Abstract
Lipoprotein (a) [Lp(a)] has been associated with both anti-fibrinolytic and atherogenic effects. However, no direct link currently exists between this atherogenic lipoprotein and intravascular coagulation. The current study examined the binding and functional effects of Lp(a), its lipoprotein constituents, apoliprotein (a) [apo(a)] and low-density lipoprotein (LDL), and lysine-plasminogen (L-PLG), which shares significant homology with apo(a), on tissue factor pathway inhibitor (TFPI), a major regulator of tissue factor-mediated coagulation. Results indicate that Lp(a), apo(a), and PLG but not LDL bound recombinant TFPI (rTFPI) in vitro and that apo(a) bound to a region spanning the last 37 amino acid residues of the c-terminus of TFPI. The apparent binding affinity for TFPI was much higher for Lp(a) (KD ∼150 nM) compared to PLG (KD ∼800 nM) and nanomolar concentrations of apo(a) (500 nM) inhibited PLG binding to TFPI. Lp(a) also inhibited in a concentration-dependent manner rTFPI activity and endothelial cell surface TFPI activity in vitro, whereas PLG had no such effect. Moreover physiologic concentrations of PLG (2 μM) had no effect on the concentration-dependent inhibition of TFPI activity induced by Lp(a). In human atherosclerotic plaque, apo(a) and TFPI immunostaining were shown to coexist in smooth muscle cell–rich areas of the intima. These data suggest a novel mechanism whereby Lp(a) through its apo(a) moiety may promote thrombosis by binding and inactivating TFPI.
Introduction
Lipoprotein (a) [Lp(a)], a complex of low-density lipoprotein (LDL) and apolipoprotein (a) [apo(a)], is an important inherited risk factor for atherosclerosis and myocardial infarction.1,2 Recently, Kronenberg and colleagues3 showed that Lp(a) concentrations predicted the risk of early atherosclerosis synergistically with LDL, whereas Lp(a) alone emerged as a leading independent risk factor for advanced atherosclerosis. The latter association with advanced atherosclerosis is of particular interest because it seems not to rely on conventional risk factors such as LDL but may be within the realm of procoagulant risk attributes contributing to plaque thrombosis.3-5Apo(a) contains multiple kringle IV–like domains, a kringle V–like domain, and a proteaselike domain that have significant homology with plasminogen (PLG).6 Lp(a) accumulates in the vessel wall and inhibits binding of PLG to the cell surface, reducing plasmin generation and subsequent clot lysis.7,8 This inhibition of PLG activation by Lp(a) also reduces active transforming growth factor-β (TGF β) production with consequent promotion of smooth muscle cell (SMC) proliferation.9 These unique structural features of Lp(a) suggest this lipoprotein has both antifibrinolytic and atherogenic potential.
The antifibrinolytic effects of Lp(a) notwithstanding, to date no mechanistic data exist to support a more direct role for this atherogenic lipoprotein in promotion of intravascular thrombosis. The lysine-binding characteristics of Lp(a) may be important in this regard, allowing the apo(a) portion of Lp(a) to bind several lysine-rich components of the coagulation system. A potential candidate for such lysine binding is tissue factor pathway inhibitor (TFPI), which has numerous lysines in its carboxy-terminal portion. TFPI is a major endogenous regulator of tissue factor (TF)–mediated coagulation,10 and its expression has been described in endothelial cells, activated monocytes, and platelets.11-14 TFPI inhibits TF/factor VIIa catalytic activity in vivo, binding in a 2-step process to factor Xa via its Kunitz 2 (K2) domain and subsequently to TF/VIIa complex via its Kunitz 1 (K1) domain to form an inhibitory quaternary complex (TF-VIIa-Xa-TFPI).10,15,16 We and others have recently described the expression and regulation of TFPI in vascular smooth muscle cells (VSMCs).17,18 We have also shown that TFPI within atherosclerotic plaque may be an important regulator of TF activity.19 These studies suggest that tissue TFPI may have a role in modification of TF activity in the vessel wall.
In the current study we hypothesized that Lp(a) may bind cell-associated TFPI. This study investigated the potential for binding between Lp(a), apo(a), Lp(a−) [LDL portion of Lp(a)] and TFPI and determined whether Lp(a) reduced TFPI anticoagulant activity. Binding studies were also performed with PLG, which shares significant amino acid homology with apo(a) and the relative binding affinities of Lp(a) and PLG for TFPI were examined. Further studies were performed to determine the region of TFPI involved in binding Lp(a) and to examine whether apo(a) and TFPI coexist in the vessel wall of human subjects.
