Abstract
The tyrosine kinase inhibitor STI571 inhibits BCR/ABL and induces hematologic remission in most patients with chronic myeloid leukemia. In addition to BCR/ABL, STI571 also inhibits v-Abl, TEL/ABL, the native platelet-derived growth factor (PDGF)β receptor, and c-KIT, but it does not inhibit SRC family kinases, c-FMS, FLT3, the epidermal growth factor receptor, or multiple other tyrosine kinases. ARG is a widely expressed tyrosine kinase that shares substantial sequence identity with c-ABL in the kinase domain and cooperates with ABL to regulate neurulation in the developing mouse embryo. As described here, ARG has recently been implicated in the pathogenesis of leukemia as a fusion partner of TEL. A TEL/ARG fusion was constructed to determine whether ARG can be inhibited by STI571. When expressed in the factor-dependent murine hematopoietic cell line Ba/F3, the TEL/ARG protein was heavily phosphorylated on tyrosine, increased tyrosine phosphorylation of multiple cellular proteins, and induced factor-independent proliferation. The effects of STI571 on Ba/F3 cells transformed with BCR/ABL, TEL/ABL, TEL/PDGFβR, or TEL/ARG were then compared. STI571 inhibited tyrosine phosphorylation and cell growth of Ba/F3 cells expressing BCR/ABL, TEL/ABL, TEL/PDGFβR, and TEL/ARG with an IC50 of approximately 0.5 μM in each case, but it had no effect on untransformed Ba/F3 cells growing in IL-3 or on Ba/F3 cells transformed by TEL/JAK2. Culture of TEL/ARG-transfected Ba/F3 cells with IL-3 completely prevented STI571-induced apoptosis in these cells, similar to what has been observed with BCR/ABL- or TEL/ABL-transformed cells. These results indicate that ARG is a target of the small molecule, tyrosine kinase inhibitor STI571.
Introduction
Small molecule drugs that can selectively inhibit tyrosine kinases are likely to be of benefit in a number of neoplastic diseases. Although tyrosine kinase inhibitors have been studied for many years, they often have had little specificity and thus were unlikely to be suitable for clinical applications. Recently, more selective tyrosine kinase inhibitors have been developed, including STI571 (formerly CGP57148B; Novartis Pharmaceuticals, Basel, Switzerland).1,2 STI571 has been shown to inhibit c-ABL, ABL oncogenes, c-KIT, and the platelet-derived growth factor (PDGF)β receptor, but it does not inhibit a wide variety of other tyrosine kinases, including members of the SRC and JAK kinase families, c-FMS, FLT3, vascular epithelial growth factor (VEGF) receptors, epidermal growth factor receptors, HER2/NEU, and many others.1-3 The restricted activity of STI571 has proven to be of value in initial clinical trials as a highly effective drug, with minimal nonhematopoietic side effects, for treating chronic myeloid leukemia (CML).4 STI571 is likely to be tested in future clinical trials in diseases in which other STI571 target kinases are activated, such as gastrointestinal sarcoma (c-KIT), glioblastomas (PDGFβ receptor). This reagent is also extremely valuable for research because of its ability to rapidly and completely inhibit tyrosine kinase activity of susceptible kinases in vitro and in vivo. Thus, identification of all kinase targets of STI571 is likely to be of both clinical and academic interest.
ARG is an ABL-relatedgene with an overall structure (SH3–SH2-kinase domain) similar to that of ABL.5 ARG is also highly related to ABL at an amino acid sequence level in the SH3, SH2, and kinase domains (89%, 90%, and 93% identity, respectively), though they are less than 30% identical in the C-terminus.6 Like ABL, ARG is a widely expressed tyrosine kinase typically detected on Western blot as a series of bands at 135 to 150 kd.7 The function of ARG is not well understood, but it is important for the proper development of the nervous system and for brain function in the adult mouse.8
The adenosine triphosphate (ATP) binding site of ARG is similar to the ATP binding site of ABL (Figure 1), suggesting the possibility that STI571 could inhibit ARG kinase activity. However, the structural features that predict for sensitivity to STI571 have not yet been defined, and there are a number of amino acid differences between ABL and ARG in the kinase domain. To determine whether STI571 inhibits ARG, an assay for ARG kinase activity was devised. There are no signaling pathways currently known to activate ARG, and no ARG substrates have been described in intact cells. Recently, the t(1;12)(q25, p13) chromosomal translocation in patients with acute leukemia has been reported to result in fusion of theTEL and ARG genes.9,10 TEL fusion with other tyrosine kinases occurs in acute and chronic leukemias, including TEL/PDGFβR, TEL/ABL, and TEL/JAK2.11,12 In each case, the TEL component oligomerizes and activates the tyrosine kinase activity of its partner, resulting in a constitutively active tyrosine kinase oncogene that transforms hematopoietic cells in vitro and in vivo. In the studies presented here, we have constructed a TEL/ARG cDNA and used it to transform the murine hematopoietic cell line Ba/F3.13 Similarly, other Ba/F3 cell lines were generated after transfection with TEL/ABL, TEL/PDGFβR, TEL/JAK2, or BCR/ABL. These cell lines were then used to compare the sensitivity of these activated tyrosine kinases to STI571 in intact cells.
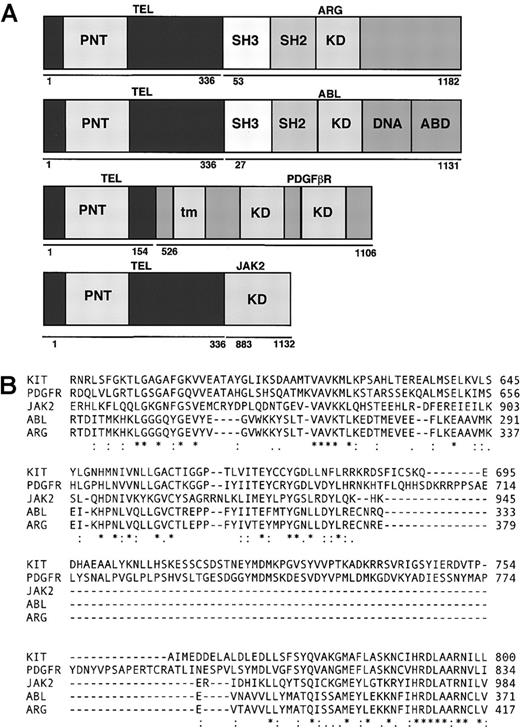
Comparison of fusion genes.
(A) Schematic representation of fusion genes. PNT indicates pointed domain; KD, tyrosine kinase domain; DNA, DNA-binding domain; ABD, actin-binding domain; tm, transmembrane domain; SH3: SRC homology 3; SH2, SRC homology 2. (B) Comparison of kinase domains of ARG, ABL, PDGFR, KIT, and JAK2.
