Abstract
Heparin is a commonly used anticoagulant drug. It functions primarily by accelerating the antithrombin inhibition of coagulation proteinases, among which factor Xa and thrombin are believed to be the most important targets. There are conflicting results as to whether anticoagulant heparins can catalyze the antithrombin inhibition of factor Xa in the prothrombinase complex (factor Va, negatively charged membrane surfaces, and calcium ion), which is the physiologically relevant form of the proteinase responsible for the activation of prothrombin to thrombin during the blood coagulation process. In this study, a novel assay system was developed to compare the catalytic effect of different molecular-weight heparins in the antithrombin inhibition of factor Xa, either in free form or assembled into the prothrombinase complex during the process of prothrombin activation. This assay takes advantage of the unique property of a recombinant mutant antithrombin, which, similar to the wild-type antithrombin, rapidly inhibits factor Xa, but not thrombin, in the presence of heparin. A direct prothrombinase inhibition assay, monitoring thrombin generation under near physiological concentrations of prothrombin and antithrombin in the presence of therapeutic doses of low- and high-molecular-weight heparins, indicates that factor Xa in the prothrombinase complex is protected from inhibition by antithrombin more than 1000 times, independent of the molecular size of heparin.
Introduction
The anticoagulant function of heparin is primarily derived from its ability to accelerate the inhibition of the blood coagulation proteinases by antithrombin.1-3 The mechanism and the extent of this acceleration have been thoroughly investigated for antithrombin inhibition of factor Xa and thrombin.4-15It is not, however, known whether heparin is effective in catalyzing the inhibition of factor Xa when the proteinase binds to factor Va on negatively charged membrane surfaces in the presence of calcium (the prothrombinase complex) to activate prothrombin to thrombin.16-24 Results of several previous studies suggest that the assembly of factor Xa into the prothrombinase complex is accompanied by the protection of factor Xa from inhibition by the antithrombin-heparin complex.18-21 Conflicting reports exist about the extent to which factor Xa in the prothrombinase complex is protected from inhibition by antithrombin in the presence of different molecular weight heparins. These reports range from support for nearly complete protection19,21 to less than 5- to 10-fold protection of factor Xa in prothrombinase from inhibition by antithrombin in the presence of high- or low-molecular-weight high-affinity heparins.22-24 As expected, these results have generated conflicting hypotheses within the scientific community as to whether the therapeutic heparins are effective in catalyzing prothrombinase inhibition by antithrombin, and if so, whether the effect is dependent on the molecular size of heparin.
These conflicting results are believed to stem from the inability of the existing assay systems to directly monitor the inhibition of prothrombinase by antithrombin in the presence of prothrombin. This is because both the enzyme and the product of the activator complex are targets for rapid inhibition by antithrombin in the presence of heparin.4,15 To overcome this problem, a mutant of human antithrombin was prepared in which the reactive site loop of the inhibitor from the P4-P4′ site (nomenclature of Schechter and Berger25) is replaced with the identical site of the second factor Xa cleavage site (Ile319-Asp-Gly-Arg-Ile-Val-Glu-Gly326) in prothrombin. This mutant is fully characterized; it is folded properly, has a normal affinity for heparin, and rapidly inhibits factor Xa, but not thrombin, in the presence of heparin. Thus, the mutant was used to evaluate, by a simple inhibition assay, the catalytic effects of heparins of different representative molecular weights in antithrombin inhibition of prothrombinase directly from the rate of thrombin generation at near physiological concentrations of the reactants. The results suggest that a physiological concentration of prothrombin protects factor Xa in the prothrombinase complex by more than 3 orders of magnitude from inhibition by the antithrombin-heparin complex independent of the molecular size of heparin.
Materials and methods
The human antithrombin mutant in which P4-P4′ residues of the reactive site loop were substituted with the corresponding residues of the second factor Xa cleavage site in prothrombin (Ile319-Asp-Gly-Arg-Ile-Val-Glu-Gly326, named HAT/Proth-2) was constructed by standard polymerase chain reaction (PCR) mutagenesis methods and expressed in human 293 cells using RSV-PL4 expression-purification vector system as previously described.26 This vector introduces a 12-residue epitope for the monoclonal antibody, HPC4, to the N-terminus of the mutant protein for easy purification.26 The antithrombin mutant was purified from cell culture supernatants to homogeneity by a combination of immuno-affinity chromatography using an HPC4 antibody linked to Affi-gel 10 (Bio-Rad, Hercules, CA) followed by HiTrap-Heparin (Amersham/Pharmacia, Piscataway, NJ) ion exchange chromatography with a gradient elution from 0.1 M to 3.0 M NaCl in 20 mM Tris-HCl, pH 7.4.
