Abstract
In allogeneic bone marrow transplantation (BMT) donor T cells are primarily responsible for antihost activity, resulting in graft-versus-host disease (GVHD), and for antileukemia activity, resulting in the graft-versus-leukemia (GVL) effect. The relative contributions of the Fas ligand (FasL) and perforin cytotoxic pathways in GVHD and GVL activity were studied by using FasL-defective or perforin-deficient donor T cells in murine parent → F1 models for allogeneic bone marrow transplantation. It was found that FasL-defective B6.gld donor T cells display diminished GVHD activity but have intact GVL activity. In contrast, perforin-deficient B6.pfp−/− donor T cells have intact GVHD activity but display diminished GVL activity. Splenic T cells from recipients of B6.gld or B6.pfp−/− T cells had identical proliferative and cytokine responses to host antigens; however, splenic T cells from recipients of B6.pfp−/− T cells had no cytolytic activity against leukemia cells in a cytotoxicity assay. In experiments with selected CD4+ or CD8+ donor T cells, the FasL pathway was important for GVHD activity by both CD4+ and CD8+ T cells, whereas the perforin pathway was required for CD8-mediated GVL activity. These data demonstrate in a murine model for allogeneic bone marrow transplantation that donor T cells mediate GVHD activity primarily through the FasL effector pathway and GVL activity through the perforin pathway. This suggests that donor T cells make differential use of cytolytic pathways and that the specific blockade of one cytotoxic pathway may be used to prevent GVHD without interfering with GVL activity.
Introduction
Allogeneic bone marrow transplantation (BMT) is an important therapeutic modality for a variety of diseases, including hematologic malignancies. The therapeutic benefits of allogeneic BMT are not only derived from the high dose of chemoradiation but also from a graft-versus-leukemia (GVL) effect.1-3 Clinical evidence for a GVL effect comes from studies demonstrating an increased relapse rate after BMT from an identical twin, autologous BMT, and T-cell depletion of the allogeneic bone marrow (BM) graft (reviewed in Truitt and Johnson3 and Antin4). Most studies indicate that the GVL effect is primarily mediated by allogeneic donor T cells, which recognize either leukemia-specific antigens or alloantigens expressed on normal and malignant cells.5-8
Graft-versus-host disease (GVHD) remains the single most important complication of allogeneic BMT and is defined as a progressive systemic illness with immunosuppression, cachexia, and specific target organ disease of the skin, liver, and intestines.9 Although the complex pathophysiology of acute GVHD involves the conditioning regimen (radiation or chemotherapy), cytokines, nitric oxide, and non-T effector cells (reviewed in Krenger et al10), the cytolytic activity of donor T cells is essential for the development of GVHD activity.
Cytolytic activity of cytotoxic T lymphocytes (CTL) is primarily mediated through 2 effector mechanisms: the Fas-FasL and perforin-granzyme pathways.11,12 Interaction of FasL, expressed on the CTL cell surface, with the Fas receptor on the target cell membrane results in the initiation of the Fas cell death pathway, which involves the activation of a caspase cascade.13 The perforin-granzyme pathway consists of the exocytosis of lytic granules, which results in the release of perforin (a pore-forming protein related to the membrane attack complex of complement) and granzymes (Ca++-dependent serine esterases that can activate the caspase cascade).14 Some studies have suggested that the expression (or secretion) of tumor necrosis factor (TNF) and tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) can also contribute to CTL cytotoxicity.15 16
Most attempts to characterize and separate GVHD and GVL activity have focused on the identification of specific GVHD or leukemia antigens, donor T-cell dose, selective donor T-cell depletion (such as CD8), delayed leukocyte infusion (DLI), in vitro polarization of donor T cells (Th1/Tc1 or Th2/Tc2), and cytokines (such as IL-2). Several studies have demonstrated the importance of the FasL and perforin pathways for the development of GVHD.17-24 However, efforts to manipulate GVHD and GVL activity through the effector pathways of T-cell–mediated cytotoxicity (FasL and perforin) have been limited.24
We hypothesized that donor T cells make differential use of their cytotoxic effector pathways to mediate GVHD and GVL activity. To test this hypothesis, we performed experimental BMT studies in 2 murine parent → F1 models for GVHD and the GVL effect and used donor T cells that were deficient for perforin or expressed defective FasL.
Materials and methods
Cell lines and reagents
The 32Dp210 (H-2k) is a myeloid leukemia cell line derived from 32Dc13 cells (of C3H/HeJ mouse origin) that were transfected with the p210 bcr/abl oncogene.25P815 (H-2d) is a mastocytoma from DBA/2 mouse origin, and LK35.2 is a murine B-cell hybridoma (both obtained from the American Type Culture Collection, Manassas, VA). DO11.10 cells, a murine T-cell hybridoma cell line, were kindly provided by Dr Barbara Osborne (University of Massachusetts, Amherst, MA). L5178Y cells, stably transfected with mFasL or vector alone, were a gift from Dr Andreas M. Hohlbaum (Boston University, Boston, MA).26
Antimurine CD16/CD32 Fc block (2.4G2), antimurine Ly-9.1-fluorescein isothiocyanate (FITC), antimurine CD4-FITC, -phycoerythrin (PE), and -PerCP (RM4-5), antimurine CD8α-FITC, -PE, and -PerCP (53-6.7), antimurine CD62L-FITC (MEL-14), antimurine CD122-FITC (TM-B1), antimurine CD44-APC (IM7), and antimurine Fas-FITC (Jo2), as well as purified NA/LE antimurine FasL (MFL3) and hamster IgG group 1 κ isotype control antibody (A19-3), were all obtained from Pharmingen (San Diego, CA). Antimurine CD3 (145-2C11) was obtained from the Monoclonal Antibody Facility at the Sloan-Kettering Institute. Antimurine CD4 (L3T4), antimurine CD8α (Ly-2), and antimurine CD45R (B220) (all coupled to microbeads) for magnetic cell separation with the MidiMACS system (Miltenyi Biotec, Auburn, CA) were obtained from Miltenyi Biotec. Ionomycin and 4β-phorbol 12-myristate 13-acetate (PMA) were obtained from Calbiochem (La Jolla, CA). Tissue culture medium consisted of RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine (as well as 50 μM 2-mercaptoethanol for the culture of L5178Y and 32Dp210 cells). Murine TNF was obtained from R&D Systems (Minneapolis, MN).
Mice and BMT
Female C57BL/6J (B6, H-2b), C57BL/6-Pfptm1Sdz (B6.pfp−/−, H-2b), B6Smn.C3H-Faslgld(B6.gld, H-2b), C3FeB6F1/J (H-2b/k), and B6D2F1/J (H-2b/d) mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and used in BMT experiments when they were between 8 and 10 weeks of age. BMT protocols were approved by the Dana Farber Cancer Institute Animal Care and Use Committee and the Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee. Bone marrow cells were removed aseptically from femurs and tibias. Donor BM was T-cell depleted by incubation with anti–Thy-1.2 for 30 minutes at 4°C, followed by incubation with low-TOX-M rabbit complement (Cedarlane Laboratories, Hornby, Ontario, Canada) for 1 hour at 37°C. Splenic T cells were obtained by purification over a nylon wool column, followed by red blood cell (RBC) removal with ammonium chloride RBC lysis buffer (Sigma, St Louis, MO). BM cells with or without splenic T cells and leukemia cells (5 × 106) were resuspended in Dulbecco modified essential medium (Life Technologies, Grand Island, NY) and transplanted by tail vein infusion (total volume, 0.25 mL) into lethally irradiated recipients on day 0. Before transplantation on day 0, recipients received 1300 cGy total body irradiation (cesium 137 source) as a split dose with 3 hours between doses (to reduce gastrointestinal toxicity). Mice were housed in sterilized micro-isolator cages and were given normal chow and autoclaved hyperchlorinated drinking water (pH 3.0).
CD4+ or CD8+ splenic T-cell separation
In some BMT experiments, splenic T cells were further selected after RBC lysis and nylon wool passage. Cells were incubated with anti-CD4 (or anti-CD8) antibodies and anti-CD45R antibodies (both coupled to microbeads) for 15 minutes at 4°C. After 2 washes with PBS (containing 1% fetal calf serum), cells were passed through Midimacs columns (Miltenyi). Samples of the negatively selected cells were subsequently analyzed for purity by flow cytometry after staining with PE-labeled anti-CD4 (or anti-CD8) antibodies. Equal numbers of purified CD4+ (or CD8+) splenic T cells were then mixed with B6 BM cells and infused into recipient animals.
Assessment of graft-versus-host disease
The severity of GVHD was assessed with a clinical GVHD scoring system as first described by Cooke et al.27 Briefly, ear-tagged animals in coded cages were individually scored every week for 5 clinical parameters on a scale from 0 to 2, including weight loss, hunched posture, decreased activity, fur ruffling, and skin lesions. A clinical GVHD index was generated by summation of the 5 criteria scores (0-10). Survival was monitored daily.
Leukemia induction and assessment of leukemic death versus death from GVHD
Animals received P815 or 32Dp210 cells intravenously in a separate injection on day 0 of BMT. Survival was monitored daily, and the cause of each death after BMT was determined as previously described.28 Briefly, we were able to distinguish lethal GVHD from leukemic death by hepatosplenomegaly (for 32Dp210 leukemia) or macroscopic liver-spleen metastases (P815) at autopsy and by clinical GVHD score (including weight loss). In our previous studies, we established hepatosplenomegaly as a highly specific criterion for death from 32Dp210 leukemia (spleen weight greater than 300 mg, liver weight greater than 1700 mg). In our experiments, we did not find hepatosplenomegaly in animals that had not received 32Dp210 cells. P815-induced death was characterized by the presence of macroscopic liver nodules, spleen nodules, or both. Death from GVHD was defined as the absence of leukemia signs and the presence of GVHD symptoms, as assessed by our clinical GVHD scoring system (described above).