Materials and methods
Materials
Lipoprotein (a) isolated from human plasma of several donors of varying apo(a) phenotypes ranging from about 300 to 600 kd molecular weight (Biomedical Technologies, Stoughton, MA) were purified via ultracentrifugation and immunoaffinity chromatography on apo(a) Sepharose yielding single bands on unreduced sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).20Highly purified human LDL (Perimmune, Rockville, MD) was used for cell culture experiments. Recombinant apo(a) was prepared as previously described.21,22 Recombinant full-length TFPI (rTFPI), truncated TFPI161, and TFPI lacking the Kunitz 3 domain (K1K2C) were as previously described.23 The antibodies to TFPI included 2 rabbit polyclonal antibodies to the whole TFPI protein and a rabbit polyclonal antibody to the carboxy-terminus of TFPI.16,24,25 A previously characterized specific goat-anti–human apo(a) antibody was obtained from Biodesign International (Kennebunk, ME).26 Antigoat horseradish peroxidase (HRP) was obtained from Calbiochem (San Diego, CA) and Amersham Life Sciences (Arlington Heights, IL). Human PLG was obtained from American Diagnostica (Greenwich, CT). IODO-GEN tubes for NaI125 radioiodination were obtained from Pierce (Rockford, IL). Genejammer for liposomal transfection was obtained from Stratagene (La Jolla, CA). All antibiotics were obtained from Gibco BRL (Rockville, MD). Synthesized c-terminus (cTFPI37) and scrambled peptides (cTFPIs) were obtained from the Mayo Protein Core facility.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were obtained from Clonetics (Walkersville, MD) at passage 2 and were subsequently cultured on 90-mm dishes as previously described.17 Cells were used for experiments between passage 4 and 6 and confirmed as endothelial cell origin by universal staining for von Willebrand factor (vWF).
Chinese hamster ovary cell line expressing TFPI
Chinese hamster ovary (CHO) cells were obtained as a gift from Dr R Cattaneo (Mayo Clinic). TFPI complementary DNA (cDNA) was digested from a pUC19 vector (pUC-TFPI) with SacI and then isolated, purified, and ligated into the BamHI site of vector pcDNA3 (Invitrogen, Carlsbad, CA). The size and orientation of the TFPI cDNA fragment was confirmed using ClaI and nucleotide sequence was verified using dideoxynucleotide chain-termination DNA sequence analysis (Mayo Clinic DNA Sequencing Facility).
The CHO cells were cultured in Dulbecco modified Eagle medium supplemented with 5% fetal calf serum (FCS), 2 mM/Ll-glutamine, 100 U/mL penicillin, and 100μg/mL streptomycin. Prior to transfection CHO cells were transferred to 6-well plates and grown for at least 24 hours to approximately 60% confluence. The cells were then transfected with 1 μL Genejammer (Stratagene) and 0.2 μg pcDNA3-TFPI plasmid containing the neomycin resistance gene. Colonies resistant to G418 (1 mg/mL, Gibco BRL) were selected as previously described,27 trypsinized, transferred to 96-well plates, and grown to confluence. Colonies were chosen based their TFPI activity and antigen levels (see below). The CHO colonies were selected for their expression of TFPI based on cell surface TFPI activity assay26 and Western blot analysis of secreted TFPI in the conditioned medium.17 Colonies with the highest levels of cell surface and secreted TFPI were selected for radioligand binding studies and compared to wild-type parental CHO cells that lacked TFPI.
Immunoblotting and ligand blotting
Both immunoblotting and ligand blotting were performed as previously described28 using primary antibody concentrations of 1 μg/mL. Either Lp(a) or rTFPI at varying concentrations were subjected to SDS-PAGE on gradient gels (4%-15% Tris-Glycine) under nonreducing conditions and electrophoretically transferred to nitrocellulose membranes. The rTFPI membranes were then ligand blotted with Lp(a) and the Lp(a) membranes ligand blotted with rTFPI. After several washes, bound Lp(a) was detected with a goat anti–human apo(a) antibody (Biodesign) and bound rTFPI detected with a rabbit anti–human TFPI antibody. Antigoat HRP (1:5000) and antirabbit HRP (1:1000), respectively, were used as secondary antibodies with chemiluminescence used for ligand-antibody complex detection. As a control in each case the immobilized protein was omitted from the protocol. Similarly, the existence of endogenous TFPI bound to Lp(a) sample following purification was excluded by running Lp(a) alone on a gel, transferring it to a membrane, and probing this blot with an anti-TFPI antibody.
Isolation of apo(a) and Lp(a−) [LDL portion of Lp(a)]
Apo(a) was isolated from Lp(a) by dithiothreitol (DTT) reduction as previously described.29 Gradient ultracentrifugation was then performed and 12 1-mL fractions selected. Each fraction was examined by dot-blot analysis for presence of apo(a) or LDL using specific goat and rabbit polyclonal primary antibodies each at 1:1000 dilution. Secondary HRP conjugates (1:10 000) and chemiluminescence were used for visualization of positive dot-blots.
Lp(a), Lp(a−), apo(a), and PLG binding studies
Recombinant TFPI (150 nM) or albumin (150 nM) was immobilized onto flat-bottomed polystyrene wells (Sarstedt, Newton, NC) as previously described.30 Immobilization was terminated by addition of phosphate-buffered saline plus Tween 0.1% (PBS-T), and washing with distilled water. Wells were then used immediately for binding assays. The binding procedures used were a modification of those described by Salonen and coworkers.30 Binding of Lp(a) or apo(a) to immobilized TFPI was performed by adding 100 μL of different concentrations of Lp(a), or apo(a) in 0.1 M NaCl, 0.0 5M Tris-HCl, pH 7.5, 0.02% (vol/vol) Tween 20 (Sigma Chemical, St Louis, MO). Incubation was performed at room temperature for 2 hours and terminated by repeated washings in PBS-T. Binding of Lp(a) and apo(a) to TFPI was determined using a polyclonal goat anti–apo(a) antibody (1 μg/mL) for 1 hour at room temperature. Binding of Lp(a−) to TFPI was determined using a polyclonal rabbit anti-LDL antibody (1 μg/mL) under similar conditions. Following several washes, Lp(a) and apo(a) wells were incubated with a HRP-conjugated anti-goat IgG (1:10 000) and Lp(a−) wells were incubated with a HRP-conjugated anti-rabbit IgG (1:5000). Both secondary antibodies had optimal dilutions established previously by dot-blotting. After further washes, duplicate wells were incubated with O-phenylenediamine dihydrochloride (OPD; Sigma) substrate for 5 minutes at room temperature, and read at an absorbance of 490 nm.