Human plasma-derived antithrombin, the active antithrombin-binding pentasaccharide fragment of heparin and full-length high-affinity heparins containing the pentasaccharide with an average molecular mass of approximately 8000 (approximately 26 saccharides) or approximately 21 000 (approximately 70-saccharides) were generous gifts from Dr Steven Olson (University of Illinois, Chicago). Concentrations of heparins were based on the antithrombin binding sites and were determined by stoichiometric titration of antithrombin with the polysaccharides, with monitoring of the interaction by changes in protein fluorescence.4,27 Phospholipid vesicles containing 80% phosphatidylcholine and 20% phosphatidylserine (PC/PS) were prepared as described.28 Human coagulation factors, factor Xa, factor Va, and prothrombin were purchased from Hematologic Technologies (Essex Junction, VT). The chromogenic substrate, Spectrozyme FXa (SpFXa), was purchased from American Diagnostica (Greenwich, CT), and S2238 was purchased from Kabi Pharmacia/Chromogenix (Franklin, OH). Unfractionated heparin from porcine intestinal mucosa, sodium salt (169.2 USP U/mg), and Polybrene were purchased from Sigma (St Louis, MO).
Kinetic methods
Catalytic effect of different molecular-weight heparins on factor Xa or prothrombinase inhibition by the antithrombin mutant was studied by 2 different assay systems under pseudo first-order conditions. In the first assay, 1 nM human factor Xa alone or in complex with a saturating concentration of human factor Va (5 nM) on 50 μM PC/PS vesicles was incubated with 500 nM antithrombin and catalytic levels of heparin (1-25 nM) in 0.1 M NaCl, 0.02 M Tris-HCl, pH 7.4 (TBS buffer) containing 1 mg/mL bovine serum albumin (BSA), 0.1% polyethylene glycol (PEG) 8000, and 2.5 mM CaCl2 as described.15,29 All reactions were carried out in 50-μL volumes in 96-well polystyrene assay plates at room temperature. At various time points (1-20 minutes), 50 μL SpFXa (500 μM) in TBS buffer containing 0.1% PEG 8000 and 1 mg/mL Polybrene (Sigma) was added to each well. The remaining enzyme activity was measured with a Vmax Kinetics Microplate Reader (Molecular Devices, Menlo Park, CA) at 405 nm. The observed pseudo first-order rate constants (kobs) were determined by computer fitting of the time-dependent change of the proteinase activity to a single exponential function. The second-order association rate constants were obtained by dividing kobs values to the concentrations of the antithrombin-heparin complexes. The antithrombin-heparin concentrations were calculated from the dissociation constant for the antithrombin-heparin interaction and total concentrations of antithrombin and heparin using the quadratic equation.27Noting the experimental conditions described above (500 nM antithrombin) and a low Kd measured for the interaction of heparin with either wild-type or mutant antithrombin (approximately 20 nM), the calculated concentrations of the antithrombin-heparin complexes were in proximity to the concentrations of free heparins.
In the second assay, similar experimental conditions were used with the exception that prothrombin was included in the reaction and the rate of factor Xa inhibition by the mutant antithrombin-heparin complex was directly measured from the inhibition of thrombin generation. In this assay, either 1 nM human factor Xa alone or 0.2 pM in complex with 5 nM human factor Va on 50 μM PC/PS vesicles in the same TBS buffer system was used to initiate the activation of 1.5 μM human prothrombin in the presence of 2.3 μM antithrombin in complex with catalytic levels of heparin (1-1000 nM). At different time points (1-20 minutes), 10 μL aliquot of the reaction was removed and added to 90 μL thrombin-specific chromogenic substrate, S2238, in TBS buffer containing 20 mM EDTA and 1 mg/mL Polybrene (Sigma) (to stop both the activation and the inhibition reactions immediately). The concentration of thrombin generated at each time point in the absence or presence of the inhibitor-heparin complex was determined from a standard curve prepared from the cleavage rate of S2238 by known concentrations of thrombin using a Vmax Kinetics Microplate Reader (Molecular Devices) as described above. Thrombin generation in the absence of heparin was normalized to 100, and the kobsvalues were determined by computer fitting of the time-dependent percentage inhibition of thrombin generation in the presence of varying concentrations of heparin to a single exponential function. The second-order association rate constants were obtained by dividing kobs values to the concentrations of the antithrombin-heparin complexes, as described above. Control experiments showed that this assay was specific for the presence of both antithrombin and heparin in the reaction because the linear rate of thrombin generation (approximately 6 nM/min by the prothrombinase complex) was not changed by the presence of either antithrombin or heparin alone for the 20-minute time-course analysis of the assays. In all assays, thrombin generation was initiated by adding factor Xa, either in free form or in complex with prothrombinase, to the reaction wells containing antithrombin, heparin, and prothrombin. In a modified form of this assay, the prothrombinase reaction was initiated under the same experimental conditions described above, with the exception that heparin was added to an ongoing prothrombinase reaction after a 1-minute progression of the reaction.