At autopsy—after fixing in 10% formalin, embedding in paraffin, and staining with hematoxylin and eosin—we submitted the spleen and liver tissue of all dead animals that did not have hepatosplenomegaly to our veterinary pathologist, Dr Hai T. Nguyen (Cornell University, New York, NY), for histopathologic examination to assess the presence or absence of leukemic cells. Although some mice were found at autopsy to be without clinical signs of GVHD or macroscopic signs of hepatosplenomegaly (32Dp210 CML) or liver metastases (P815), microscopic examination revealed extensive leukemic infiltration.
Cells and serum
As described before, splenic T cells were obtained by purification over a nylon wool column; this was followed by RBC removal with ammonium chloride RBC lysis buffer, which resulted in greater than 75% purity. On day 14 after BMT, splenocytes from recipients used in cytotoxicity and proliferation assays were first pretreated with ammonium chloride to remove RBCs, and percentages of CD4+and CD8+ T cells from donor origin were subsequently determined by 2-color flow cytometry. Splenocytes from naive animals were pretreated with ammonium chloride RBC lysis buffer only before use. Peripheral blood was obtained by retro-orbital or intracardiac puncture after general anesthesia with methoxyflurane (Schering-Plough Animal Health, Union City, NJ).
Flow cytometric analysis
Splenocytes or murine leukemia cell lines were washed in FACS buffer (PBS/2% BSA/0.1% azide), and 106 cells/mL were incubated for 30 minutes at 4°C with CD16/CD32 Fc block. Subsequently, cells were incubated for 30 minutes at 4°C with primary antibody or antibodies (1 μg/mL) and washed twice with FACS buffer. Stained cells were resuspended in FACS buffer and analyzed on a Facscan flow cytometer (Becton Dickinson, San Jose, CA) with Cellquest (Becton Dickinson) software.
Intracellular cytokine staining
Briefly, cells were incubated for 4 hours (for PMA-ionomycin stimulation) or for 12 to 15 hours (for MLR) with Brefeldin A (10 μg/mL). Then they were harvested, washed, and stained with primary (surface) fluorochrome (FITC, PerCP, and APC)-conjugated antibodies, fixed and permeabilized with the Cytofix/Cytoperm Kit (Pharmingen, San Diego, CA), and subsequently stained with secondary (intracellular cytokine) PE-conjugated antibody. FACS analysis was conducted by gating for the designated populations. Flow cytometer and software were used as mentioned before.
Chromium 51 release assays
Target cells were labeled with 100 μCi chromium 51 (51Cr) at 2 × 106 cells/mL for 2 hours at 37°C and 5% CO2. After 3 washes, labeled targets were plated at 104 to 2 × 104 cells/well in U-bottom plates (Costar, Cambridge, MA). Effector cells were prepared (details below) and added at various effector-to-target ratios in a final volume of 200 μL and were incubated for 4 hours (allogeneically stimulated T cells) or 8 hours (activated DO11.10 and mFasL-L5178Y cells) at 37°C and 5% CO2. Subsequently, 100 μL supernatant was removed from each well and counted in a gamma counter to determine experimental release (Cobra, Meriden, CT). Spontaneous release was obtained from wells receiving target cells and medium only, and total release was obtained from wells receiving 1% Triton X-100. Spontaneous release was less than 15% of total release. Percentage cytotoxicity was calculated by the following formula: % cytotoxicity = 100 × (experimental release − spontaneous release)/(total release − spontaneous release).
To determine FasL-mediated lysis by activated DO11 cells, 96-well plates were coated with antimurine CD3 (145-C11) at 1 μg/mL in PBS for 24 hours at 4°C. Subsequently, DO11.10 cells were incubated with FasL-antibody (MFL3) or isotype control (A19-3) (both obtained from Pharmingen) in these antibody-coated 96-well plates for 3 hours and subsequently coincubated with labeled target cell lines as described above. For allogeneic stimulation, splenocytes (8 × 104) were incubated with irradiated C3FeB6F1 splenocytes (4 × 104) in 96-well plates, and 10 U/mL human IL-2 (Chiron, Emoryville, CA) was added on day 3. Cells were used as effectors on day 7 against lipopolysaccharide (LPS)–stimulated C3FeB6F1 splenocytes. LPS-stimulated cells were obtained by incubating whole splenocytes for 72 hours with 1.6 to 4 μg/mL LPS (Sigma).
Proliferation assay
Splenic T cells (4 × 105 cells/well; prepared as described above) were incubated for 3 days with irradiated (2000 cGy) C3FeB6F1 splenocytes as stimulators (2 × 105 cells/well) in 96-well plates. Cultures were pulsed during the final 18 hours with 1 μCi/well [3H] thymidine, and DNA was harvested on a Harvester 96 (Tomtec, Hamden, CT).
Tumor necrosis factor enzyme-linked immunosorbent assay
The TNF enzyme-linked immunosorbent assay (ELISA) kit was obtained from R&D Systems. Assays were performed according to the manufacturer's protocol. Briefly, serum samples were diluted at ratios of 1:2 or 1:4 and were incubated in wells coated with specific anti-TNF antibody. After several washes, wells were incubated with a secondary antibody coupled to biotin. The biotin-labeled assays were developed with streptavidin and substrate and were read at 450 nm with a microplate reader (Bio-Rad, Hercules, CA). Recombinant murine TNF was used as a standard. Samples and standards were run in duplicate, and the sensitivity of the assays was 10 to 20 pg/mL.
Statistics
All values are expressed as mean ± SEM. Statistical analysis of clinical GVHD index scores and weight losses was performed with the nonparametric unpaired Mann-Whitney U test, whereas the Mantel-Cox log rank-test was used for survival data.P < .05 was considered statistically significant.
Results
B6 → C3FeB6F1: murine BMT model for GVHD and the GVL effect
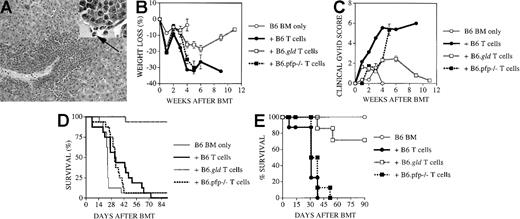
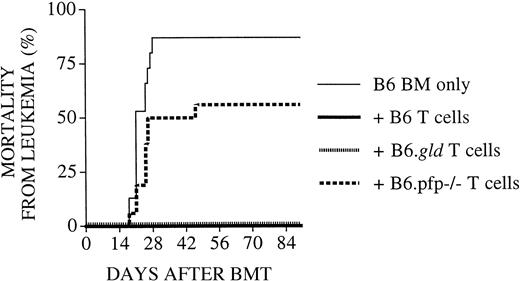
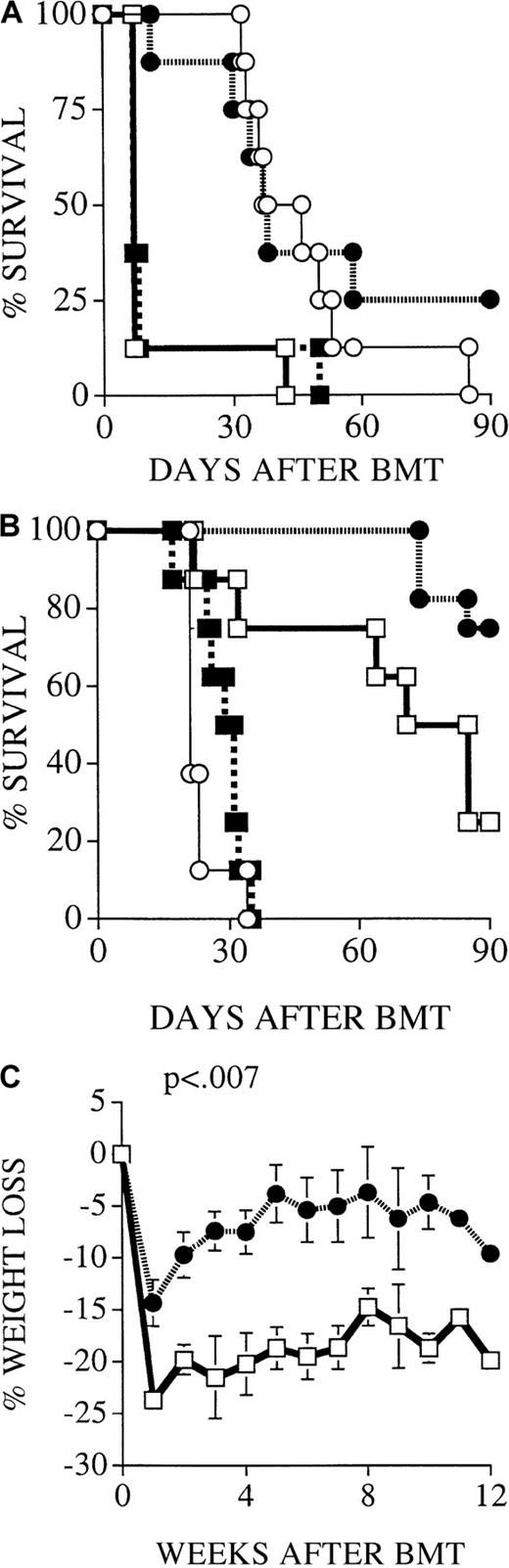
