Abstract
Clonal expansion of activated T cells is controlled by homeostatic mechanisms leading to cell death of a large proportion of the cells. The CD3/TcR pathway induces cell death, mostly when triggered in the absence of costimulatory signal. The unique T cell–specific chemokine of the C class, lymphotactin (Lptn), has recently been shown to inhibit the production of Th1-type lymphokines in human CD4+ T cells. The present study shows the ability of Lptn to costimulate the death of CD4+ T lymphocytes triggered through CD3/TCR. The Lptn-mediated increased cell death exhibited characteristic features of apoptosis, as mainly determined by DNA fragmentation and exposure of an apoptotic-specific mitochondrial antigen. This apoptosis was dependent on Fas/FasL signaling, was not rescued by addition of interleukin 2, and proceeded with a predominant processing of both initiator procaspase-9 and effector procaspase-7. These caspase activities were further evidenced by specific cleavage of poly(ADP-ribose) polymerase (PARP) and CD3/TCR ζ-chain, but not DNA fragmentation factor (DFF45). This study demonstrates that the functional repertoire of Lptn in the regulation of human CD4+ T-lymphocyte activation includes the ability to costimulate apoptosis.
Introduction
Chemokines play a crucial role in immune and inflammatory reactions by causing both the recruitment and activation of leukocytes.1,2 With the exception of lymphotactin (Lptn), most chemokines share a conserved 4-cysteine motif in their N-terminus. Based on the arrangement of this motif and on both genetic and functional criteria, chemokines are subdivided into 4 subfamilies, designated as CXC (or α), CC (β), C (γ), CX3C (δ).1,2 Although the number of both CXC and CC chemokines is continually growing, the C and CX3C are single representatives, named lymphotactin, Lptn/SCM-1/ATAC3-5 and fractalkine/neurotactin,6,7 respectively. CXC chemokines mainly target neutrophils and T cells; CC chemokines generally attract monocytes, eosinophils, basophils, and T cells. In contrast, both C and CX3C chemokines have a specificity mainly restricted to T cells. Both in vitro and in vivo, Lptn is chemotactic toward human CD8+ and CD4+ T cells, but only modestly toward natural killer (NK) cells.3,8-11 As already demonstrated for most chemokine receptors,12-14 the receptor for Lptn, XCR1, also belongs to G protein–coupled receptors.15 CC and CXC chemokines play a role in T-cell activation events such as proliferation, production of cytokines, induction of immunoglobulin production, cytotoxicity, adhesion to extracellular matrix proteins, and apoptosis.16-23 Until our recent work,24chemotaxis was the only reported function ascribed to Lptn.
In physiologic situations, peripheral T cells respond to CD3/TCR complex triggering by proliferating, producing cytokines and receptors, undergoing death, or becoming anergic. A variety of factors, including the type of antigen, the magnitude of TCR stimulation, the coordinate engagement of costimulatory molecules such as CD28, or the production of T-cell growth factors such as interleukin 2 (IL-2), are known to influence the destiny of T cells. IL-2 or CD28 signals cooperate with TCR engagement to induce optimal T-cell clonal expansion, production of cytokines,25,26 including CC chemokines,27 and expression of cytokine receptors such as IL-2 receptor (IL-2R).28 29 The lack of these costimulatory signals during TCR triggering results in anergic or apoptotic response of T cells.
Apoptosis is an active form of cell death that is crucial to maintain self-tolerance of the peripheral immune system, by down-regulating the cellular expansion of activated T cells.30,31 Among molecular mechanisms involved in this process, the Fas/Fas ligand (FasL) system is implicated as a major mediator.32 FasL can be cleaved from the cell surface via a metalloproteinase, releasing a soluble form.33,34 This latter form (sFasL) has been shown not only to be less active than the membrane-bound form (mFasL), regarding their proapoptotic activity,35 but also to inhibit the activity of mFasL.36 The apoptotic program includes characteristic morphologic and biochemical changes. Caspases (cysteine aspartate-specific proteases) are critical mediators of apoptosis characterized by their ability to cleave their substrates after aspartic residues.37 Caspase activity results in the cleavage of several cellular substrates38 such as the poly(ADP-ribose) polymerase (PARP),39-41 the DNA fragmentation factor 45 (DFF45),42,43 the CD3/TCR ζ-chain,44 as well as caspases themselves. A current model suggests that apoptotic stimuli like death receptors activate initiator caspases such as caspase-8 or -9, that in turn cleave and activate the engagement of effector caspases, such as caspase-3, -6, and -7.37 45
Our recent work provided evidence that Lptn was optimally produced in response to TCR engagement alone, in the absence of CD28 costimulation, in human CD4+ T cells.46 This expression was accompanied by an inhibitory effect of Lptn on CD4+ T cell proliferation through the inhibition of Th1-type lymphokine production.24 In the work reported herein, we describe the enhancement of TCR-induced apoptosis of CD4+ T cells by Lptn, which might also explain the reported inhibition of proliferation.24 This increase in apoptosis occurred through Fas/FasL interaction as supported by complete inhibition in the presence of antagonist anti-Fas monoclonal antibody (mAb), by up-regulation of mFasL, and down-regulation of sFasL expression, both induced by Lptn. This Lptn-mediated apoptosis was mostly dependent on Fas/FasL signaling, was not completely rescued by addition of IL-2, and proceeded with a strong processing of the initiator procaspase-9 and the effector procaspase-7. These Lptn-induced caspase activities resulted in specific cleavage of PARP, CD3/TCR ζ-chain, but not DFF45.
Materials and methods
CD4+T-cell purification and activation
The T cells were isolated from mononuclear cells as described elsewhere28 and then purified by negative selection using antimouse IgG magnetic immunobeads that were coupled with an anti-CD8 mAb (10B4.6 [D. Olive, Marseille, France]), as recommended by the manufacturer (Beckman, Immunotech, Marseille, France). They were more than 90% CD4+ pure before activation, remaining at this degree of purity over the entire stimulation period, as controlled by labeling with specific mAbs. These cells were activated with the following stimuli: anti-CD3 mAb, singly or in combination with the recombinant human chemokines, Lptn or the control RANTES (Promocell GmbH, Heidelberg, Germany), used as previously described.24 In all types of experiments, a unique dose of 1 μg/mL Lptn was used, except for both TUNEL assays and 7A6 antigen expression analyses, where the use of 500 ng/mL was tested and gave similar effects, as previously described.24 In some experiments, a soluble anti-CD28 mAb or recombinant human IL-2 (Chiron; France) was used in combination with anti-CD3 mAb to activate the cells.24 The neutralizing polyclonal rabbit antiserum specific for Lptn (Promocell GmbH) was added at 25 μg/mL at the initiation of the cultures.24 In the indicated experiments, the following reagents were added to the cells 15 minutes before stimulation: ZB4 and 7C11 anti-Fas mAbs (Immunotech) acting, respectively, as inhibitor and inducer of Fas-mediated apoptosis, both used at 1 μg/mL; z-VAD-fmk ([Z-V-A-D-(OMe)-CH2F]; France Biochem, Meudon, France) a cell-permeable and irreversible pan-caspase inhibitor, used at 25 μM.