Results
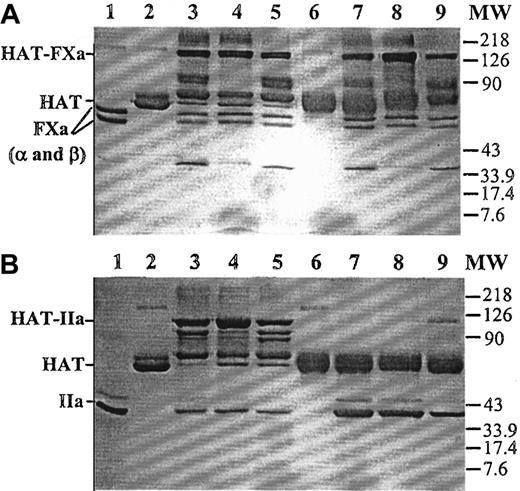
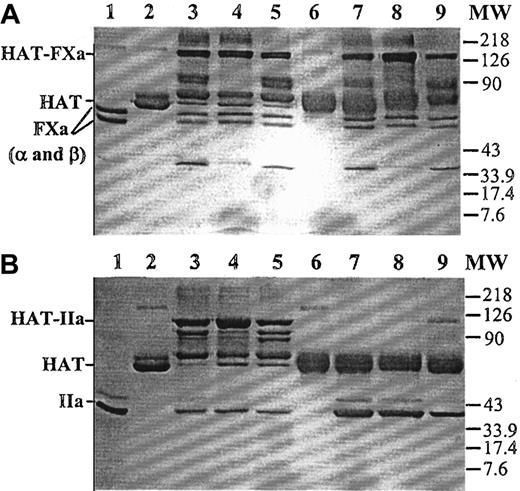
A mutant of human antithrombin (HAT/Proth-2) was prepared in which the reactive site loop of the inhibitor from the P4-P4′ site was replaced with the identical site of the factor Xa cleavage site (Ile319-Asp-Gly-Arg-Ile-Val-Glu-Gly326) in prothrombin. The mutant was purified to homogeneity by a combination of an HPC4 immuno-affinity and a HiTrap-Heparin (Amersham/Pharmacia) ion exchange column chromatography, as described in “Materials and methods.” Similar to the wild-type antithrombin, the mutant was eluted from the HiTrap-Heparin column at approximately 0.8 M NaCl (data not shown). Sodium dodecyl sulfate–polyacrylamide gel (SDS-PAGE) analysis, under nonreducing conditions, suggested that the mutant had a similar relative molecular mass as the plasma-derived antithrombin (Figure 1). Similar to plasma antithrombin, the mutant formed stable complexes with factor Xa in both the absence and the presence of heparin (Figure 1A). However, unlike plasma antithrombin, the mutant did not form a stable complex with thrombin in either the absence or the presence of low-molecular-weight heparin (Figure 1B). In the presence of a full-length (approximately 70-saccharides) high-affinity heparin, a small fraction of the mutant antithrombin appeared to form a stable complex with thrombin (Figure 1B, lane 9). The lack of stable mutant antithrombin-thrombin complexes was not due to thrombin recognizing the mutant as a substrate because no cleavage product was detected in either the absence or the presence of heparin (Figure 1B, lanes 6-9). Consistent with these data, kinetic analysis suggested that the mutant inhibited factor Xa only approximately 5 to 10 times more slowly than the wild-type antithrombin in the absence and the presence of heparin and the pentasaccharide fragment of heparin, but it did not react with thrombin at a detectable rate in either the absence or the presence of pentasaccharide. Its reactivity with thrombin was impaired by approximately 5 orders of magnitude in the presence of a full-length (approximately 70-saccharides) high-affinity heparin (Table1). Similar to the wild-type antithrombin, the mutant bound to high-affinity heparins with aKd of approximately 20 nM, as determined from changes in intrinsic protein fluorescence on binding to heparin27 (data not shown). Because the heparin-catalyzed proteinase inhibition by the mutant was minimally affected for factor Xa but virtually abolished for thrombin, the mutant was used to evaluate the catalytic effect of heparins of various molecular sizes in antithrombin inhibition of prothrombinase during prothrombin activation directly from the inhibition of thrombin generation.
Nonreducing SDS-PAGE analysis of the stable factor Xa and thrombin complexes with plasma or mutant antithrombin.
(A) Human plasma or mutant antithrombin (2 μM) was incubated with an equimolar concentration of factor Xa for 30 minutes in the absence of heparin or for 5 minutes in the presence of pentasaccharide or approximately 70-saccharide high-affinity heparin (4 μM) in 25 μL reactions in TBS buffer at room temperature. Five microliters 5× nonreducing sample buffer was added to each reaction, and the samples were boiled for 5 minutes. Twenty-five microliters of each reaction was loaded on 10% gel. The stable proteinase-antithrombin complexes migrated as the highest molecular weight bands (HAT-FXa), followed by degraded complexes. Lane 1, factor Xa (FXa in α and β forms). Lane 2, human plasma antithrombin (HAT). Lanes 3 to 5, plasma antithrombin incubated with factor Xa in the absence of heparin, in the presence of pentasaccharide, or in the presence of approximately 70-saccharide heparin, respectively. Lane 6, mutant antithrombin. Lanes 7 to 9, mutant antithrombin incubated with factor Xa in the absence of heparin, in the presence of pentasaccharide, or in the presence of approximately 70-saccharide heparin, respectively. MW, molecular weight marker. (B) Identical to panel A, except that thrombin (IIa) was used to initiate the reactions.