We used a well-established parent → F1 murine model for allogeneic BMT with a full mismatch for major histocompatibility complex (MHC) class I and II (B6 [H-2b] into C3FeB6F1/J [H-2b/k]) to study the roles of the FasL and perforin effector pathways in GVHD and GVL activity. The advantages of this BMT model are that GVL and GVHD activity has been well defined in this model,30 that it allows the use of CML 32Dp210 (H-2k),25 and that FasL-defective and perforin-deficient mice on a B6 background can be used as donors. In all experiments, we used 5 × 106 T-cell–depleted B6 donor BM cells and 1300 cGy split-dose lethal total body irradiation. In our initial experiments, lethally irradiated C3FeB6F1/J recipients received T-cell–depleted BM (TCD-BM) with or without 2 × 106 donor B6 splenic T cells. On day 0, 32Dp210 leukemia cells (103 cells) were given with the donor cell inoculum. T-cell depletion consistently prevented GVHD, but all animals succumbed to leukemia (Figure 1A). The addition of T cells to the TCD-BM inoculum induced severe GVHD with high mortality, but leukemia did not develop in the GVHD survivors, demonstrating the GVL activity of the donor T cells as previously described.28
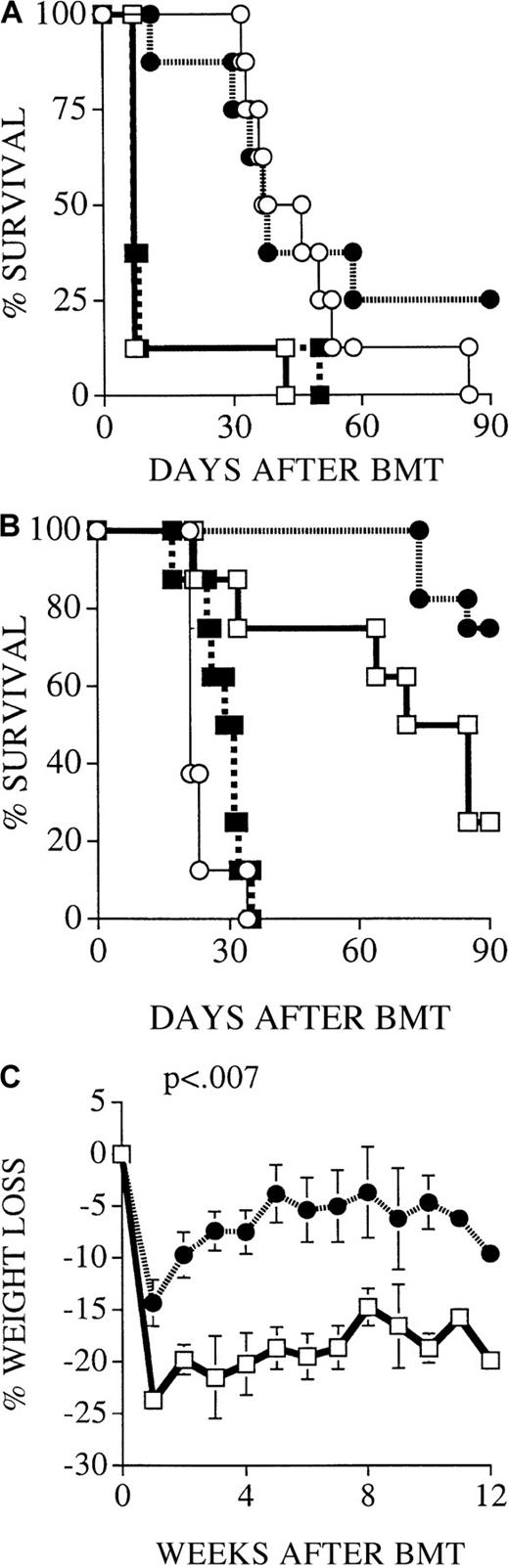
Recipients of FasL-defective B6.gld donor T cells develop significantly less GVHD but do not die of leukemia.
All C3FeB6F1/J recipients received, on day 0, lethal irradiation (1300 cGy) followed by intravenous injection of B6 TCD-BM cells (5 × 106) and 32Dp210 leukemia cells (103). In addition, some recipients received splenic T cells (2 × 106) from normal B6, B6.gld, or B6.pfp−/− donors. (A) Recipients of B6 TCD-BM succumbed to leukemia, and hepatosplenomegaly was observed at autopsy. Hepatic tissue was preserved in formalin (10%) and embedded in paraffin, and tissue sections were stained with hematoxylin and eosin. Histopathologic examination revealed extensive periportal leukemic infiltration (large picture; original magnification, 100×), with many large anaplastic cells with pleomorphic, multilobular large nuclei and frequent mitotic figures (arrow, inset; original magnification, 1000×). Recipients of B6 TCD-BM only or B6 TCD-BM + B6.gld T cells developed less GVHD as determined by weight loss (B) and clinical GVHD score (C). The overall survival in recipients of TCD-BM + B6.gld was significantly better than in all other groups (D) (recipients of B6.gld T cells vs other recipients, P < .0001). Data from 2 combined experiments with 16 recipients per group are shown. This experiment was repeated without the inoculation of leukemia (E), and the survival of recipients of B6.gld was again significantly better than the survival of recipients of B6 T cells or B6.pfp−/− T cells. Each group consisted of 8 animals.
Recipients of FasL-defective B6.gld donor T cells develop significantly less GVHD but do not die of leukemia.
All C3FeB6F1/J recipients received, on day 0, lethal irradiation (1300 cGy) followed by intravenous injection of B6 TCD-BM cells (5 × 106) and 32Dp210 leukemia cells (103). In addition, some recipients received splenic T cells (2 × 106) from normal B6, B6.gld, or B6.pfp−/− donors. (A) Recipients of B6 TCD-BM succumbed to leukemia, and hepatosplenomegaly was observed at autopsy. Hepatic tissue was preserved in formalin (10%) and embedded in paraffin, and tissue sections were stained with hematoxylin and eosin. Histopathologic examination revealed extensive periportal leukemic infiltration (large picture; original magnification, 100×), with many large anaplastic cells with pleomorphic, multilobular large nuclei and frequent mitotic figures (arrow, inset; original magnification, 1000×). Recipients of B6 TCD-BM only or B6 TCD-BM + B6.gld T cells developed less GVHD as determined by weight loss (B) and clinical GVHD score (C). The overall survival in recipients of TCD-BM + B6.gld was significantly better than in all other groups (D) (recipients of B6.gld T cells vs other recipients, P < .0001). Data from 2 combined experiments with 16 recipients per group are shown. This experiment was repeated without the inoculation of leukemia (E), and the survival of recipients of B6.gld was again significantly better than the survival of recipients of B6 T cells or B6.pfp−/− T cells. Each group consisted of 8 animals.
FasL-defective B6.gld donor T cells display diminished GVHD activity but have intact GVL activity
We used splenic T cells (2 × 106) from FasL-defective B6.gld and perforin-deficient B6.pfp−/− mice as donor T-cell inoculum to examine whether donor T-cell–mediated GVL and GVHD activity can be differentiated by their cytotoxic pathways. B6.gld(generalized lymphoproliferative disease) mice have a spontaneous point mutation in the FasL gene that renders the protein nonfunctional.31 They have a defect in peripheral tolerance and activation-induced cell death that results in progressive lymphoid hyperplasia (adenopathy and splenomegaly) and auto-antibody production.13 B6.pfp−/− mice have a defect in CTL- and natural killer cell–mediated cytotoxicity32,33and decreased tumor surveillance.34 Figure 1B-D demonstrates the development of GVHD and leukemia as determined by weight loss (Figure 1B), clinical GVHD score (Figure 1C), and overall survival (Figure 1D). Recipients of B6 TCD-BM only did not have GVHD, but all animals died of leukemia (Figure 1A). Survival in recipients of TCD-BM + FasL-defective B6.gld T cells was significantly better than in the other groups, with only a 6% mortality rate from GVHD and no leukemic deaths (recipients of B6.gld T cells vs other recipients;P < .0001). In contrast, recipients of TCD-BM + perforin-deficient B6.pfp−/− T cells had lethal GVHD, comparable to that in recipients of TCD-BM + normal B6 T cells. None of these recipients of B6 T cells or B6.pfp−/− T cells had leukemia.
We repeated these experiments without leukemia inoculation at the time of BMT to eliminate any effects leukemia cells could have on the development of GVHD. We found again that recipients of B6.gld T cells had significantly less GVHD than recipients of B6 T cells or B6.pfp−/− T cells, all of which died of GVHD (Figure 1E). From these data in this GVHD/GVL model with MHC class I and II mismatch, we conclude that the Fas/FasL cytotoxic pathway is important for the development of GVHD but is not required for GVL activity and that the perforin pathway is not an absolute requirement for the development of GVHD.