Because of the close homology between PLG and apo(a), similar binding experiments were performed substituting lysine-PLG (L-PLG; American Diagnostica) for recombinant apo(a). PLG-TFPI binding was performed in the presence and absence of ε-amino caproic acid (EACA, 0.2 M). Furthermore, inhibition of PLG binding to TFPI was examined using nanomolar concentrations of apo(a). In all experiments either the soluble or solid-phase proteins were replaced with buffer or bovine serum albumin (BSA) as a control. Furthermore when the specific primary antibody was replaced with similar concentrations of goat IgG no reaction occurred.
Assessment of apo(a) binding regions within rTFPI
To identify the region of TFPI involved in binding Lp(a), apo(a) binding was performed in the presence of identical nanomolar concentrations of immobilized full-length rTFPI and TFPI161, which lacks the K3 domain and carboxy-terminus. Similar binding of Lp(a) to full-length rTFPI and TFPI lacking the K3 domain and the intervening sequence between K2 and K3 (K1K2C) were also performed. Previous immobilization of equivalent concentrations of radiolabeled rTFPI and TFPI161 and K1K2C showed identical immobilization of all 3 forms of TFPI on polystyrene (data not shown).
Further experiments on apo(a) binding were performed after immobilization of a 37–amino acid peptide spanning the c-terminus region of TFPI (cTFPI37). To determine whether apo(a)-cTFPI37 binding was lysine dependent, experiments were performed in the presence and absence of excess EACA (0.2 M). Lp(a) binding to cTFPI37 and a scrambled form of that peptide, cTFPIs, was also performed to examine whether the specific TFPI c-terminus sequence was required for Lp(a) binding.
Radioligand binding of Lp(a) and PLG to CHO-TFPI cells
Lipoprotein (a) and L-PLG (1 μg of each) were radiolabeled in IODO-GEN tubes with 1 mCi (37 MBq) NaI125 as previously described.27 A TFPI-expressing CHO line was grown to 70% confluence on 96-well Lockwell plates (Nunc, Naperville, IL) and washed once in binding buffer (11 mM n-2-hydroxyethylpiperazine-n-2-ethansulfonic acid,137 mM NaCl, 4 mM KCl, 11 mM glucose pH 7.4 supplemented with 3 mM CaCl2, 1 mM MgCl2, and 5 mg/mL BSA). Cells were then incubated at 4°C for 30 minutes with increasing concentrations of both radiolabeled proteins diluted in binding buffer in the presence or absence of 200 mM EACA to identify nonspecific binding. Duplicate wells were analyzed in a series of at least 6 experiments for each protein. Specific binding was calculated by subtracting nonspecific binding from total binding. Total cell counts per well were generated by hemocytometer counts based on 4 wells seeded at the same density. Binding data were analyzed by the PRISM program from GraphPad Software (San Diego, CA) using a nonlinear regression curve for a one-site binding model. Specific activity equaled 2881.5 cpm/fmol. In separate experiments specific binding of Lp(a) (5 nM) was assessed in CHO-TFPI and parental CHO cells.
Surface TFPI and TF activity of Lp(a)-treated HUVECs
For cell surface TFPI and TF activity assays, HUVECs were grown on 96-well plates and then incubated with serum-free medium (100 μL) supplemented with varying concentrations (0, 1.6, 6.25, 12.5, 25, 50 nM) of Lp(a) in the presence or absence of 2 μM L-PLG for 1 hour at 37°C. Following removal of the medium the cells were washed twice in triethanolamine-buffered saline (TBS) buffer and assayed for cell surface TFPI or TF activity.
For cell surface TFPI activity assays, cells were incubated with 20 μL TBS buffer (Tris HCL 50 mM, NaCl 100mM, pH 7.5) containing 15 mM CaCl2. Twenty microliters of factor VIIa (50 ng/mL; American Diagnostica) and TF (6.6 ng/mL; American Diagnostica) were added to the cells and incubated at 37°C for 30 minutes followed by the addition of 20 μL factor X (10 μg/mL; American Diagnostica) for a further 15 minutes at 37°C. The reaction was stopped with 20 μL 0.2 M EDTA. Then, 20 μL Spectrozyme factor Xa chromogenic substrate (American Diagnostica) was added and release of p-nitroalanine (pNA) chromophore measured at an absorbance of 405 nm. TFPI activity was determined from a standard curve of known plate concentrations of recombinant TFPI, which were processed in a similar fashion to the cells.
