Abstract
Secondary rearrangements of immunoglobulin gene segments that generate a new antibody repertoire in peripheral B cells have been described as receptor revision and occur by as yet unknown mechanisms. To determine the importance of recombination activating gene (RAG) expression in receptor revision, heterozygous rag1/green fluorescent protein (gfp) knockin mice were used to examine the location of RAG1 expression in the germinal centers (GCs) of lymphoid follicles after immunization with a variety of T-cell–dependent antigens. Immunization of rag1/gfp heterozygous mice orrag1 homozygous knockout mice reconstituted withrag1/gfp heterozygous spleen cells caused the down-regulation of RAG1/GFP signal in GCs. Although some RAG1/GFP+ cells appeared in regions surrounding the peanut agglutinin (PNA)+GL-7+ GC area, RAG1/GFP+ cells did not accumulate in the central region. In addition, the stimulation of spleen B cells with anti-μ antibody plus interleukin-4 (IL-4) or with anti-CD40 monoclonal antibody plus IL-7 did not induce GFP signals at detectable levels in vitro. These results clearly demonstrate that RAG1 re-expression either does not occur or is at extremely low levels in antigen-driven B cells in GCs of secondary lymphoid follicles, suggesting that other mechanisms may mediate the gene rearrangements observed in receptor revision.
Introduction
B cells are generated in the fetal liver or the adult bone marrow (BM) after immunoglobulin gene rearrangements that create a primary repertoire of random antibody (Ab) specificity.1-3 B cells that react to various self-antigens (Ags) at immature stages are thought to experience anergy after encountering Ags or to be eliminated by apoptotic mechanisms in the BM microenvironment.4-6 It has been suggested that a substantial proportion of B cells reactive to self-Ags undergoes a process known as receptor editing in which secondary immunoglobulin gene rearrangements replace variable (V) gene segments, generating a new Ab specificity as a part of the primary Ab repertoire.7-11 B cells recruited to the circulation are stimulated by Ags and helper-T (Th) cells in the T cell area of secondary lymphoid organs.12-14 Such Ag-driven B cells subsequently undergo rapid proliferation to become centroblasts located in the dark zone of germinal centers (GCs) and then arrest cell cycling and become small centrocytes in the light zone.15-20
Recent studies proposed an interesting model in which mature B cells also undergo a similar process, termed receptor editing, in GCs of secondary lymphoid follicles after stimulation by T-dependent (TD) Ags.21-25 This notion has been supported by the detection of rag1/rag2 transcripts and by the evidence of immunoglobulin gene rearrangements in situ, suggesting that receptor editing occurs in the GC light zone.21 In addition, stimulation with anti-CD40 monoclonal antibody (mAb) plus interleukin-4 (IL-4), or lipopolysaccharide (LPS) plus IL-4, induced the up-regulation of rag1/rag2 transcripts and immunoglobulin gene rearrangements in mature surface immunoglobulin M (sIgM)+ B cells in vitro.22 24-26 These results suggested that mature B cells frequently undergo receptor editing in GCs after stimulation with Ags. The receptor editing mechanism might provide a more flexible and diverse Ab specificity response than the one produced solely by the somatic mutation mechanism. The receptor editing in GCs could be a requisite molecular event for an immediate and effective response producing high-affinity Abs against various antigenic stimuli. Alternatively, receptor editing could salvage B cells carrying anti-self specificities.
The reactivation of rag gene expression has been suggested as one mechanism for the immunoglobulin gene rearrangements that occur in receptor revision.27 28 To demonstrate direct in vivo evidence of the induction of RAG molecules in GCs and to measure the frequency of cells undergoing receptor editing (or receptor revision), we examined lymphoid organs for RAG1/green fluorescent protein (GFP) signal using immunized or unimmunized rag1/gfp knockin mice. Unimmunized heterozygous rag1/gfp knockin mice express GFP that can be detected at high sensitivity by microscopic observation of splenic sections. Surprisingly, when we examined GCs of immunized mice, we observed that the RAG1/GFP signal was not significantly induced in GCs after stimulation with TD-Ags. This observation suggests that the RAG proteins do not play a major role in receptor revision during the clonal expansion of antigen-reactive B cells at the GC area after stimulation by TD-Ags.
Materials and methods
Cells and cell culture
Spleen B cells were purified essentially as described.29 Spleen cells, dispersed in a plastic culture dish with complete culture medium of RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (Dainippon Pharmaceutical, Osaka, Japan), 2 mM L-glutamine (Biowhittaker, Walkersville, MD), 50 mM 2-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin (Wako, Osaka, Japan) were incubated for 1.5 hours at 37°C in a 5% CO2 humidified incubator. Nonadherent cells were harvested and incubated for 20 minutes at 4°C with anti-Thy1.2 coated Dynabeads (Dynal, Oslo, Norway). The B-cell fraction remaining after immunodepletion of Thy1.2 cells contained more than 95% B220+ B cells, as shown by flow cytometric analysis.
Mice
The rag1/gfp knockin mice were generated as described previously.30 The rag1 knockout mice (C57BL background) used for reconstitution ofrag1+/gfp+ spleen cells were purchased from the Jackson Laboratory (Bar Harbor, ME) and were bred under specific pathogen-free conditions.
Immunization with TD-Ags
Heterozygous rag1/gfp knockin mice were immunized with 200 μL prewashed sheep red blood cell (SRBC) (Nippon Bio-Test Laboratories, Tokyo, Japan) in phosphate-buffered saline, 25 μg dinitrophenyl-keyhole limpet hemocyanin (DNP-KLH) in complete Freund's adjuvant (CFA) emulsion, or 100 μg nitrophenyl-chicken gamma globulin (NP-CGG) (provided by Dr Hitoshi Ohmori, Okayama University, Japan) in CFA emulsion. We introduced Ags by intraperitoneal injection for analysis of spleens or by injection into each hind footpad with 20 μg NP-CGG in CFA for analysis of lymph nodes.