Nonreducing SDS-PAGE analysis of the stable factor Xa and thrombin complexes with plasma or mutant antithrombin.
(A) Human plasma or mutant antithrombin (2 μM) was incubated with an equimolar concentration of factor Xa for 30 minutes in the absence of heparin or for 5 minutes in the presence of pentasaccharide or approximately 70-saccharide high-affinity heparin (4 μM) in 25 μL reactions in TBS buffer at room temperature. Five microliters 5× nonreducing sample buffer was added to each reaction, and the samples were boiled for 5 minutes. Twenty-five microliters of each reaction was loaded on 10% gel. The stable proteinase-antithrombin complexes migrated as the highest molecular weight bands (HAT-FXa), followed by degraded complexes. Lane 1, factor Xa (FXa in α and β forms). Lane 2, human plasma antithrombin (HAT). Lanes 3 to 5, plasma antithrombin incubated with factor Xa in the absence of heparin, in the presence of pentasaccharide, or in the presence of approximately 70-saccharide heparin, respectively. Lane 6, mutant antithrombin. Lanes 7 to 9, mutant antithrombin incubated with factor Xa in the absence of heparin, in the presence of pentasaccharide, or in the presence of approximately 70-saccharide heparin, respectively. MW, molecular weight marker. (B) Identical to panel A, except that thrombin (IIa) was used to initiate the reactions.
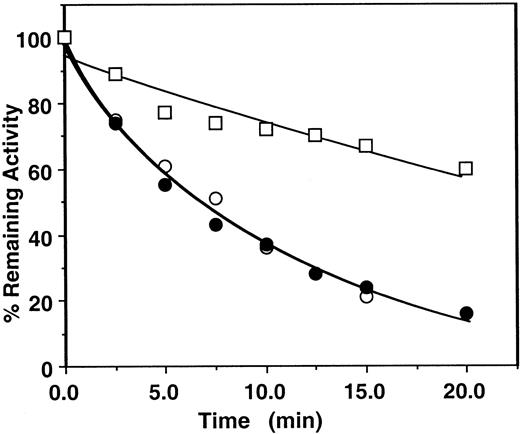
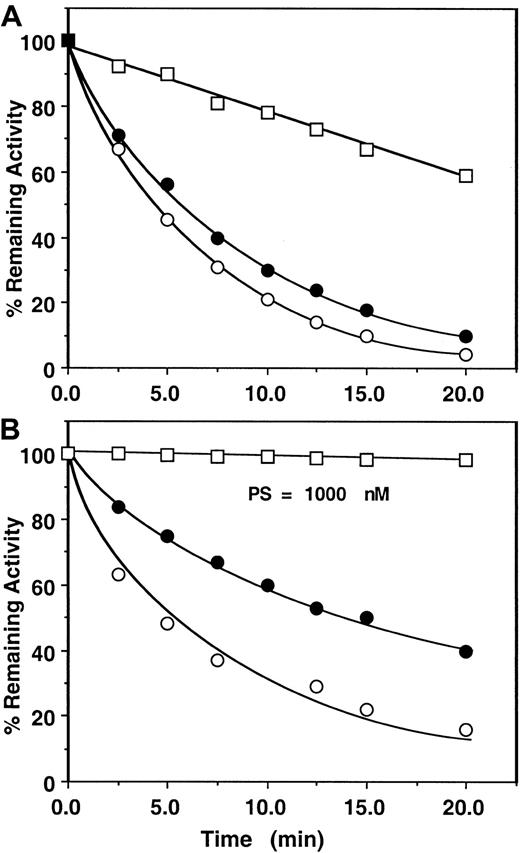
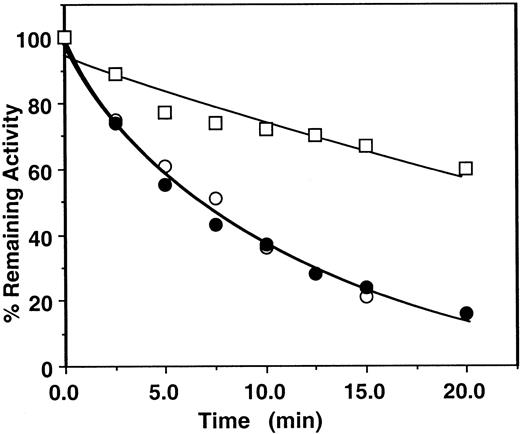
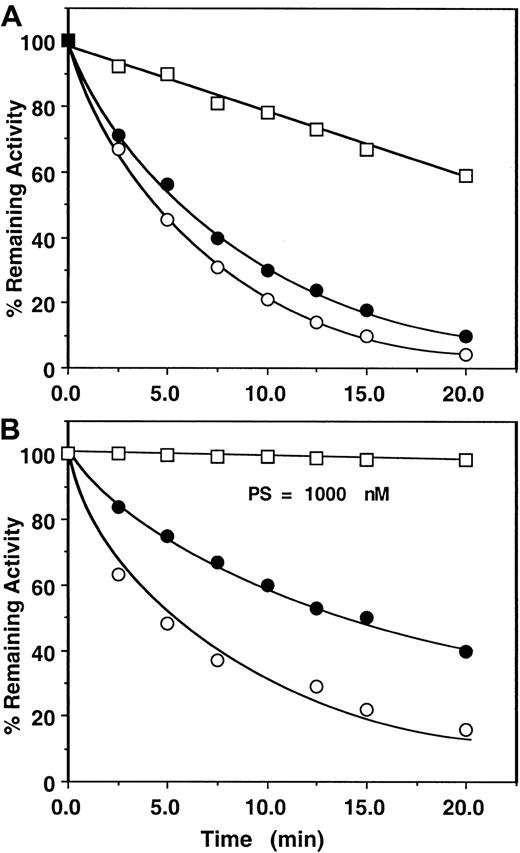
First, the assay system was validated by studying the kinetics of factor Xa or prothrombinase inhibition by the mutant antithrombin in the presence of heparins by an amidolytic activity assay that monitored hydrolysis of SpFXa and compared the results with those obtained for the wild-type antithrombin under identical conditions. Thus, in Figure2, the pentasaccharide-mediated wild-type antithrombin inhibition of factor Xa was measured in either the absence or the presence of factor Va on PC/PS vesicles from remaining amidolytic activities toward cleavage of the chromogenic substrate SpFXa (first assay), as described in “Materials and methods.” In this assay, second-order rate constants of approximately 6 × 105 M−1 second−1 for the pentasaccharide-mediated antithrombin inhibition of factor Xa in the absence and the presence of PC/PS vesicles and approximately 8 × 104 M−1 second−1 for the inhibition of factor Xa in complex with factor Va on PC/PS vesicles were observed. Under the same