Cell surface TF activity was measured using a modification of the TF Actichrome activity assay (American Diagnostica). Briefly 20 μL TBS containing 15 mM CaCl2 was added to TBS-washed HUVECs as before. Twenty microliters of factor VII (18 nM/L; American Diagnostica) was added and the plate incubated at 37°C for 10 minutes. Spectrozyme factor VIIa was then added and substrate cleavage measured spectrophotometrically. TF activity was determined from a standard curve of known plate concentrations of TF, which were processed in a similar fashion to the cells. This assay measures the peptidyl activity of TF and its ability to form a complex with factor VII. TF/VII complex becomes allosterically activated and TF activity is measured by the ability of this complex to cleave Spectrozyme factor VIIa, a highly specific chromogenic substrate for TF/VIIa complexes. In the case of each assay duplicate wells were examined in 3 separate experiments.
Effect of Lp(a), LDL, and L-PLG on fluid-phase rTFPI activity
Recombinant TFPI (50 ng/mL) was incubated in a glass tube in TBS buffer (Tris HCl 50 mM/L, NaCL 150 mM, pH 7.4) supplemented with 15 mM CaCl2 in the presence of increasing concentrations of Lp(a) in the presence or absence of physiologic concentrations of L-PLG (2 μM) for 30 minutes at 37°C. Aliquots of 20 μL were then transferred to an assay plate and TFPI activity measured using an Actichrome activity assay (American Diagnostica) as previously described.26 rTFPI activity was also assessed in the absence of Lp(a) or L-PLG.
In separate experiments rTFPI (50 ng/mL) was incubated in a glass tube in TBS/CaCL2 buffer for 30 minutes at 37°C in the presence or absence of LDL (total volume of 100 μL) and TFPI activity was again measured by Actichrome activity assay as described above.
Immunocytochemistry
Human atherosclerotic plaque specimens were obtained from 4 patients undergoing coronary atherectomy. Each specimen was freeze-embedded in OCT compound (Tissue-Tek, Sakura Finetek, Torrance, CA) in liquid nitrogen-cooled isopentane.17,31 Briefly, mouse anti–human actin, goat anti–apo(a) (1:250), rabbit anti–human TFPI (1:200), or mouse anti–human plasminogen (1:100) was incubated at room temperature for 1 hour. Secondary biotinylated antimouse (1:1000) antigoat (1:1000), and antirabbit (1:200) antibodies were incubated for 30 minutes at room temperature. Streptavidin-alkaline phosphatase (1:300) was incubated for 40 minutes at room temperature. A reaction product was then visualized with fast-red substrate. In each case a mouse IgG, goat IgG, or rabbit IgG was used as a negative control at a similar concentration to the matched antiactin, anti–apo(a), anti-TFPI, or anti-PLG antibody. Double immunolabeling was also performed as previously described.32
Statistical analysis
Unless otherwise stated, data are presented as the mean ± SEM. Dose comparisons between the groups treated with Lp(a), LDL, and L-PLG were made using one-way ANOVA. Comparisons between other groups were made using an unpaired Student t test (2-tailed). AP < .05 was considered statistically significant.
Results
Lp(a) and apo(a) but not Lp(a−) bind TFPI
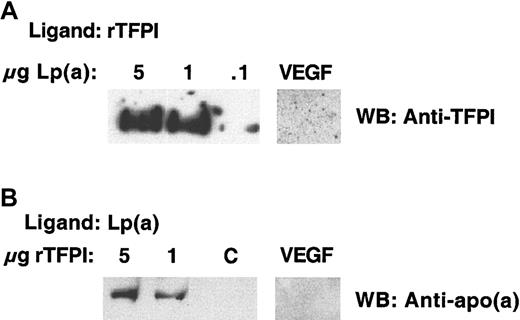
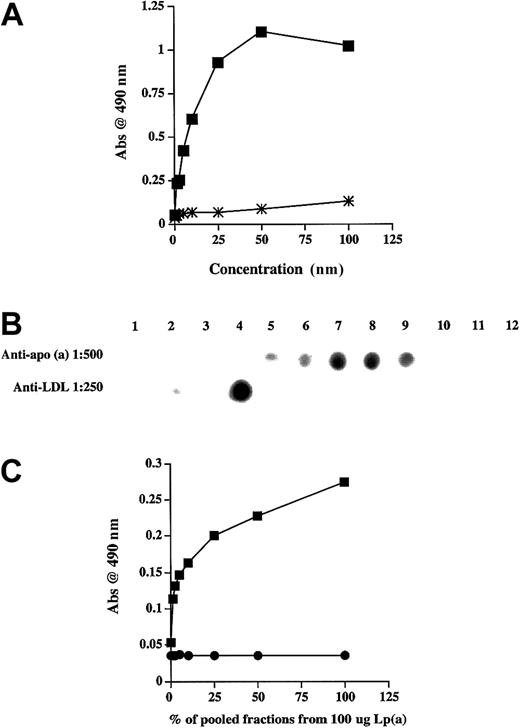
The possibility of a binding interaction between Lp(a) and TFPI was explored using ligand blot analysis and solid-phase binding studies. Experiments using Lp(a) immobilized on nitrocellulose and rTFPI as the ligand confirmed that TFPI bound Lp(a) (Figure1A). Conversely Lp(a) as ligand bound rTFPI immobilized on nitrocellulose (Figure 1B). These data supported a binding interaction between Lp(a) and rTFPI. Nonspecific binding was excluded by failure of either L(a) or TFPI to bind an irrelevant immobilized protein vascular endothelial growth factor (VEGF).