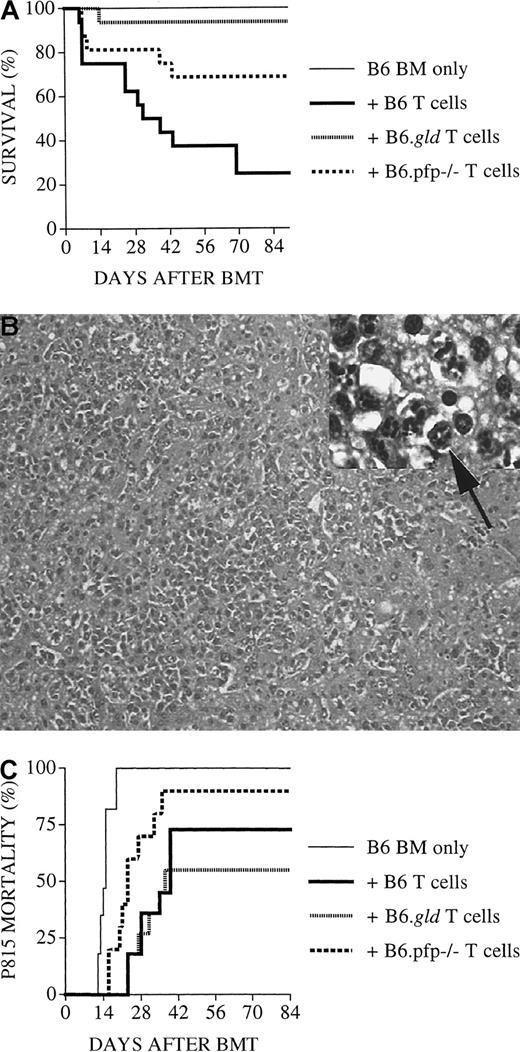
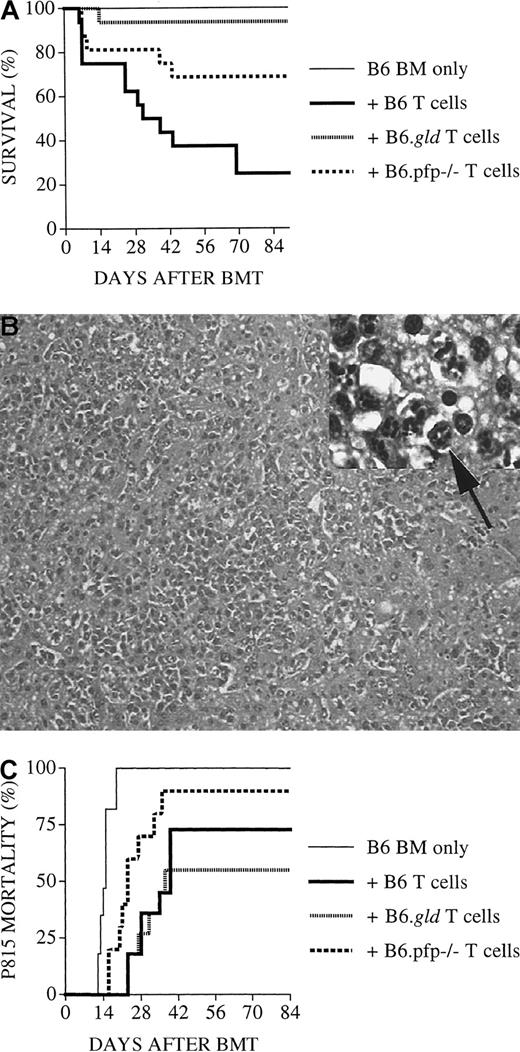
Perforin-deficient B6.pfp−/− donor T cells have intact GVHD activity but display diminished GVL activity
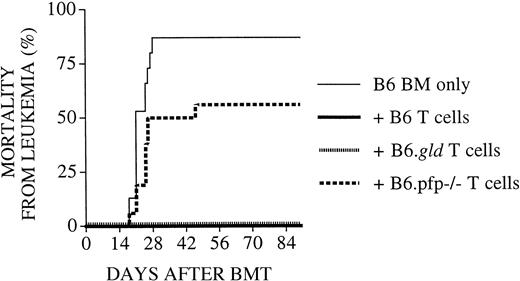
To better determine the role of the FasL and perforin effector pathways in GVL activity, we subsequently performed experiments (Figure2) with an increased number of tumor cells (5 × 103) and a decreased number of donor T cells (1 × 106). As expected, lethal GVHD developed in few animals: 3 of 16 recipients of B6 T cells, 0 of 16 recipients of B6.gld T cells, and 1 of 16 recipients of B6.pfp−/− T cells. However, 50% (8 of 16) of recipients of TCD-BM + B6.pfp−/− T cells succumbed to leukemia, whereas none of the recipients of TCD-BM + B6 T cells or B6.gld T cells had leukemia These results indicate that in this GVHD/GVL model, the perforin pathway is important for donor T-cell–mediated GVL activity, whereas FasL-defective donor T cells displayed intact GVL activity, even at a higher tumor dose. Interestingly, donor B6 or B6.gld T cells could mediate sufficient GVL activity, even at a dose that resulted in few GVHD deaths.
Recipients of perforin-deficient B6.pfp−/−donor T cells develop leukemia.
All C3FeB6F1/J recipients underwent transplantation, as described in Figure 1. However, the leukemia cell dose was increased to 5000 cells/mouse, and the donor T-cell dose was decreased to 1 × 106 cells/mouse. Percentages of animals that died of leukemia from combined experiments with 16 recipients per group are shown. Recipients of TCD-BM + B6.pfp−/− T cells displayed a significantly higher mortality rate from leukemia (56%) than recipients of TCD-BM + B6 (or B6.gld) T cells (0%).
Recipients of perforin-deficient B6.pfp−/−donor T cells develop leukemia.
All C3FeB6F1/J recipients underwent transplantation, as described in Figure 1. However, the leukemia cell dose was increased to 5000 cells/mouse, and the donor T-cell dose was decreased to 1 × 106 cells/mouse. Percentages of animals that died of leukemia from combined experiments with 16 recipients per group are shown. Recipients of TCD-BM + B6.pfp−/− T cells displayed a significantly higher mortality rate from leukemia (56%) than recipients of TCD-BM + B6 (or B6.gld) T cells (0%).
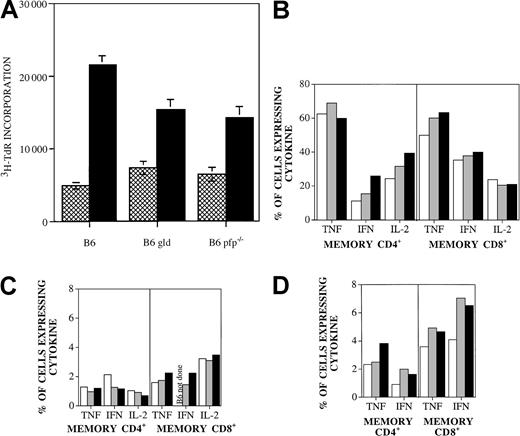
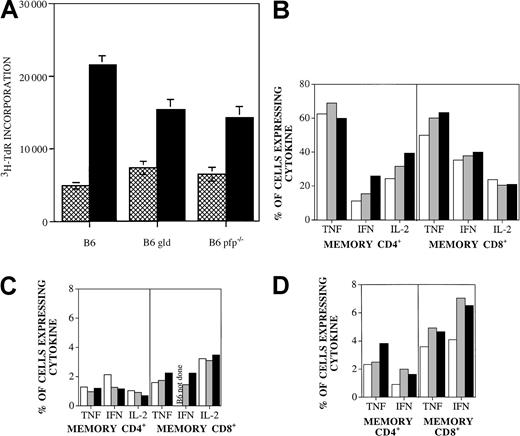
B6.gld and B6.pfp−/−donor T cells display intact proliferative and cytokine response to C3FeB6F1 host antigens
To determine whether the remarkable loss in GVHD activity of B6.gld T cells was due to a decrease in proliferative response to host antigens, we determined the proliferation of splenic T cells from recipients on day 14 after BMT against C3FeB6F1/J antigens in an MLR (Figure 3A). Splenic T cells from recipients of both B6.gld and B6.pfp−/− T cells displayed identical specific proliferative responses to host antigens that were diminished compared to wild-type B6 cells. Moreover, we found comparable numbers of donor T cells in the spleen 14 days after BMT (data not shown). Therefore, B6.gld and B6.pfp−/− T cells seem to be capable of a proliferative response to C3FeB6F1 host antigens.
Proliferative response and cytokine profile of B6.pfp−/− and B6.gld T cells are intact.
(A) Proliferative response of splenic T cells from recipients of TCD-BM with B6, B6.gld, or B6.pfp−/− T cells with (▪) or without (▩) irradiated (20 Gy) C3FeB6F1/J splenic stimulator cells (“Materials and methods”). (B) Splenic T cells from B6 (■), B6.gld (▪), and B6.pfp−/− (░) mice were incubated for 4 hours with PMA (10 ng/mL) and ionomycin (2 μM). Brefeldin A (10 μg/mL) was added after the first hour of incubation. Intracellular cytokine expression in CD4 memory cells (CD4+, CD62L−, CD44+) and CD8 memory cells (CD8+, CD122+, CD44+) was measured by flow cytometric analysis. Cytokine expression in unstimulated controls was less than 7% (data not shown). (C) Splenic T cells from B6 (■), B6.gld (▪), and B6.pfp−/− (░) mice were incubated with equal amounts of T-cell–depleted, irradiated (20 Gy) C3FeB6F1/J splenic stimulator cells in 24-well plates for 16 hours. Brefeldin A (10 μg/mL) was added after the first hour of incubation. Intracellular cytokine expression in CD4 memory cells (CD4+, CD62L−, CD44+) and CD8 memory cells (CD8+, CD122+, CD 44+) was measured by flow cytometric analysis. (D) Splenic T cells from B6 (■), B6.gld (▪), and B6.pfp−/− (░) mice were incubated with equal amounts of irradiated (20 Gy) C3FeB6F1/J splenic stimulator cells in 24-well plates for 5 days. Cells were harvested and restimulated with T-cell–depleted, irradiated (20 Gy) C3FeB6F1/J splenic stimulator cells for 16 hours. Brefeldin A (10 μg/mL) was added after the first hour of incubation. Intracellular cytokine expression in CD4 memory cells (CD4+, CD62L−, CD44+) and CD8 memory cells (CD8+, CD122+, CD44+) was measured by flow cytometric analysis.