Proliferationassays
The T cells (1 × 106/mL) were plated in flat-bottom 24- or 96-well culture dishes (Costar, Cambridge, MA), previously coated with the above-mentioned stimuli. After 96 hours of culture, wells were pulsed with 1μCi [3H]-thymidine (Amersham France, Les Ulis, France) for the remaining 18 hours and then harvested onto glass fiber filters. Thymidine incorporation was measured in a direct beta counter (Matrix 9600, Packard Instruments, Rungis, France).
Cell viability and hypodiploid assessment
The CD4+ T cells were activated in 24-well plates for the indicated days, as described above. Trypan blue exclusion and propidium iodide (PI) staining were assessed by light microscopy and flow cytometry, respectively. For PI assays, the cells were stained for 30 minutes at 4°C with a fluorescein isothiocyanate ( FITC)–conjugated anti-CD4 mAb, or a nonbinding matched isotype control mAb (Immunotech), washed twice in phosphate-buffered saline (PBS), and then fixed in PBS plus ethanol 70% (1 vol/5 vol) at −20°C. After washing, they were resuspended in PI/RNAse A (10μg/250μg/mL) buffer and incubated 30 minutes at 37°C. Samples were analyzed using a Becton Dickinson (Mountain View, CA) FACScan cytometer. Ten thousand events were acquired and dead cells were excluded using forward- and side-scatter gating. Percent of cells was determined in the different phases of cell cycle, as previously described.47
Immunofluorescence staining and flow cytometric analysis (fluorescence-activated cell sorter)
For estimation of surface expression of Fas, cells were stained with FITC-conjugated anti-Fas mAb (clone UB2) or a nonbinding matched isotype control mAb (both from Immunotech).
The expression of 7A6 antigen, a mitochondrial membrane protein that is exposed on cells undergoing apoptosis, was analyzed by staining with a PE-coupled Apo2.7 mAb (clone 2.7A6A3, Immunotech), after activation of the cells followed by digitonin permeabilization, as recommended by the manufacturer. Fluorescence-activated cell sorter (FACS) analyses were performed as described above. In some experiments, prior to activation, cells were either left untreated or pretreated at 37°C for 15 minutes with pertussis toxin (PT), an inhibitor of G protein–coupled receptor signal transduction.
Enzyme-linked immunosorbent assay detection of mFasL and sFasL
Quantitative determination of human FasL protein in both lysates and supernatants from activated CD4+ T cells was performed with a commercial “sandwich” enzyme immunoassay using mouse mAbs (Oncogene Research Products, France Biochem), according to the manufacturer's instructions. Lysates and supernatants were prepared or collected, respectively, from the activated cells harvested at different times after activation between 3 and 120 hours, and were then frozen at −80°C until analysis. Serial dilutions of the supernatants and lysates were performed to ensure measuring in the linear range, and the sensitivity of the enzyme-linked immunosorbent assay (ELISA) assay was 0.02 ng/mL.
DNA fragmentation analysis
DNA fragmentation was detected by the TUNEL method using an in situ cell death detection kit (Boehringer Mannheim, Roche Diagnostics, Meylan, France) according to the manufacturers' instructions. Briefly, CD4+ T cells were stimulated for overnight to 4 days, as described above. They were then daily harvested and washed twice in PBS. After centrifugation, cell pellets were resuspended in 200 μL PBS containing 4% paraformaldehyde for 1 hour at room temperature, and washed twice again. Permeabilization was conducted using 100 μL 0.1% Triton X-100 and 0.1% sodium citrate in PBS. After washing, cells were resuspended in TUNEL reaction mixture containing FITC-dUTP and terminal-deoxy-nucleotidyl-transferase (TdT). Control cells were incubated in TUNEL reaction mixture containing FITC-dUTP without TdT. Incubation was performed for 1 hour at 37°C before washing cells twice. Fluorescein labels incorporated in DNA strand breaks were detected by flow cytometry, as described above.
Westernblots
The CD4+ T cells (2 × 106/point) were harvested at different times between 3 and 120 hours after stimulation and washed twice in PBS. Pellets were boiled 5 minutes in lysis buffer (10 mM Tris-HCl, pH 7.4, containing 1% sodium dodecyl sulfate [SDS] and 1 mM sodium vanadate), and then frozen at −20°C. Protein lysates were separated by appropriate percentages of SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to Immobilon-P membranes (Millipore, Bedford, MA) as recommended by the manufacturer. Membranes were probed with the following antibodies directed against caspase-3 (MBL, France Biochem, Nagoya, Japan), caspase-4 (Immunotech), caspases-7, -8, -9 (MBL), DFF45 (MBL), (TCR) ζ-chain (6B10.2; Santa Cruz Biotechnology, Santa Cruz, CA), PARP (Chemicon International, Euromedex, Souffleweyersheim, France), that were used according to the manufacturer's instructions. The protein bands were detected by enhanced chemiluminescence (ECL, Amersham Life Science,) at different times. To ensure that the expression of the different proteins could be compared between the samples, blots were reprobed with a polyclonal antibody directed against the p85 subunit of phosphatidyl-inositol-3-OH kinase (UBI, Euromedex), which displays a uniform level of expression in T lymphocytes (not shown). The intensity of bands was quantified using a laser densitometer (Ultroscan LKB, Pharmacia). The level of expression of a specific band was given as the ratio (optical density of the band/optical density of p85) and normalized results were expressed in arbitrary units.
Caspase-9 colorimetric assay
The enzymatic activity of caspase-9 was determined in CD4+ T cells activated for the indicated days via CD3, singly or in combination with Lptn, or its neutralizing Ab, using a commercial kit (R&D Systems Europe, Abingdon, England). The assays were performed according to the manufacturer's protocol.
Results
Lptn inhibits and induces both cell growth and death of human CD4+T cells, respectively
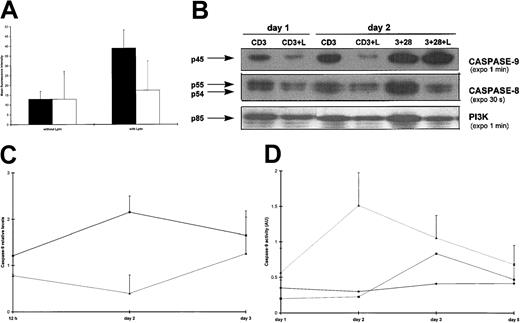
We previously demonstrated that Lptn inhibited the production of Th1-type lymphokines (IL-2, interferon-γ [IFN-γ]) by CD3/TCR-activated CD4+ T cells, as well as their proliferation.24 To determine whether this reduced proliferation was due to decreased cell division or increased cell death (or both), viability and cell cycle analysis for determination of DNA content were assessed by trypan blue exclusion (Figure1A) and PI staining (Figure 1B,C), respectively. We observed a progressive increase in the number of living lymphocytes on CD3 stimulation alone, which was abrogated by Lptn (Figure 1A). A similar increase on CD3 stimulation, in the presence of either anti-Lptn Ab (Figure 1A) or the CC chemokine RANTES (not shown), indicated both specificity and selectivity of the Lptn inhibitory effect. Under identical conditions, PI staining revealed that the presence of Lptn not only induced a reduction in the percent of cells at the G2/M interface (Figure 1B) but also increased the percent of subdiploid cells (Figure 1B,C). The accumulation of subdiploid cells was abrogated by the addition of anti-Lptn Ab (Figure 1B,C). Thus, the inhibition of CD4+T-cell proliferation induced by Lptn could result from both arrest in cell growth and increase in cell death.
