Viral interleukin-6 (vIL-6) encoded by Kaposi sarcoma (KS)–associated herpesvirus (KSHV) has been detected in 3 acquired immunodeficiency syndrome (AIDS)-related disorders: KS, primary effusion lymphoma, and multicentric Castleman disease (MCD).1,2 Similar to its human counterpart, vIL-6 supports the growth and survival of certain mouse and human cell lines in vitro.1,3 When expressed in mice, recombinant vIL-6 induced marked lymph-node plasmacytosis similar in morphology to the plasmacytosis noted in the plasma-cell variant of human MCD.4 MCD is a lymphoproliferative disorder featuring systemic manifestations of inflammation and B-cell hyperreactivity. Virtually all HIV-positive cases of MCD and nearly 50% of HIV-negative cases are infected with KSHV, and all KSHV-positive MCD tissues were found to express vIL-6.1 In contrast to localized Castleman disease that typically remits after surgical removal of the localized mass, the multicentric form, occurring in a small percentage of patients, behaves more aggressively and is frequently fatal.5 Immunohistochemical studies demonstrated abundant human IL-6 (hIL-6) expression in the germinal centers of MCD lymph nodes, and there is evidence for a pathogenetic role of hIL-6 in MCD.1 KSHV vIL-6 is expressed by immunoblastic cells present among the mantle zone of MCD.1 The expression of vIL-6 in MCD lesions and the functional similarities of cellular and vIL-6 raised the possibility that vIL-6 may also play a role in the pathogenesis of MCD. But the role of vIL-6, if any, in MCD or other KSHV-associated disorders has not been fully examined. As a step toward further understanding a potential pathogenetic role of vIL-6, we measured circulating vIL-6 levels in an HIV-positive patient at the onset of MCD.
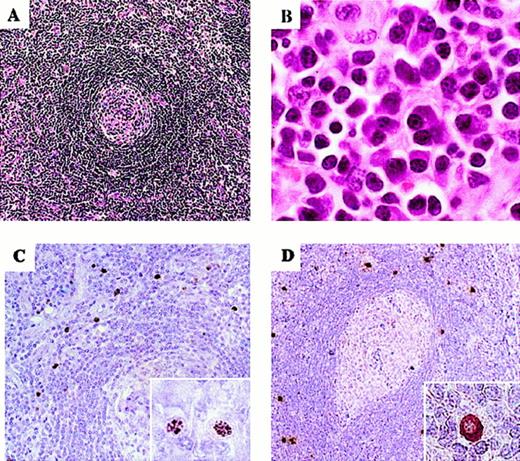
A 40-year-old HIV-positive homosexual man presented with a sore throat, nonproductive cough, night sweats, fever, diarrhea, marked fatigue, and weight loss in June 1999. The patient had received highly active antiretroviral therapy (HAART) since July 1996, including lamivudine (3TC; 150 mg × 2/d), stavudine (d4T; 40 mg × 2/d), and nelfinavir (NFV; 2250 mg × 1/d). HIV-RNA load had been stable at less than 1200 copies/mL since 1996. CD4+ T-cell counts had remained over 300 cells/mm3. On physical examination, the patient had lymphadenopathy, abdominal distention with hepatosplenomegaly, abdominal tenderness, and skin rash, but no evidence of KS. Oral administration of prednisone (30 mg/d) was started, to relieve systemic symptoms. A cervical lymph-node biopsy displayed the typical features of mixed plasma cell/hyaline vascular type of MCD:5 concentric layers of small lymphocytes surrounding the germinal centers and plasma cell infiltration in the interfollicular areas (Figure 1A-B). KSHV infection in the patient was confirmed by immunohistochemical detection of KSHV–latency-associated nuclear antigen (KSHV-LANA) in a lymph-node biopsy specimen (Figure 1C). A vIL-6–specific monoclonal antibody6 detected vIL-6 expression in the same specimen by immunohistochemistry (Figure 1D). The KSHV-LANA–positive and vIL-6–positive cells, similar in frequency, localized predominantly to the mantle zone of the lymph node. By contrast, expression of hIL-6, which has previously been detected in the germinal centers of certain MCD cases, was not detectable by immunohistochemistry in this lymph node (data not shown). Due to a dramatic improvement of systemic symptoms, prednisone was tapered after 10 days, and administration of the antiherpesvirus agent foscarnet (7g × 2/day) started, followed by splenectomy in September 1999. Fifteen months later, the patient continued to be well, in remission, on occasional maintenance chemotherapy.
Microscopic morphology of representative lymph node sections and immunohistochemical detection of KSHV infection.
(A) Small hyalinized germinal center surrounded by concentric layers of small lymphocytes (hematoxylin and eosin stain; × 100). (B) Interfollicular sheets of plasma cells are shown (hematoxylin and eosin stain; × 1000). (C) KSHV-LANA antigen detection in the lymph node mantle zone visualized by immunohistochemical staining with monoclonal antibody (× 200). The inset shows the characteristic speckled nuclear pattern (× 1000). (D) vIL-6 detection in immunoblastic cells in the lymph node mantle zone (× 100). The insets show specific cytoplasmic staining (× 1000).