Proliferative response and cytokine profile of B6.pfp−/− and B6.gld T cells are intact.
(A) Proliferative response of splenic T cells from recipients of TCD-BM with B6, B6.gld, or B6.pfp−/− T cells with (▪) or without (▩) irradiated (20 Gy) C3FeB6F1/J splenic stimulator cells (“Materials and methods”). (B) Splenic T cells from B6 (■), B6.gld (▪), and B6.pfp−/− (░) mice were incubated for 4 hours with PMA (10 ng/mL) and ionomycin (2 μM). Brefeldin A (10 μg/mL) was added after the first hour of incubation. Intracellular cytokine expression in CD4 memory cells (CD4+, CD62L−, CD44+) and CD8 memory cells (CD8+, CD122+, CD44+) was measured by flow cytometric analysis. Cytokine expression in unstimulated controls was less than 7% (data not shown). (C) Splenic T cells from B6 (■), B6.gld (▪), and B6.pfp−/− (░) mice were incubated with equal amounts of T-cell–depleted, irradiated (20 Gy) C3FeB6F1/J splenic stimulator cells in 24-well plates for 16 hours. Brefeldin A (10 μg/mL) was added after the first hour of incubation. Intracellular cytokine expression in CD4 memory cells (CD4+, CD62L−, CD44+) and CD8 memory cells (CD8+, CD122+, CD 44+) was measured by flow cytometric analysis. (D) Splenic T cells from B6 (■), B6.gld (▪), and B6.pfp−/− (░) mice were incubated with equal amounts of irradiated (20 Gy) C3FeB6F1/J splenic stimulator cells in 24-well plates for 5 days. Cells were harvested and restimulated with T-cell–depleted, irradiated (20 Gy) C3FeB6F1/J splenic stimulator cells for 16 hours. Brefeldin A (10 μg/mL) was added after the first hour of incubation. Intracellular cytokine expression in CD4 memory cells (CD4+, CD62L−, CD44+) and CD8 memory cells (CD8+, CD122+, CD44+) was measured by flow cytometric analysis.
We then determined the cytokine profiles of alloreactive T cells from B6, B6.gld, and B6.pfp−/− mice. We developed a multicolor flow cytometric protocol that allowed quantitative analysis of the intracytoplasmic cytokine expression in naive, activated, and memory CD4+ (or CD8+) alloreactive T cells of donor origin in a MLR. We first analyzed whether splenic T cells from B6.gld and B6.pfp−/− mice had intrinsic differences in their cytokine responses to nonspecific activation with PMA and ionomycin. As shown in Figure 3B, we found no significant differences in the numbers of CD4+ and CD8+memory cells that express TNF, IFN-γ, or IL-2. Moreover, thelevel of intracytoplasmic cytokine production (reflected in the fluorescence intensity) in reactive cells was also comparable in B6, B6.gld, and B6.pfp−/− mice (data not shown).
We then determined the cytokine response of alloreactive splenic T cells by analyzing donor splenic T cells from B6, B6.gld, or B6.pfp−/− mice incubated with irradiated host stimulator cells in an MLR for 1 or 5 days. Again, we did not detect any significant differences between the cytokine profiles of CD4+ or CD8+ memory T cells from B6, B6.gld, or B6.pfp−/− mice (Figure 3C-D).
In addition, we could not detect any significant expression of IL-4 or IL-10 in donor splenic T cells from B6, B6.gld, or B6.pfp−/− mice after 1 or 5 days of allogeneic stimulation (data not shown). We conclude that cytokine profiles from alloreactive T cells are not significantly affected by FasL or perforin deficiency and therefore cannot explain the differences in GVHD and GVL activity we have observed.
Antileukemic cytolytic activity from splenic T cells of BMT recipients is dependent on the perforin effector pathway
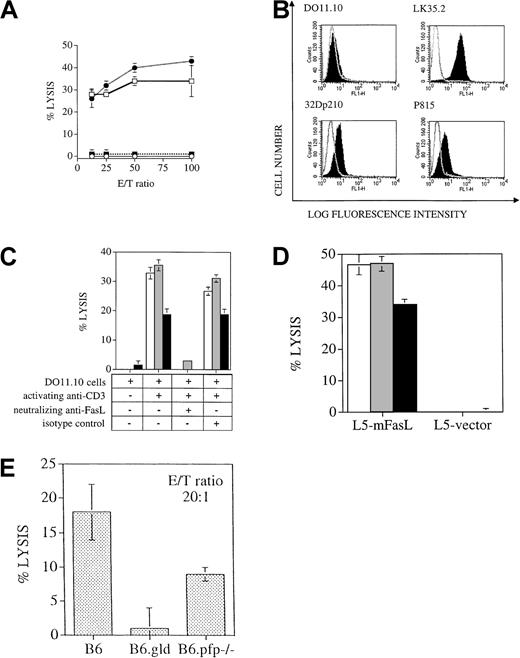
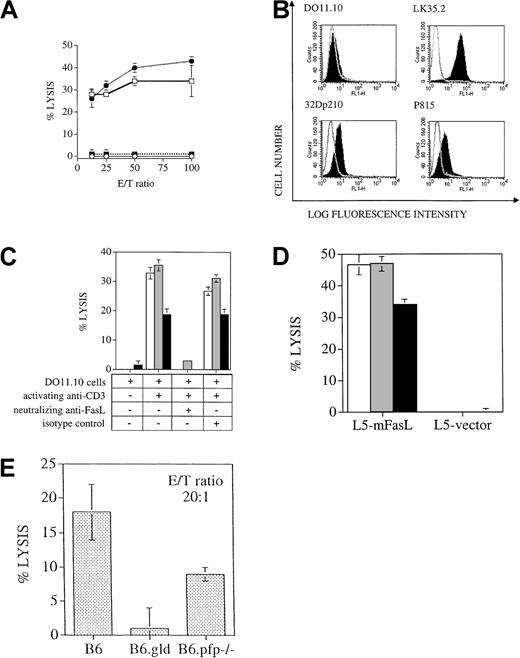
Several studies in murine BMT models have indicated that donor T-cell expansion occurs in the first weeks after allogeneic BMT, with maximal donor T-cell expansion in the spleen by day 10 to 14 after BMT (reviewed in Hakim and Mackall35). To analyze the antileukemia activity of the donor T cells after BMT, we performed in vitro cytotoxicity assays with splenic donor T cells 14 days after BMT. Virtually all splenic T cells recovered from animals 14 days after BMT were of donor origin, as determined by flow cytometric analysis with antibodies against Thy1.2 (pan T-cell marker) and Ly9.1 (present on hematopoietic cells of C3FeB6F1/J host origin but not on B6 donor cells). We found significant lysis of 32Dp210 leukemia by donor T cells from recipients of TCD-BM + B6 T cells or B6.gld T cells, whereas splenic T cells from recipients of TCD-BM only or TCD-BM + B6.pfp−/− T cells showed no cytolytic activity against leukemic target cells (Figure4A). These results support our in vivo data that cytolytic activity of donor T cells against 32Dp210 leukemic cells is mediated through the perforin effector pathway and not through the FasL pathway.
T cells use the perforin, but not the FasL, pathway to lyse the 32Dp210 leukemia cell line, even though the leukemic cell lines express Fas and are in vitro sensitive to FasL-induced cell death.
(A) Cytolytic response of splenic T cells that were incubated as effector cells in a cytotoxicity assay with 51Cr labeled 32Dp210 target cells, as described in “Materials and methods.” Percentages of donor T cells in this experiment were 18% in recipients of B6 T cells (■), 34% in recipients of B6.gld T cells (●), and 26% in recipients of B6.pfp−/− T cells (▪). B6 bone marrow only, ○. (The effector/target (E/T) ratio was corrected for the percentage of donor splenic T cells in each group. (B) Compared to isotype control (nonfilled overlay curve), FACS staining with anti-Fas antibody (filled curve) demonstrates expression of Fas on the tumor cell lines 32Dp210 and P815 and on the positive control LK35.2, a B-cell hybridoma with known high expression of Fas, but not on the T cell hybridoma cell line DO11.10, which has previously been shown to be Fas negative in its resting state. (C)51Cr-labeled tumor cell lines 32Dp210 (░) and P815 (▪) and the B-cell hybridoma LK35.2 (with known FasL sensitivity; ■) were coincubated with resting or activated FasL-expressing DO11.10 cells for 8 hours, and cell death was measured by 51Cr release. Lysis is FasL specific, as shown by the inhibition of cell death by neutralizing FasL antibody, but not by isotype control antibody. (D)51Cr-labeled tumor cell lines 32Dp210 (░) and P815 (▪) and the B-cell hybridoma LK35.2 (with known FasL sensitivity; ■) were coincubated for 8 hours with L5178Y cells, stably transfected with membrane-bound FasL or with vector alone. Significant lysis as measured by 51Cr release occurred in the presence of membrane-bound FasL but not with vector-only transfected cells. (E)51Cr-labeled LPS-stimulated C3FeB6F1/J splenocytes were coincubated for 4 hours with B6, B6.gld, or B6.pfp−/−splenocytes, which had previously been cocultured for 7 days in the presence of irradiated C3FeB6F1/J splenocytes. Cytolytic activity of allogeneically stimulated splenocytes was measured against51Cr-labeled LPS blasts.