Ligand blot analysis showing rTFPI ligand binds to immobilized Lp(a) and Lp(a) ligand binds to immobilized rTFPI.
(A) Purified Lp(a) at varying concentrations was subjected to nonreducing SDS-PAGE and transferred to nitrocellulose. Membranes were then incubated overnight at 4°C with rTFPI ligand. After washing, bound rTFPI ligand was identified using a primary anti-TFPI antibody and secondary antirabbit-HRP conjugate. The antigen-antibody complex was visualized using chemiluminescence. An irrelevant protein VEGF was immobilized on a separate membrane under similar conditions to Lp(a) and the membrane ligand blotted with rTFPI ligand as above. (B) rTFPI at varying concentrations electrophoresed and transferred to nitrocellulose under similar nonreducing conditions and subjected to ligand blotting with Lp(a) overnight at 4°C. Bound Lp(a) ligand was identified with primary anti-apo(a) and secondary antigoat antibodies, respectively. The antigen-antibody complex was visualized using chemiluminescence. An irrelevant protein VEGF was immobilized under similar conditions to TFPI and the membrane ligand blotted with Lp(a) as described above. C refers to a control lane where the rTFPI was omitted.
Ligand blot analysis showing rTFPI ligand binds to immobilized Lp(a) and Lp(a) ligand binds to immobilized rTFPI.
(A) Purified Lp(a) at varying concentrations was subjected to nonreducing SDS-PAGE and transferred to nitrocellulose. Membranes were then incubated overnight at 4°C with rTFPI ligand. After washing, bound rTFPI ligand was identified using a primary anti-TFPI antibody and secondary antirabbit-HRP conjugate. The antigen-antibody complex was visualized using chemiluminescence. An irrelevant protein VEGF was immobilized on a separate membrane under similar conditions to Lp(a) and the membrane ligand blotted with rTFPI ligand as above. (B) rTFPI at varying concentrations electrophoresed and transferred to nitrocellulose under similar nonreducing conditions and subjected to ligand blotting with Lp(a) overnight at 4°C. Bound Lp(a) ligand was identified with primary anti-apo(a) and secondary antigoat antibodies, respectively. The antigen-antibody complex was visualized using chemiluminescence. An irrelevant protein VEGF was immobilized under similar conditions to TFPI and the membrane ligand blotted with Lp(a) as described above. C refers to a control lane where the rTFPI was omitted.
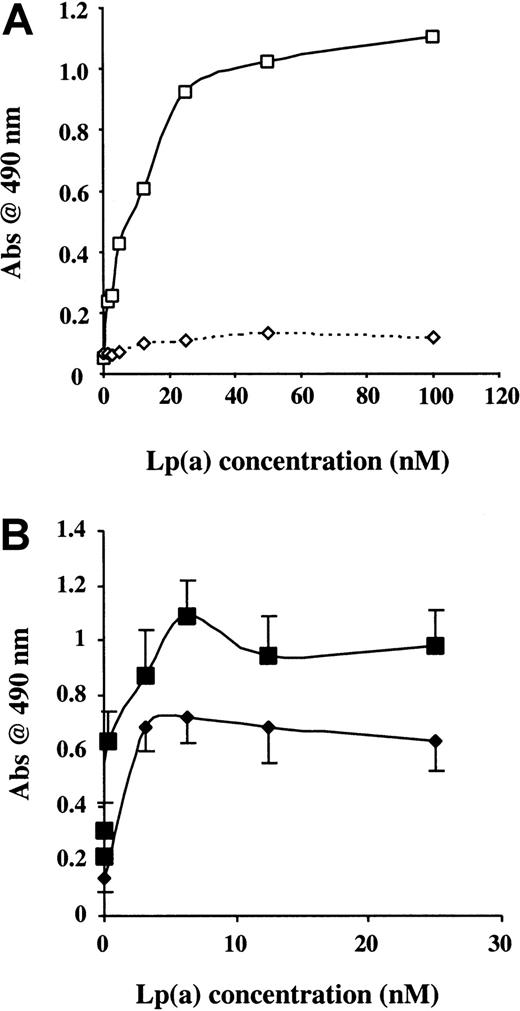
To examine further this in vitro binding between Lp(a) and TFPI, albumin and rTFPI were immobilized separately on polystyrene and incubated with purified Lp(a) at increasing concentrations. Lp(a) bound to immobilized TFPI but not to immobilized albumin in a concentration-dependent and saturable manner (Figure2A). Lp(a−) isolated by DTT reduction of Lp(a) and gradient ultracentrifugation did not bind to immobilized rTFPI, suggesting that binding was not due to the LDL component of Lp(a) (Figure 2B,C). Apo(a) isolated in the same gradient ultracentrifugation run bound to rTFPI in a similar concentration-dependent manner as Lp(a) (Figure 2B,C).
The apo(a) portion of Lp(a) binds TFPI.
(A) Binding of Lp(a) to rTFPI (▪) and albumin (∗) immobilized on polystyrene wells. (B) Representative dot-blot analysis of Lp(a) ultracentrifugation fractions following DTT reduction, showing Lp(a−) in fraction 4 and apo(a) in fractions 6 to 9. (C) TFPI binding of apo(a) (▪) and Lp(a−) (●) fractions obtained from 100 μg Lp(a) by DTT reduction and gradient ultracentrifugation. Data presented are the mean values from 3 separate experiments.