Microscopic morphology of representative lymph node sections and immunohistochemical detection of KSHV infection.
(A) Small hyalinized germinal center surrounded by concentric layers of small lymphocytes (hematoxylin and eosin stain; × 100). (B) Interfollicular sheets of plasma cells are shown (hematoxylin and eosin stain; × 1000). (C) KSHV-LANA antigen detection in the lymph node mantle zone visualized by immunohistochemical staining with monoclonal antibody (× 200). The inset shows the characteristic speckled nuclear pattern (× 1000). (D) vIL-6 detection in immunoblastic cells in the lymph node mantle zone (× 100). The insets show specific cytoplasmic staining (× 1000).
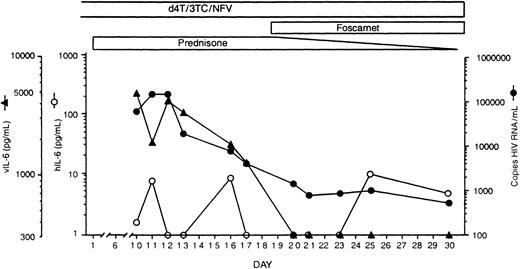
Serum vIL-6 was initially detected at 4756 pg/mL (Figure2). But vIL-6 decreased to undetectable (less than 300 pg/mL) levels over the next 10 days and subsequently remained undetectable. By contrast, serum levels of hIL-6 fluctuated at low levels (range, < 1.0-10.8 pg/mL) throughout this period. The HIV-RNA load presented marked changes during this period. For approximately 3 years prior to the onset of MCD, the HIV-RNA load in this patient had remained at less than 1200 copies/mL. After MCD was diagnosed, the HIV-RNA load peaked at 146 460 copies/mL, followed by a rapid decrease to 792 copies/mL on day 21 of treatment (Figure 2). Of note, the patient continued to receive the same antiretroviral drug regimen he had received during the previous 3 years. Thus vIL-6 and HIV-RNA serum levels displayed parallel decreases over a 20-day period of observation following initiation of steroid treatment for MCD (Rho = .9: P = .005, Spearman test).
Detection of vIL-6, hIL-6, and HIV RNA in serial serum samples from an HIV-positive patient with MCD.
On day 1, prednisone treatment was initiated. vIL-6 levels were measured by a vIL-6–specific enzyme-linked immunosorbent assay (ELISA) established in our laboratory.6 hIL-6 was measured by a commercially available ELISA kit (R&D Systems, Minneapolis, MN) that does not detect vIL-6.6 The lower limit of ELISAs sensitivity in serum was calculated to be 300 pg/mL of vIL-6 and 1.0 pg/mL of hIL-6. HIV-RNA load was measured by Amplicor HIV-1 reverse transcriptase–polymerase chain reaction test (Roche Diagnostic Systems, Basel, Switzerland), kindly performed by Dr Timothy Alcorn, Laboratory Corporation of America. 3TC indicates lamivudine; d4T, stavudine; and NFV, nelfinavir.
Detection of vIL-6, hIL-6, and HIV RNA in serial serum samples from an HIV-positive patient with MCD.
On day 1, prednisone treatment was initiated. vIL-6 levels were measured by a vIL-6–specific enzyme-linked immunosorbent assay (ELISA) established in our laboratory.6 hIL-6 was measured by a commercially available ELISA kit (R&D Systems, Minneapolis, MN) that does not detect vIL-6.6 The lower limit of ELISAs sensitivity in serum was calculated to be 300 pg/mL of vIL-6 and 1.0 pg/mL of hIL-6. HIV-RNA load was measured by Amplicor HIV-1 reverse transcriptase–polymerase chain reaction test (Roche Diagnostic Systems, Basel, Switzerland), kindly performed by Dr Timothy Alcorn, Laboratory Corporation of America. 3TC indicates lamivudine; d4T, stavudine; and NFV, nelfinavir.
The widespread use of HAART has led to a substantial decrease in the incidence of KS,7 perhaps due to KS cell growth stimulation by certain HIV-derived proteins,8 but the impact of HAART on AIDS-related B-cell disorders is unclear.7 In this patient, it is unlikely that either the increase or the decrease in HIV-RNA load is attributable to HAART because the antiretroviral regimen had not changed. Rather, it is more likely that factors derived from or induced by MCD lesions, including vIL-6, may have activated HIV replication in this patient. Although the pathogenesis of MCD is likely complex, the remarkable association between circulating vIL-6 levels and MCD status shown here, combined with previous information on the biologic activities of this virokine, further support a role of vIL-6 in the pathogenesis of AIDS-MCD.