Cell surface staining, flow cytometric analysis, and cell sorting
Cell surface staining was performed as described previously31 with various mAbs. Phycoerythrin (PE)–anti-B220 mAb (RA3-6B2; Pharmingen, San Diego, CA), biotinylated–anti-IgM mAb (R6-60.2; Pharmingen), biotinylated–anti-Fas mAb (Jo2; Pharmingen), and PE–anti-IgD mAb (11-26; Southern Biotechnology Associates, Birmingham, AL) were purchased. Anti-CD43 mAb (S7) and anti–heat-stable antigen (HSA) mAb (M1/69) were purified from the culture supernatants of hybridoma cells by protein-G Sepharose column chromatography and were biotinylated as described previously.31 For 5-parameter analyses of individual cells, streptavidin-R670 (Gibco-BRL, Rockville, MD) was used as the secondary reagent for mAbs. Cells were analyzed by flow cytometry (Facscan; Becton Dickinson, San Jose, CA) with lymphoid gating using side and forward scatters.
Detection of rag messenger RNA
Total RNAs from FACS-sorted GFP+ cells of therag1+/gfp+ spleen cells were extracted using RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacture's protocol. The possible contamination of genomic DNA in polymerase chain reaction (PCR) assay was avoided by DNase I (Boehringer Mannheim, Mannheim, Germany) treatment as described.32 The complementary DNAs (cDNAs), synthesized from RNAs with MuLV reverse transcriptase (PerkinElmer, Foster City, CA), were used for PCR assay using Ampli Taq Gold (PerkinElmer) with gene-specific primers for rag1 messenger RNAs (mRNAs) as described previously.33,34 The amplification was carried out as follows: 10 minutes at 95°C; 35 cycles of 40 seconds at 94°C, 40 seconds at 60°C, 60 seconds at 72°C; and 10 minutes at 72°C. PCR products were resolved on 2% agarose gels and stained with ethidium bromide.32 As the control for RNA preparation, hypoxanthine-guanine phosphoribosyltransferase (HPRT) amplification was compared. PCR primers were as follows: 5′-TGCAGACATTCTAGCACTCTGG-3′ and 5′-ACATCTGCCTTCACGTCGAT-3′ for RAG1, 5′-CCATCCTGGTCGAGCTGGAC-3′ and 5′-GCTCAGGTAGTGGTTGTCGG-3′ for GFP, 5′-GCTCAGGTAGTGGTTGTCGG-3′ and 5′-GCTGGTGAAAAGGACCTC-3′ for HPRT, and 5′-CCTAAGGCCAACCGTGAAAAG-3′ and 5′-TCTTCATGGTGCTAGGAGCCA-3′ for β-actin.
Reconstitution of spleen cells into rag1knockout mice
Spleen cells (1 × 107 cells) from heterozygousrag1/gfp-knockin mice (9 weeks after birth) were resuspended in 0.3 mL RPMI 1640 medium and injected into rag1-knockout mice intravenously. The mice, 14 days after reconstitution, were used for immunization with various TD-Ags, or the spleen cells were obtained for in vitro culture with various stimuli.
Detection of anti-DNP Abs
Ninety-six–well plates coated with DNP-chicken egg ovalbumin were incubated with serially diluted serum samples, and bound Abs were detected with biotinylated-goat–antimouse immunoglobulin Abs (isotype-specific for IgM and IgG1, respectively, obtained from Southern Biotechnology Associates), followed by streptavidin-alkaline phosphatase using a substrate of p-nitrophenyl phosphate disodium tablet (Sigma). After each incubation step of 3 hours at room temperature, the plates were washed 3 times with washing buffer Tris-buffered saline containing 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2% bovine serum albumin, and 0.05% Tween-20. Enzyme reaction was carried out as described elsewhere and was measured using the Bio-Rad enzyme-linked immunosorbent assay (ELISA) reader at 410 nm. The average of triplicate wells is shown.
In vitro stimulation
B cells were recovered from spleens of rag1 knockout mice that had been reconstituted with spleen cells (GFP-SPL) fromrag1/gfp knockin mice. These recovered B cells were cultured with RPMI-1640 complete medium with various combinations of the following stimulatory reagents: 10 μg/mL affinity-purified F(ab′)2 goat antimouse μ-chain–specific Ab (ICN, Costa Mesa, CA), 20 μg/mL LPS (Sigma Chemical, St Louis, MO), 1 μg/mL rat antimouse CD40 mAb (LB429),29 500 U/mL recombinant mouse IL-4 (provided by Dr K. Nakanishi, Hyogo College of Medicine, Nishinomiya, Japan), or 100 U/mL recombinant mouse IL-7 (provided by Dr F. Melchers, Basel Institute for Immunology, Switzerland) for various periods. After stimulation in vitro, cells were recovered and GFP signal was detected by flow cytometry. For control in experiments in which we attempted RAG1/GFP induction, we prepared in vitro cell lines from the BM of heterozygous rag1/gfp knockin mice using a BM culture method.35 After culturing for 3 weeks, B220+GFP+ cells, B220+GFPlow cells, and B220+GFP− cells could be observed. These cells were used for comparison of the GFP signal. Cellular responses to various stimulatory reagents were examined by the incorporation of [3H]-thymidine (TdR; Amersham, Buckinghamshire, United Kingdom). Cells (1 × 105 cells per well) were cultured in 96-well microtiter plates in culture medium with various stimulatory reagents for 48 hours and were pulsed with [3H]-TdR during the last 16 hours. After pulse labeling, cells were harvested using a cell harvester, and [3H]-TdR incorporation was measured by scintillation counter. The average of triplicate wells is shown.
