Transduction of murine stem cells with a multidrug-resistance 1 gene (MDR1) retrovirus results in dramatic ex vivo and in vivo expansion of repopulating cells accompanied by a myeloproliferative disorder. Given the use ofMDR1-containing vectors in human trials, investigations have been extended to nonhuman primates. Peripheral blood stem cells from 2 rhesus monkeys were collected, CD34-enriched, split into 2 portions, and transduced with eitherMDR1 vectors or neo vectors and continued in culture for a total of 10 days before reinfusion. At engraftment, the copy number in granulocytes was extremely high from bothMDR vectors and neo vectors, but the copy number fell to 0.01 to 0.05 for both. There were no perturbations of the leukocyte count or differential noted. After 3 cycles of stem cell factor/granulocyte colony-stimulating factor, there were no changes in the levels of MDR1 vector– or neovector–containing cells. There was no evidence for expansion ofMDR1 vector–transduced cells. Long-term engraftment with MDR1 vector– and neo vector–transduced cells occurred despite prolonged culture.
Introduction
The introduction of drug-resistance genes into hematopoietic stem cells (HSCs) has been pursued as a strategy to protect patients from chemotherapy-induced myelosuppression and to allow in vivo selection of transduced cells.1 The most extensively studied is the multidrug-resistance 1 gene (MDR1) encoding P-glycoprotein (P-gp), a transmembrane pump that effluxes anthracyclines, vinca alkaloids, and paclitaxel. Murine studies demonstrated that HSCs containing MDR1 vectors had preferential survival in vivo after treatment withMDR1-pumped drugs.2 Recently we reported that murine HSCs transduced with a Harvey murine sarcoma virus vector (HaMDR1) expressing a splice-corrected MDR1 complementary DNA (cDNA) were able to expand remarkably in vitro, and when transplanted, the cells continued to expand in vivo, resulting in a polyclonal myeloproliferative syndrome with blastic transformation in some animals.3 Even without prolonged ex vivo expansion, the same phenomenon with longer latency resulted when transduced cells were given without expansion, suggesting thatMDR1-overexpressing HSCs have an intrinsic survival or proliferative advantage.4 Helper virus was not detected, and other Harvey vectors with identical backbones have not induced the same syndrome, thereby implicating the overexpression of P-gp as the etiology of the HSC effect. The underlying mechanism is being actively investigated, but it still remains unclear.
This finding has resulted in concerns regarding the safety of clinical trials employing MDR1 vectors. Several trials have resulted in low-level and often transient engraftment with vector-containing cells, and there is no convincing evidence for selection of transduced cells with post-transplantation chemotherapy.5-8 The most recent trial did report increased levels of vector-containing cells in some patients after chemotherapy.9 Although there has been no observed myeloproliferation, HSC transduction was inefficient, and expression of functional P-gp was hampered by a cryptic splice site in theMDR1 cDNA used.10 We have now used the rhesus macaque to investigate this phenomenon in a model with more direct relevance to human HSC biology.11 Such studies are important to complete before considering clinical trials usingMDR1 vectors optimized for expression.
Study design
Animal care and cell collection
Rhesus macaques (Macaca mulatta) were handled in accordance with published guidelines (DHSS Publication No NIH 85-23), and the protocol was approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute (NHLBI), the National Institutes of Health, Bethesda, MD. Daily doses of 200 μg/kg recombinant human stem cell factor (rHSCF) (Amgen Inc, Thousand Oaks, CA) and 10 μg/kg rHG-CSF (granulocyte colony-stimulating factor) (Amgen) were given subcutaneously (SQ) for 5 days, and mobilized blood cells were harvested by apheresis on day 6 as described.12
Transduction and expansion
Mononuclear cells were isolated by gradient centrifugation (LSM; Organon Teknika, Durham, NC) and enriched for CD34+cells using the magnetic-activated cell sorter (MACS) system (Miltenyi Biotech, Auburn, CA) and the 12.8 anti-CD34 antibody.13The G1Na and HaMDR1 amphotropic vector supernatants were produced from PA 317 clones cultured in Dulbecco modified Eagle medium (DMEM) and 10% fetal calf serum (FCS), and the supernatants had biologic titers of approximately 5 × 106 particles per mL. CD34-enriched cells were split into 2 equal aliquots for G1Na versus HaMDR1 transduction and cultured on flasks coated with Retronectin (Takara Shuzo, Otsu, Japan) in the presence of vector supplemented with 8 μg/mL protamine sulfate (Sigma Chemical Co, St Louis, MO), 20 ng/mL rHIL-3 (interleukin-3) (Sandoz, East Hanover, NJ), 50 ng/mL rHIL-6 (Sandoz), 100 ng/mL rHSCF (Amgen), and 100 ng/mL rHflt-3 ligand (Immunex, Seattle, WA).14 At the conclusion of the 96-hour transduction, cells were replated in fresh DMEM with 10% FCS and cytokines. Media and cytokines were replenished at day 7, and culture volume was adjusted to keep the cell concentration at less than 5 × 105 cells per mL. On day 10 the cells were collected and infused intravenously after the animal received 2 doses of 500 rads total body irradiation (TBI).