Enhancement of both arrest in cell growth and increase in cell death by Lptn.
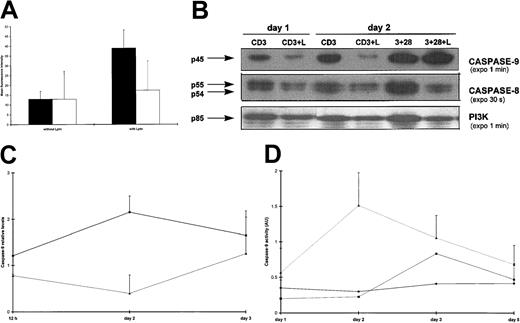
(A) The kinetics of viable cell recovery, as measured by trypan blue exclusion, of purified CD4+ T cells activated for the indicated days with plate-bound CD3 mAb, in the presence or absence of either Lptn or RANTES. At day 0, 4 × 106 cells/well were plated in each activation condition. Similar cell counts were obtained in the presence of RANTES, alone or in conjunction with its specific Ab, and are not shown. The figure represents the mean values ± SD (error bars) of 3 independent experiments. ▪, CD3; ♦, CD3 plus Lptn; ▴, CD3 plus Lptn plus anti-Lptn. (B) A typical flow cytometric profile of PI-stained CD4+ T cells. Cells were activated with CD3 mAb in the presence or absence of Lptn. At day 2 after activation, the different stages of cell cycle were analyzed for nuclei DNA content after PI uptake. The percentages of cells in the different stages (subdiploid [< 2n DNA], G0/G1 [2n DNA], and S+G2+M [> 2n DNA]) are indicated. In parallel, CD4 staining ensured a 90% purity of the cultured cells (not shown). (C) Quantitative expression of the data shown in panel A. CD4+ T cells were activated for 2 days with the indicated stimuli. Each graph represents the mean values ± SD of 3 independent experiments.
Enhancement of both arrest in cell growth and increase in cell death by Lptn.
(A) The kinetics of viable cell recovery, as measured by trypan blue exclusion, of purified CD4+ T cells activated for the indicated days with plate-bound CD3 mAb, in the presence or absence of either Lptn or RANTES. At day 0, 4 × 106 cells/well were plated in each activation condition. Similar cell counts were obtained in the presence of RANTES, alone or in conjunction with its specific Ab, and are not shown. The figure represents the mean values ± SD (error bars) of 3 independent experiments. ▪, CD3; ♦, CD3 plus Lptn; ▴, CD3 plus Lptn plus anti-Lptn. (B) A typical flow cytometric profile of PI-stained CD4+ T cells. Cells were activated with CD3 mAb in the presence or absence of Lptn. At day 2 after activation, the different stages of cell cycle were analyzed for nuclei DNA content after PI uptake. The percentages of cells in the different stages (subdiploid [< 2n DNA], G0/G1 [2n DNA], and S+G2+M [> 2n DNA]) are indicated. In parallel, CD4 staining ensured a 90% purity of the cultured cells (not shown). (C) Quantitative expression of the data shown in panel A. CD4+ T cells were activated for 2 days with the indicated stimuli. Each graph represents the mean values ± SD of 3 independent experiments.
Lptn-mediated increase in CD4+T-cell death occurs through an apoptotic-like mechanism
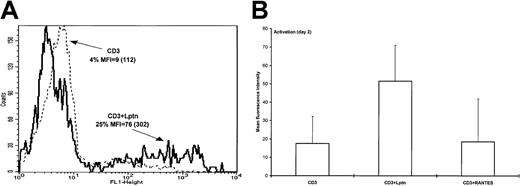
In a attempt to determine whether an apoptotic-like mechanism could characterize the Lptn-mediated increase in CD4+T-cell death, both DNA fragmentation and detection of the 7A6 mitochondrial protein at the cell surface were used. DNA fragmentation was evaluated by terminal deoxynucleotidyl transferase-mediated nick-end labeling assay (TUNEL). Positive controls were systematically included in each activation condition by treatment of the cells with DNase I. The flow cytometric profiles presented in Figure2A show an enhanced TUNEL staining, when Lptn was present together with CD3/TCR stimulation. The observed increase in the percent of apoptotic cells and mean fluorescence intensity (MFI) peaked at day 2 (not shown). Contrasting with the effect of Lptn, RANTES did not noticeably affect the intensity of TUNEL staining, indicating the selectivity of Lptn (Figure 2B).
DNA fragmentation as a characteristic of Lptn-mediated CD4+ T-cell death.
(A) DNA fragmentation was evaluated by enzymatic in situ labeling of DNA strand breaks (TUNEL assay), as described in “Materials and methods.” Briefly, CD4+ T cells were activated for 2 days with the indicated stimuli and subjected to labeling with FITC-dUTP and the TdT enzyme, followed by flow cytometric analysis. FACS profiles are representative of one out of 5 independent experiments. (B) Quantitative determination of TUNEL-based DNA fragmentation intensity performed as described in panel A. In the case of RANTES, 2 experiments were performed.
DNA fragmentation as a characteristic of Lptn-mediated CD4+ T-cell death.
(A) DNA fragmentation was evaluated by enzymatic in situ labeling of DNA strand breaks (TUNEL assay), as described in “Materials and methods.” Briefly, CD4+ T cells were activated for 2 days with the indicated stimuli and subjected to labeling with FITC-dUTP and the TdT enzyme, followed by flow cytometric analysis. FACS profiles are representative of one out of 5 independent experiments. (B) Quantitative determination of TUNEL-based DNA fragmentation intensity performed as described in panel A. In the case of RANTES, 2 experiments were performed.
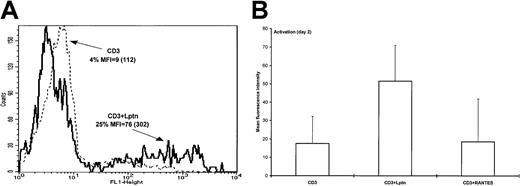
To confirm that cell death induced by Lptn was due to apoptosis, we performed immunodetection of 7A6 mitochondrial protein, which is specifically expressed on apoptotic cells.48 In correlation with the TUNEL assay results, the 7A6 expression increased on CD3 stimulation in the presence of Lptn, as indicated by the percentage of positive cells (Table 1), and more variably by MFI (not shown). This increase was totally abrogated by the addition of anti-Lptn Ab or PT (Table 1), supporting specificity and coupling to Gα type of G proteins of the Lptn-mediated signaling, respectively. Interestingly, in both types of experiments, the addition of exogenous IL-2 was not able to reduce the intensity of the Lptn-mediated CD4+ T-cell death to the control level (CD3 stimulation alone), suggesting that the strong IL-2 deprivation induced by Lptn in such activation conditions24did not completely account for the apoptotic effect of Lptn (Table 1). Similar results were obtained from TUNEL-based experiments (not shown).
Lptn-induced increase in CD4+T-cell apoptosis is mostly Fas dependent
Fas signaling is the best known inducer of apoptosis in CD4+ T cells.49,50 To examine whether this signaling could contribute to the Lptn-induced cell death, we first examined the effects of agonistic (7C11) and antagonist (ZB4) anti-Fas mAbs on CD4+ T-cell proliferation. For this purpose, T cells were preincubated with the antibodies and thymidine uptake was then measured, as described.24 As shown in Figure 3A, a slight reduction in the CD3-mediated proliferation was induced by the agonistic 7C11 mAb, whereas no effect of the antagonist ZB4 mAb was detected. In contrast, this latter antibody completely reversed to the control level (CD3 stimulation alone) the Lptn-mediated inhibition of proliferation, whereas the agonistic anti-Fas mAb had no significant effect (Figure 3A).