Preparation of tissue sections, immunohistochemistry, and confocal laser microscopic analysis
Lymphoid organs from rag1/gfp knockin mice or littermates embedded in OCT compound (Tissue-Tek, Sakura Finetechnical, Tokyo, Japan) were rapidly frozen in liquid nitrogen. Tissues were prepared for histochemical analysis after they were sectioned at 10 μm, mounted on glass slides, and subsequently treated with 2% 3-amino-propyltriethoxysilane (Sigma) in acetone. Sections were processed immediately for confocal laser scan microscopy (Olympus, Tokyo, Japan) to detect the fluorescent signal emitted from GFP. Frozen specimens were prepared in a rectangular shape by cutting to the rim of the organs using a cryostat, and then serial sections were mounted onto glass slides. Immediately after drying the fixed sections, the fluorescence pictures were captured under confocal laser microscopy. Multiple marks were made at the edge of the each glass slide to identify a starting point. The turns of the microscope knobs from this starting point in both vertical and horizontal directions were counted. Using these counts from the initial start point of each slide, the microscopic focal area was oriented precisely onto the same GC without observing the view. By this method, the same GC on adjacent sections was easily identified. Using this method, the GFP signals from 2 contiguous slides could be compared and then identified with the corresponding GC by hematoxylin–eosin (H&E) or PNA staining. For marking of GCs, the mounted section was fixed with pure acetone and stained with biotinylated PNA (Molecular Probes, Eugene, OR). Biotinylated PNA was developed with diaminobenzidine (DAB) staining using streptavidin–horseradish peroxidase (HRP; Kierkegaard & Perry Laboratories, Gaithersburg, MD). For staining of GL-7, nonconjugated rat mAb GL-7 (Ly-77; Pharmingen) was used in combination with biotinylated-mouse antirat κ chain-specific mAb (MRK-1; Pharmingen) and developed with DAB staining as described above. H&E staining was conducted as described elsewhere.
Results
RAG1/GFP+ B cells in secondary lymphoid organs
In previous reports, expression of RAG1 and RAG2 was demonstrated in the whole area of PNA+ GCs, especially at the region of centrocytes, after immunization with various TD-Ags.21,22,24 25 Typically, a priming injection of NP-CGG with CFA at a footpad induced RAG1 expression in GCs at days 5, 12, and 21.
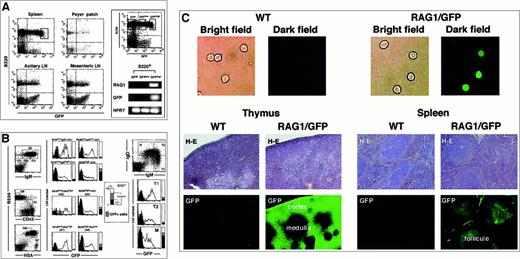
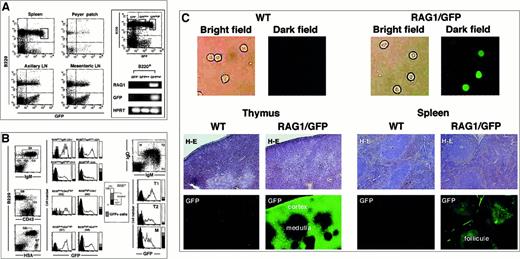
To examine the pattern of RAG1 expression in greater detail,rag1/gfp knockin mice were used to localize the expression of RAG1 at the single-cell level in vivo after immunization with TD-Ags. When peripheral B cells were examined, the GFP signal was observed in B220+ populations from spleen, Peyer patch, and lymph nodes (Figure 1A). Because most splenic B cells do not normally express RAG proteins or transcripts, our observation of RAG1/GFP signal could potentially be explained by the persistence of GFP protein that had been expressed in the pre–B-cell stage of development under the control of therag1 gene transcriptional elements. Indeed, RAG1/GFP signal generated from pro-B/pre-B cells of heterozygous rag1/gfpknockin mice was previously shown to be clearly detectable.30 The spleen contained nearly 20% GFP+B220+ cells, most of which were probably freshly recruited from the BM and could express low levels of RAGs in normal conditions.36,37 To investigate further, B220+ cells were separated into 3 populations based on GFP signal. Expression of rag1 mRNA was detected in the population with highest GFP expression by PCR analysis (Figure 1A, right panel), though it was not detected in the cells with weak or no GFP expression. The rag mRNA+ fraction with the highest GFP expression contained 5% of the B220+ cells and was presumably made up of freshly recruited cells. Similar GFPhigh+ cells were much rarer in the Peyer patch, axillary lymph nodes, and mesenteric lymph nodes. Recent reports used similar approaches to look for RAG molecules using mice expressing RAG1/RAG2 transgenes in a bacterial artificial chromosome vector (RAG1/RAG2 transgenic mice)36,37 or expressing RAG2/GFP fusion protein (RAG2/GFP knockin mice).38 Percentages of the GFP+ cells were between 5% to 20%, similar to the results of RAG1 transgenic (TG) mice, whereas RAG2/GFP knockin mice showed fewer GFP+ cells. Percentages of GFP+ spleen cells in heterozygous rag1/gfp knockin mice were comparable to previous results from RAG1 TG mice using similar gating (Figure 1B).
Expression of RAG1/GFP signal in the peripheral lymphoid organs.
(A) Flow cytometric analysis of the GFP expression in B220+cells of the spleen, Peyer patch, axillary LN, and mesenteric LN of heterozygous rag1/gfp knockin mouse (left). Five percent of splenic B cells expressing the highest level of GFP are shown in the gated area on the left of panel A. Next, splenic B220+ B cells were separated into GFP−, GFPlow, and GFPhigh populations by cell sorter using gates shown in the left panel. The RNA isolated from each cell population was used for reverse transcriptase–PCR (RT-PCR) with gene specific primers for RAG1, GFP, and HPRT, respectively (right). Only the GFPhighB cells showed rag1 mRNA. (B) Flow cytometric analysis of the splenic B cells. GFP signal was compared in B220+ cell fractions further separated by staining with sIgM (G1, G2, G3, and G4), CD43 (G5 and G6), and HSA+ (G7 and G8). GFP signals in splenic B cells were also analyzed in different fractions stained with sIgM+ and sIgD+ (T1, T2, and M). For regions G1 to G8, the vertical bars to the right of each histogram show the percentage of B220+ cells represented by each gate that were GFP+. (C) Fluorescence microscopy analysis of GFP signal. The upper panels show GFP signals of purified splenic cells sorted with B220+ from WT or RAG1/GFP knockin mice. The middle panels show tissue sections from thymus and spleen of WT or RAG1/GFP mice analyzed for GFP expression. The bottom panels show the same sections stained with H&E (middle panels). Original magnification, 100 ×.
Expression of RAG1/GFP signal in the peripheral lymphoid organs.