Statistical analysis
Blood samples for molecular analysis and complete blood count determinations were collected at the time of engraftment, weekly for 1 month, and then monthly. After 3 months both animals were given a total of 3 monthly cycles of G-CSF/SCF daily for 5 days. One year after transplantation, bone marrow was collected; CD34-enriched with the 12.8 antibody as described above; and then further purified by fluorescence-activated cell sorter (FACS) after staining with a second CD34 antibody, anti-CD34 murine immunoglobulin (Ig)G1 (clone 563; gift from G. Gaudernack, The National Hospital, Oslo, Norway), which was conjugated to allophycocyanin as described.12 Purity was greater than 96%. DNA was extracted using the QIAamp kit (Qiagen, Chatsworth, CA), and RNA was extracted using the RNA STAT 60 (Tel-Test Inc, Friendswood, TX).
Polymerase chain reaction (PCR) for neo sequences and β-actin sequences were performed as described.15 16 PCR for HaMDR1 was performed using primers 5′-GTCTCCTACTTTAGTGCT-3′ and 5′-GCCCACATCATCATGATC-3′ for 28 cycles at 94°C, 54°C, and 72°C for 1 minute each. Reverse transcriptase (RT)-PCR was performed on 1 μg RNA using the GeneAmp RT-PCR Core Kit (Perkin Elmer, Branchburg, NJ) with random hexamers, and the cDNA product was amplified for 45 cycles using the same primers and conditions described above. Phosphorous-32–labeled dicytidine 5′-triphosphate (32P-dCTP) was added to all PCR reactions to allow imaging and quantitation of the resultant products following gel electorphoresis using a Phosphoimager (Molecular Dynamics, Sunnyvale, CA).
Results and discussion
Transduction and culture conditions for rhesus CD34-enriched cells were chosen to be as similar to the murine model as possible.3 The identical HaMDR1 vector was used and packaged amphotropically. CD34-enriched peripheral blood stem cell samples from 2 monkeys were split into equal halves and transduced either with the HaMDR1 vector or a control neo marking vector (G1Na) to assess the competitive repopulation ability of cells transduced with the MDR1 versus the control vector.17 Using monkey Nos. 95E038 and 95E120, 47 and 34 million CD34-enriched cells, respectively, were obtained, transduced, and then expanded as described above. At the end of culture, the total cell number had expanded 40- and 37-fold for the HaMDR1-transduced cells, and 47- and 67-fold for the G1Na-transduced cells, with similar degrees of expansion for colony-forming unit granulocyte-macrophage (CFU-GM).