Comparison of fusion genes.
(A) Schematic representation of fusion genes. PNT indicates pointed domain; KD, tyrosine kinase domain; DNA, DNA-binding domain; ABD, actin-binding domain; tm, transmembrane domain; SH3: SRC homology 3; SH2, SRC homology 2. (B) Comparison of kinase domains of ARG, ABL, PDGFR, KIT, and JAK2.
Materials and methods
Cell lines and cell culture
The IL-3–dependent murine hematopoietic cell line Ba/F3 was transfected with a pGD vector containing p210BCR/ABL (B2A2) cDNA to generate the Ba/F3.p210 cell line.14,15 STI571 was obtained from Elisabeth Buchdunger of Novartis Pharmaceuticals and was dissolved in water. Ba/F3 cell lines expressing TEL/ABL (approximately 180 kd), TEL/PDGFβR (approximately 80 kd), and TEL/JAK2 (approximately 65-70 kd) have been described.15-17 pcDNA3 TEL/ARG plasmid was provided by Dr Yuko Sato (International Medical Center of Japan, Tokyo) and was used to express TEL/ARG (approximately 170 kd) (Iijima Y, et al, manuscript in preparation).9Four independent sublines of Ba/F3 expressing TEL/ARG were generated by electroporation, as previously described. Transfected cells were selected with G418 (1 mg/mL) in media containing 10% WEHI-conditioned medium as a source of IL-3. Transfected cells were cultured at 37°C with 5% CO2 at a concentration of 2 × 105 to 5 × 105 cells/mL in RPMI 1640 medium (Mediatech, Herndon, VA) with 10% fetal calf serum. Untransfected parental Ba/F3 cells were cultured at 37°C with 5% CO2 at a concentration of 2 × 105 to 5 × 105cells/mL in RPMI 1640 medium (Mediatech) with 10% fetal calf serum and 15% WEHI-conditioned medium as a source of IL-3.
Cell viability determination
After staining with trypan blue (Sigma, St Louis, MO), viable cells were counted using a hemacytometer. Cell concentrations were calculated as number of cells × 10 000/mL. Cell viability was reported as percentage control (untreated) cells.
Annexin-propidium iodide staining
Cell viability and apoptosis in STI571-treated cells were assessed using the Annexin-V–Fluos Staining Kit (Boehringer Mannheim, Indianapolis, IN). Briefly, 1 × 106 cells cultured in the presence or absence of STI571 were washed once with 1× phosphate-buffered saline (PBS) and centrifuged at 1500 rpm for 5 minutes. Cell pellets were resuspended in 100 μL 20% Annexin-V–fluorescein labeling reagent and 20% propidium iodide (PI) in Hepes buffer. Cells were incubated for 15 minutes at room temperature, diluted in 0.8 mL Hepes buffer, and analyzed by flow cytometry. In addition, as controls, cells were stained with Annexin-V–fluorescein labeling reagent alone or PI alone or were left unstained.
Antibodies
Anti-pTyr monoclonal antibody (mAb) #4G10 was provided by Dr Brian Druker (University of Oregon Health Sciences Center, Portland) and was diluted 1:2500 for immunoblot. Monoclonal anti-actin (clone AC-15; Sigma) was diluted 1:1000 for immunoblot. Rabbit polyclonal anti-ARG, directed against the SH2 and SH3 domains of ARG, was a gift from Dr Anthony Koleske (Yale University, New Haven, CT) and diluted 1:1000 for immunoblot. Anti-ARG, goat polyclonal antibody (pAb) clone c-20, directed against the carboxy terminus of ARG, was obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and used for immunoprecipitation. Anti-ABL (clone 3F12), which is directed against the SH2 domain of c-ABL, was developed by Dr Ravi Salgia and Dr James Griffin (Dana Farber Cancer Institute, Boston, MA) and was used at 1:500 for immunoblotting. Anti-PAC1, goat pAb clone c-20, targeted against a peptide mapping at the carboxy terminus of protein tyrosine phosphatase PAC1 of human origin, was obtained from Santa Cruz Biotechnology and used for immunoprecipitation.
Immunoblotting and immunoprecipitation
Cells were lysed in lysis buffer containing 0.02 M Tris, pH 8.0, 0.15 M NaCl, 10% glycerol, 1% NP-40 (wt/vol), 0.1 M NaF, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 40 μg/mL leupeptin, and 20 μg/mL aprotinin. Cell lysates were incubated on ice for 25 minutes, with vortexing every 5 minutes, and then centrifuged at 12 000g for 15 minutes. Protein yields were determined in the supernatants by Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA). Equal amounts of protein were then loaded onto gel lanes or used for immunoprecipitation experiments. For immunoprecipitation, cell lysates were incubated with anti-ARG antibody (clone C-20) or anti–PAC-1 antibody (clone C-20) and protein G Sepharose overnight at 4°C. As a control, cell lysates were also incubated with protein G Sepharose beads alone. After incubation, immune complexes were washed twice with lysis buffer, twice with 1× PBS, and were dissolved in Laemmeli's sample buffer by boiling for 5 minutes. For immunoprecipitation and immunoblotting, immune complexes and whole cell lysates, respectively, were resolved on a sodium dodecyl sulfate (SDS)–7.5% polyacrylamide gel. Protein was then electrophoretically transferred to a Protran nitrocellulose transfer and immobilization membrane (Schleicher and Schuell, Dassel, Germany). The membrane was blocked for either 1 hour at 25°C or overnight at 4°C with 5% nonfat dry milk in 1× TBS (10 mM Tris-HCl, pH 8.0, 150 mM NaCl) and then probed for 2 hours at 25°C or overnight at 4°C with antibody in 1× TBST buffer (10 mM Tris-HCl, pH 8.0,150 mM NaCl, 0.05% Tween 20). After 3 washes with 1× TBST, membranes were incubated for 1 hour at 25°C with anti-mouse immunoglobulin, horseradish peroxidase–linked whole antibody (from sheep) (Amersham Life Science, Arlington Heights, IL) or anti-rabbit immunoglobulin, and horseradish peroxidase–linked whole antibody (from donkey) (Amersham Life Science). The membrane was washed 5× for 5 minutes/wash in 1× TBST buffer, and bound antibodies were detected with enhanced luminol and oxidizing reagent as specified by the manufacturer (NEN Life Science Products, Boston, MA). Filters were stripped with stripping buffer (2% SDS, 0.0625 M Tris, pH 6.8, and 0.7% 2-mercaptoethanol) for 30 minutes at 50°C before they were probed with additional antibodies.