(A) Flow cytometric analysis of the GFP expression in B220+cells of the spleen, Peyer patch, axillary LN, and mesenteric LN of heterozygous rag1/gfp knockin mouse (left). Five percent of splenic B cells expressing the highest level of GFP are shown in the gated area on the left of panel A. Next, splenic B220+ B cells were separated into GFP−, GFPlow, and GFPhigh populations by cell sorter using gates shown in the left panel. The RNA isolated from each cell population was used for reverse transcriptase–PCR (RT-PCR) with gene specific primers for RAG1, GFP, and HPRT, respectively (right). Only the GFPhighB cells showed rag1 mRNA. (B) Flow cytometric analysis of the splenic B cells. GFP signal was compared in B220+ cell fractions further separated by staining with sIgM (G1, G2, G3, and G4), CD43 (G5 and G6), and HSA+ (G7 and G8). GFP signals in splenic B cells were also analyzed in different fractions stained with sIgM+ and sIgD+ (T1, T2, and M). For regions G1 to G8, the vertical bars to the right of each histogram show the percentage of B220+ cells represented by each gate that were GFP+. (C) Fluorescence microscopy analysis of GFP signal. The upper panels show GFP signals of purified splenic cells sorted with B220+ from WT or RAG1/GFP knockin mice. The middle panels show tissue sections from thymus and spleen of WT or RAG1/GFP mice analyzed for GFP expression. The bottom panels show the same sections stained with H&E (middle panels). Original magnification, 100 ×.
To determine the maturation stage of the GFP-expressing splenic B cells, we looked at several markers that correlated with B-cell maturation. The maturation of splenic B cells was accompanied by increased expression of sIgM—as shown in G1, G2, G3—and finally reached the B220high stage as shown in the G4 fraction. The least mature G1 population expressed the highest level of GFP signal, and further maturation was accompanied by the gradual decrease of the signal. The expression of CD43 also identifies the immature cells39,40 seen in fraction G5, which expressed a higher GFP signal than the CD43− G6 fraction. The correlation of higher GFP expression with less mature stages of B-cell maturation was also observed with the HSA marker because HSAhigh G7 shows higher GFP signal than the HSAlow G8 fraction,41 and the relatively immature sIgMhighsIgDlow cells (T1 fraction) express the highest GFP signal.36,37,42 The RAG1/GFP signal in the BM was characterized in a previous study that showed the pro-B, pre-B, and immature B-cell fractions characterized by the expression of sIgM, B220, CD43, HSA, and c-kit showed GFP signals to various extents.30 These expression patterns were basically similar to those reported for RAG2/GFP TG mice reported by others,36,37 but the level of GFP signal was markedly higher than that reported in a RAG2/GFP model.38 However, RAG2 expression is more strictly regulated than the expression of RAG1 in the process of B-cell maturation from the earliest precursor cells. One caveat is that the rag1/gfp knockin mouse has aneo gene on the rag1 locus. Despite any obvious effect on gene regulation, this may subtly compromise proper gene regulation. In spite of such concerns, these mice have the distinct advantage of producing GFP signals sufficient to be detected in situ on GC sections using histochemical analysis. A trace amount ofgfp transcript originating under rag1transcriptional regulation leads to the accumulation of GFP protein in lymphoid cells, providing a strong signal readily detectable by confocal laser microscopic analysis. The rag1/gfp knockin mice showed a strong GFP signal on the sections obtained for histochemical analysis, as shown in the thymus and spleen and in isolated spleen B cells (Figure 1C). Using this technique, we studied the appearance or the increase of GFP signal in GCs inrag1/gfp knockin mice after immunization with various TD-Ags.
Localization of RAG1/GFP+ cells in peripheral lymphoid organs after immunization
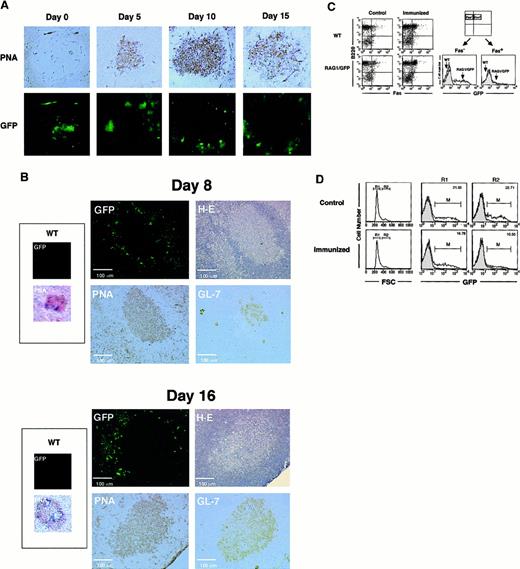
After a single injection of SRBC, GCs were clearly visible by PNA staining on days 5, 10, and 15 (Figure2A); however, there was no up-regulation of GFP signal in the central region of GCs until day 15, in comparison to the other follicular regions. Other TD-Ags, DNP-KLH, and NP-CGG showed similar results (data not shown). The linear-shape accumulation of GFP signal was observed in the spleen sections shown in Figures 1C and 2A (on day 0). Because no similar GFP fluorescence was detected on the sections of control wild-type (WT) mice, we believe that the signal was generated by the RAG1/GFP knockin in B-lineage cells. These cells are presumably recruited from the BM under nonimmunized conditions.
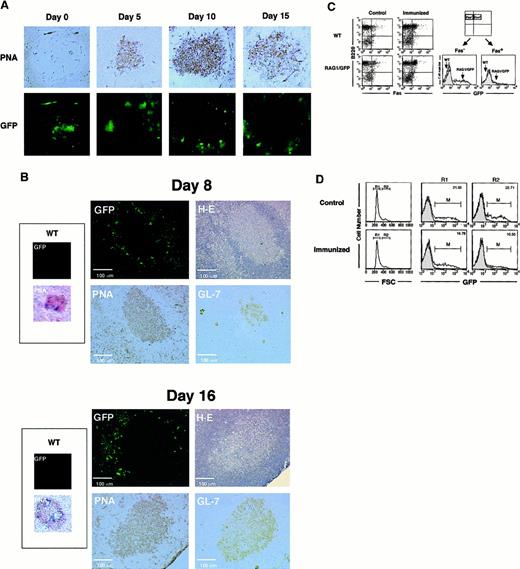
Localization of RAG1/GFP signal after immunization with TD-Ags in rag1/gfp knockin mice.