The apo(a) portion of Lp(a) binds TFPI.
(A) Binding of Lp(a) to rTFPI (▪) and albumin (∗) immobilized on polystyrene wells. (B) Representative dot-blot analysis of Lp(a) ultracentrifugation fractions following DTT reduction, showing Lp(a−) in fraction 4 and apo(a) in fractions 6 to 9. (C) TFPI binding of apo(a) (▪) and Lp(a−) (●) fractions obtained from 100 μg Lp(a) by DTT reduction and gradient ultracentrifugation. Data presented are the mean values from 3 separate experiments.
PLG and apo(a) binding to TFPI
Because apo(a) shares significant homology with PLG, the ability of L-PLG to bind rTFPI was also examined. L-PLG bound rTFPI in a saturable and dose-dependent manner and this binding was significantly inhibited by EACA (Figure 3A). L-PLG–TFPI binding could also be inhibited completely by apo(a) at a nanomolar concentration (500 nM; Figure 3B). Further experiments were performed to exclude any cross-reactivity between the anti-po(a) and anti-PLG antibodies. Western blotting confirmed complete lack of cross-reactivity between these antibodies (Figure 3C).
PLG binds TFPI and apo(a) inhibits this binding.
(A) Binding of varying concentrations of L-PLG to rTFPI in the presence (⋄) or absence (■) of EACA (0.2 M). (B) Binding of varying concentrations of L-PLG to rTFPI in the presence (♦) or absence (■) of apo(a) (500 nM). (C) Western blot showing lack of cross-reactivity between anti-PLG and anti-apo(a) antibodies. In panels A and B data presented are the mean values from 3 separate experiments.
PLG binds TFPI and apo(a) inhibits this binding.
(A) Binding of varying concentrations of L-PLG to rTFPI in the presence (⋄) or absence (■) of EACA (0.2 M). (B) Binding of varying concentrations of L-PLG to rTFPI in the presence (♦) or absence (■) of apo(a) (500 nM). (C) Western blot showing lack of cross-reactivity between anti-PLG and anti-apo(a) antibodies. In panels A and B data presented are the mean values from 3 separate experiments.
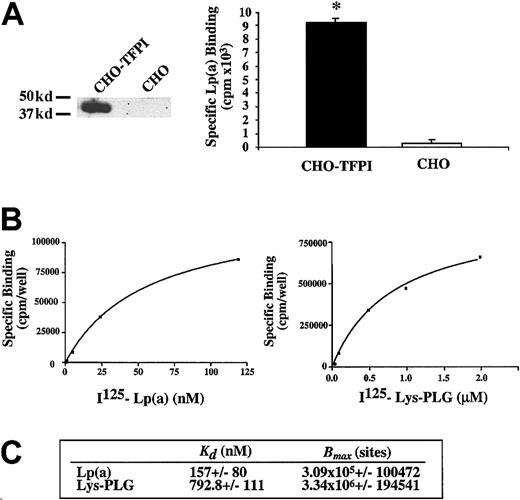
Binding affinities of Lp(a) and L-PLG for cell-associated TFPI
To determine the relative binding affinities and relevance of Lp(a) and PLG binding to TFPI in a cellular environment, a CHO cell line was stably transfected with pcDNA3-TFPI plasmid so that TFPI was expressed on the surface of these cells. Expression of full-length biologically active TFPI on stably transfected CHO cells was confirmed by Western blot analysis (Figure 4A) and cell surface TFPI activity (0.09 ± 0.15 U/well), whereas TFPI was undetectable in the parental CHO cell line. Moreover specific binding of Lp(a) (5 nM) to CHO TFPI cells was significantly greater (> 25-fold) than the negligible Lp(a) binding seen in nontransfected parental CHO cells (Figure 4A; P < .01). Radioligand binding of 125I-labeled Lp(a) and L-PLG was then performed on CHO-TFPI cells. Both Lp(a) and L-PLG bound in a dose-dependent and saturable manner to CHO-TFPI cells with dissociation constants of 157 ± 80 nM and 793 ± 111 nM, respectively (Figure 4B,C). The relative binding capacity (Bmax) for both ligands is shown in Figure 4C.
Radiolabeled Lp(a) binds with higher affinity than L-PLG to CHO cell surface TFPI.
(A) Left panel shows Western blot evidence of TFPI protein expression in CHO cells stably transfected with pcDNA3-TFPI plasmid compared to absent TFPI expression in parental nontransfected CHO cell line. Right panel shows specific binding of Lp(a) (5 nM) to CHO-TFPI cells compared to negligible binding of Lp(a) to a parental nontransfected CHO cell line. (B) Representative radioligand binding curves for Lp(a) and L-PLG to cell surface TFPI expressed on CHO-TFPI cells. (C) Apparent binding affinities (KD) and binding capacities (Bmax) of CHO cell surface TFPI for Lp(a) and L-PLG. Data presented are the means of at least 4 separate experiments performed in duplicate and the asterisk indicates P < .01.
Radiolabeled Lp(a) binds with higher affinity than L-PLG to CHO cell surface TFPI.