Lptn-mediated apoptosis occurs through Fas/FasL signaling pathway.
(A) Effects of ZB4 and 7C11 anti-Fas mAbs on the Lptn-induced inhibition of proliferation. Proliferation was assessed by [3H]-thymidine incorporation during the last 18 hours of 96-hour culture of CD4+ T cells activated via CD3, in the presence or absence of Lptn. Both anti-Fas mAbs (used at 1 μg/mL) were preincubated 1 hour before activation. Results are expressed as the mean count/min values ± SD of 3 separate experiments. (B) Protection by ZB4 mAb against Lptn-induced increase in DNA fragmentation. CD4+ T cells were preincubated with 1μg/mL of the antagonist ZB4 anti-Fas mAb prior to CD4+ T-cell stimulation for 2 days, as described in “Materials and methods.” Cells were then washed and subjected to the TUNEL assay, according to manufacturer's protocol. For each sample, an aliquot was treated for 10 minutes with DNase I, serving as positive control. After FITC-dUTP and TdT staining, analyses were performed by flow cytometry. Data represent values of mean fluorescence intensity ± SD (error bars) calculated from at least 3 independent experiments. ▪, CD3; ■, CD3 plus ZB4.
Lptn-mediated apoptosis occurs through Fas/FasL signaling pathway.
(A) Effects of ZB4 and 7C11 anti-Fas mAbs on the Lptn-induced inhibition of proliferation. Proliferation was assessed by [3H]-thymidine incorporation during the last 18 hours of 96-hour culture of CD4+ T cells activated via CD3, in the presence or absence of Lptn. Both anti-Fas mAbs (used at 1 μg/mL) were preincubated 1 hour before activation. Results are expressed as the mean count/min values ± SD of 3 separate experiments. (B) Protection by ZB4 mAb against Lptn-induced increase in DNA fragmentation. CD4+ T cells were preincubated with 1μg/mL of the antagonist ZB4 anti-Fas mAb prior to CD4+ T-cell stimulation for 2 days, as described in “Materials and methods.” Cells were then washed and subjected to the TUNEL assay, according to manufacturer's protocol. For each sample, an aliquot was treated for 10 minutes with DNase I, serving as positive control. After FITC-dUTP and TdT staining, analyses were performed by flow cytometry. Data represent values of mean fluorescence intensity ± SD (error bars) calculated from at least 3 independent experiments. ▪, CD3; ■, CD3 plus ZB4.
To further confirm the involvement of the Fas/FasL system, we examined the expression of both the soluble and membrane-bound forms of FasL in the same ELISA assays, the results of which are presented in Table2. In absence of Lptn, the amount of sFasL progressively accumulated to reach approximately 10 ng/mL between days 3 and 5, whereas in presence of Lptn, a very constant lower level (around 2 ng/mL) persisted during the same time (Table 2). This 5-fold reduction in the amount of sFasL in supernatants of the Lptn-treated cells was probably not due to the decrease in cell number induced by Lptn, because this reduced amount of sFasL was detected before the decrease in cell number. In sharp contrast with this, a constant low level (1-2 ng/mL) of mFasL expression was measured in the absence of Lptn, as opposed to a 3-fold higher level in the presence of Lptn (Table 2). This increase in mFasL expression peaked at day 2, concomitant with the peak of Lptn-induced cell death. The expression of Fas that progressively increased on CD3 stimulation was additionally up-regulated by Lptn, at early times after activation (not shown). The inefficiency of Lptn to enhance cell death, on CD3 stimulation in the presence of IL-2, was associated with an opposite pattern of mFasL expression, because this expression was down-regulated by the presence of Lptn (not shown).
Finally, to more directly investigate the implication of Fas signaling in the Lptn-induced apoptosis, we examined the effect of the antagonist anti-Fas ZB4 mAb on DNA fragmentation using the TUNEL assay. As shown in Figure 3B, preincubation of the cells with ZB4 mAb did not affect the intensity of TUNEL staining on CD3 stimulation alone, whereas it reduced staining intensity in the presence of Lptn, nearly to the levels detected in the absence of Lptn. A comparable inhibitory effect of ZB4 mAb was observed on the Lptn-induced increase in 7A6 antigen expression (data not shown). Collectively, these results supported a role of the Fas/FasL pathway in Lptn-mediated apoptosis.
Selective activation of both initiator and effector caspases in response to Lptn-induced apoptosis
Because caspase activation is a common final step required for many apoptotic stimuli, we examined the effect of a pan-caspase inhibitor, z-VAD-fmk, on DNA fragmentation in CD3-activated T cells in the presence or absence of Lptn. As shown in Figure4A, z-VAD-fmk completely inhibited the Lptn-mediated increase in TUNEL staining, when compared with TUNEL positivity obtained by CD3 stimulation alone. In addition, the reduced thymidine uptake in Lptn-treated cells was also overcome by this inhibitor, whereas that of untreated cells was not affected (not shown). Because z-VAD-fmk has been shown to titrate a large number of caspases, we further investigated the contribution of specific initiator and effector caspases in Lptn-mediated apoptosis. The processing of these 2 sets of caspases was analyzed by Western blots, performed at different times on CD3 stimulation in the presence or absence of Lptn. With respect to the initiator caspases, a clear processing of procaspase-9 was observed in Lptn-treated cells as opposed to untreated cells (Figure 4B). This processing peaked at day 2 (Figure 4B,C) and returned to the control level (Lptn-untreated cells) at day 3 (Figure 4C). With regard to the expression of procaspase-8, a transient increase was observed at day 2 in both activation conditions (not shown). The procaspase-8 levels seemed, however, to be steadily lower (from 0.5-5 days) in the presence of Lptn (Figure 4B and not shown). As an internal positive control, a clear processing of the procaspase-8 was detected in the presence of Lptn, on CD3 and CD28 costimulation (Figure 4B). Because no cleaved fragment of caspase-9 could be detected even after long exposures of the blots, the activity of this caspase was investigated at different times on CD3 stimulation in the presence or absence of Lptn, using LEHD, a synthetic tetrapeptide substrate in colorimetric assays, as described in “Materials and methods.” As seen in Figure 4D, a 6-fold increase in caspase-9 activity, peaking at day 2, was measured in Lptn-treated cells, as compared with either untreated cells or Lptn-treated cells in conjunction with the anti-Lptn Ab. Consequently, the kinetics of increase in caspase-9 activity correlated closely with the kinetics of both decrease in caspase-9 proform expression and increase in DNA fragmentation, observed in response to Lptn.
Selective activation of initiator caspases in Lptn-induced apoptosis.