T cells use the perforin, but not the FasL, pathway to lyse the 32Dp210 leukemia cell line, even though the leukemic cell lines express Fas and are in vitro sensitive to FasL-induced cell death.
(A) Cytolytic response of splenic T cells that were incubated as effector cells in a cytotoxicity assay with 51Cr labeled 32Dp210 target cells, as described in “Materials and methods.” Percentages of donor T cells in this experiment were 18% in recipients of B6 T cells (■), 34% in recipients of B6.gld T cells (●), and 26% in recipients of B6.pfp−/− T cells (▪). B6 bone marrow only, ○. (The effector/target (E/T) ratio was corrected for the percentage of donor splenic T cells in each group. (B) Compared to isotype control (nonfilled overlay curve), FACS staining with anti-Fas antibody (filled curve) demonstrates expression of Fas on the tumor cell lines 32Dp210 and P815 and on the positive control LK35.2, a B-cell hybridoma with known high expression of Fas, but not on the T cell hybridoma cell line DO11.10, which has previously been shown to be Fas negative in its resting state. (C)51Cr-labeled tumor cell lines 32Dp210 (░) and P815 (▪) and the B-cell hybridoma LK35.2 (with known FasL sensitivity; ■) were coincubated with resting or activated FasL-expressing DO11.10 cells for 8 hours, and cell death was measured by 51Cr release. Lysis is FasL specific, as shown by the inhibition of cell death by neutralizing FasL antibody, but not by isotype control antibody. (D)51Cr-labeled tumor cell lines 32Dp210 (░) and P815 (▪) and the B-cell hybridoma LK35.2 (with known FasL sensitivity; ■) were coincubated for 8 hours with L5178Y cells, stably transfected with membrane-bound FasL or with vector alone. Significant lysis as measured by 51Cr release occurred in the presence of membrane-bound FasL but not with vector-only transfected cells. (E)51Cr-labeled LPS-stimulated C3FeB6F1/J splenocytes were coincubated for 4 hours with B6, B6.gld, or B6.pfp−/−splenocytes, which had previously been cocultured for 7 days in the presence of irradiated C3FeB6F1/J splenocytes. Cytolytic activity of allogeneically stimulated splenocytes was measured against51Cr-labeled LPS blasts.
32Dp210 and P815 leukemia cells express the Fas receptor and are sensitive to Fas-induced apoptosis
To evaluate whether the preferential use of the perforin pathway for GVL activity by donor T cells was influenced by an intrinsic resistance to Fas-mediated apoptosis of leukemia cells, we analyzed the expression of the Fas receptor and the sensitivity to Fas-mediated apoptosis of 32Dp210 and P815 (a mastocytoma model for the GVL effect that will be further described below) cells. The assessment of Fas sensitivity of Fas-expressing cells has been a topic of much debate. Recent studies have indicated that activation of the Fas receptor by FasL expressed on the surfaces of effector cells will result in a stronger signal more likely to induce apoptosis than Fas activation with soluble FasL or anti-Fas antibodies.26 36-40
We first confirmed that both leukemia cell lines express the Fas receptor as described previously by us and others (Figure4B).24 28 Subsequently, we determined Fas sensitivity of our leukemia cell lines using 2 effector cell lines that express FasL on their cell surfaces.
First, we used a T-cell hybridoma cell line (DO11.10) that expresses FasL upon activation through the TCR/CD3 complex and that has been used by us and others as a FasL+ cell line.41 We found that both leukemia cell lines were lysed by FasL-expressing TCR/CD3-activated DO11.10 cells but not by unstimulated DO11.10 cells (Figure 4C). We confirmed that this cytolysis was FasL-dependent by the inhibition of apoptosis with neutralizing anti-FasL antibodies.
In addition, we used a lymphoma cell line, L5178Y, that was stably transfected with membrane-bound FasL. When our leukemia cells were exposed to these FasL+ effector cells, we again observed significant cytolysis, whereas leukemia cells incubated with control L5178Y cells were not affected (Figure 4D). Interestingly, the percentage of cytolysis of each leukemia cell line was comparable to that of a highly Fas-sensitive control cell line, LK35.2.
These data clearly demonstrate that our leukemia cell lines are Fas-sensitive. Therefore, donor T cells have the opportunity to use the FasL pathway to mediate GVL activity in both our experimental BMT models for GVHD and the GVL effect, but our experiments indicate that they preferentially use the perforin pathway for GVL activity.
B6.pfp−/− splenocytes can develop in vitro cytolytic activity against C3FeB6F1 antigens
To evaluate whether B6.pfp−/− T cells can develop cytotoxic activity against allogeneic C3FeB6F1/J cells, we incubated splenocytes from B6, B6.gld, and B6.pfp−/−mice for 7 days with irradiated B6FeC3F1/J splenocytes as stimulators and subsequently used these cells as effector cells against51Cr-labeled LPS-stimulated splenocytes from B6FeC3F1 mice. Effector cells from B6.gld mice had no cytolytic activity against the allogeneic targets, whereas effector cells from B6 and B6.pfp−/− mice could lyse allogeneic targets (Figure 4E). These in vitro data correlate with our in vivo observations that B6 and B6.pfp−/− T cells have cytolytic activity against cells from the recipient and can cause GVHD, whereas B6.gld T cells are unable to exert cytolytic activity against host cells and cause less GVHD.
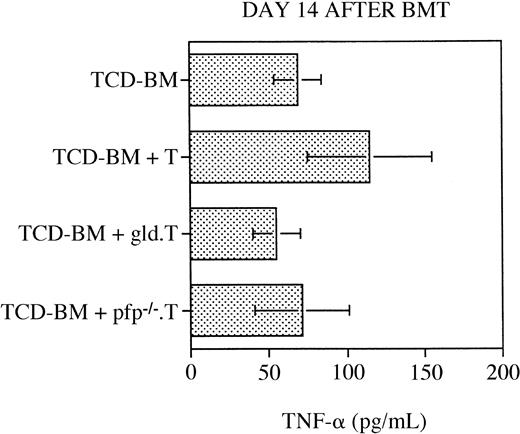
Serum tumor necrosis factor levels do not significantly differ in any of the transplantation groups
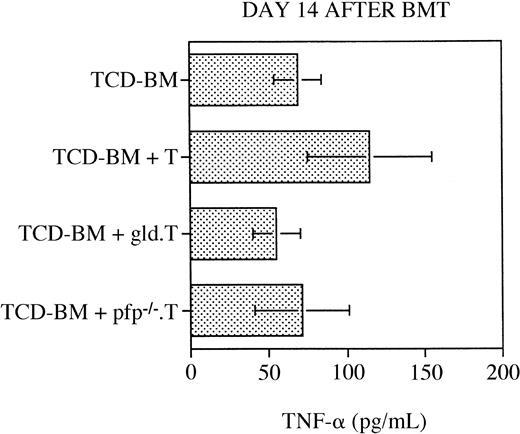
As discussed in the “Introduction,” a number of studies have determined that TNF is a (third) important cytolytic pathway in the pathogenesis of GVHD (reviewed in Hattori et al,23 Herve et al,42 Holler et al,43 Piguet et al,44 Speiser et al,45 and Stuber et al46). To analyze the relative contribution of TNF to the development of GVHD in our murine BMT model, we measured serum TNF levels by ELISA at 14 (or 15) days after BMT in the recipients. As shown in Figure 5, we did not detect any significant differences in the serum TNF levels between the recipients of TCD-BM only, TCD-BM + B6 T cells, TCD-BM + B6.gld T cells, or TCD-BM + B6.pfp−/− T cells. However, these data do not exclude the possibility that membrane-bound TNF on GVHD-GVL effector cells or soluble TNF at the site of inflammation in GVHD organs could play a role in the development of GVHD and the GVL effect.
Serum TNF levels are not significantly increased in any of the transplantation groups.
All C3FeB6F1 recipients underwent transplantation, as illustrated in Figure 1, and serum was obtained on day 14 or 15 after BMT. Serum TNF levels were determined by ELISA, as described in “Materials and methods.” Mean serum TNF level (±SEM) for each group is depicted, and no statistically significant differences between groups were observed. Combined data consisted of 7 to 11 sera per group, and these samples were collected from 3 different BMTs. ELISA analysis was repeated 3 times in duplicate, and results from a representative analysis are shown.
Serum TNF levels are not significantly increased in any of the transplantation groups.
All C3FeB6F1 recipients underwent transplantation, as illustrated in Figure 1, and serum was obtained on day 14 or 15 after BMT. Serum TNF levels were determined by ELISA, as described in “Materials and methods.” Mean serum TNF level (±SEM) for each group is depicted, and no statistically significant differences between groups were observed. Combined data consisted of 7 to 11 sera per group, and these samples were collected from 3 different BMTs. ELISA analysis was repeated 3 times in duplicate, and results from a representative analysis are shown.