(A) Left panel shows Western blot evidence of TFPI protein expression in CHO cells stably transfected with pcDNA3-TFPI plasmid compared to absent TFPI expression in parental nontransfected CHO cell line. Right panel shows specific binding of Lp(a) (5 nM) to CHO-TFPI cells compared to negligible binding of Lp(a) to a parental nontransfected CHO cell line. (B) Representative radioligand binding curves for Lp(a) and L-PLG to cell surface TFPI expressed on CHO-TFPI cells. (C) Apparent binding affinities (KD) and binding capacities (Bmax) of CHO cell surface TFPI for Lp(a) and L-PLG. Data presented are the means of at least 4 separate experiments performed in duplicate and the asterisk indicates P < .01.
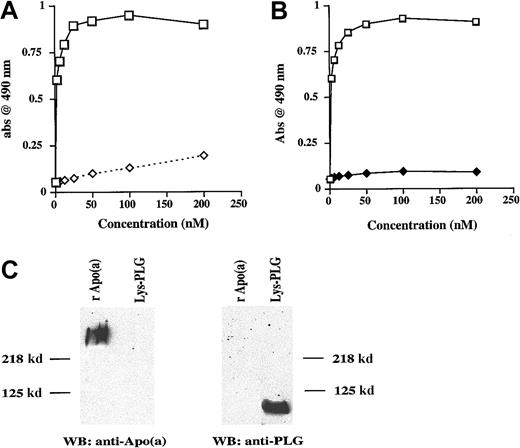
Lp(a) binds the c-terminus region of TFPI
To determine what region of TFPI was involved in Lp(a) and apo(a) binding, a series of experiments were performed with full-length and mutated forms of TFPI. Lp(a) bound to full-length immobilized rTFPI in a concentration-dependent manner (Figure5A), whereas no Lp(a) dose-dependent binding was seen when TFPI161 lacking the K3 domain and c-terminus was immobilized instead of rTFPI (Figure 5A). A similar lack of binding to TFPI161 was seen for apo(a) (data not shown). These data suggested that either the K3 domain or c-terminus of TFPI may be involved in Lp(a) and apo(a) binding. Further experiments were performed to examine whether deletion of the K3 domain had any effect on Lp(a) binding to TFPI. There was no significant difference in Lp(a) binding to rTFPI and to a mutant form of TFPI lacking the K3 domain and the intervening sequence between K2 and K3 (K1K2C) (Figure 5B). These data suggested that the c-terminus may be more important than the K3 domain for Lp(a) binding.
Lp(a) binds full-length but not truncated TFPI161.
(A) Binding of varying concentrations of Lp(a) to immobilized rTFPI (■) and to immobilized TFPI161 (⋄). Data presented are the mean values from 3 separate experiments. (B) Binding of varying concentrations of Lp(a) to immobilized rTFPI (▪) and to immobilized TFPI-K1K2C (♦). Data presented are the means values from 4 separate experiments.
Lp(a) binds full-length but not truncated TFPI161.
(A) Binding of varying concentrations of Lp(a) to immobilized rTFPI (■) and to immobilized TFPI161 (⋄). Data presented are the mean values from 3 separate experiments. (B) Binding of varying concentrations of Lp(a) to immobilized rTFPI (▪) and to immobilized TFPI-K1K2C (♦). Data presented are the means values from 4 separate experiments.
To identify whether the c-terminus of TFPI alone could bind recombinant apo(a), a peptide of 37 amino acid residues representing the entire c-terminal portion of TFPI (cTFPI37) was synthesized and then immobilized at the same nanomolar concentration to rTFPI. Binding of recombinant apo(a) to cTFPI37 occurred in a similar concentration-dependent and saturable manner as previously seen with rTFPI (Figure 6A). Apo(a) binding to cTFPI37 was significantly inhibited by preincubation with EACA (P < .05; Figure 6A). Furthermore the inhibitory concentration of 50% (IC50) for EACA inhibition of apo(a) binding to rTFPI was 3 mM (Figure 6B), similar to that observed for apo(a) binding proteins in other studies.33 To determine whether a specific amino acid sequence in the 37-mer peptide was responsible for Lp(a) binding, a comparison was made between cTFPI37 and a peptide with a scrambled sequence containing the same 37 amino acid residues (cTFPIs). No difference in the Lp(a) binding was seen between the standard and scrambled TFPI c-terminal sequence (Figure 6C).
Apo(a) binds the c-terminus of TFPI and apo(a) binding is inhibited by EACA (0.2 M).
(A) Binding of recombinant apo(a) to cTFPI37 in the presence (⋄) or absence of EACA (■). (B) Dose-dependent inhibition of apo(a) binding to rTFPI with EACA. (C) Binding of varying concentrations of Lp(a) to cTFPI37 (▪]) or cTFPIs (⋄). Data presented are the means values of 3 separate experiments.
Apo(a) binds the c-terminus of TFPI and apo(a) binding is inhibited by EACA (0.2 M).
(A) Binding of recombinant apo(a) to cTFPI37 in the presence (⋄) or absence of EACA (■). (B) Dose-dependent inhibition of apo(a) binding to rTFPI with EACA. (C) Binding of varying concentrations of Lp(a) to cTFPI37 (▪]) or cTFPIs (⋄). Data presented are the means values of 3 separate experiments.