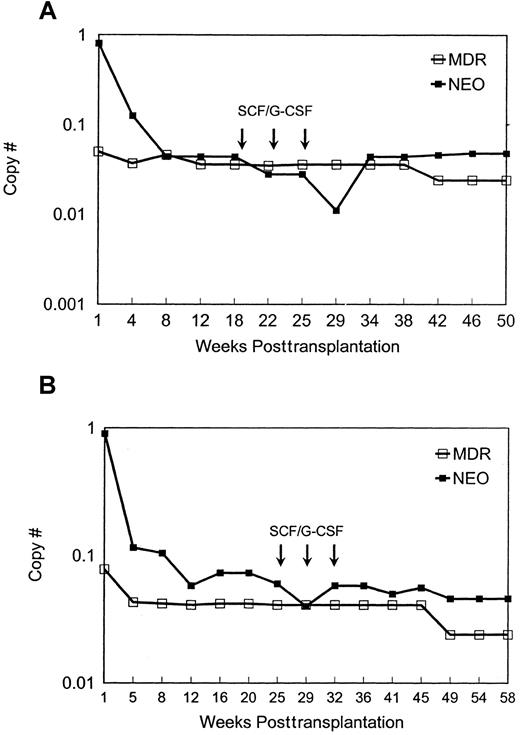
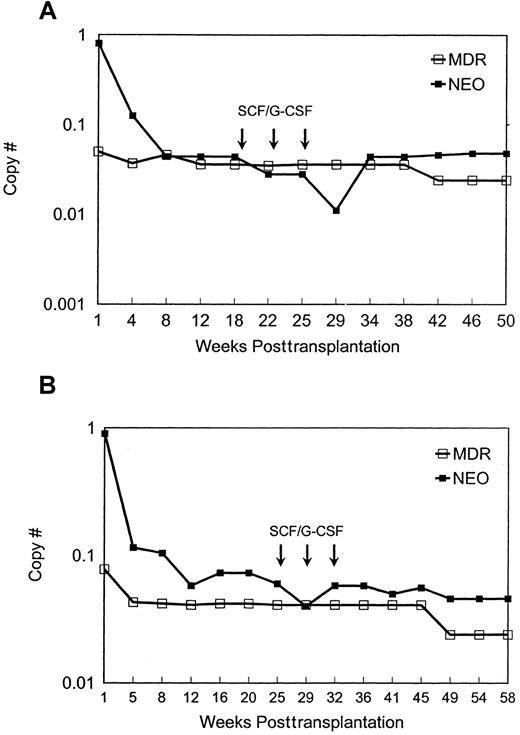
Both animals engrafted rapidly, with neutrophil counts of more than 500 cells per μL by day 10, despite receiving only cells cultured for prolonged periods ex vivo. The level of marked cells containing either vector was initially very high in both animals (Figures 1 and2) and then dropped to stable levels of a few percent, assuming one insertion of vector per cell. There was no evidence for selective expansion of MDR1 compared toneo transduced cells. The animals were treated with 3 monthly cycles of SCF and G-CSF more than 5 months after transplantation in an attempt to accelerate any in vivo advantage forMDR1-transduced cells; these cytokines were effective at decreasing the latency of the stem cell expansion and myeloproliferative syndrome in the murine model.4 We observed no effect on the relative levels of circulating vector-containing cells (Figures 1 and 2). With follow-up of more than one year, this pattern has persisted, and other than the cytokine treatment periods, the animals have maintained normal leukocyte counts and differentials. To document that the MDR1 vector was actually expressing in vivo, RT-PCR was performed, and transcription ofMDR1 driven by the vector long-term repeat sequence was detected in mature circulating cells and in CD34+-purified progenitors (Figure 1B).
Molecular analysis of
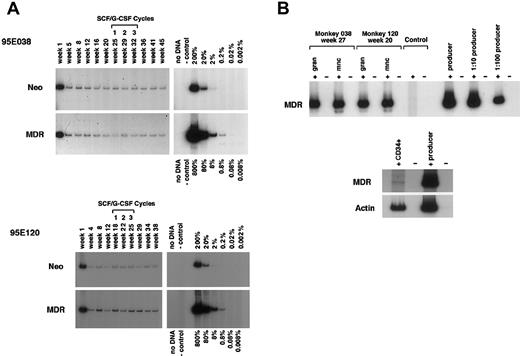
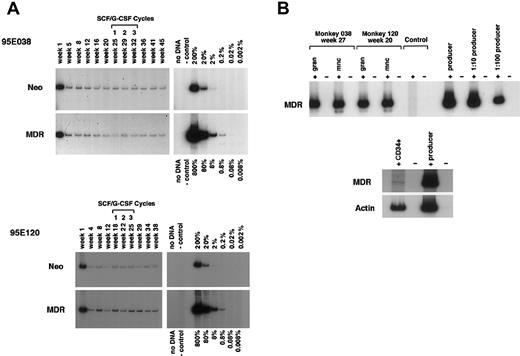
MDR1 vector and neo vector copy number and expression. (A) DNA analysis by PCR for MDR1 vector versus control neo vector sequences in purified granulocytes from animal Nos. 95E038 and 95E120 at various time points after transplantation. Both animals were treated with monthly cycles of SCF and G-CSF as shown, with the timing as related to sampling marked over the lanes. The negative control lane contains normal rhesus peripheral blood DNA, and the positive controls consist of known copy number–transduced cell DNA diluted into normal rhesus peripheral blood DNA. A copy number of one would correspond to one vector genome per cell.(B) RT-PCR MDR1 vector expression analysis of purified peripheral blood granulocytes (gran), mononuclear cells (mnc), and CD34+ cells. The negative lanes indicate that there was no addition of RT. Control: normal rhesus peripheral blood mononuclear cell RNA. Producer: positive control RNA was extracted from theMDR1 retroviral producer line, and serial dilutions of the RNA into normal rhesus mononuclear cell RNA were made prior to the RT reaction.
