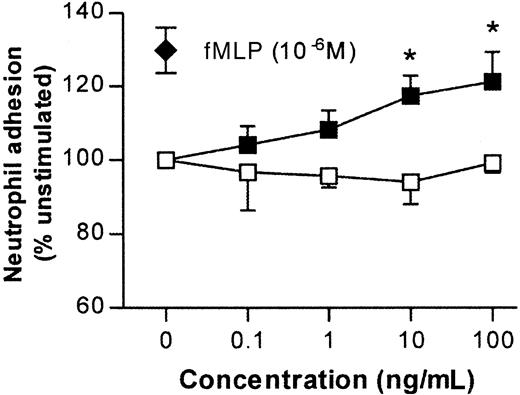
TNFα can rapidly stimulate murine neutrophils in vitro
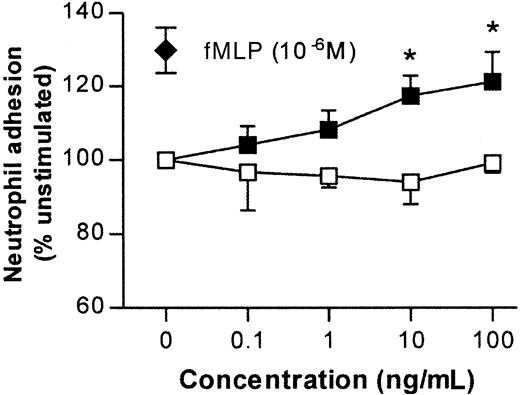
Because leukocyte transmigration induced by IL-1β but not TNFα was suppressed in PECAM-1–deficient mice (Figure 2), we hypothesized that perhaps in the present murine model TNFα was acting via stimulation of neutrophils and thus bypassing a requirement for endothelial cell PECAM-1. In this context, we have previously shown that in contrast to responses elicited by IL-1β, leukocyte transmigration through rat mesenteric venules induced by the chemoattractant fMLP is PECAM-1–independent.19 35 Hence, the ability of TNFα, compared with IL-1β, to stimulate murine neutrophils was assessed in an in vitro adhesion assay. As can be seen in Figure 6, TNFα induced a dose-dependent increase in neutrophil adhesion, responses that were comparable to that induced by the chemoattractant fMLP. In contrast, IL-1β had no such effect.
Effects of IL-1β and TNFα on adhesion of murine neutrophils in vitro.
Fluorochrome-labeled purified murine neutrophils were incubated with IL-1β or TNFα at the concentrations shown in wells coated with BSA. The ability of IL-1β (■) and TNFα (▪) to stimulate firm adhesion is expressed as percentage of adhesion levels detected in unstimulated neutrophils (24.7% ± 1.5%). The effect of a single dose of fMLP (10−6 M) is shown for comparison (♦). These data represent mean ± SEM (n = 3; P < .001 for overall difference between the 2 curves; *P < .05 for difference between IL-1β and TNFα at equivalent doses).
Effects of IL-1β and TNFα on adhesion of murine neutrophils in vitro.
Fluorochrome-labeled purified murine neutrophils were incubated with IL-1β or TNFα at the concentrations shown in wells coated with BSA. The ability of IL-1β (■) and TNFα (▪) to stimulate firm adhesion is expressed as percentage of adhesion levels detected in unstimulated neutrophils (24.7% ± 1.5%). The effect of a single dose of fMLP (10−6 M) is shown for comparison (♦). These data represent mean ± SEM (n = 3; P < .001 for overall difference between the 2 curves; *P < .05 for difference between IL-1β and TNFα at equivalent doses).
Discusssion
The final stage in migration of leukocytes from the vascular lumen to the extravascular tissue involves penetration of the vessel wall, a response involving 2 distinct but sequential cellular events: (1) migration of leukocytes across the endothelial cell lining and (2) migration of leukocytes through the perivascular basement membrane. Although a number of adhesion molecules have been implicated in leukocyte transendothelial cell migration, very little is known about the molecular events that mediate the passage of leukocytes through the perivascular basement membrane.2-4,12 PECAM-1 appears to be unique in that in vitro and in vivo evidence has indicated a role for it in migration of leukocytes across both barriers.31,34,35 As these studies have been carried out largely with the use of anti–PECAM-1 antibodies, an experimental approach that has often been subject to criticism related to possible nonspecific effects of reagents,42 the aim of the present study was to use mice that have been genetically engineered to lack PECAM-1 to further elucidate the role of this molecule in leukocyte transmigration in vivo. Duncan et al38 recently gave details of the normal development of these mice and reported on their normal levels of leukocyte infiltration in several models of acute inflammation. Although there was some suggestion of a defect in leukocyte migration through the perivascular basement membrane, the study did not address the temporal or stimulus specificity of this observation. In light of this, the aim of the present study was to use intravital and electron microscopy to extend the findings of Duncan et al by investigating leukocyte migration through cremasteric venules of PECAM-1–deficient mice at the cellular level. We hypothesized that with this experimental approach, a more detailed analysis of leukocyte behavior could be achieved, and hence more subtle defects in leukocyte migration might be detected and quantified. Indeed, although our findings provide conclusive evidence for the involvement of PECAM-1 in neutrophil migration through the perivascular basement membrane, the role of PECAM-1 appears to be as a regulatory molecule in that its requirement is transient and cytokine-specific, mediating neutrophil migration induced by IL-1β, but not TNFα , in this model.