FasL pathway is important for GVHD activity by donor CD4+ and CD8+ T cells but is not required for GVL activity by donor CD8+ T cells
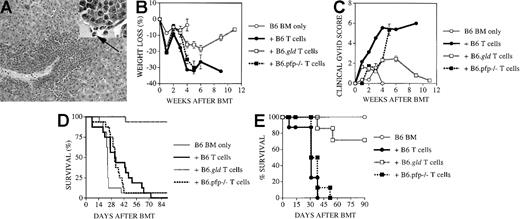
To analyze the relative contributions of CD4+ and CD8+ donor T cells and their cytolytic pathways in GVHD and the GVL effect, we performed experiments with CD4+ and CD8+ selected T cells. Highly purified donor T cells were obtained by enriching donor splenocytes by RBC lysis and nylon wool passage. Subsequently, we purified CD4+ and CD8+ T cells by negative selection after incubation with anti-B220 (CD45R) and anti-CD4 (or anti-CD8) antibodies coupled to microbeads. We included anti-B220 antibodies in this negative selection step not only to further deplete B cells, but especially to deplete B220+CD3+CD4−CD8− T cells from B6.gld splenic T cells. After this purification, the CD4+ or CD8+ selected T cells were analyzed by flow cytometry before infusion to ensure that in each group animals received equal numbers of purified CD4+ or CD8+T cells.
Lethal GVHD developed rapidly in all recipients of B6 CD4+T cells (106) and recipients of B6.pfp−/−CD4+ T cells (Figure6A). Most recipients of B6.gld CD4+ T cells did not develop lethal GVHD, but they succumbed to leukemia (Figure 6A). All recipients of CD8+ T cells (106) developed less severe (nonlethal) GVHD, but recipients of B6.pfp−/− CD8+ T cells died of leukemia (data not shown). We then increased the number of CD8+ selected donor T cells from 106 to 4 × 106 and repeated these experiments. Recipients of B6 CD8+ T cells did develop moderate to severe GVHD resulting in morbidity and mortality (Figure 6B-C), whereas recipients of B6.gldCD8+ T cells developed significantly less (and nonlethal) GVHD. Again, all recipients of B6.pfp−/− CD8+T cells died of leukemia. We conclude from these experiments that in our BMT model, CD4+ donor T cells displayed stronger GVHD activity than CD8+ donor T cells, and this CD4-mediated GVHD activity was exerted through the FasL pathway. However, our data indicate that CD8+ T cells in this BMT model can also exhibit GVHD activity, as was shown previously by Fowler et al,30 and though the FasL pathway seems to play a role in CD8-mediated GVHD activity, it is not required for CD8-mediated GVL activity. In addition, the perforin pathway was not important for CD4-mediated GVHD activity but was required for CD8-mediated GVL activity.
FasL pathway is important for GVHD activity by donor CD4+ and CD8+ T cells but is not required for GVL activity by donor CD8+ T cells.
(A) All C3FeB6F1/J recipients underwent transplantation, as illustrated in Figure 1, with 106 purified donor CD4+splenic T cells (see “Materials and methods”) and a leukemia dose of 5000 cells/mouse. Survival is depicted as a Kaplan-Meier curve. We determined by necropsy that all recipients of B6 CD4+ T cells (■) and of B6.pfp−/− CD4+ T cells (▪) died of GVHD, whereas only one recipient of B6.gldCD4+ T cells (●) developed lethal GVHD. All other deaths in recipients of B6 BM only (○) or B6.gld CD4+T cells were from leukemia. (B) All C3FeB6F1/J recipients underwent transplantation, as illustrated in Figure 1, with 4 × 106 purified donor CD8+ splenic T cells (see “Materials and methods”) and a leukemia dose of 5000 cells/mouse. Survival is depicted as a Kaplan-Meier curve. We determined by necropsy that 5 of 6 deaths in recipients of B6 CD8+ T cells (■) were from GVHD. One of the 8 recipients of B6.pfp−/− CD8+ T cells (▪) and one recipient of B6.gld CD8+ T cells (●) died of GVHD, whereas all other deaths could be attributed to leukemia. All deaths among recipients of B6 BM only (○) were from leukemia. (C) Weight loss (as a measurement of GVHD morbidity) was determined weekly in all recipients of B6 CD8+ T cells (■) and B6.gld CD8+ T cells (●) from the BMT experiment described in panel B. Recipients of B6.gldCD8+ T cells had significantly less weight loss than recipients of B6 CD8+ T cells (P < .007). All experimental groups consisted of 8 animals, and representative results of 3 experiments with CD4+ T cells and 5 experiments with CD8+ T cells are shown.
FasL pathway is important for GVHD activity by donor CD4+ and CD8+ T cells but is not required for GVL activity by donor CD8+ T cells.
(A) All C3FeB6F1/J recipients underwent transplantation, as illustrated in Figure 1, with 106 purified donor CD4+splenic T cells (see “Materials and methods”) and a leukemia dose of 5000 cells/mouse. Survival is depicted as a Kaplan-Meier curve. We determined by necropsy that all recipients of B6 CD4+ T cells (■) and of B6.pfp−/− CD4+ T cells (▪) died of GVHD, whereas only one recipient of B6.gldCD4+ T cells (●) developed lethal GVHD. All other deaths in recipients of B6 BM only (○) or B6.gld CD4+T cells were from leukemia. (B) All C3FeB6F1/J recipients underwent transplantation, as illustrated in Figure 1, with 4 × 106 purified donor CD8+ splenic T cells (see “Materials and methods”) and a leukemia dose of 5000 cells/mouse. Survival is depicted as a Kaplan-Meier curve. We determined by necropsy that 5 of 6 deaths in recipients of B6 CD8+ T cells (■) were from GVHD. One of the 8 recipients of B6.pfp−/− CD8+ T cells (▪) and one recipient of B6.gld CD8+ T cells (●) died of GVHD, whereas all other deaths could be attributed to leukemia. All deaths among recipients of B6 BM only (○) were from leukemia. (C) Weight loss (as a measurement of GVHD morbidity) was determined weekly in all recipients of B6 CD8+ T cells (■) and B6.gld CD8+ T cells (●) from the BMT experiment described in panel B. Recipients of B6.gldCD8+ T cells had significantly less weight loss than recipients of B6 CD8+ T cells (P < .007). All experimental groups consisted of 8 animals, and representative results of 3 experiments with CD4+ T cells and 5 experiments with CD8+ T cells are shown.
GVHD activity is mediated through the FasL effector pathway, and GVL activity is mediated through the perforin pathway in B6D2F1/J recipients
To further test our hypotheses regarding the roles of the FasL and perforin pathways in the GVL effect and GVHD, we used another well-established parent → F1 murine model for allogeneic BMT with a full mismatch for MHC class I and II (B6 → B6D2F1). In our first experiments, B6D2F1/J recipients received lethal irradiation (1300 cGy) and were transplanted with B6 TCD-BM (5 × 106 cells) with or without splenic T cells from normal B6, B6.gld, or B6.pfp−/− mice. In this case, B6D2F1 recipients of B6.pfp−/− T cells had a 31% mortality rate from GVHD, significantly lower than that of recipients of normal B6 T cells (75%;P < .04) but significantly higher than that of recipients of B6.gld T cells (6%; P < .04) (Figure7A). These results suggest that in this BMT model for GVHD, both cytotoxic pathways are required for maximal GVHD activity by donor T cells, though the FasL pathway seems to be most important. In additional experiments, we inoculated B6D2F1 recipients with P815 mastocytoma cells (1000 cells/mouse) at the time of BMT and found a significant delay in deaths from leukemia in recipients of B6 T cells or B6.gld T cells compared to recipients of TCD-BM only, but not in recipients of B6.pfp−/− T cells (Figure 7B-C). This indicates again a role for the perforin pathway in GVL activity, whereas FasL-defective B6.gld donor T cells displayed GVL activity comparable to that of normal B6 T cells.
B6D2F1/J recipients of perforin-deficient B6.pfp−/− donor T cells display less GVL activity against P815 mastocytoma.
(A) On day 0, all B6D2F1 recipients received lethal irradiation (1300 cGy) and then B6 TCD-BM cells (5 × 106). In addition, some recipients received splenic T cells (2 × 106) from normal B6, B6.gld, or B6.pfp−/− donors. Death from GVHD is shown as a Kaplan-Meier survival curve. Recipients of B6.pfp−/− T cells had significantly fewer deaths than recipients of B6 T cells (P < .04) and more deaths than recipients of B6.gld T cells (P < .04). Combined data from 2 experiments with 16 animals per group are shown. (B, C) B6D2F1/J mice received transplants as in panel A, but all recipients received 1000 P815 mastocytoma cells in addition on day 0 after the lethal irradiation. Recipients of B6 TCD-BM succumbed to P815 mastocytoma, and hepatomegaly and liver metastases were observed at autopsy. Liver tissue was preserved in formalin (10%) and embedded in paraffin, and tissue sections were stained with hematoxylin and eosin. Histopathologic examination revealed extensive tumor infiltration with a loss of liver architecture (large picture; original magnification, 100 ×), many large round to polyhedral cells with small basophilic cytoplasmic granules, and empty vesiculated nuclei with large nucleoli and frequent mitotic figures (arrow, inset: original magnification, 1000 ×). Mortality of GVHD survivors from P815 mastocytoma is shown in panel C. Results from 3 combined experiments with 10 to 17 animals per group are shown. Recipients of B6.pfp−/− T cells had significantly more deaths than recipients of B6 T cells (P < .03) or B6.gld T cells (P = .02).