Results
Comparison of the amino acid sequence around the ATP binding site of ABL, ARG, KIT, PDGFβR, and JAK2 kinases
A diagram showing the structure of TEL/ARG, TEL/ABL, TEL/PDGFβR, and TEL/JAK2 is presented in Figure 1A. Amino acid sequences of human ABL, ARG, KIT, PDGFβR, and JAK2 were obtained from GenBank (gi:2 144 425, 6 382 062, 125 472, 66 816, and 7 446 414, respectively). Approximately 135 amino acids from c-ABL were aligned with corresponding sequences from the other kinases using the ClustalW Program (http://www2.ebi.ac.uk/clustalw/) (Figure 1B), starting 10 amino acids N-terminal of the consensus ATP binding site (G-X-G-X-F/Y-G-X-V-X). KIT and PDGFβR have inserts of approximately 100 amino acids that are lacking in ABL, ARG, or JAK2. ABL, KIT, and PDGFβR are known to be inhibited by STI571 at submicromolar concentrations in vivo, whereas JAK2 is more than 100-fold less sensitive. Over this span, there were 9 positions in which the amino acids matched between ABL, ARG, KIT, and PDGFβR, but not in JAK2 (ABL positions R239, K274, E281, L301, L302, T306, T315, N358, and A366).
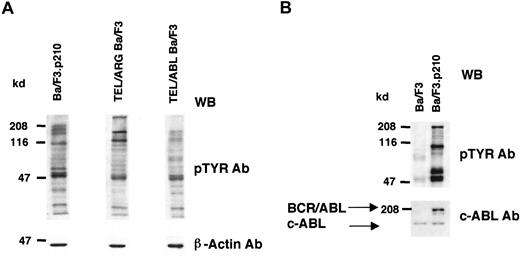
Expression of TEL/ARG increases tyrosine phosphorylation of cellular proteins in Ba/F3 cells
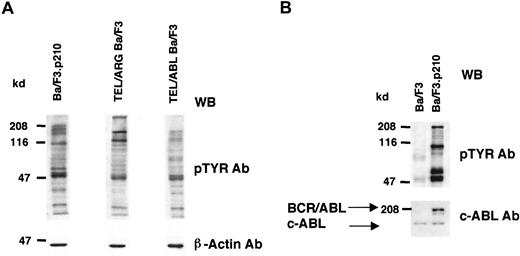
Cell lines expressing TEL/ARG, TEL/ABL, or BCR/ABL were generated as described in “Materials and methods,” and the overall tyrosine phosphorylation of cellular proteins was evaluated by immunoblotting with an anti-phosphotyrosine monoclonal antibody (Figure2). Compared to Ba/F3 cells, each of the TEL-kinase fusion protein-transfected cell lines showed elevated tyrosine phosphorylation of multiple cellular proteins.
Comparison of cellular tyrosine phosphorylation patterns in fusion protein-transfected Ba/F3 cells and untransfected Ba/F3 cells.
(A) Comparison of cellular tyrosine phosphorylation patterns in Ba/F3.p210 cells, TEL/ARG Ba/F3 cells, and TEL/ABL Ba/F3 cells. Anti–β-actin was used as a loading control. The first and second lanes shown (Ba/F3.p210 and TEL/ARG Ba/F3, respectively) are derived from the same Western blot; the third lane shown (TEL/ABL Ba/F3) is derived from an independent Western blot performed in parallel with the first blot. (B) Comparison of cellular tyrosine phosphorylation patterns in Ba/F3.p210 cells and Ba/F3 cells. An anti-ABL antibody was used to measure levels of ABL in both cell types and to show the expression of BCR/ABL in Ba/F3.p210 cells. WB indicates Western blot.
Comparison of cellular tyrosine phosphorylation patterns in fusion protein-transfected Ba/F3 cells and untransfected Ba/F3 cells.
(A) Comparison of cellular tyrosine phosphorylation patterns in Ba/F3.p210 cells, TEL/ARG Ba/F3 cells, and TEL/ABL Ba/F3 cells. Anti–β-actin was used as a loading control. The first and second lanes shown (Ba/F3.p210 and TEL/ARG Ba/F3, respectively) are derived from the same Western blot; the third lane shown (TEL/ABL Ba/F3) is derived from an independent Western blot performed in parallel with the first blot. (B) Comparison of cellular tyrosine phosphorylation patterns in Ba/F3.p210 cells and Ba/F3 cells. An anti-ABL antibody was used to measure levels of ABL in both cell types and to show the expression of BCR/ABL in Ba/F3.p210 cells. WB indicates Western blot.
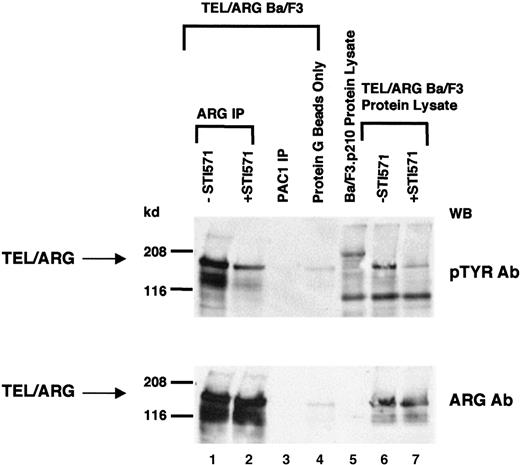
Inhibition of TEL/ARG autophosphorylation by STI571
Because TEL/ARG increased cellular tyrosine phosphorylation and most tyrosine kinase oncogenes are autophosphorylated, tyrosine phosphorylation of TEL/ARG was examined. TEL/ARG was immunoprecipitated from TEL/ARG-transfected Ba/F3 cells using a purified goat polyclonal ARG antibody (clone C-20) directed against the C-terminus of ARG (Figure 3). Immunoblotting with an anti-phosphotyrosine antibody revealed tyrosine phosphorylation of TEL/ARG. Exposure of cells to 1 μM STI571 for 24 hours before lysis resulted in a reduction of cellular tyrosine phosphorylation in general and of TEL/ARG specifically. Immunoblotting with a rabbit anti-ARG antibody directed against the SH2 and SH3 domains of ARG revealed similar levels of TEL/ARG protein in untreated and STI571-treated cells (Figure 3, lower panel). To confirm that the approximately 170-kd phosphorylated band observed after ARG immunoprecipitation was, indeed, specific for TEL/ARG, immunoprecipitation was performed with a purified goat polyclonal antibody directed against the phosphatase PAC1 (Figure3). No band sizes comparable to that of TEL/ARG were detected, suggesting that ARG antibody-precipitated bands were specific. These results suggest that autophosphorylation of TEL/ARG is inhibited by STI571.
Inhibition of TEL/ARG autophosphorylation by STI571.
