Abstract
Using sensitive, automated immunoassays, increased concentrations of either κ or λ free light chains (and abnormal κ/λ ratios) were detected in the sera of 19 of 28 patients with nonsecretory multiple myeloma. Four other patients had suppression of one or both light chains, and the remaining 5 sera had normal or raised free light-chain concentrations with substantially normal κ/λ ratios. Six of the patients with an elevated single free light chain, who were studied during follow-up, had changes in disease activity that were reflected by the changes in free light-chain concentrations. It is concluded that quantification of free light chains in serum should prove useful for the diagnosis and monitoring of many patients with nonsecretory myeloma.
Introduction
Nonsecretory multiple myeloma (NSM) is characterized by the absence of detectable monoclonal proteins in serum and urine and accounts for 1% to 5% of all patients with multiple myeloma.1 In approximately 85% of patients with NSM, immunohistochemical studies demonstrate the presence of cytoplasmic M-proteins within the plasma cells of the bone marrow indicating immunoglobulin synthesis.2 Using sensitive techniques, monoclonal free light chains (flc) can be detected in the urine of some of these patients,2 but the usual serum and urine electrophoretic assays are inadequate.3 Thus, the diagnosis and monitoring of patients with NSM depends on clinical assessment and bone marrow biopsies. This study evaluated the use of sensitive, serum flc immunoassays in the diagnosis and management of patients with NSM.
Study design
Collection and storage of serum from patients with NSM
We obtained archived sera from patients studied in the United Kingdom, Medical Research Council, Myeloma Trials between 1983 and 1999. From a total of 2323 patients, 64 (3.6%) had NSM of which 28 were selected for study because of complete clinical data and adequate serum samples. The clinical diagnosis of multiple myeloma was based on the plasma cell content of bone marrow aspirates or trephine biopsies together with the clinical features of multiple myeloma, including lytic bone lesions or fractures. At the time of diagnosis, none of the patients were considered to have significant monoclonal proteins in their serum or urine (3 had minimal monoclonal urine flc; Table1) by the routine laboratory tests. These comprised analysis of serum and urine by electrophoresis together with immunoelectrophoresis or immunofixation electrophoresis (IFE). Six of the 28 patients were further studied to assess the role of flc assays for disease monitoring. In these individuals, flc were measured in sera collected at presentation, plateau phase (asymptomatic, stable hemoglobin, and normal serum β2-microglobulin) and at relapse, each phase having been established by the clinical features and bone marrow biopsies. All sera had been stored, long-term, at −20°C with sodium azide as preservative.
Immunoassays for measuring free κ and free λ light chains
The flc were measured using latex-enhanced, immunoassays4 on a Behring nephelometric analyzer (Dade Behring, Deerfield, IL). These quantitative assays could detect less than 1.0 mg/L κ and λ compared with 150 to 500 mg/L by IFE and 500 to 2000 mg/L by serum protein electrophoresis.4,5Calibration was against purified flc that had been quantified by amino acid analysis.6 Results were compared with 100 normal (blood donor) sera in which the mean concentration of free κ was 8.4 mg/L and free λ, 14.5 mg/L, with a mean κ/λ ratio of 0.6 (range, 0.36-1.0). Because IFE techniques have improved since the patients first presented, all sera were tested by IFE at 1:2 dilution against whole κ and λ antisera and neat by IFE against free κ and λ antisera. No urine specimens were available for evaluation. An assessment of flc molecular weight was made on sera from patients 5 and 7 by subjecting them to size separation chromatography on Sephadex G100 (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) and polyacrylamide gel electrophoresis (PAGE) using reducing and nonreducing gels. Sera from patients 1, 3, 5, 7, and 8 were assessed by immunodiffusion in agarose gels against antisera specific for IgGFc, whole κ and free κ to confirm the presence of flc.
Results and discussion
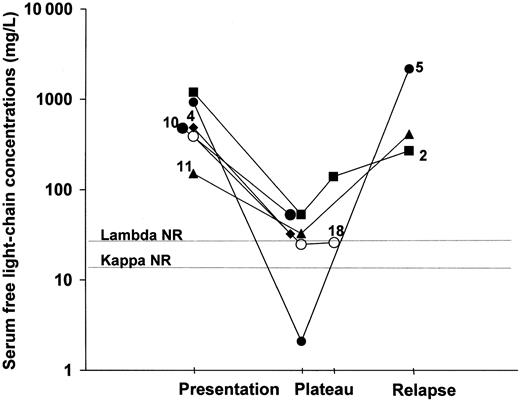
Table 1 shows the serum flc concentrations from the 28 patients together with the κ/λ concentration ratios, the bone marrow plasma cell content at clinical presentation, and other positive laboratory results. In 19 of the sera, concentrations of either κ or λ were highly elevated and κ/λ concentration ratios were abnormal with 6 having suppression of the other light chain. On the basis of increased serum flc levels these patients could be classified as oligosecretory myelomas.1 Four other patients had suppression of one or both flc with little evidence of light-chain clonality. The remaining 5 samples had normal/borderline κ/λ ratios of which 2 contained elevated concentrations and 3, normal concentrations of both flc. Presumably, some of these patients truly have nonproducer myelomas, but we have no immunohistochemical data on bone marrow plasma cells to confirm this suggestion. These results compare with 15% nonproducer myelomas reported in immunohistochemical studies.1 The 6 patients studied during the course of their disease (5 κ and 1 λ) showed changes in concentrations of flc that were in accordance with their clinical progress (Figure 1). In patient 2 an increase of flc concentrations occurred before the patient was judged to have relapsed by the existing clinical criteria.