(A) Site of RAG1 expression in the spleen. GFP signal under confocal laser microscopy was compared to the immunohistochemical analysis of the GC area of the spleen after immunization with SRBCs. SRBCs were injected into the peritoneal cavity of heterozygous rag1/gfpknockin mice (9 weeks old). On days 0, 5, 10, and 15 after immunization, spleens were obtained and processed as described in “Materials and methods.” GFP signal (green) was examined under laser microscopy. The same section was then stained with biotinylated-PNA followed by streptavidin-HRP with DAB staining (brown). Original magnification, 100 ×. (B) Site of RAG1 expression in lymph nodes. Sizes of GCs and localization of GFP signals were compared on the same or serial sections of lymph nodes 8 or 16 days after Ag immunization. NP-CGG (20 μg) in CFA was injected into foot pads of rag1/gfp knockin mice (6 weeks old), and cryosections were prepared from popliteal lymph nodes after 8 and 16 days. First, GFP signal was analyzed using laser microscopy. Then the same section was stained with H&E, and serial sections were analyzed using biotinylated-PNA or rat anti–GL-7 mAb, respectively. Expression of PNA and GL-7 was visualized with DAB staining using streptavidin-HRP (brown). More than 50 GCs from spleens and lymph nodes were observed after immunization with TD-Ags, but none of the GCs showed positive GFP signals within the GC area when analyzed as shown in this figure. (C) Flow cytometric analysis of GFP expression in the spleen. Heterozygousrag1/gfp knockin mice (9 weeks old) and WT mice were immunized with TD-Ags, and the spleen cells were examined after surface staining for B220 and Fas. GFP signal was compared in B220+Fas+ cells before and after the immunization. (D) Flow cytometric analysis of GFP signal in the large cells after immunization (15 days). Heterozygous rag1/gfpknockin mice (9 weeks old) were untreated or immunized with TD-Ags, and splenic B220+ B cells were gated using forward scatter (FSC) to separate R1 (small cell) and R2 (large cell) populations. For comparison, the GFP signal for the RAG1/GFP splenocytes was superimposed over the contour of wild-type splenocytes.
Localization of RAG1/GFP signal after immunization with TD-Ags in rag1/gfp knockin mice.
(A) Site of RAG1 expression in the spleen. GFP signal under confocal laser microscopy was compared to the immunohistochemical analysis of the GC area of the spleen after immunization with SRBCs. SRBCs were injected into the peritoneal cavity of heterozygous rag1/gfpknockin mice (9 weeks old). On days 0, 5, 10, and 15 after immunization, spleens were obtained and processed as described in “Materials and methods.” GFP signal (green) was examined under laser microscopy. The same section was then stained with biotinylated-PNA followed by streptavidin-HRP with DAB staining (brown). Original magnification, 100 ×. (B) Site of RAG1 expression in lymph nodes. Sizes of GCs and localization of GFP signals were compared on the same or serial sections of lymph nodes 8 or 16 days after Ag immunization. NP-CGG (20 μg) in CFA was injected into foot pads of rag1/gfp knockin mice (6 weeks old), and cryosections were prepared from popliteal lymph nodes after 8 and 16 days. First, GFP signal was analyzed using laser microscopy. Then the same section was stained with H&E, and serial sections were analyzed using biotinylated-PNA or rat anti–GL-7 mAb, respectively. Expression of PNA and GL-7 was visualized with DAB staining using streptavidin-HRP (brown). More than 50 GCs from spleens and lymph nodes were observed after immunization with TD-Ags, but none of the GCs showed positive GFP signals within the GC area when analyzed as shown in this figure. (C) Flow cytometric analysis of GFP expression in the spleen. Heterozygousrag1/gfp knockin mice (9 weeks old) and WT mice were immunized with TD-Ags, and the spleen cells were examined after surface staining for B220 and Fas. GFP signal was compared in B220+Fas+ cells before and after the immunization. (D) Flow cytometric analysis of GFP signal in the large cells after immunization (15 days). Heterozygous rag1/gfpknockin mice (9 weeks old) were untreated or immunized with TD-Ags, and splenic B220+ B cells were gated using forward scatter (FSC) to separate R1 (small cell) and R2 (large cell) populations. For comparison, the GFP signal for the RAG1/GFP splenocytes was superimposed over the contour of wild-type splenocytes.
The observation of no RAG1/GFP signal in the centrocyte region of the GCs was surprising because others had reported RAG re-expression after immunization.21-25 Loss of GFP expression in GCs could be due to differences of Ags or the immunization protocol. Therefore, we immunized mice with the same batch of NP-CGG and by the same immunization protocol described previously.24 Figure 2B shows the results in the draining lymph nodes after the administration of NP-CGG with CFA in footpads. On days 8 and 16 after immunization, no RAG1/GFP signal appeared in the central area of GCs, which are identified by H&E staining and positive staining with PNA and GL-7.18 43-45 These results demonstrated the down-regulation of RAG1 expression in most of Ag-driven B cells at the central area of GCs.
Although RAG1/GFP signal was obviously down-regulated in the whole GC area, we saw slightly positive GFP signals on the periphery of GCs that were clearly demarcated by H&E, PNA, and GL-7 (Figure 2B). The GFP+ cells seen at the margins of GCs might receive signals for further maturation and activation in the process of Ag immunization in vivo. GFP+ cells, though they are not present in the centrocyte area, seem to be present in the region surrounding the GC architecture. RAG1/GFP+ cells may appear at the extra-GC area of lymphoid organs after Ag immunization in vivo. However, these results did not demonstrate the re-expression of RAG1 in the centrocyte area of GCs. The GFP signal was compared between Fas+B220+ GC B-cell fractions before and after immunization with TD-Ags (Figure 2C).46 There is no up-regulation of GFP signal in the Fas+ GC B cells after Ag (SRBC) immunization, whereas the Fas−B220+(non-GC) B cells showed the apparent GFP signal. We further characterized GFP expression in the larger cell population that most likely contains blast-like cells induced after Ag (SRBC) immunization (Figure 2D). The larger cell population showed a decreased level (median intensity of fluorescence, from 6.48 to 4.18) and number (from 22.7% to 10.6%) of GFP+ cells after immunization in vivo (15 days after immunization).