TEL/ARG was immunoprecipitated from TEL/ARG-transfected Ba/F3 cells cultured in the absence or presence of STI571 for 24 hours (lanes 1 and 2, respectively). An anti-PAC1 polyclonal goat antibody was used to immunoprecipitate the phosphatase PAC1 as a nonspecific control (lane 3). Protein lysate from TEL/ARG-Ba/F3 cells was also incubated in the presence of protein G beads alone, as a nonspecific control (lane 4). Immunoblotting was performed on all samples with anti-pTYR (upper panel) and anti-ARG (lower panel). Whole cell lysates from TEL/ARG-Ba/F3 cells cultured in the absence and presence of STI571 (lanes 6 and 7, respectively) were run in parallel with the immunocomplexes, and immunoblotting was similarly performed on these samples with anti-pTyr (upper panel) and anti-ARG (lower panel). Whole cell lysate from Ba/F3.p210 cells was run alongside whole cell lysates from TEL/ARG-Ba/F3 cells for comparison (lane 5). IP indicates immunoprecipitation.
Inhibition of TEL/ARG autophosphorylation by STI571.
TEL/ARG was immunoprecipitated from TEL/ARG-transfected Ba/F3 cells cultured in the absence or presence of STI571 for 24 hours (lanes 1 and 2, respectively). An anti-PAC1 polyclonal goat antibody was used to immunoprecipitate the phosphatase PAC1 as a nonspecific control (lane 3). Protein lysate from TEL/ARG-Ba/F3 cells was also incubated in the presence of protein G beads alone, as a nonspecific control (lane 4). Immunoblotting was performed on all samples with anti-pTYR (upper panel) and anti-ARG (lower panel). Whole cell lysates from TEL/ARG-Ba/F3 cells cultured in the absence and presence of STI571 (lanes 6 and 7, respectively) were run in parallel with the immunocomplexes, and immunoblotting was similarly performed on these samples with anti-pTyr (upper panel) and anti-ARG (lower panel). Whole cell lysate from Ba/F3.p210 cells was run alongside whole cell lysates from TEL/ARG-Ba/F3 cells for comparison (lane 5). IP indicates immunoprecipitation.
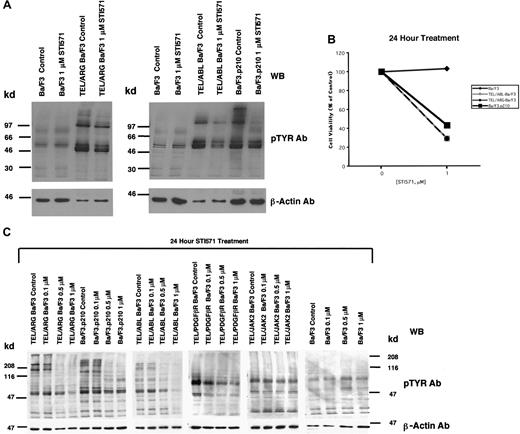
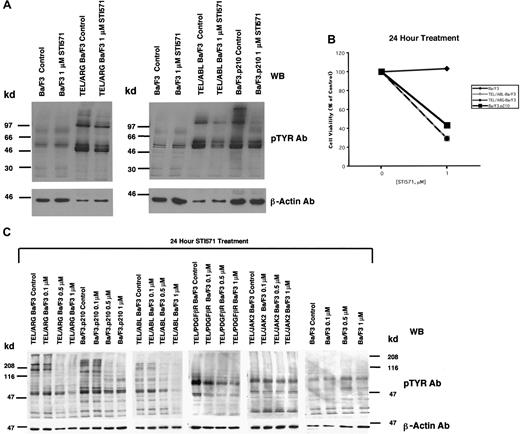
Comparison of the effects of STI571 on cellular tyrosine phosphorylation in Ba/F3 cells expressing BCR/ABL, TEL/ABL, TEL/ARG, TEL/PDGFβR, or TEL/JAK2
The apparent inhibition of TEL/ARG kinase activity shown in Figure 3 was further evaluated by comparing the effects of STI571 (0-1 μM for 24 hours) on total cellular tyrosine phosphorylation in Ba/F3 cells transformed by BCR/ABL, TEL/ABL, and TEL/ARG (Figure4A). Total cellular tyrosine phosphorylation was elevated in Ba/F3.p210, TEL/ABL-Ba/F3, and TEL/ARG-Ba/F3 cells in comparison with untransfected Ba/F3 cells, and it was inhibited by 1 μM STI571 after 24 hours of treatment in each of these cell lines (Figure 4A). The growth and proliferation of the Ba/F3 cells expressing BCR/ABL, TEL/ABL, and TEL/ARG was similarly inhibited by the compound after 24 hours (Figure 4B). Total cellular tyrosine phosphorylation was inhibited in Ba/F3 cells expressing BCR/ABL, TEL/ABL, TEL/ARG, and TEL/PDGFβR at concentrations of STI571 < 1 μM, and there was no inhibition in cells expressing TEL/JAK2 or untransfected Ba/F3 cells (Figure 4C). Two independent TEL/JAK2 Ba/F3 lines were tested for responsiveness to STI571 in terms of tyrosine phosphorylation and both yielded similar results (data shown for only one of the 2 cell lines in Figure 4C). The IC50 (50% inhibitory concentration) was approximately 0.5 μM STI571 for each of the sensitive cell lines, though TEL/PDGFβR-Ba/F3 cells were slightly more sensitive to the kinase inhibitor.
Comparison of the effects of STI571 on cellular tyrosine phosphorylation in Ba/F3 cells expressing BCR/ABL, TEL/ABL, TEL/ARG, TEL/PDGFR, or TEL/JAK2.
(A) An anti-pTYR immunoblot showing cellular tyrosine phosphorylation in untransfected Ba/F3 cells and TEL/ARG-Ba/F3 cells cultured in the presence and absence of STI571 (left panel). An anti-pTYR immunoblot showing cellular tyrosine phosphorylation in untransfected Ba/F3 cells, TEL/ABL-Ba/F3, and Ba/F3.p210 cells cultured in the presence and absence of STI571 (right panel). Immunoblots were stripped and incubated with anti–β-actin as a loading control. (B) Effect of 24-hour STI571 treatment on viability of cells analyzed in part A. (C) Anti-pTYR immunoblots showing cellular tyrosine phosphorylation in TEL/ABL-Ba/F3, TEL/ARG-Ba/F3, Ba/F3.p210, TEL/PDGFR-Ba/F3, TEL/JAK2-Ba/F3, and untransfected Ba/F3 cells treated with increasing concentrations of STI571. Immunoblots were stripped and incubated with anti–β-actin as a loading control.
Comparison of the effects of STI571 on cellular tyrosine phosphorylation in Ba/F3 cells expressing BCR/ABL, TEL/ABL, TEL/ARG, TEL/PDGFR, or TEL/JAK2.