Changes in serum flc concentrations and clinical status in 6 patients with NSM.
NR indicates upper limit of normal range; numbers refer to patients in Table 1.
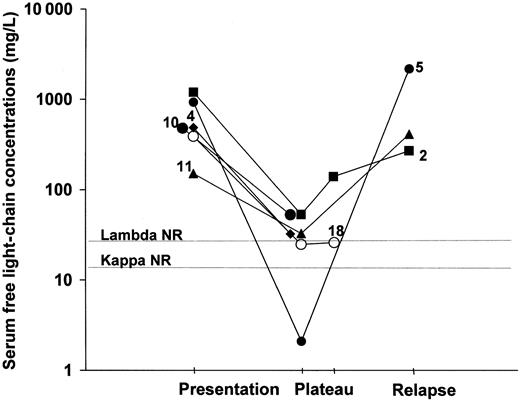
Changes in serum flc concentrations and clinical status in 6 patients with NSM.
NR indicates upper limit of normal range; numbers refer to patients in Table 1.
Table 1 shows that some of the samples with high flc concentrations were weakly positive when reassessed by sensitive IFE. The monoclonal bands detected in the 4 κ-producing myelomas were weak and diffuse, whereas the bands in 3 λ-producing myelomas were narrow and clearly visible by IFE using flc-specific antibodies. In the other patients with a single elevated flc, no monoclonal bands were detected by IFE even though the concentrations measured by nephelometry were much greater than the detection limit of IFE (∼100 mg/L). When 5 of these samples were further analyzed by gel immunodiffusion, all contained substantial amounts of flc (seen as immunoprecipitation nonidentity lines between IgGFc and flc antisera), although the amounts could not be quantified in these assays. Samples 5 and 7 were investigated for polymerized light chains. Sample 5 contained κ immunoreactivity at sizes ranging from 40 to 200 kd with peaks at approximately 42 kd, 96 to 107 kd, and 200 kd by chromatography and PAGE but none at 25 kd (monomeric flc). Sample 7 contained large molecular weight free κ light chains at 150 to 200 kd but little at lower molecular weights. Thus, these 2 representative sera contained variably polymerized flc, which may be structurally abnormal, as previously suggested.7 The polymerization could account for the diffuse immunoprecipitation observed with most of the samples by IFE. Such large polymers would have minimal renal clearance compared with monomeric flc and this could account for the negative flc urine tests in NSM. In addition, the more frequent occurrence of diffuse IFE bands in the κ NSM indicates that variable polymerization is more common than for λ NSM and could account for the 4:1 ratio of κ/λ NSM that is reported in the literature.1
Table 1 shows the concentrations and ranges of flc in normal sera. These results were obtained from individuals who were younger (18-54 years) than the patients and indicates that an age-matched control group is required for future studies. It is of note that the normal flc concentrations (which were based on standardization against pure proteins6) showed κ values lower than λ values. Most investigations have recorded the inverse, but higher normal λ levels were reported in a recent study.8 Although there are approximately twice the number of κ-producing compared with λ-producing plasma cells in the body, κ is normally monomeric (25 kd), whereas λ is largely dimeric (50 kd) and is cleared more slowly by the kidney. In a study on the clearance of dextran polymers, it was shown that molecules of 20 kd were cleared 3.2 times faster than 37-kd molecules.9 The slower clearance of λ molecules might partly explain the inverse serum κ/λ ratio that was observed.
The assays described here do not measure the absolute amount of flc present in the sera and no accurate reference method is available. It is possible that the nephelometric immunoassays overestimate the true concentrations. Polymers of flc containing multiple epitopes could enhance the kinetics of immunoprecipitation resulting in relatively high results compared with the same concentrations of monomeric flc. A more complete analysis of these issues will be the subject of further studies. Nevertheless, the results from the current study indicate that the sensitive quantification of serum flc should prove useful for the diagnosis and monitoring of many patients with NSM.
We thank The Binding Site for the provision of the immunoassay kits. The myeloma sera were provided by the MRC Adult Leukaemia Working Party.
Supported in part by a grant from the Department of Trade and Industry (grant no. WMR/26799/SP).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
A. R. Bradwell, Division of Immunity and Infection, The Medical School, University of Birmingham, Birmingham B15 2TT, England.