(A) The Lptn-induced increase in DNA fragmentation is blocked by z-VAD-fmk; 25 μM of z-VAD-fmk (▪) or control (■) DMSO was preincubated with the cells prior to CD3 stimulation for 2 days, in the presence or absence of Lptn, followed by TUNEL staining. The panel is representative of 2 independent experiments. (B) Respective strong and weak processings of procaspases-9 and -8 in Lptn-mediated apoptosis. CD4+ T-cell lysates were made after the indicated times and conditions of activation and analyzed by Western blots that were sequentially probed for detection of expression of caspases-9 and -8, and the invariant PI3K control, using the antibodies described in “Materials and methods.” Arrows indicate the procaspases. No or little increased expression of procaspase-9, and decreased expression (2- to 3-fold) of procaspase-8 are observed on CD3 and CD28 costimulation in the presence of Lptn. This typical experiment is a representative of 3. (C) Kinetics of down-regulation of procaspase-9 expression by Lptn. The graph represents the relative levels of the caspase-9/PI3K signal ratio induced by the indicated stimuli, with error bars corresponding to SD. The results are representative of 3, 4, and 3 independent experiments at 0.5, 2, and 3 days, respectively. (D) An increase in caspase-9 activity is induced by Lptn. Cell lysates were prepared after the indicated times and conditions of activation, and analyzed in a caspase-9 specific colorimetric assay, as described in “Materials and methods.” For each condition, the graph represents the mean values from 2 independent experiments.
Selective activation of initiator caspases in Lptn-induced apoptosis.
(A) The Lptn-induced increase in DNA fragmentation is blocked by z-VAD-fmk; 25 μM of z-VAD-fmk (▪) or control (■) DMSO was preincubated with the cells prior to CD3 stimulation for 2 days, in the presence or absence of Lptn, followed by TUNEL staining. The panel is representative of 2 independent experiments. (B) Respective strong and weak processings of procaspases-9 and -8 in Lptn-mediated apoptosis. CD4+ T-cell lysates were made after the indicated times and conditions of activation and analyzed by Western blots that were sequentially probed for detection of expression of caspases-9 and -8, and the invariant PI3K control, using the antibodies described in “Materials and methods.” Arrows indicate the procaspases. No or little increased expression of procaspase-9, and decreased expression (2- to 3-fold) of procaspase-8 are observed on CD3 and CD28 costimulation in the presence of Lptn. This typical experiment is a representative of 3. (C) Kinetics of down-regulation of procaspase-9 expression by Lptn. The graph represents the relative levels of the caspase-9/PI3K signal ratio induced by the indicated stimuli, with error bars corresponding to SD. The results are representative of 3, 4, and 3 independent experiments at 0.5, 2, and 3 days, respectively. (D) An increase in caspase-9 activity is induced by Lptn. Cell lysates were prepared after the indicated times and conditions of activation, and analyzed in a caspase-9 specific colorimetric assay, as described in “Materials and methods.” For each condition, the graph represents the mean values from 2 independent experiments.
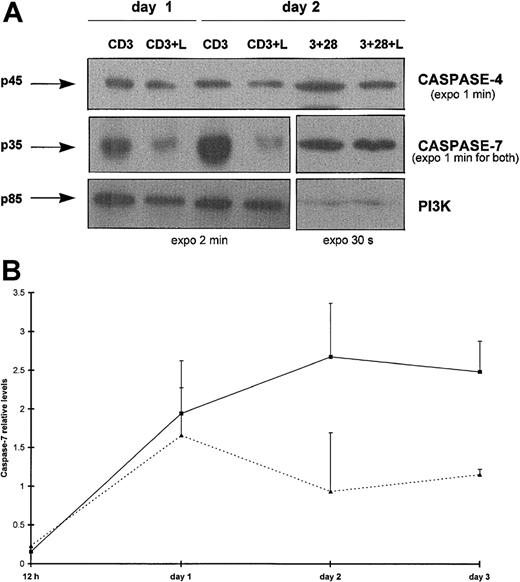
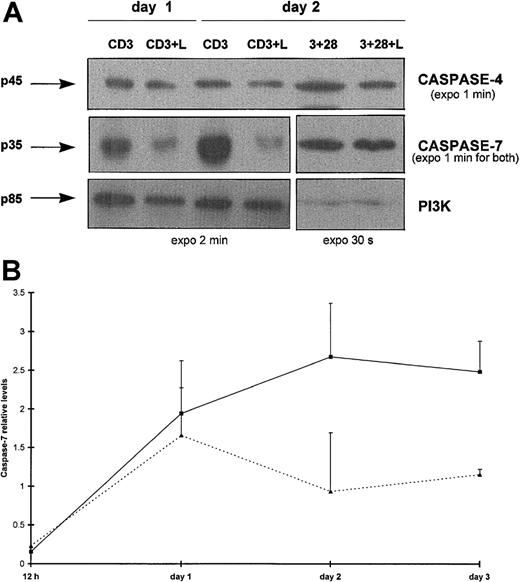
We next considered whether some effector caspases could also be specifically processed by Lptn under identical conditions. By Western blotting, a marked decrease in levels of the caspase-7 proenzyme was detected from day 1 on CD3 stimulation, in the presence but not in the absence of Lptn (Figure 5A). This decrease peaked from day 2 to at least day 3 (Figure 5B, and not shown for day 4). As an internal control, no significant variation in caspase-7 proenzyme expression was detected on CD3 and CD28 costimulation, irrespective of the presence or absence of Lptn (Figure5A and not shown). The processing of caspase-7 appeared to be selective because caspase-3 (not shown) and caspase-4 (Figure 5A) proenzymes remained at constant levels in the presence of Lptn. For caspase-3, a 9-fold increased expression was observed at day 2 on CD3 stimulation alone (not shown). With regard to procaspase-4, expression profiles were similar in the presence or absence of Lptn (Figure 5A and not shown) following CD3 stimulation, but not on CD3 and CD28 costimulation (3-fold increase in absence of Lptn) (Figure 5A).
Selective processing of effector caspases in Lptn-induced apoptosis.
(A) Total proteins of CD4+ T cells activated for days 0.5 to 4 with the indicated stimuli were separated by SDS-PAGE and analyzed by Western blotting for detection of expression of caspases-4 and -7, and the PI3K control, using the antibodies described in “Materials and methods.” Arrows indicate the full-length forms of the caspases. Positive controls of expression of procaspase-7 (no variation with or without Lptn) and procaspase-4 (around 3-fold reduction with Lptn) are shown on CD3 and CD28 costimulation, as a representative experiment of 2. (B) Kinetics of procaspase-7 processing induced by Lptn. The graph represents the relative levels of the caspase-7/PI3K signal ratio induced by the indicated stimuli, with error bars corresponding to SD. These mean levels are representative of 2, 3, 5, and 2 independent experiments at 0.5, 1, 2, and 3 days, respectively. ▪, CD3; ▴, CD3 plus Lptn.
Selective processing of effector caspases in Lptn-induced apoptosis.
(A) Total proteins of CD4+ T cells activated for days 0.5 to 4 with the indicated stimuli were separated by SDS-PAGE and analyzed by Western blotting for detection of expression of caspases-4 and -7, and the PI3K control, using the antibodies described in “Materials and methods.” Arrows indicate the full-length forms of the caspases. Positive controls of expression of procaspase-7 (no variation with or without Lptn) and procaspase-4 (around 3-fold reduction with Lptn) are shown on CD3 and CD28 costimulation, as a representative experiment of 2. (B) Kinetics of procaspase-7 processing induced by Lptn. The graph represents the relative levels of the caspase-7/PI3K signal ratio induced by the indicated stimuli, with error bars corresponding to SD. These mean levels are representative of 2, 3, 5, and 2 independent experiments at 0.5, 1, 2, and 3 days, respectively. ▪, CD3; ▴, CD3 plus Lptn.