experimental conditions, the same values for inhibition by the mutant antithrombin were 1.1 × 105M−1 second−1 for free factor Xa, 8.2 × 104 M−1 second−1 for factor Xa on PC/PS vesicles, and 1.8 × 104M−1 second−1 for factor Xa in complex with factor Va on PC/PS vesicles (Figure 3A, Table 2). These results suggested that the complex formation of factor Xa with factor Va on PC/PS vesicles in the absence of the natural substrate prothrombin confers approximately 4- to 8-fold protection against inhibition by either the wild-type or the mutant antithrombin-pentasaccharide complex under these experimental conditions. Previously, a similar factor Va-mediated protective effect against the pentasaccharide-catalyzed24or heparin-catalyzed23 factor Xa inhibition was observed using wild-type antithrombin in similar or slightly different assay systems. Similarly, the mutant antithrombin was an efficient inhibitor of factor Xa both in solution and on PC/PS vesicles in the absence of factor Va if the natural substrate prothrombin was used to monitor the inhibition reaction (Figure 3B, Table 3). However, in the presence of factor Va and prothrombin on PC/PS vesicles, no inhibitory effect for the antithrombin mutant was detected for the 20-minute duration of the assay (less than 10 M−1second−1), even if the concentration of pentasaccharide was increased to 1000 nM (Figure 3B open squares, Table 3). The observation that both assays—using either the chromogenic substrate SpFXa (Figure 3A, Table 2) or the natural substrate prothrombin (Figure3B, Table 3)—yielded similar k2 values in the absence of factor Va is indicative of the reliability of these assays.
Time course of the pentasaccharide-catalyzed inhibition of factor Xa or prothrombinase by human plasma antithrombin.
Factor Xa (1 nM) was incubated with 500 nM plasma antithrombin in complex with 5 nM pentasaccharide in the absence (○) or the presence of 50 μM PC/PS minus (●) or plus 5 nM factor Va (■) in TBS buffer containing 1 mg/mL BSA, 0.1% PEG 8000, and 2.5 mM Ca++. At the indicated time points, aliquots of the reaction were removed to 500 μM SpFXa containing 1 mg/mL Polybrene (Sigma), and the remaining enzyme activity was determined by an amidolytic activity assay using Spectrozyme Fxa (American Diagnostica). Solid lines are nonlinear regression fit of data to a pseudo first-order rate equation, as described in “Materials and methods.”
Time course of the pentasaccharide-catalyzed inhibition of factor Xa or prothrombinase by human plasma antithrombin.