(A) An anti-pTYR immunoblot showing cellular tyrosine phosphorylation in untransfected Ba/F3 cells and TEL/ARG-Ba/F3 cells cultured in the presence and absence of STI571 (left panel). An anti-pTYR immunoblot showing cellular tyrosine phosphorylation in untransfected Ba/F3 cells, TEL/ABL-Ba/F3, and Ba/F3.p210 cells cultured in the presence and absence of STI571 (right panel). Immunoblots were stripped and incubated with anti–β-actin as a loading control. (B) Effect of 24-hour STI571 treatment on viability of cells analyzed in part A. (C) Anti-pTYR immunoblots showing cellular tyrosine phosphorylation in TEL/ABL-Ba/F3, TEL/ARG-Ba/F3, Ba/F3.p210, TEL/PDGFR-Ba/F3, TEL/JAK2-Ba/F3, and untransfected Ba/F3 cells treated with increasing concentrations of STI571. Immunoblots were stripped and incubated with anti–β-actin as a loading control.
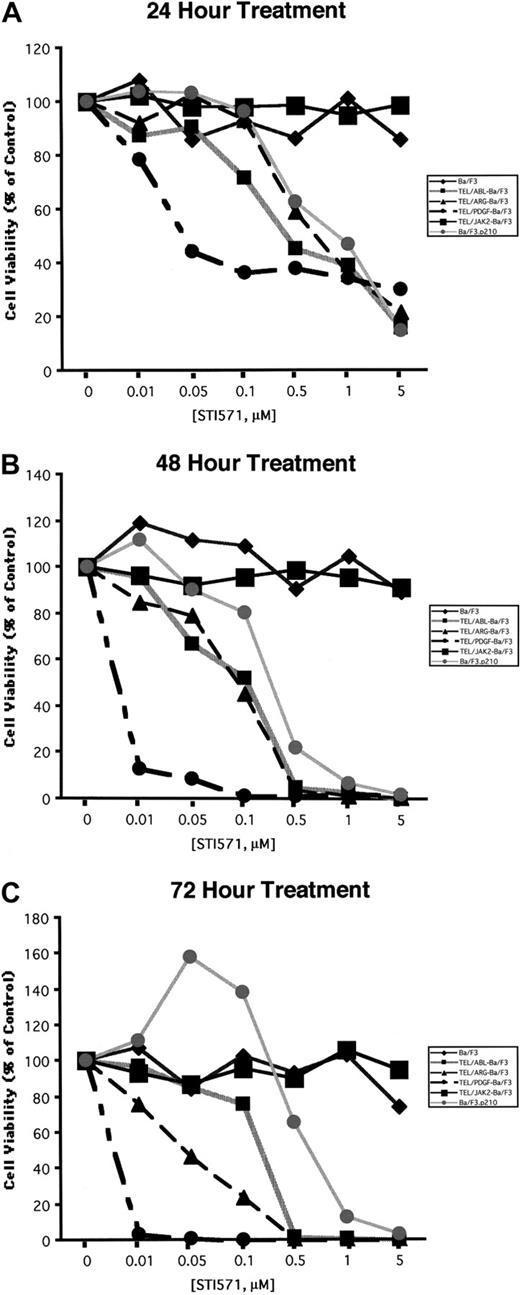
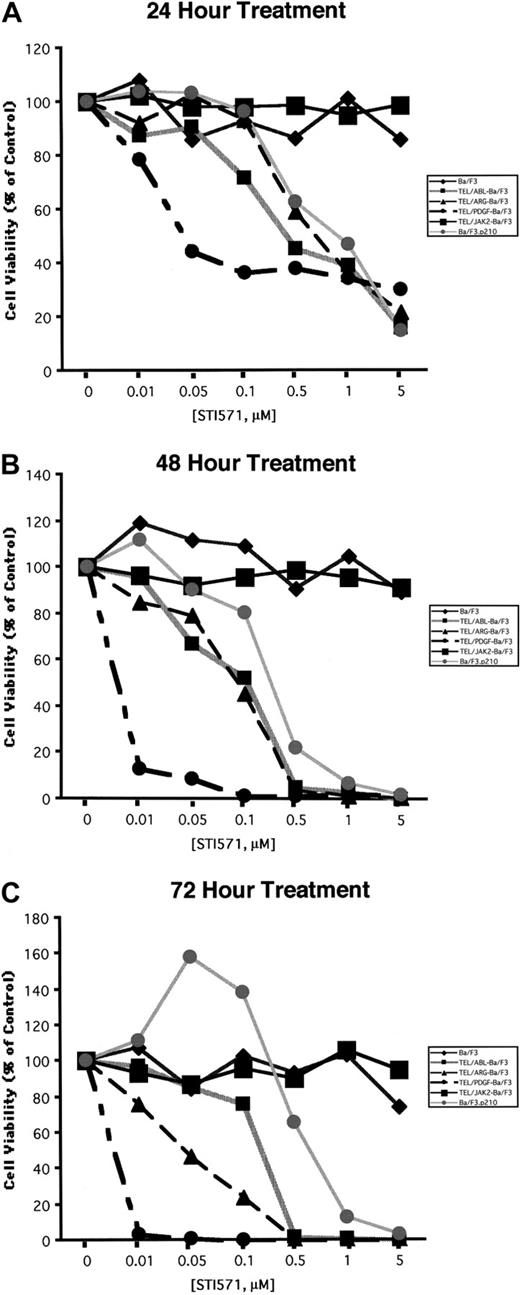
Comparison of the effects of STI571 on cellular proliferation and viability in Ba/F3 cells expressing BCR/ABL, TEL/ABL, TEL/ARG, TEL/PDGFβR, or TEL/JAK2
Ba/F3 cell lines were exposed to various concentrations of STI571, and cell number and viability were measured after 24, 48, and 72 hours (Figure 5). Proliferation of Ba/F3.p210, TEL/ARG Ba/F3, and TEL/ABL Ba/F3 cells was inhibited by STI571 to an approximately similar degree at each time point, whereas TEL/PDGFβR-Ba/F3 cells were slightly more sensitive. Concentrations of up to 1 μM STI571 did not inhibit the proliferation of untransformed Ba/F3 cells growing in IL-3 or of TEL/JAK2-Ba/F3 cells. Two independent TEL/JAK2 Ba/F3 lines were tested for responsiveness to STI571 in terms of cell proliferation, and both yielded similar results (data shown for only one of the 2 cell lines in Figure 5).
Comparison of the effects of STI571 on cellular proliferation and viability in Ba/F3 cells expressing BCR/ABL, TEL/ARG, TEL/ABL, TEL/PDGFR, or TEL/JAK2.
(A) Growth of cells cultured in the presence of increasing concentrations of STI571 (0.01-5 μM) for 24 hours. (B) Growth of cells cultured in the presence of increasing concentrations of STI571 (0.01-5 μM) for 48 hours. (C) Growth of cells cultured in the presence of increasing concentrations of STI571 (0.01-5 μM) for 72 hours. For all cell lines, values obtained for the 24-hour time points were calculated as the average of 3 independent experiments, shown as percentage untreated (control) cells. Values obtained for the 48- and 72-hour time points, respectively, were calculated as the average of 2 independent experiments, shown as percentage untreated (control) cells. Each cell line is represented by a different symbol, as shown in the figure legends.