Specific cleavage of caspase substrates in response to Lptn-induced apoptosis
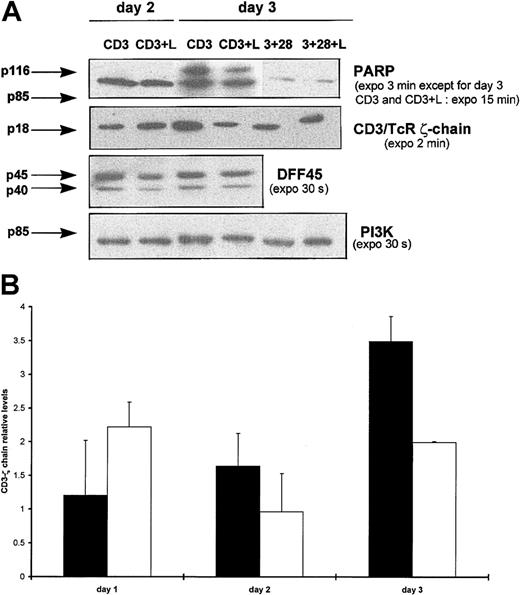
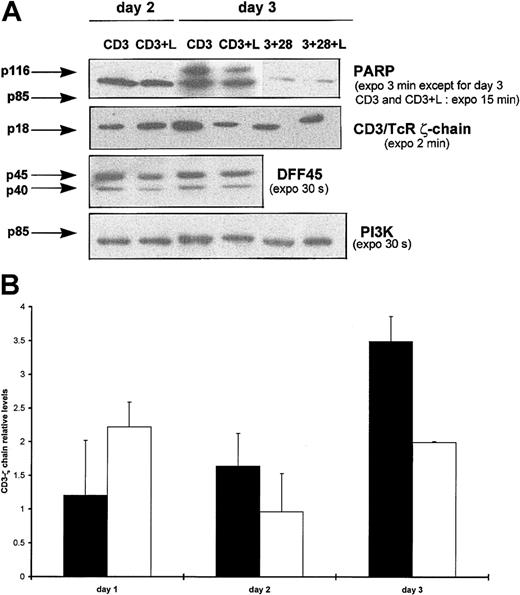
Because the effector caspase-7 was specifically processed after CD3 triggering in the presence of Lptn, we next examined whether caspase substrates such as PARP, CD3/TCR ζ-chain, or DFF45, which have been shown as targets for caspase-7,40,41,43,44 could be involved in our Lptn-dependent apoptotic process. As shown in Figure6A, on CD3 stimulation, the PARP (p116) protein appeared to be more efficiently cleaved in Lptn-treated cells than in untreated cells, mainly as the result of a stronger reduction in expression of the p116 precursor than of an increase in expression of the p85 cleaved product. Such an effect peaked around day 3 (Figure6A). As an additional substrate undergoing reduction in its expression during apoptosis,44 the CD3/TCR ζ-chain also underwent a transient loss of expression on CD3 stimulation, in the presence of Lptn, with a peak around day 3, in contrast with a progressive increase in expression in the absence of Lptn (Figure 6A,B). Finally, because the cleavage of DFF45, an inhibitor of caspase-activated deoxyribonuclease (CAD)42 43 is known to play a critical role in apoptotic DNA fragmentation, we analyzed DFF45 expression profile in the cell extracts used for expression analyses of PARP, CD3 ζ-chain, and caspases. Results shown in Figure 6A showed that DFF45 did not appear to be cleaved in the presence of Lptn, a result in agreement with the absence of detection of caspase-3 processing in these conditions (not shown). Altogether, these results indicate that the apoptogenic effect of Lptn on CD3-activated CD4+ T cells was accompanied by a selective processing of both caspases and substrates.
PARP and CD3/TCR ζ-chain, but not DFF45, are cleaved during Lptn-induced apoptosis.
(A) Total proteins of cells activated for days 0.5 to 4 with the indicated stimuli were analyzed by Western blotting for detection of substrates and PI3K control expression, using the antibodies, as already described. Arrows indicate the full-length forms of the substrates, except for p85 of PARP corresponding to a cleaved product. No expression variation for both PARP and CD3 ζ-chain were observed on CD3 and CD28 costimulation, in presence or absence of Lptn, and are not shown. This experiment was performed at least 3 times, except for 2 times in the case of DFF45. As a positive control, on CD3 and CD28 costimulation, the presence of Lptn induced a 2-fold reduction in DFF45 expression. (B) Kinetics of CD3/TCR ζ-chain processing by Lptn. The graph represents the relative levels of the ζ-chain/PI3K signal ratio induced by the indicated stimuli, with SD indicated by error bars. Each mean level is representative of 4 independent experiments. ▪, CD3; ■, CD3 plus Lptn.
PARP and CD3/TCR ζ-chain, but not DFF45, are cleaved during Lptn-induced apoptosis.
(A) Total proteins of cells activated for days 0.5 to 4 with the indicated stimuli were analyzed by Western blotting for detection of substrates and PI3K control expression, using the antibodies, as already described. Arrows indicate the full-length forms of the substrates, except for p85 of PARP corresponding to a cleaved product. No expression variation for both PARP and CD3 ζ-chain were observed on CD3 and CD28 costimulation, in presence or absence of Lptn, and are not shown. This experiment was performed at least 3 times, except for 2 times in the case of DFF45. As a positive control, on CD3 and CD28 costimulation, the presence of Lptn induced a 2-fold reduction in DFF45 expression. (B) Kinetics of CD3/TCR ζ-chain processing by Lptn. The graph represents the relative levels of the ζ-chain/PI3K signal ratio induced by the indicated stimuli, with SD indicated by error bars. Each mean level is representative of 4 independent experiments. ▪, CD3; ■, CD3 plus Lptn.
Discussion
Apoptosis of T cells plays an important role in controlling immune responses and is influenced by cytokines/growth factors. This report clearly provides the first demonstration of the direct involvement of Lptn, the unique member of the C chemokine subfamily, in human CD4+ T-cell death, as an additional mechanism to explain the Lptn-induced antiproliferative effects.24 In conjunction with CD3/TCR signaling, Lptn behaves like a death costimulator for recently activated CD4+ T cells. The peak of Lptn-induced apoptosis is observed around day 2, time that is probably required for the cells to express the receptor for Lptn or to become susceptible to activation-induced cell death. The abrogation of the Lptn-mediated apoptosis by PT is in accordance with both the nature of the receptor for Lptn15 and the previously demonstrated PT sensitivity of the inhibition of Th1-type lymphokine production by Lptn.24 In contrast with Lptn, our TUNEL-based results showed no effect of the CC chemokine RANTES on CD4+ T-cell apoptosis. So far, procytotoxic or proapoptotic effects have only been reported for CXC or CC chemokines in the context of human immunodeficiency virus (HIV)–suppressing activities.21-23Whereas the apoptotic effect of Lptn toward primary CD4+ T cells was direct, that of SDF-1 toward primary CD8+ T cells only occurred in the presence of macrophages.21
The expression of Fas and FasL, known as critical mediators of elimination of activated T cells, requires activation signals through CD3/TCR complex.50 FasL has been shown to be processed and shed from the surface of human T cells.34 Although sFasL is less active than mFasL in killing T lymphocytes, it can also inhibit the activity of mFasL.35 36 We demonstrate here that Lptn both increased the expression of mFasL and reduced that of sFasL. We hypothesize that the combination of these events might contribute to enhance the apoptotic activity of FasL signaling through its receptor and therefore might account for the increase in CD4+ T-cell death induced by Lptn. Such a conclusion is also supported by the following observations: (1) the antagonist anti-Fas mAb abrogated the Lptn-mediated apoptosis, as determined by DNA fragmentation and surface expression of the 7A6 mitochondrial antigen; (2) Lptn and agonistic anti-Fas mAbs did not display additive effects when mixed together at optimal doses; and (3) using a metalloproteinase inhibitor, we observed a correlation between the increase in 7A6 apoptotic antigen expression and the inhibition of sFasL (not shown).