GFP signal in RAG1/GFP+ spleen cells adoptively transferred into RAG1-deficient mice
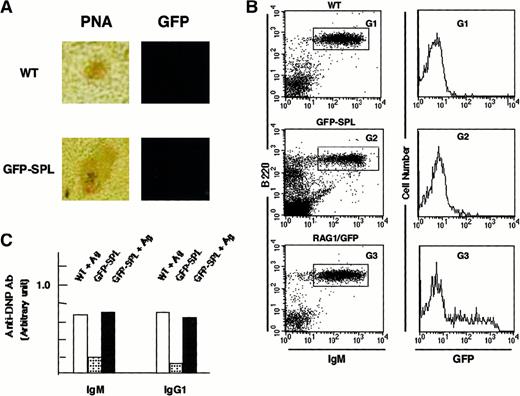
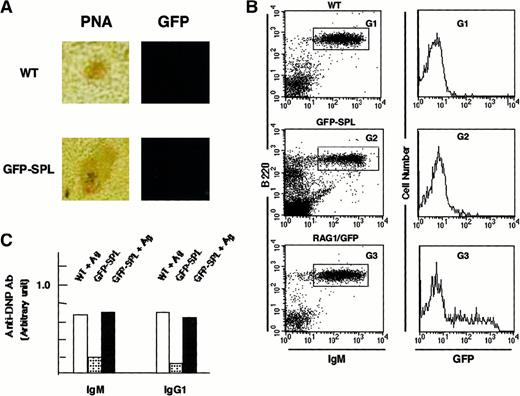
We observed GFP+ cells in the follicular area under nonimmunized conditions, which made it difficult to determine whether the RAG1/GFP signal originated from B cells newly arriving from the BM or from Ag-driven B cells that re-express RAG1. To further investigate this question, we reconstituted rag1 knockout mice with spleen cells from heterozygous rag1/gfp mice (GFP-SPL mice). Because rag1 knockout mice generate neither B nor T cells in the periphery,47,48 any mature lymphocytes detected in reconstituted mice would originate from the RAG1/GFP cells. The spleens of these reconstituted GFP-SPL mice contained only a small number of GFP+ mature lymphoid cells 7 days after transplantation. We immunized the GFP-SPL mice with DNP-KLH and observed no up-regulation of RAG1/GFP signal in the GC-like region (Figure3A). On FACS analysis, no obvious increase of GFP signal appeared in sIgM+ mature B cells after immunization (Figure 3B), whereas the immunization induced Ab production (Figure 3C). Only a limited number of B cells expressed faint GFP signals. Control spleens of unmanipulated heterozygous mice (RAG1/GFP) demonstrated GFP expression, presumably from newly arrived B cells from the BM (G3), as shown in Figure 1A. These results are in accord with the results of similar transfer experiments reported previously by Nussenzweig and colleagues.36
Reconstitution of rag1+/gfp+ spleen cells into rag1 knockout mice.
(A) Site of RAG1 expression in the reconstituted mice. Inducibility of RAG1/GFP signal was examined by confocal laser microscopy and flow cytometry with heterozygous rag1+/gfp+spleen cells (9-week-old mice) reconstituted into rag1knockout mice. Ten million splenocytes from heterozygousrag1/gfp knockin mice were injected into rag1knockout mice intravenously. These mice were identified as GFP-SPL. After 14 days, mature lymphocytes resided in the peripheral lymphoid organs of GFP-SPL reconstituted rag1 knockout mice. These reconstituted mice were then used for intraperitoneal Ag immunization with DNP-KLH (100 μg) in CFA. The fluorescence signal was compared with PNA staining on an adjacent serial section for WT and GFP-SPL mice. Original magnification, 100 ×. (B) Flow cytometric analysis of GFP signal. On days 8 and 16, spleen cells from WT, GFP-SPL, and RAG1/GFP mice were characterized by flow cytometry using PE–anti-B220 mAb and biotinylated–-IgM mAb plus streptavidin-R670. Representative results on day 16 after Ag immunization are shown. GFP fluorescence in B220+ sIgM+ fractions of WT (G1), GFP-SPL (G2), and RAG1/GFP nonimmunized control (G3) are compared in the histogram. (C) Immune response to TD-Ag. Serum samples were also kept and examined using an ELISA method that detected Ag-specific Ab of IgM and IgG1 classes. GFP-SPL mice with or without Ag immunization showed the Ag-specific response.
Reconstitution of rag1+/gfp+ spleen cells into rag1 knockout mice.
(A) Site of RAG1 expression in the reconstituted mice. Inducibility of RAG1/GFP signal was examined by confocal laser microscopy and flow cytometry with heterozygous rag1+/gfp+spleen cells (9-week-old mice) reconstituted into rag1knockout mice. Ten million splenocytes from heterozygousrag1/gfp knockin mice were injected into rag1knockout mice intravenously. These mice were identified as GFP-SPL. After 14 days, mature lymphocytes resided in the peripheral lymphoid organs of GFP-SPL reconstituted rag1 knockout mice. These reconstituted mice were then used for intraperitoneal Ag immunization with DNP-KLH (100 μg) in CFA. The fluorescence signal was compared with PNA staining on an adjacent serial section for WT and GFP-SPL mice. Original magnification, 100 ×. (B) Flow cytometric analysis of GFP signal. On days 8 and 16, spleen cells from WT, GFP-SPL, and RAG1/GFP mice were characterized by flow cytometry using PE–anti-B220 mAb and biotinylated–-IgM mAb plus streptavidin-R670. Representative results on day 16 after Ag immunization are shown. GFP fluorescence in B220+ sIgM+ fractions of WT (G1), GFP-SPL (G2), and RAG1/GFP nonimmunized control (G3) are compared in the histogram. (C) Immune response to TD-Ag. Serum samples were also kept and examined using an ELISA method that detected Ag-specific Ab of IgM and IgG1 classes. GFP-SPL mice with or without Ag immunization showed the Ag-specific response.