Comparison of the effects of STI571 on cellular proliferation and viability in Ba/F3 cells expressing BCR/ABL, TEL/ARG, TEL/ABL, TEL/PDGFR, or TEL/JAK2.
(A) Growth of cells cultured in the presence of increasing concentrations of STI571 (0.01-5 μM) for 24 hours. (B) Growth of cells cultured in the presence of increasing concentrations of STI571 (0.01-5 μM) for 48 hours. (C) Growth of cells cultured in the presence of increasing concentrations of STI571 (0.01-5 μM) for 72 hours. For all cell lines, values obtained for the 24-hour time points were calculated as the average of 3 independent experiments, shown as percentage untreated (control) cells. Values obtained for the 48- and 72-hour time points, respectively, were calculated as the average of 2 independent experiments, shown as percentage untreated (control) cells. Each cell line is represented by a different symbol, as shown in the figure legends.
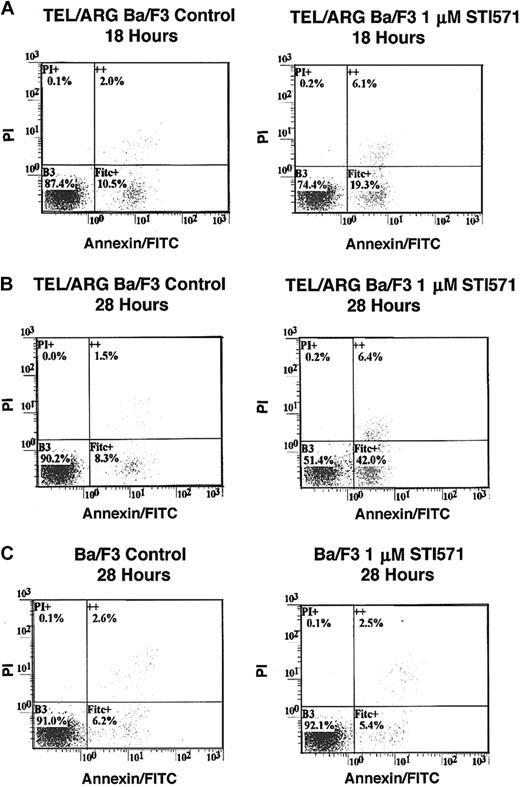
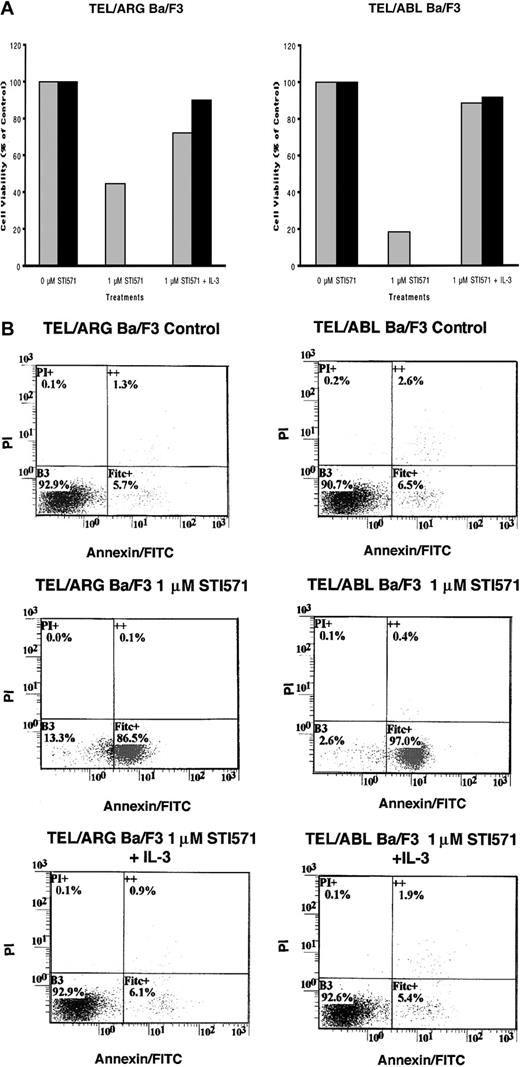
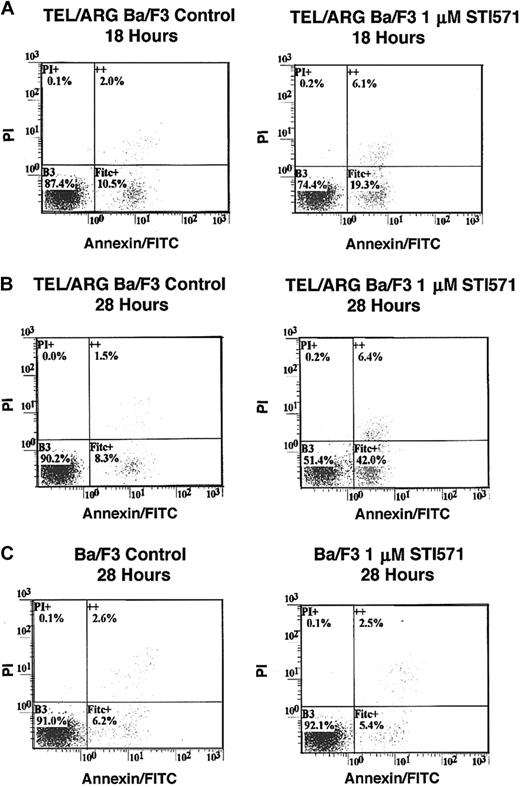
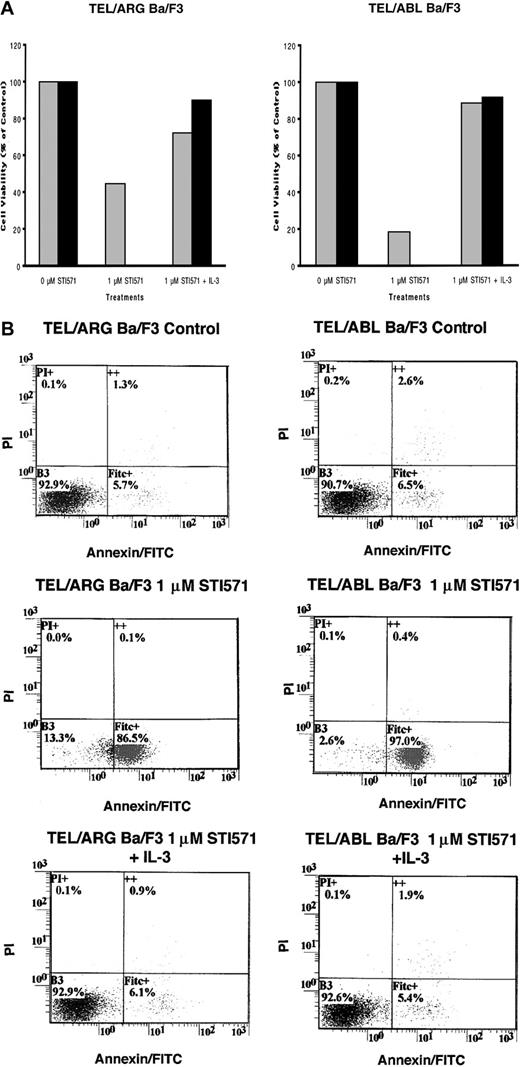
To determine whether the decrease in cell number observed in Figure 5was due to the induction of apoptosis, Annexin V–PI staining was performed on TEL/ARG, TEL/ABL, TEL/PDGFβR, and BCR/ABL-Ba/F3 cells treated for 8, 18, and 28 hours, respectively, with 1 μM STI571. Although STI571 did not induce apoptosis in untransfected Ba/F3 cells and TEL/ARG-Ba/F3 cells after 8 hours of treatment (data not shown), STI571 increased the early and late apoptotic fractions of cells from TEL/ARG-Ba/F3 after 18 and 28 hours of treatment, respectively (Figure6). STI571 had no effect on untransfected Ba/F3 cells after either 18 hours (data not shown) or 28 hours (Figure6). The results indicate that STI571 can induce rapid apoptosis in cells expressing tyrosine kinase oncogenes sensitive to this inhibitor. It has been reported that BCR/ABL-, TEL/ABL-, and TEL/PDGFβR-transformed Ba/F3 cells can be rescued from the cytotoxic effects of STI571 by IL-32 18; we asked whether TEL/ARG-transformed Ba/F3 cells likewise could be rescued by IL-3. Indeed, cell death induced by STI571 at 24 or 72 hours of treatment of TEL/ABL-Ba/F3 and TEL/ARG-Ba/F3 cells was completely abrogated by co-culture of both cell lines with WEHI-conditioned medium, a source of IL-3 (Figure 7). These results suggest functional overlap of the viability signals induced by TEL/ARG and IL-3.
Effects of STI571 on cell viability and apoptosis in Ba/F3 cells expressing TEL/ARG and untransfected Ba/F3 cells, as determined by annexin-PI staining.
(A) Eighteen-hour treatment of TEL/ARG-Ba/F3 cells with vehicle (left panel) or 1 μM STI571 (right panel). (B) Twenty-eight–hour treatment of TEL/ARG-Ba/F3 cells with vehicle (left panel) or 1 μM STI571 (right panel). (C) Twenty-eight–hour treatment of untransfected Ba/F3 cells with vehicle (left panel) or 1 μM STI571 (right panel). FITC indicates fluorescein isothiocyanate.
Effects of STI571 on cell viability and apoptosis in Ba/F3 cells expressing TEL/ARG and untransfected Ba/F3 cells, as determined by annexin-PI staining.
(A) Eighteen-hour treatment of TEL/ARG-Ba/F3 cells with vehicle (left panel) or 1 μM STI571 (right panel). (B) Twenty-eight–hour treatment of TEL/ARG-Ba/F3 cells with vehicle (left panel) or 1 μM STI571 (right panel). (C) Twenty-eight–hour treatment of untransfected Ba/F3 cells with vehicle (left panel) or 1 μM STI571 (right panel). FITC indicates fluorescein isothiocyanate.
IL-3 rescue of TEL/ARG-Ba/F3 cells and TEL/ABL-Ba/F3 cells.
(A) Growth curves for TEL/ARG-Ba/F3 cells treated with STI571 for 24 (░) and 72 hours (▪), respectively, and cultured in the absence or presence of 15% WEHI-conditioned medium, used as a source of IL-3 (left panel). Growth curves for TEL/ABL-Ba/F3 cells treated as described for TEL/ARG-Ba/F3 cells (right panel). Experiments were performed in duplicate for each cell line, and viability counts were determined by trypan blue exclusion and shown as percentage control. (B) Annexin-PI analysis of TEL/ARG-Ba/F3 cells (left panel) and TEL/ABL-Ba/F3 cells (right panel), treated as described in panel A.