Molecular analysis of
MDR1 vector and neo vector copy number and expression. (A) DNA analysis by PCR for MDR1 vector versus control neo vector sequences in purified granulocytes from animal Nos. 95E038 and 95E120 at various time points after transplantation. Both animals were treated with monthly cycles of SCF and G-CSF as shown, with the timing as related to sampling marked over the lanes. The negative control lane contains normal rhesus peripheral blood DNA, and the positive controls consist of known copy number–transduced cell DNA diluted into normal rhesus peripheral blood DNA. A copy number of one would correspond to one vector genome per cell.(B) RT-PCR MDR1 vector expression analysis of purified peripheral blood granulocytes (gran), mononuclear cells (mnc), and CD34+ cells. The negative lanes indicate that there was no addition of RT. Control: normal rhesus peripheral blood mononuclear cell RNA. Producer: positive control RNA was extracted from theMDR1 retroviral producer line, and serial dilutions of the RNA into normal rhesus mononuclear cell RNA were made prior to the RT reaction.
Quantitation of
MDR1 vector versus neo vector copy number in granulocytes. The two animals used were designated nos. 95E038 (A) and 95E120 (B). The copy number of MDR1 vector and neo vector sequences in peripheral blood granulocytes at different time points after transplantation is shown. Values were normalized for amplifiable DNA content by concurrent PCR for β-actin sequences, and copy number was estimated using a standard curve generated from concurrent amplification of known vector copy number DNA diluted into normal rhesus DNA.
Quantitation of
MDR1 vector versus neo vector copy number in granulocytes. The two animals used were designated nos. 95E038 (A) and 95E120 (B). The copy number of MDR1 vector and neo vector sequences in peripheral blood granulocytes at different time points after transplantation is shown. Values were normalized for amplifiable DNA content by concurrent PCR for β-actin sequences, and copy number was estimated using a standard curve generated from concurrent amplification of known vector copy number DNA diluted into normal rhesus DNA.
There are a number of explanations for the lack of an observable phenotype in the rhesus compared to the murine model. Stem cell kinetics in mice versus primates may be quite different. In general, mice receiving transduced cells have oligoclonal reconstitution, suggesting that (1) a relatively small number of stem cells contribute to hematopoiesis, (2) each stem cell therefore may be mitotically very active, and (3) differences in proliferative or survival potential may manifest quickly.3 In contrast, we have shown that primates appear to reconstitute polyclonally, and 40 or more transduced clones contribute to reconstitution for more than one year.18 Therefore, any effect could require longer latency. Second, in primates the expression level of the MDR1 protein in HSCs or progenitors may be inadequate to produce the effect. In the murine studies, each MDR1- or control-transduced primitive cell contributing to hematopoiesis appeared to have multiple vector insertions.3,4 In primates we have rarely found evidence for more than one vector insertion per cell, presumably resulting in a lower expression level.18 Third, the effect may be specific to murine cells due to specific interactions ofMDR1 with murine pathways affecting proliferation or survival of cells. Although these data are reassuring in some respects regarding the use of MDR1 vectors in past and future clinical trials, follow-up of these animals should be continued, and caution should be exercised until more information regarding the mechanism of the effect in the murine cellsis obtained.
Whether extended ex vivo culture can “expand” short-term or long-term engrafting cells is a critical issue for gene therapy and clinical transplantation applications. Previously we reported that transduced cells expanded for 10-14 days lost almost all repopulating ability, even short-term, compared to cells cultured for only 4 days.15 Thus, we were surprised that in the current study, engraftment with transduced expanded cells occurred at encouraging levels up to 5% long-term. These cultures included the CH-296 carboxy-terminal fibronectin fragment, and this may have supported viability and cycling of engrafting cells better than standard supernatant culture or culture on marrow stromal cells.19 20 We are now directly examining this finding in the macaque by comparing the engraftment of cells cultured and transduced for 10-14 days in the presence of fibronectin to cells cultured and transduced for 4 days.
We wish to thank Immunex (Seattle, WA) for supplying flt-3 ligand, Amgen (Thousand Oaks, CA) for supplying stem cell factor and G-CSF, and Takara Shuzo (Otsu, Japan) for supplying Retronectin.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Cynthia E. Dunbar, Hematology Branch, National Heart, Lung, and Blood Institute, Bldg 10, Rm 7C103, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: dunbarc@nhlbi.nih.gov.