Absence of increased expression of RAG1/GFP signal in mature spleen B cells stimulated in vitro
Previous reports demonstrated the re-expression of RAG1 and RAG2 in spleen B cells by various B-cell activators, including LPS plus IL-4 and anti-CD40 mAb plus IL-7 in vitro.22,24-26 To study whether B-cell antigen receptor (BCR) cross-linking or any of these other combinations induces RAG1/GFP signal, spleen cells obtained from GFP-SPL mice were stimulated by similar methods. BCR cross-linking with anti-μ Ab plus IL-4 did not obviously up-regulate the expression of RAG1/GFP+ cells, suggesting that RAG1 expression is hardly detectable after stimulation with Ags. LPS plus IL-4 or stimulation with anti-CD40 mAb plus IL-7 could not induce an apparent increase of RAG1/GFP+ cells (Figure 4). To demonstrate that these stimulatory reagents were effective on the purified B cells in vitro, proliferative responses to the same stimulatory reagents were examined in vitro. As the control for RAG1/GFP signal, pre-B cells maintained by the Whitelock and Witte35 method were used. Line 5 shows intermediate GFP signal, and line 7 shows a mixture of bright positive cells and cells with various levels of GFP signal during culture in the presence of IL-7. We also examined whether the same stimuli, reported previously by others,22,24-26 can induce the expression of endogenousrag1 mRNA in vitro. Stimulation with any combinations did not induce rag1 transcripts in spleen cells obtained from GFP-SPL mice (Figure 4). As the comparison, freshly obtained spleen cells from the WT mice were also stimulated in vitro. Fresh spleen cells do express the rag1 mRNA detected by the RT-PCR method, as described previously,36 38 but there is no further induction by the stimulation in vitro. The stimulation with anti-μ Ab + IL-4 or anti-μ Ab + IL-7 down-regulated therag1 mRNA induction in culture (data not shown). Our results demonstrated that peripheral B-lineage cells seldom induce the expression of RAG1 by stimulation.
In vitro stimulation of reconstituted spleen B cells from GFP-SPL.
After 14 days of reconstitution, spleen B cells were enriched, as described in “Materials and methods.” Purified B cells were cultured in RPMI-1640 complete medium with various combinations of anti-μ Ab (10 μg/mL), LPS (20 μg/mL), rat antimouse CD40 mAb (1 μg/mL), mouse IL-4 (500 U/mL), or mouse IL-7 (100 U/mL). Cell proliferation by the same stimulatory reagents was examined by the incorporation of [3H]-TdR, as described in “Materials and methods.” As a control for GFP signal in B-lineage cells, we used the BM-derived pre-B cell lines from RAG1/GFP mice cultured in vitro using the method described by Whitelock and Witte.35Endogenous rag1 transcripts after stimulation of the spleen cells in vitro. Spleen cells from the GFP-SPL mice were stimulated in vitro as above, and the rag1 transcripts were monitored by RT-PCR with primers, as described in “Materials and methods.”
In vitro stimulation of reconstituted spleen B cells from GFP-SPL.
After 14 days of reconstitution, spleen B cells were enriched, as described in “Materials and methods.” Purified B cells were cultured in RPMI-1640 complete medium with various combinations of anti-μ Ab (10 μg/mL), LPS (20 μg/mL), rat antimouse CD40 mAb (1 μg/mL), mouse IL-4 (500 U/mL), or mouse IL-7 (100 U/mL). Cell proliferation by the same stimulatory reagents was examined by the incorporation of [3H]-TdR, as described in “Materials and methods.” As a control for GFP signal in B-lineage cells, we used the BM-derived pre-B cell lines from RAG1/GFP mice cultured in vitro using the method described by Whitelock and Witte.35Endogenous rag1 transcripts after stimulation of the spleen cells in vitro. Spleen cells from the GFP-SPL mice were stimulated in vitro as above, and the rag1 transcripts were monitored by RT-PCR with primers, as described in “Materials and methods.”
Discussion
Many reports from several investigators have shown that BCR cross-linking or stimulation with LPS could induce receptor editing, as evidenced by the up-regulation of rag1/rag2 transcripts and immunoglobulin gene rearrangements in spleen B cells.22 24-26 These in vitro experiments could not determine the localization of B cells undergoing receptor editing. Although the expression of PNA should have been a good marker for GC B cells, it would have been difficult to decide whether therag1/rag2 mRNA was induced in mature sIgM+ B cells by stimulation with BCR cross-linking or simply that the in vitro stimulation maintained the survival of rag1+ B lineage cells that have been freshly recruited from the BM. Mice stimulated with multiple injections of TD-Ags would still receive a supply of virgin B cells from the BM into regional lymph nodes through the hematogenous route. B cells with high-affinity receptors will participate in effective Ab production, but most of the B cells would re-enter the next round of maturation acquiring further hyper-mutation at V gene segments of immunoglobulin genes. Vigorous consumption of B cells in the periphery might accelerate the generation and recruitment of fresh B cells from the BM.
The results demonstrated that BCR cross-linking does not induce the up-regulation of RAG1/GFP signal in the centrocyte area of GCs. Although the data cannot rule out the possibility that a very low number (below 0.1%) of total cells would participate in receptor editing in Ag-driven B cells in the spleen, the peripheral BCR+ B cells will seldom undergo secondary immunoglobulin gene rearrangements under normal conditions. Two independent analyses using RAG1/RAG2 TG mice and RAG2/GFP knockin mice studied the expression of RAGs in the secondary lymphoid tissues on immunization with TD-Ags.36,38 These studies did not unequivocally determine whether re-expression of RAG molecules occurs in Ag-stimulated B cells. The results of RAG1/RAG2 TG mice showed a nonresponsiveness of RAG induction after stimulation both in vivo and in vitro.36 In RAG2/GFP knockin mice, 2% to 20% of splenic B cells expressed GFP after immunization, whereas less than 1% of these cells expressed GFP in unimmunized mice.38 Most GFP+ cells in the spleen were phenotypically identical to pre-B and immature B cells.38 Our experiments studied the expression of RAG1/GFP in GCs of the Ag-immunized spleen. GFP expression was not detected in the central region of GCs, nor was it down-regulated. Regardless of whether GFP+B220+cells were in the central GC area, the GFPLow+ cells surrounding the GC area (Figure 2) might have been reported previously as RAG+ cells.38
Similar RAG+ B cells were also observed in a human tonsillar population.49 Human GC B cells are classified by surface markers of the CD38+sIgD− population.Rag mRNA was detected in some of these cells, especially the CD38+CD77− centrocytes, which also express λ-like and VpreB, a pre-BCR component. It was mentioned that some CD38−IgD+ non-GC B cells express VpreB, suggesting that their origin might be from recent BM immigrants. Chronic infection around the laryngeal region causes the continuous activation and consumption of tonsillar B cells, which might also require the supply of newly generated B cells. Although the structure of human tonsillar GCs is somewhat different from the GCs in the follicles of Ag-immunized mice,50 neither follicular mantle B cells (CD38−sIgD+) nor GC B cells (CD38+sIgD−) revealed the apparent re-expression of rag mRNA during in vitro culture with IL-2, IL-4, and IL-10 in the presence of CD40L.