IL-3 rescue of TEL/ARG-Ba/F3 cells and TEL/ABL-Ba/F3 cells.
(A) Growth curves for TEL/ARG-Ba/F3 cells treated with STI571 for 24 (░) and 72 hours (▪), respectively, and cultured in the absence or presence of 15% WEHI-conditioned medium, used as a source of IL-3 (left panel). Growth curves for TEL/ABL-Ba/F3 cells treated as described for TEL/ARG-Ba/F3 cells (right panel). Experiments were performed in duplicate for each cell line, and viability counts were determined by trypan blue exclusion and shown as percentage control. (B) Annexin-PI analysis of TEL/ARG-Ba/F3 cells (left panel) and TEL/ABL-Ba/F3 cells (right panel), treated as described in panel A.
Discussion
The use of small molecule drugs to inhibit specific oncogenes in leukemia promises to improve outcome and reduce side effects compared to traditional chemotherapy. Tyrosine kinase oncogenes are likely to be excellent drug targets for several reasons. First, tyrosine kinases are frequently activated by chromosomal translocations in leukemia. In addition to ABL, tyrosine kinases known to be activated in leukemias include KIT, FLT3, JAK2, PDGFβR, and ARG.9,10,12,19-22Second, there have been many recent advances in the understanding and development of drugs that inhibit tyrosine kinases. For example the tyrphostin AG-490 was shown to induce apoptosis of acute lymphoblastic leukemia cells by inhibiting the JAK2 tyrosine kinase,23and the anticancer agent SU5416 is being tested in clinical trials as a potent inhibitor of VEGF signaling through the FLK-1 receptor, a receptor tyrosine kinase involved in tumor angiogenesis.24Finally, the biologic activities of tyrosine kinase oncogenes make them attractive drug targets. Specifically, there is increasing evidence that tyrosine kinase oncogenes generate strong viability signals and that turning off a kinase leads to rapid apoptosis. This effect has been nicely demonstrated for BCR/ABL both in vitro and in vivo.25,26 Thus, the inhibition of kinase activity in patients with CML would be expected to have potent anti-leukemia activity. A potential concern in the development of kinase inhibitors for clinical trials has been specificity. Earlier tyrosine kinase inhibitors such as genistein had relatively broad inhibitory activity, and only recently have inhibitors with more restricted effects on kinases been developed. STI571 was generated in a search for drugs that could inhibit the PDGFβ receptor. This phenylaminopyrimidine derivative was found to inhibit the PDGFβ receptor at subnanomolar concentrations and to inhibit ABL oncogenes and c-KIT.1-3Other kinases expressed in hematopoietic cells were not inhibited, including c-FMS, JAK2, FLT3, and SRC-family kinases.2Selective inhibition of the growth of cells expressing TEL-ABL, TEL-PDGFβR, p210BCR/ABL, and p190BCR/ABL by STI571 has been demonstrated,18,27,28 and STI571 selectively induces apoptosis in BCR/ABL-positive cells.29
STI571 is believed to interact with the ATP binding site of kinases and to block the binding of ATP.2 The characteristics that distinguish sensitive from resistant kinases are partially understood. Based on a crystal structure of the catalytic domain of ABL complexed to a variant of STI571, initial drug binding requires that the kinase be in an “inactive” conformation in which the “activation loop” is unphosphorylated.30 The inactive conformation of the loop is, contrary to the active conformation, diverse among protein kinases.30 One factor possibly contributing to the specificity of STI571 for ABL was reported to be the preservation of an ion pair between lysine and glutamine residue side chains in the inactive conformation of ABL that appears to contribute to drug-substrate interaction. Inactive conformations of SRC tyrosine kinases are characterized by a disrupted ion pair.30Threonine residue 315 (T315) was also suggested to be necessary for the interaction of ABL interaction with STI571.30 In accordance with this, T315 in ABL is conserved in STI571 substrates ARG, PDGFβR, and KIT but is replaced by a methionine in the STI571-insensitive JAK2.