B6D2F1/J recipients of perforin-deficient B6.pfp−/− donor T cells display less GVL activity against P815 mastocytoma.
(A) On day 0, all B6D2F1 recipients received lethal irradiation (1300 cGy) and then B6 TCD-BM cells (5 × 106). In addition, some recipients received splenic T cells (2 × 106) from normal B6, B6.gld, or B6.pfp−/− donors. Death from GVHD is shown as a Kaplan-Meier survival curve. Recipients of B6.pfp−/− T cells had significantly fewer deaths than recipients of B6 T cells (P < .04) and more deaths than recipients of B6.gld T cells (P < .04). Combined data from 2 experiments with 16 animals per group are shown. (B, C) B6D2F1/J mice received transplants as in panel A, but all recipients received 1000 P815 mastocytoma cells in addition on day 0 after the lethal irradiation. Recipients of B6 TCD-BM succumbed to P815 mastocytoma, and hepatomegaly and liver metastases were observed at autopsy. Liver tissue was preserved in formalin (10%) and embedded in paraffin, and tissue sections were stained with hematoxylin and eosin. Histopathologic examination revealed extensive tumor infiltration with a loss of liver architecture (large picture; original magnification, 100 ×), many large round to polyhedral cells with small basophilic cytoplasmic granules, and empty vesiculated nuclei with large nucleoli and frequent mitotic figures (arrow, inset: original magnification, 1000 ×). Mortality of GVHD survivors from P815 mastocytoma is shown in panel C. Results from 3 combined experiments with 10 to 17 animals per group are shown. Recipients of B6.pfp−/− T cells had significantly more deaths than recipients of B6 T cells (P < .03) or B6.gld T cells (P = .02).
Discussion
Studies using mice with defects or deficiencies in the perforin/granzyme pathway or the Fas/FasL pathway have demonstrated that the perforin/granzyme pathway is important in the CTL and natural killer cell response against virally infected cells and malignant cells,34,47,48 whereas the FasL pathway is mostly implicated in the regulation of the immune response. For example, perforin-deficient mice display increased vulnerability to a variety of tumors and infections, whereas both Fas-deficient and FasL-defective mice develop a lymphoproliferative disorder with autoantibody production, lymphoid hyperplasia, and defects in peripheral tolerance and activation-induced cell death.13 Although pfp−/− mice display no obvious immunologic disorders during normal development, recent studies of pfp−/− mice infected with LCMV and patients with familial hemophagocytic lymphohistiocytosis (who have mutations in the perforin gene) have demonstrated an immune regulatory defect resulting in the accumulation of activated macrophages and lymphocytes (predominantly CD8+) and an overproduction of inflammatory cytokines (such as TNF).49-51
Several authors have proposed that 3 cytotoxic pathways are involved in the pathogenesis of GVHD: FasL/Fas, perforin/granzyme, and TNF/TNFR.17-24,44,45,52,53 The importance of the Fas/FasL pathway in GVHD was demonstrated in spleen cell transfer models (parent → F1 or B6 → Balb/c) into unconditioned recipients, in which FasL-defective gld donor T cells or treatment with neutralizing anti-FasL antibodies could ameliorate GVHD, as measured by host lymphoid depletion, myelosuppression, weight loss, and death.19,20,23,53 Studies by Baker et al17,18using FasL-defective gld donor T cells in an MHC-matched mHAg-mismatched allogeneic BMT model with lethally irradiated recipients showed diminished cutaneous and hepatic GVHD and lymphoid hypoplasia but no effect on cachexia and death. In contrast, Graubert et al21 found delayed death in an allogeneic BMT model with MHC class II disparity and lethally irradiated recipients.
Several studies with pfp−/− donor T cells in various murine BMT models with disparity for MHC class I,21 class I and II,22 or mHAg17,18 have demonstrated improved survival, indicating that GVHD activity can be mediated through the perforin pathway. Interestingly, the use of pfp−/− donor T cells did not result in diminished GVHD target organ abnormalities of liver, skin, and intestines.17
Evidence for the role of the TNF/TNFR pathway in GVHD comes from studies in murine BMT models demonstrating that the administration of anti-TNF antibody to recipients can ameliorate GVHD24,44and that the TNFR p55-deficient recipients of allogeneic BM with T cells had lower mortality rates, though TNF release and T-cell proliferation and cytotoxicity were similar to levels in control recipients.45 Clinical studies have shown that patients with GVHD have elevated serum TNF levels,43 and phase I/II trials with anti-TNF antibody treatment in patients with refractory GVHD demonstrated some improvement (especially in intestinal GVHD).42
We found in both BMT models, across MHC class I and II disparity, that the FasL pathway is primarily responsible for GVHD activity. In C3FeB6F1/J recipients, the use of FasL-defective gld donor T cells resulted in a virtual elimination of GVHD deaths, whereas B6D2F1/J recipients had significantly prolonged survival times. In contrast, the use of pfp−/− donor T cells had no effect (in C3FeB6F1/J recipients) or a moderate effect (in B6D2F1/J recipients) on the development of GVHD in both strain combinations. Previously only the studies of Graubert et al21 had demonstrated such a dominant role for the FasL pathway in the development of GVHD, and this was in a strain combination with an MHC class II disparity. Our studies with CD4+ and CD8+ selected donor T cells indicate that CD4+T cells are more important than CD8+ T cells for GVHD activity in our MHC class I and II disparate model and that they exert most of their GVHD activity through the FasL pathway. This is in agreement with a number of studies showing that CD4+ T cells preferentially use the FasL pathway for cytolytic activity.11,54 55 Interestingly, our experiments with CD8+ selected donor T cells demonstrate for the first time that the FasL pathway can also play a role in CD8-mediated GVHD activity.
Our studies demonstrate that the FasL pathway is not important for GVL activity in either model tested, though we found in both models that leukemia cells displayed Fas on their surfaces and could undergo apoptosis in vitro when exposed to FasL+ effector cells. In both models, we found that the perforin pathway was important for GVL activity. We also demonstrated in vitro a concomitant loss of antitumor cytotoxicity by perforin-deficient lymphocytes. These findings are in agreement with our previous data regarding the importance of the perforin pathway in GVL activity by G-CSF–mobilized donor T cells56 and with the proposed role for the perforin pathway in tumor immunosurveillance, as demonstrated by the decreased tumor surveillance of perforin-deficient mice.34 Our data suggest that alloreactive donor T cells, through an unknown mechanism, select the FasL pathway for their GVHD activity but not for their GVL activity.
Thus far, only one other study has evaluated the role of the effector pathways in GVHD and the GVL effect. Tsukada et al24 used the B6 → B6D2F1/J model with B6, B6.gld, or B6.pfp−/− whole splenocytes as T-cell inoculum. The TNF pathway was inhibited by anti-TNF antibody administration, and the DBA/2 leukemia L1210 and P815 were used to study the GVL effect. The authors found a similar reduction in lethal GVHD when B6.gldor B6.pfp−/− cells were used. However, their data regarding the role of the FasL and perforin pathways in GVL were inconclusive. The authors describe uncertainty regarding the cause of death (GVHD vs tumor) in all recipients of leukemia and B6.gld T cells. They show only overall survival data that diminish the ability to draw conclusions regarding the specific role of the FasL and perforin pathways in GVHD and GVL activity. In contrast, as we have described previously,28 we were able to distinguish lethal GVHD from leukemic death by hepatosplenomegaly (for 32Dp210 leukemia) or macroscopic liver/spleen metastases (P815) at autopsy, by histopathologic examination of liver and spleen, and by clinical GVHD score (including weight loss).
Moreover, recipients in the study by Tsukada et al24received TCD-BM from B6.gld mice that resulted in reconstitution with a FasL-defective hematopoietic system. This could result in the development of the B6.gld-related autoimmune and lymphoproliferative defects in the recipient with potentially lethal outcomes. In our experiments, we elected to use in all transplantation groups TCD-BM from normal B6 mice to avoid the confounding variable of hematopoietic reconstitution with FasL-defective or perforin-deficient stem cells.
In conclusion, the in vitro and in vivo data presented here support the hypothesis that donor T cells make differential use of their cytotoxic pathways for GVHD and GVL activity. In the 2 murine BMT models tested, the FasL effector pathway was required for GVHD activity (but not for GVL activity), whereas the perforin pathway was important for GVL activity. This suggests that selective inhibition of a cytolytic pathway may represent a novel strategy for the separation of GVL from GVHD activity.
We thank Dr Geoffrey R. Hill for helpful discussions, Dr Miguel Perales for help with intracellular cytokine staining, Dr Andreas M. Hohlbaum and Dr Ann Marshak-Rothstein for providing the mFasL-transfected L5178Y cell line, and Dr Hai T. Nguyen for histopathologic examination of tissue specimens.
Supported by National Institutes of Health program project grant 5PO1 CA39542 (S.J.B.). M.R.M.v.d.B. is the recipient of a research award from the Amy Strelzer Manasevit Scholars Program for the study of posttransplantation complications, funded by The Marrow Foundation in cooperation with the National Marrow Donor Program.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marcel R. M. van den Brink, Department of Medicine, Memorial Sloan-Kettering Cancer Center, Kettering 1118, Mailbox 111, 1275 York Ave, New York, NY 10021.