Factor Xa (1 nM) was incubated with 500 nM plasma antithrombin in complex with 5 nM pentasaccharide in the absence (○) or the presence of 50 μM PC/PS minus (●) or plus 5 nM factor Va (■) in TBS buffer containing 1 mg/mL BSA, 0.1% PEG 8000, and 2.5 mM Ca++. At the indicated time points, aliquots of the reaction were removed to 500 μM SpFXa containing 1 mg/mL Polybrene (Sigma), and the remaining enzyme activity was determined by an amidolytic activity assay using Spectrozyme Fxa (American Diagnostica). Solid lines are nonlinear regression fit of data to a pseudo first-order rate equation, as described in “Materials and methods.”
Time course of the pentasaccharide-catalyzed inhibition of factor Xa or prothrombinase by the mutant of antithrombin.
(A) Factor Xa (1 nM) was incubated with 500 nM mutant antithrombin in complex with 25 nM pentasaccharide in the absence (○) or the presence of 50 μM PC/PS minus (●) or plus 5 nM factor Va (■) in TBS buffer containing 1 mg/mL BSA, 0.1% PEG 8000, and 2.5 mM Ca++. At the indicated time points, aliquots of the reaction were removed to 500 μM SpFXa containing 1 mg/mL Polybrene (Sigma), and the remaining enzyme activity was determined by an amidolytic activity assay using Spectrozyme FXa (American Diagnostica), as described in “Materials and methods.” (B) Factor Xa (1 nM) in the absence (○) or the presence of 50 μM PC/PS (●) or 0.2 pM factor Xa in complex with 5 nM factor Va on 50 μM PC/PS (■) was incubated with 1.5 μM prothrombin in the same TBS buffer system. Time course of thrombin generation in the absence and the presence of 2.3 μM antithrombin mutant in complex with 25 nM (○, ●) or 1000 nM (■) pentasaccharide was monitored from hydrolysis of S2238. Thrombin generation in the absence of heparin was normalized to 100, and the percentage inhibition of thrombin generation (y-axis) was plotted as a function of varying concentrations of the antithrombin-heparin complex (x-axis), as described in “Materials and methods.” Solid lines are nonlinear regression fit of data to a pseudo first-order rate equation.
Time course of the pentasaccharide-catalyzed inhibition of factor Xa or prothrombinase by the mutant of antithrombin.
(A) Factor Xa (1 nM) was incubated with 500 nM mutant antithrombin in complex with 25 nM pentasaccharide in the absence (○) or the presence of 50 μM PC/PS minus (●) or plus 5 nM factor Va (■) in TBS buffer containing 1 mg/mL BSA, 0.1% PEG 8000, and 2.5 mM Ca++. At the indicated time points, aliquots of the reaction were removed to 500 μM SpFXa containing 1 mg/mL Polybrene (Sigma), and the remaining enzyme activity was determined by an amidolytic activity assay using Spectrozyme FXa (American Diagnostica), as described in “Materials and methods.” (B) Factor Xa (1 nM) in the absence (○) or the presence of 50 μM PC/PS (●) or 0.2 pM factor Xa in complex with 5 nM factor Va on 50 μM PC/PS (■) was incubated with 1.5 μM prothrombin in the same TBS buffer system. Time course of thrombin generation in the absence and the presence of 2.3 μM antithrombin mutant in complex with 25 nM (○, ●) or 1000 nM (■) pentasaccharide was monitored from hydrolysis of S2238. Thrombin generation in the absence of heparin was normalized to 100, and the percentage inhibition of thrombin generation (y-axis) was plotted as a function of varying concentrations of the antithrombin-heparin complex (x-axis), as described in “Materials and methods.” Solid lines are nonlinear regression fit of data to a pseudo first-order rate equation.