The studies shown here indicate the c-ARG kinase is sensitive to STI571 at a level comparable to that of c-ABL, at least when activated as part of the TEL/ARG oncogene. Direct testing of c-ARG for sensitivity to STI571 in an intact cell will require discovery of a normal signaling pathway that activates ARG. However, because the TEL/ARG fusion protein would not be expected to alter the structure of the ARG kinase domain, it is highly likely that c-ARG and TEL/ARG will be equally sensitive to STI571.
The high homology between the SH2 domain and the kinase domain of ARG and ABL suggests that both proteins may share common substrates. Indeed, an in vitro study showed that both ABL and ARG catalyze the tyrosine phosphorylation of the C-terminal repeat domain of RNA polymerase II.31 In another study, the substrate preferences for c-ABL and c-ARG were compared using synthetic peptides, and considerable similarity in substrate selection was reported.32 In contrast, BCR/ABL and TEL/ABL were more promiscuous and phosphorylated a number of peptides that are poor substrates for either ABL or ARG.32 In general, however, in vitro kinase assays may not be accurate at predicting in vivo substrates. This has been observed repeatedly when comparing c-ABL and BCR/ABL, where most of the known substrates for c-ABL are nuclear proteins and most of the known substrates for BCR/ABL are cytoplasmic proteins. Similarly, subcellular localization of ARG and ABL is distinct: whereas ABL is located in the nucleus and cytoplasm, ARG is located only in the cytoplasm.33 Although not a goal of the current studies, it is clear from examining the phosphotyrosine immunoblots shown in Figures 2 and 4 that the overall patterns of cellular phosphorylation induced by TEL/ARG, TEL/ABL, and BCR/ABL in Ba/F3 cells are not the same.
Although ARG is widely expressed,7 its only known function is in the central nervous system. ARG is abundant in the brain and is concentrated in synapses.8 ARG knockout mice develop normally but have neuronal dysfunction manifested by multiple behavioral disorders.8 Interestingly, ABL/ARG double knockout mice have severe defects in neurulation, including failure to close the neural tube.8 Because these defects are more significant than in either single knockout mouse, it is likely that ABL and ARG cooperate during development of the nervous system, perhaps in regulating cytoskeletal structure or function. ARG is not known to be required for normal hematopoiesis and, unlike c-ABL, has not been linked to the regulation of DNA repair processes.
Fusion of the TEL and ARG genes has been reported in 2 patients with leukemia, with a specific chromosomal translocation, t(1;12)(q25;p13).9,10 In both cases, the fusions included the Pointed (PNT) domain of TEL (ETV6) and the SH3, SH2, and kinase domains of ARG. The reciprocal ARG/TEL transcript was detected in one patient but not in the other. One patient had acute myeloblastic leukemia (AML)-M39 and also had the t(15;17) translocation characteristic of acute promyelocytic leukemia. The second patient had AML-M4.10 Interestingly, cells from both patients displayed eosinophilic differentiation, suggesting that activation of ARG might modulate the differentiation potential of leukemic cells. Based on previous studies with TEL/PDGFβR, TEL/JAK2, and TEL/ABL, it would be anticipated that the TEL/ARG fusion protein would increase tyrosine kinase activity. Results presented here confirm that hypothesis and show that TEL/ARG can confer factor-independent proliferation to Ba/F3 cells. The TEL/ARG construct generated here was essentially identical to the TEL/ARG sequences found in the 2 leukemia patients. As noted above, the ETV6/TEL gene has been reported to fuse with multiple partners, including 5 tyrosine kinases—PDGFβR, JAK2, ABL, TRKC, and ARG.9,10,23,34-37The PNT domain of ETV6/TEL mediates oligomerization and, thereby, activation of the tyrosine kinases.35 In this respect, theTEL gene functions like the coiled-coil “oligomerization” domain of BCR, which is required for the high level of kinase activity of BCR/ABL.38 Fusions between TEL and ABL have been associated with both chronic and acute leukemias.35,36,39 40
Overall, the results presented here extend the spectrum of kinases known to be inhibited by STI571 to a fourth kinase, c-ARG. STI571 appears in early trials to represent a major advance in the therapy of CML and other ABL-oncogene related leukemias. Patients in chronic phase are reported to have high rates of hematologic response and significant rates of cytogenetic response. Patients in more advanced stages also have high rates of at least short-term responses. The remarkable lack of serious side effects suggests either that the PDGFβ receptor, c-KIT, c-ABL, and c-ARG are not important for organ function in adults or that the inhibition of these kinases is partial and that the residual activity is sufficient for normal homeostasis in adults. The latter interpretation seems more likely because gene targeting studies in mice have shown that complete loss of function of each of these genes is associated with severe developmental and functional abnormalities of multiple organ systems. It is also possible that long-term administration or higher concentrations of the kinase inhibitor or its use in patients other than adults may reveal a different spectrum of drug effects. In either event, the activity of STI571 is impressive, and, at least as currently used, side effects are modest in comparison to standard chemotherapy for CML.
K.O. is supported by Grants in Aid (no. 11671011) from the Ministry of Education, Science, and Culture of Japan.
K.O. and E.W. contributed equally to the manuscript.
J.D.G. has declared a financial interest in Novartis Pharmaceuticals.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
James D. Griffin, Department of Adult Oncology, Dana Farber Cancer Institute, 44 Binney Street, Boston, MA 02115; e-mail: james_griffin@dfci.harvard.edu.