Various apoptotic pathways triggered by distinct stimuli include a final step of caspase activation, involving particular caspases that can be tissue specific but also stimulus specific within the same cell type. The identification of specific caspases and their substrates could therefore be useful to propose specific targets for inhibition of apoptosis.44 Initiator caspases with long prodomains like caspases-8, -9, and -10 usually cleave and activate effector caspases like caspases-3 and -7.37 In this study, we clearly demonstrated the activation of caspase-9 as a predominant initiator caspase implicated in Lptn-mediated apoptosis of CD4+ T cells. We cannot exclude, however, a minor or later contribution of caspase-8 to the apoptotic process of both Lptn-treated or untreated cells, on CD3 stimulation. Effectively, according to the study of Droin and colleagues,51 the up-regulation of procaspase-8 expression at day 2 could be associated with a subsequent phase of apoptosis. Our results may, therefore, suggest either a regulatory role of caspase-8 in a caspase-9–driven apoptosis,52 or alternatively, an involvement of caspase-8 in proliferation or other T-cell activation-related events, as already reported.53,54 Our inability to detect a processing of caspase-10 did not exclude its potential contribution to the Lptn-mediated apoptotic process, as well as for other nontested caspases or different proteases, as for instance calpain.55
We demonstrate a processing of caspase-7, but not of the caspases-3 and -4, in Lptn-mediated apoptosis. Interestingly, caspase-4 activation has been more implicated in generation of proinflammatory cytokines56 than in apoptosis.57 Our observation is thus in accordance with one of the most important features of the CD28 costimulation, namely, its ability to increase the production of such cytokines.25,26 Alam and coworkers54 showed that the activation of caspases-6, -7, and -3, was required as a downstream event for T-cell proliferation. In line with this, CD3-stimulated T cells, which exhibit a more vigorous proliferation in absence than in presence of Lptn, display a dramatic increase in caspase-3 proenzyme expression.
Several proteins, like PARP and DFF45, which play a role in DNA repair or maintenance, are cleaved by caspases in apoptotic cells.58 Although almost all caspases can cleave PARP in vitro, its in vivo cleavage is more likely attributed to caspases-3 and -7.39-41 The observed cleavage of PARP in CD3-activated cells in the presence of Lptn is in accordance with the increase in procaspase-7 processing. The effect of caspase-3 on the cleavage of DFF45 has been shown to be more effective than that of caspase-7.43 We also observed a simultaneous absence of DFF45 cleavage and caspase-3 activation in response to Lptn, which is in complete agreement with the previous proposition of caspase-3 being the major regulator of DFF45 proteolysis.43 A reduction in the expression of CD3/TCR ζ-chain due to proteolysis by caspase-3 or caspase-7 appears as a common feature of T-cell apoptosis.44 Our data agreed with these studies because we demonstrated a correlation between the processing of caspase-7 and the reduced expression of the CD3/TCR ζ-chain in the presence of Lptn. Whether the reduced expression of CD3/TCR ζ-chain by Lptn may be associated with T-cell signaling abnormalities deserves further investigation. Altogether our data suggest a model in which the enhancement of CD4+ T-cell apoptosis by Lptn involves activation of at least the initiator caspase-9, which in turn activates the effector caspase-7, resulting in cleavage of substrates like the ζ-chain of CD3/TCR, PARP, but not DFF45.
Although IL-2 is a T-cell survival factor, it can also confer susceptibility to apoptosis to TCR-stimulated T cells,30by increasing the expression of death molecules like Fas/FasL. We have previously shown that a strong IL-2 deprivation was induced by Lptn, on CD3 stimulation.24 This phenomenon, however, is probably not responsible for the increased apoptosis mediated by Lptn. This notion could be supported by the following observations: (1) the addition of IL-2, even at high doses, was unable to completely rescue the CD3-activated T cells from the Lptn-induced apoptosis, and (2) a reciprocal pattern expression of both mFas and sFasL was induced by Lptn in CD3-activated cells in the presence of IL-2. This suggests an inhibitory effect of IL-2 on the particular Fas-dependent Lptn-mediated CD4+ T-cell death pathway. The deprivation of the same lymphokines that drive T cells into cycle such as IL-2 can induce a death different from apoptosis, with no role of FasL.30 In contrast, as shown by the present work, Lptn is able to directly exert a feedback control of T-cell activation by apoptosis.
By demonstrating the apoptotic function of Lptn, which was previously shown by us to inhibit proliferation of CD4+ T cells, through abrogation of Th1-type lymphokine production,24 we further emphasize the role of Lptn in the in vitro clearance of CD3-activated CD4+ T cells, by proposing Lptn as a crucial modulator of TCR signals. During thymic development, the elimination of self-reactive immature T cells is ensured through clonal deletion via apoptosis, and it would be thus interesting to analyze the potential role of Lptn in such a deletional mechanism. Finally, the induction of in vivo peripheral deletion of inappropriately activated mature CD4+ T cells by Lptn-mediated apoptotic signals could be considered as a basis for the elaboration of new therapies using selective immunosuppression.
Supported by INSERM and by grants from the Fédération Nationale des Centres de Lutte Contre le Cancer and the Association pour la Recherche sur le Cancer (ARC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Chantal Cerdan, INSERM U119, UniversitéMéditerranée, 27, Boulevard Leı̈ Roure, 13009 Marseille, France; e-mail: cerdan@marseille.inserm.fr.