Intravital microscopy was used to compare and quantify dynamic leukocyte responses in IL-1β– and TNFα–activated cremasteric venules of PECAM-1–deficient mice. In control wild-type animals, intrascrotal administration of both cytokines resulted in increased leukocyte firm adhesion and transmigration. In PECAM-1–deficient mice, although both cytokines elicited comparable firm adhesion, leukocyte transmigration induced by IL-1β, but not TNFα, was significantly suppressed (67% inhibition). Additional time-course experiments revealed that this suppression, detected 4 hours postinjection of the cytokine, was not evident at 24 hours postadministration of IL-1β. These results suggest that absence of PECAM-1 results in a delay, as opposed to a total inhibition, of leukocyte migration through the venular wall. To determine the stage of leukocyte transmigration at which this delay occurred, we analyzed 4-hour IL-1β–stimulated cremasteric venules by transmission electron microscopy. These studies demonstrated a significant increase in the number of neutrophils within the vessel wall of PECAM-1–deficient mice, ie, neutrophils that had successfully penetrated the endothelium but not the perivascular basement membrane. Hence, in this clean model of genetic deletion, our results suggest that PECAM-1–independent adhesion pathways can compensate for lack of PECAM-1 with respect to leukocyte transendothelial cell migration, and we provide conclusive evidence for a role for PECAM-1 in neutrophil migration through the perivascular basement membrane. An important aspect of our findings is that PECAM-1 deletion appears to exert a transient inhibitory effect on neutrophil movement through the perivascular basement membrane, suggesting a regulatory role, as opposed to an absolute requirement, for PECAM-1 in neutrophil transmigration. These findings are in agreement with the observations of Duncan et al38 and may explain the apparently normal leukocyte infiltration into inflamed peritoneal and air pouch cavities of PECAM-1–deficient mice that they reported.
The mechanism by which PECAM-1 regulates migration of neutrophils through the perivascular basement membrane remains to be clarified. Because PECAM-1 has not been reported to interact directly with molecules within this structure, it seems likely that this mechanism will depend on the signaling properties of the molecule, as we have previously discussed.35 For example, the following explanations are possible:
At endothelial cell junctions, PECAM-1/PECAM-1 interactions may transduce signals that result in enhanced migration of neutrophils through the vessel wall, thus accelerating the process of leukocyte transmigration. Of relevance, using an in vitro flow model, Rainger et al43 have shown that the migration velocity of neutrophils is significantly greater on PECAM-1–coated surfaces compared with albumin- or ICAM-1–coated plates, demonstrating the ability of PECAM-1 to regulate neutrophil motility.
The ligation of PECAM-1 at endothelial cell junctions may facilitate the movement of neutrophils through the perivascular basement membrane by triggering the expression of molecules such as β1integrins that may then aid the direct interaction of leukocytes with components of the perivascular basement membrane such as laminin and collagen IV. In this context, PECAM-1–dependent interactions can activate β1 integrin, as well as β2 and β3 integrin functions.21-23
The ligation of PECAM-1 may regulate the cell-surface expression of leukocyte proteolytic enzymes, such as neutrophil elastase, that may facilitate the movement of neutrophils through the perivascular basement membrane. Of relevance, neutrophils are known to express proteolytic enzymes at their leading edge during transendothelial migration,44 and protease inhibitors have been shown to reduce migration of neutrophils through a basement-membrane–like structure in vitro.45 The association of PECAM-1 ligation with cell-surface expression of neutrophil elastase is under investigation in our laboratory.
A surprising finding of our study was that although IL-1β–induced neutrophil transmigration was suppressed in PECAM-1–deficient mice, the response elicited by TNFα appeared to be totally PECAM-1–independent. As we have previously found that anti–PECAM-1 antibodies can suppress leukocyte transmigration through rat mesenteric venules induced by IL-1β but not by the direct leukocyte chemoattractant fMLP,19,35 we hypothesized that TNFα may be acting as a chemoattractant via stimulation of neutrophils in our murine model. Indeed, TNFα, but not IL-1β, directly stimulated the adhesion of murine neutrophils to protein-coated plates. In addition, local inhibition of protein synthesis can suppress leukocyte transmigration induced by IL-1β but not by TNFα (unpublished observations). In combination, these results strongly suggest that TNFα, but not IL-1β, can act as a murine neutrophil chemoattractant via its ability to stimulate murine neutrophils, and that with respect to some acute inflammatory reactions, the principal target cell of TNFα for the induction of neutrophil migration may be the neutrophil itself. This property of TNFα may be associated with the rapid release of preformed protein or lipid mediators such as LTB4, following TNFα–induced neutrophil adhesion. Gamble et al46 demonstrated that TNFα could activate both human neutrophils and cultured human endothelial cells to induce their adhesion, though the effect on neutrophils was rapid (5 minutes) and protein-synthesis–independent and the effect on endothelial cells was slow (4 hours) and dependent on de novo protein generation. Furthermore, in an in vivo study, the kinetics of neutrophil accumulation in rabbit skin elicited by TNFα and IL-1β were very different.47 Specifically, TNFα–induced neutrophil accumulation was fast (maximum within 30 minutes), similar to responses induced by the chemoattractants fMLP or LTB4, and the response elicited by IL-1β was slow (maximum within 3 to 4 hours) and protein-synthesis–dependent.47 48
In summary, with the use of PECAM-1–deficient mice, the results of the present study clearly demonstrate a role for PECAM-1 as a regulatory molecule in leukocyte migration through vessel walls at sites of inflammation. However, our findings also highlight the fact that PECAM-1–independent leukocyte migration can occur in a temporal- and/or stimulus-specific manner.
Supported by grants from the Medical Research Council (United Kingdom), the Wellcome Trust, and the British Heart Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sussan Nourshargh, BHF Cardiovascular Medicine Unit, Imperial College School of Medicine at the National Heart and Lung Institute, Hammersmith Hospital, Du Cane Rd, London W12 0NN, United Kingdom; e-mail: s.nourshargh@ic.ac.uk.