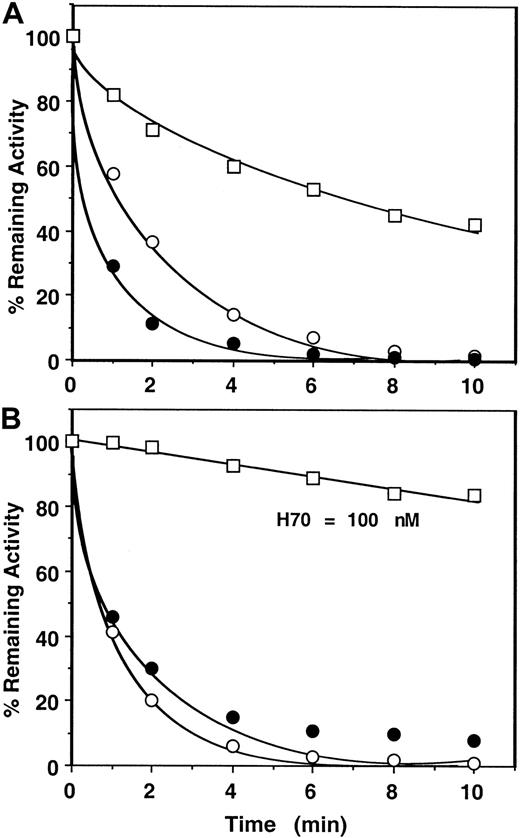
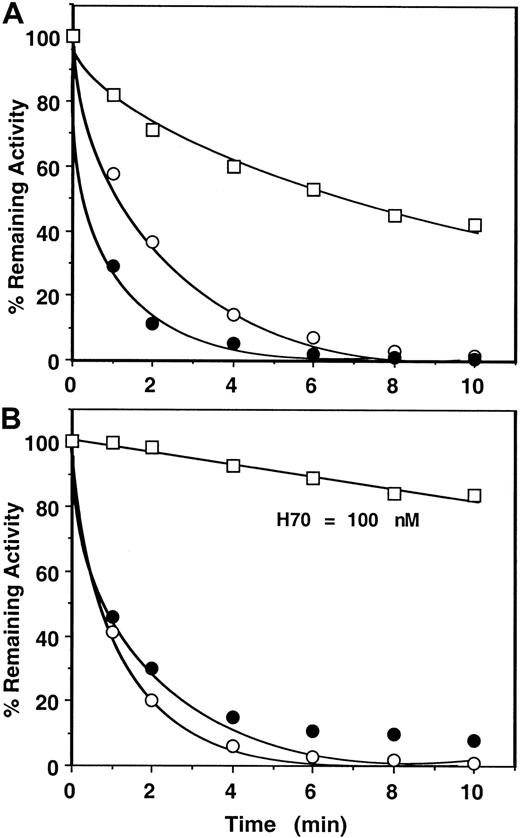
Next, the catalytic effects of longer chain heparins (approximately 26 and approximately 70-saccharides high-affinity heparins or unfractionated [UFH] heparin) in antithrombin inhibition of factor Xa and prothrombinase were evaluated in both assay systems (Figure4, shown for H70 only). Comparisons of the rate constants derived from the first assay (monitoring the cleavage of SpFXa) for these studies suggested that binding of factor Xa to factor Va on PC/PS vesicles in the absence of prothrombin confers only an approximately 2-fold (H26), approximately 6-fold (H70), or approximately 12-fold (UFH) protection against inhibition by the mutant of antithrombin (Table 2). However, these compounds were virtually ineffective in catalyzing the inhibition of prothrombinase during prothrombin activation even if their concentrations were increased by up to 2 orders of magnitude (Table 3, Figure 4B shown for H70 only). In a slightly modified form of this assay, when heparins were added to an ongoing prothrombinase reaction after a 1-minute progression of the reaction, similarly no inhibition of thrombin generation was observed with pentasaccharide (1000 nM), and approximately 0.5% to 1% (per minute) inhibition of thrombin generation was observed with the longer chain, high-affinity heparins (100-200 nM). Whether this minor inhibition of thrombin generation by the antithrombin mutant in the presence of the longer chain heparins was due to the inhibition of free factor Xa that might have been in dissociable equilibrium with factor Xa in the prothrombinase complex or due to the inhibition of prothrombinase is unknown. Based on such results, k2(app) values of less than 10 M−1 second−1 (pentasaccharide); approximately 2 × 102 M−1second−1 (H26); and approximately 3 × 103 M−1 second−1 (both H70 and UFH) for prothrombinase inhibition by the mutant antithrombin-heparin complexes were estimated (Table 3). These results suggest that prothrombin protects factor Xa in the prothrombinase complex more than 1000-fold from the heparin-catalyzed inhibition by antithrombin independent of the molecular size of heparin.
Time course of approximately 70-saccharide (H70) high-affinity heparin-catalyzed inhibition of factor Xa or prothrombinase by the mutant of antithrombin.
(A) Conditions and symbols are the same as those described for Figure3A, except that 1 nM H70 was used in the reactions. (B) The same as Figure 3B except that 1 nM H70 in the absence (○) or the presence of 50 μM PC/PS (●) and 100 nM H70 in the presence of 50 μM PC/PS plus 5 nM factor Va (fully assembled prothrombinase) (■) was used in the reactions.
Time course of approximately 70-saccharide (H70) high-affinity heparin-catalyzed inhibition of factor Xa or prothrombinase by the mutant of antithrombin.
(A) Conditions and symbols are the same as those described for Figure3A, except that 1 nM H70 was used in the reactions. (B) The same as Figure 3B except that 1 nM H70 in the absence (○) or the presence of 50 μM PC/PS (●) and 100 nM H70 in the presence of 50 μM PC/PS plus 5 nM factor Va (fully assembled prothrombinase) (■) was used in the reactions.