The evidence in our model mouse provides critical information regarding receptor editing in the secondary lymphoid follicles. Our results show an absence of RAG1 expression at the centrocyte stage. Centrocytes, generated from the Ag-driven centroblasts with arrested cell cycling, will be selected to enrich the B cells with high-affinity BCR through the follicular dendritic cells network. Because this selection process could be a critical filter to eliminate the self-reactive B-cell clones, it seems reasonable to suppress the re-expression of RAGs, which could cause receptor editing events at the centrocyte stage of the GCs. This may also be beneficial to maintain the allelic exclusion of immunoglobulin genes in the mature B cells of the peripheral lymphoid organs, as suggested by Nussenzweig and colleagues.36
We thank Dr Ohmori, Department of Biotechnology, Faculty of Engineering, Okayama University, for providing us NP-CGG and the immunization protocol routinely performed in his laboratory. We thank Drs Michelle Nussenzweig, and Garnett Kelsoe for the critical discussion and the kind help to prepare the manuscript. We thank Drs Marjorie Shapiro and Ejaz Shamim for critical reading of the manuscript. We thank Drs Seiji Inui and Kazuhiko Kuwahara in our laboratory for the help on the ELISA assay and Ms Y. Mukohmatsu (Department of Cell Differentiation) for cell sorting.
Supported by grants from The Ministry of Education, Culture, Sports, Science, and Technology. Supported in part by a grant from Novartis Foundation for the Promotion of Science.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nobuo Sakaguchi, Department of Immunology, Kumamoto University School of Medicine, 2-2-1, Honjo, Kumamoto 860-0811, Japan; e-mail: nobusaka@kaiju.medic.kumamoto-u.ac.jp.

![Fig. 4. In vitro stimulation of reconstituted spleen B cells from GFP-SPL. / After 14 days of reconstitution, spleen B cells were enriched, as described in “Materials and methods.” Purified B cells were cultured in RPMI-1640 complete medium with various combinations of anti-μ Ab (10 μg/mL), LPS (20 μg/mL), rat antimouse CD40 mAb (1 μg/mL), mouse IL-4 (500 U/mL), or mouse IL-7 (100 U/mL). Cell proliferation by the same stimulatory reagents was examined by the incorporation of [3H]-TdR, as described in “Materials and methods.” As a control for GFP signal in B-lineage cells, we used the BM-derived pre-B cell lines from RAG1/GFP mice cultured in vitro using the method described by Whitelock and Witte.35Endogenous rag1 transcripts after stimulation of the spleen cells in vitro. Spleen cells from the GFP-SPL mice were stimulated in vitro as above, and the rag1 transcripts were monitored by RT-PCR with primers, as described in “Materials and methods.”](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2680/6/m_h80910973004.jpeg?Expires=1767701799&Signature=muGa~5sMr1kaQ6LM~sHTbBkpwZNI66Slc0oSXG~q5RDmROZkn~~VG~B5vs3PvweBWjFI~b~Uz5gwYHs3Gz7mG-CgBWaUQoZ8zIx3wuHyG8FX44~YkX7rIJAg8eRg9FyUUvHivcJLI6cxR8IUOisFrDl~-NbICHrYMlhpc8xwdt4DB~lZYZp-ix6WkgFjlH~CUDY5dijLAkA4HE2~j4pOoG2eRyPpNiuAGuV0~bmX0IeSeEv31sgeJRwG70~D8afthm~tv-y4dF52cnpsQowMtdhbJlkVoL5R9CpT6ZyH2B~nnmp~f0i0e3dqFknCLZ~yhyDyx7o-cBkyqr9tNYcESA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 4. In vitro stimulation of reconstituted spleen B cells from GFP-SPL. / After 14 days of reconstitution, spleen B cells were enriched, as described in “Materials and methods.” Purified B cells were cultured in RPMI-1640 complete medium with various combinations of anti-μ Ab (10 μg/mL), LPS (20 μg/mL), rat antimouse CD40 mAb (1 μg/mL), mouse IL-4 (500 U/mL), or mouse IL-7 (100 U/mL). Cell proliferation by the same stimulatory reagents was examined by the incorporation of [3H]-TdR, as described in “Materials and methods.” As a control for GFP signal in B-lineage cells, we used the BM-derived pre-B cell lines from RAG1/GFP mice cultured in vitro using the method described by Whitelock and Witte.35Endogenous rag1 transcripts after stimulation of the spleen cells in vitro. Spleen cells from the GFP-SPL mice were stimulated in vitro as above, and the rag1 transcripts were monitored by RT-PCR with primers, as described in “Materials and methods.”](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2680/6/m_h80910973004.jpeg?Expires=1767701800&Signature=VDFtpYgwWP4Y~2-ri9sDz~eemCOZnUBaRJlYa2isyqIb5O9AZXU9TuMG96qciO88CGnC7nR7pD3ahYU04LSyXlJ7Qt~c7mLArwSfwF5qdn~bUnIBiXtNbGMPB9LnlX5ZajZQfx7wKsHdfp09KCSFKrpcd0NELjy4MUgxHKHx7vrNzi~strbf7bfr3udIZtZRD0B78pJ0vz16GT7lGfmRQSsWPR9xCV5qdr7E9CVBBxwWmGYEdDBu000hdPlSC94aYmk-0MPdCY26UMXxC0u4yuRN-8p1lOr9MIJEhet60lw-h4g9pfcGifGtlEkKTdvPScJoF6L7jc~KC-GSQOe04g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)