![Fig. 1. Enhancement of both arrest in cell growth and increase in cell death by Lptn. / (A) The kinetics of viable cell recovery, as measured by trypan blue exclusion, of purified CD4+ T cells activated for the indicated days with plate-bound CD3 mAb, in the presence or absence of either Lptn or RANTES. At day 0, 4 × 106 cells/well were plated in each activation condition. Similar cell counts were obtained in the presence of RANTES, alone or in conjunction with its specific Ab, and are not shown. The figure represents the mean values ± SD (error bars) of 3 independent experiments. ▪, CD3; ♦, CD3 plus Lptn; ▴, CD3 plus Lptn plus anti-Lptn. (B) A typical flow cytometric profile of PI-stained CD4+ T cells. Cells were activated with CD3 mAb in the presence or absence of Lptn. At day 2 after activation, the different stages of cell cycle were analyzed for nuclei DNA content after PI uptake. The percentages of cells in the different stages (subdiploid [< 2n DNA], G0/G1 [2n DNA], and S+G2+M [> 2n DNA]) are indicated. In parallel, CD4 staining ensured a 90% purity of the cultured cells (not shown). (C) Quantitative expression of the data shown in panel A. CD4+ T cells were activated for 2 days with the indicated stimuli. Each graph represents the mean values ± SD of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2205/5/m_h80810924001.jpeg?Expires=1767713705&Signature=nw5LO7Su5GVGMDb8K240LyG0KJcqE-eeFW4ucoC7bbHF~iuBSw4nuVmzHAMz~p7shXwbJ~jI-hRhQ-AJ6u~02lqTwgtiagcQB-P4GnY~LgUD5fhfWQMgCMAOHZUGYGuDI~1FJwRABNPDe~hAEtnpYP5kD54My9qANtTIhgnIJ4RHK0XvmuxCgr6C53ETjqQDSToFB4C5-qhOq3w2cdSgEgpp5dfhP5ASFjFgqkYinFGUVyjLQHLON~NawxauN67kYXAgicqNBGl9E5ooKDGIiOglYkO33TCg3hPx9n4PlHEir2Y7sRukWvCGeSTNu4mvAR5HcWshVEaU75FnHdRw7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Lptn-mediated apoptosis occurs through Fas/FasL signaling pathway. / (A) Effects of ZB4 and 7C11 anti-Fas mAbs on the Lptn-induced inhibition of proliferation. Proliferation was assessed by [3H]-thymidine incorporation during the last 18 hours of 96-hour culture of CD4+ T cells activated via CD3, in the presence or absence of Lptn. Both anti-Fas mAbs (used at 1 μg/mL) were preincubated 1 hour before activation. Results are expressed as the mean count/min values ± SD of 3 separate experiments. (B) Protection by ZB4 mAb against Lptn-induced increase in DNA fragmentation. CD4+ T cells were preincubated with 1μg/mL of the antagonist ZB4 anti-Fas mAb prior to CD4+ T-cell stimulation for 2 days, as described in “Materials and methods.” Cells were then washed and subjected to the TUNEL assay, according to manufacturer's protocol. For each sample, an aliquot was treated for 10 minutes with DNase I, serving as positive control. After FITC-dUTP and TdT staining, analyses were performed by flow cytometry. Data represent values of mean fluorescence intensity ± SD (error bars) calculated from at least 3 independent experiments. ▪, CD3; ■, CD3 plus ZB4.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2205/5/m_h80810924003.jpeg?Expires=1767713705&Signature=DBkeAUCIzWuTSLj9CEs9WndqMxjGKZ9BOrZe4Nss3MfBRBjwFWJf9OAosTPKq2hXhDvoRIvRcJk6ob94L3eAHX8Uw2cTVncnIHrN91JV55e5kKQ8PH4OI2pP4uBHWNhAd9Mmb3~M-csArC5yXPBqA-BZN3QfI7RSb6t9BMq61Ll6JLyJOxyJDGHYLU94R5UEg~7dQxECd~Lh5G4FECuu6bBnstpOzzzSv6Je8Qb-JR52dS8tyryjvYqeGE9UH1ZqbsZmimEl9BwTg6p3o9BtQtQKPZ5FmQ8-jD1A2-tbMrCxc954htXZDHDYEwREXQObqAZOyh1rDw-eIeOwezMbVg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. Enhancement of both arrest in cell growth and increase in cell death by Lptn. / (A) The kinetics of viable cell recovery, as measured by trypan blue exclusion, of purified CD4+ T cells activated for the indicated days with plate-bound CD3 mAb, in the presence or absence of either Lptn or RANTES. At day 0, 4 × 106 cells/well were plated in each activation condition. Similar cell counts were obtained in the presence of RANTES, alone or in conjunction with its specific Ab, and are not shown. The figure represents the mean values ± SD (error bars) of 3 independent experiments. ▪, CD3; ♦, CD3 plus Lptn; ▴, CD3 plus Lptn plus anti-Lptn. (B) A typical flow cytometric profile of PI-stained CD4+ T cells. Cells were activated with CD3 mAb in the presence or absence of Lptn. At day 2 after activation, the different stages of cell cycle were analyzed for nuclei DNA content after PI uptake. The percentages of cells in the different stages (subdiploid [< 2n DNA], G0/G1 [2n DNA], and S+G2+M [> 2n DNA]) are indicated. In parallel, CD4 staining ensured a 90% purity of the cultured cells (not shown). (C) Quantitative expression of the data shown in panel A. CD4+ T cells were activated for 2 days with the indicated stimuli. Each graph represents the mean values ± SD of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2205/5/m_h80810924001.jpeg?Expires=1767713706&Signature=1urGpAGc3OhiywcNmL1QMvVoEp-eZAzM3U~~N8LS1QfbXQYXrynapsUkqyksU68H8g9ZUb00PRz1bWv6cXwZnLGaZL-sufY2SN1Duj7Buv-SdSoxMi6PHgjjkHrtulwdbzuBA098dpxohL0ahpU~WLyg85nhFgV94q2QvY5qrZvCln9oPIPCdLE1XjC~QjwEfnfrxt7Y-msXdN0QMTYDyCxu2JV-u7ovuA182XSSZzgIxA-vB-Ukfa8vT-oh5LKWmY9oc7QbPb3x-aYaISGSOhAarzONiIwAxXiyXxhnxN-8mfqUatO~eZ~pBP-4T2H62XETVm0Lzm~dMe8UcvUbrA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Lptn-mediated apoptosis occurs through Fas/FasL signaling pathway. / (A) Effects of ZB4 and 7C11 anti-Fas mAbs on the Lptn-induced inhibition of proliferation. Proliferation was assessed by [3H]-thymidine incorporation during the last 18 hours of 96-hour culture of CD4+ T cells activated via CD3, in the presence or absence of Lptn. Both anti-Fas mAbs (used at 1 μg/mL) were preincubated 1 hour before activation. Results are expressed as the mean count/min values ± SD of 3 separate experiments. (B) Protection by ZB4 mAb against Lptn-induced increase in DNA fragmentation. CD4+ T cells were preincubated with 1μg/mL of the antagonist ZB4 anti-Fas mAb prior to CD4+ T-cell stimulation for 2 days, as described in “Materials and methods.” Cells were then washed and subjected to the TUNEL assay, according to manufacturer's protocol. For each sample, an aliquot was treated for 10 minutes with DNase I, serving as positive control. After FITC-dUTP and TdT staining, analyses were performed by flow cytometry. Data represent values of mean fluorescence intensity ± SD (error bars) calculated from at least 3 independent experiments. ▪, CD3; ■, CD3 plus ZB4.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2205/5/m_h80810924003.jpeg?Expires=1767713706&Signature=M2CjsdGTL-FgIrEQmdwTVnRCFG4sH6ImQDhlQ2dP42v0B-wPOEbHn77QWZ4maPUg6ETD2on5ZviQ7uIrurHTbXxjjl4S1~m2Xw8tvtEel8yftuG9xbQuoFpbspiEPYB595PXVvDFCFlXeM8bGYaYDelRgkxPtQHIRx5IoomJZtVXtiRIK47OGCQ7cJlaoEyV6CvM8IKDPi0E9p0K0vfDqATnClmYZvFbpg4vk0SDJ4ZfpYvQL7MQ6tSRGGmhvJdAzyx3rHJrWYRCOwuhbG2tPTVoHiE4HyCCtMx-abBfIsQtzumBKUJhg4nDeVXw3rudbrzh87dyRmUzUN81PUhLQg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)