Discussion
Assembly of factor Xa into the prothrombinase complex is required for the generation of thrombin during the blood coagulation process.16,17 Complex formation not only enhances the rate of thrombin generation approximately 3 × 105-fold, it is also accompanied by protection of factor Xa from inhibition by antithrombin.16,17 Because factor Xa is an important target for heparin during anticoagulant therapy, knowing the extent to which prothrombinase complex formation protects the proteinase from heparin-catalyzed inhibition by antithrombin is crucial for treating patients with thrombosis. However, attempts to quantitatively address this question have resulted in inconsistent and conflicting data in the literature.19,21-24 This problem is believed to arise primarily from the inherent complexity of the assay systems needed to measure the rate of prothrombinase inhibition by antithrombin. This is because factor Xa and thrombin, the proteinase and the product of prothrombinase, are both inhibited at near diffusion-limited rates by the antithrombin-heparin complex.4,15,30 Thus, in some previous studies, prothrombin had to be excluded from the assays to measure the factor Va-mediated protective effect against inhibition by the antithrombin-heparin complex by a simple amidolytic activity assay.23,24 Results of the current study suggest that the inhibition rates obtained from such assays do not represent the heparin-catalyzed inhibition of a fully assembled prothrombinase complex in the presence of prothrombin. In one previous study, the heparin-catalyzed inhibition of prothrombinase was studied in the presence of prothrombin. However, complicated mathematical models had to be developed for data analysis because the rate of prothrombinase inhibition had to be calculated from the initial rate of thrombin generation, which itself is concomitantly and rapidly inhibited by the inhibitor-heparin complex.22 In this previous study, the prothrombinase complex formation resulted in an approximately 5-fold protection against inhibition by the heparin-antithrombin complex. Results of current direct prothrombinase inhibition assays do not support the reliability of these previous findings.
Another factor that might have contributed to the discrepancies in the literature is that results of some of the previous studies mentioned above have been analyzed based on the traditional view that a heparin-induced conformational change in the reactive site loop of antithrombin is solely responsible for the cofactor effect of heparin in factor Xa inhibition. Thus, heparin-catalyzed prothrombinase inhibition by antithrombin was measured in the presence of Ca++ and compared to that of free-factor Xa inhibition in the absence of Ca++. However, recently the existence of a binding site for heparin on the proteinase domain of factor Xa in a region homologous to heparin binding exosite 2 of thrombin was reported.15,29 It was demonstrated that heparin binding to this site accelerates factor Xa inhibition by antithrombin several hundred-fold more if the Gla-domain of the proteinase is neutralized by physiological concentrations of Ca++.15,29This increased acceleration was mediated by a bridging mechanism based on the bell-shaped dependence of the additional reaction rate enhancement on heparin concentration.15,29 30 It is likely that in some of these previous studies, the additional template cofactor effect of high-molecular-weight heparins has been wrongly attributed to the ability of longer chain heparins to overcome the protective effect of prothrombinase complex formation against inhibition by antithrombin.
The mechanistic basis for resistance of prothrombinase to inhibition by the antithrombin-heparin complexes in the presence of prothrombin was not investigated in detail. It is known, however, that the assembly of factor Xa into the prothrombinase complex dramatically lowers the Km of prothrombin (less than 1 μM) for binding to prothrombinase.31 In contrast, antithrombin, free or in complex with low-molecular-weight heparins, has a very high dissociation constant (approximately 200 μM) for binding to factor Xa.32 It follows, therefore, that in the presence of prothrombin, antithrombin may not effectively compete with the substrate prothrombin for binding to the catalytic center of factor Xa in the prothrombinase complex. Although binding of full-length heparin chains to an exosite on factor Xa can significantly improve the affinity of factor Xa for binding to antithrombin in the presence of physiological Ca++,15,29,30 previous kinetic data nevertheless suggest that the heparin-binding exosite of factor Xa overlaps with the binding site for factor Va, prothrombin, or both in prothrombinase.29 Thus, therapeutic doses of heparin cannot bind to this site to accelerate the prothrombinase inhibition by antithrombin because the affinity of factor Xa for factor Va–prothrombin is several orders of magnitude higher than that for the polysaccharide, and very high concentrations of heparin would be required to dissociate factor Xa from the prothrombinase complex.29,31 33
In summary, the results of this study suggest that prothrombin confers more than 3 orders of magnitude of protection for prothrombinase against inhibition by antithrombin in complex with either low- or high-molecular-weight heparins. The clinical ramifications of this observation are that the high-affinity pentasaccharide and other similarly low-molecular-weight heparins possessing predominantly anti–factor Xa activity may only be effective anticoagulant drugs for prophylactic purposes. Once prothrombinase is assembled on injured endothelium or on activated platelets, it is resistant to heparin-catalyzed inhibition by antithrombin in the presence of plasma concentrations of prothrombin. Thus, for the management of ongoing thrombosis, heparin therapy may have to be directed to the inhibition of thrombin with appropriate molecular-size heparins.
I would like to thank Dr Steven Olson for critical reading, Dr Akash Mathur for assistance with Figure 1, and Audrey Rezaie for proofreading the manuscript.
Supported by grant R01 HL 62565 from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alireza R. Rezaie, Department of Biochemistry and Molecular Biology, St Louis University School of Medicine, 1402 S Grand Blvd, St Louis, MO 63104; e-mail: rezaiear@slu.edu.