Effects of Lp(a), L-PLG, and LDL on fluid-phase TFPI activity
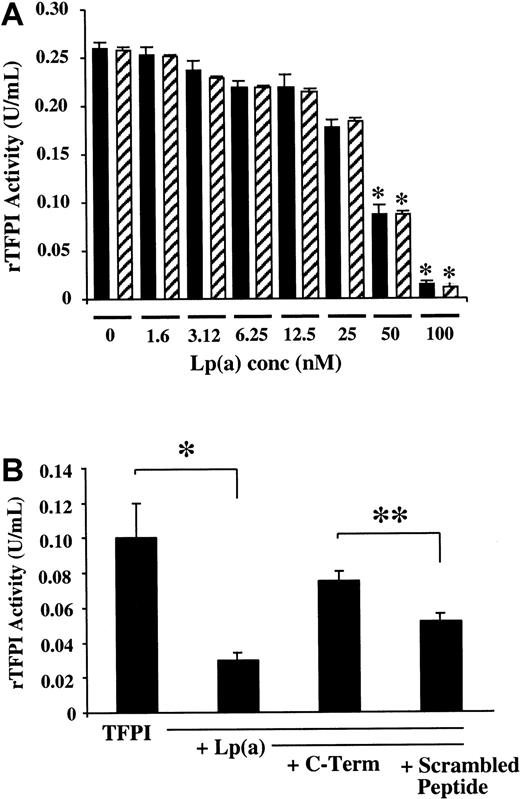
To examine whether coincubation with Lp(a) had any effect on the biologic activity of rTFPI, a series of experiments were performed. The dose-dependent inhibitory effects of Lp(a) on rTFPI activity in the presence or absence of physiologic concentrations of L-PLG (2 μM) was initially assessed. Lp(a) caused a dose-dependent inhibition of TFPI activity in the presence or absence of physiologic concentrations of L-PLG (Figure 7;P < .01). Furthermore PLG at a similar physiologic concentration (2 μM) in the absence of Lp(a) had no effect on fluid-phase rTFPI activity (Figure 7A). Similar experiments using the same incubation conditions (time and temperature) showed that LDL had no effect on the rTFPI activity in vitro (data not shown).
Lp(a) decreases rTFPI activity in fluid phase.
(A) Lp(a) decreases rTFPI activity in a dose-dependent manner in the presence (shaded bars) or absence of 2 μM L-PLG (filled bars) following coincubation for 30 minutes at 37°C. (B) Normal sequence cTFPI37 (c-term) attenuates Lp(a) inhibition of rTFPI activity to a significantly greater extent than cTFPIs (scrambled peptide). Data presented are the means from at least 3 separate experiments; * indicates P < .01; ** indicatesP < .05.
Lp(a) decreases rTFPI activity in fluid phase.
(A) Lp(a) decreases rTFPI activity in a dose-dependent manner in the presence (shaded bars) or absence of 2 μM L-PLG (filled bars) following coincubation for 30 minutes at 37°C. (B) Normal sequence cTFPI37 (c-term) attenuates Lp(a) inhibition of rTFPI activity to a significantly greater extent than cTFPIs (scrambled peptide). Data presented are the means from at least 3 separate experiments; * indicates P < .01; ** indicatesP < .05.
To further explore whether sequence specificity of the c-terminus of TFPI was important for the functional interaction with Lp(a), a series of fluid-phase incubations of rTFPI and Lp(a) were performed in the presence and absence of 100 molar excess concentrations of cTFPI37 or cTFPIs. The normal sequence cTFPI37attenuated the functional inhibitory effects of Lp(a) on rTFPI activity to a significantly greater extent than equimolar concentrations of the scrambled peptide cTFPIs (Figure 7B; P < .05).
Effects of Lp(a), L-PLG, and LDL on cell surface TFPI activity
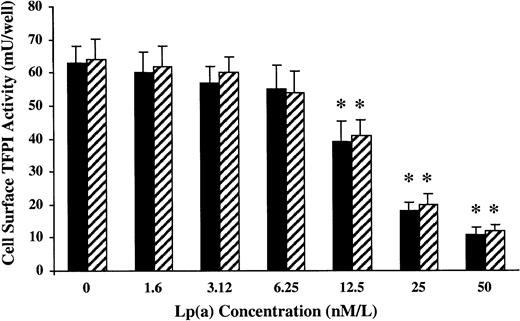
To examine whether Lp(a) had any effects on cell-associated TFPI activity, a series of concentration response experiments were performed using HUVECs in culture. Cell surface TFPI activity was measured following a 1-hour incubation with varying concentrations of Lp(a) in the presence or absence of L-PLG (2 μM). Lp(a) induced a concentration-dependent decrease in cell surface TFPI activity with an IC50 of about 20 nM in the presence and absence of physiologic concentrations of L-PLG (Figure8; P < .01). Neither LDL nor L-PLG alone across a similar concentration range had any significant effect on cell surface TFPI activity (data not shown).
Cell surface TFPI activity.
Lp(a) decreases endothelial cell surface TFPI activity in a dose-dependent manner in vitro in the presence (shade bars) or absence (filled bars) of L-PLG (2 μM). TFPI activity on human endothelial cells was measured following incubation for 1 hour at 37°C in the presence of varying concentrations of Lp(a). Following incubation the cells were washed with TBS buffer and cell surface TFPI activity assessed. All data presented are the means of 3 separate experiments and the asterisk indicates P < .01.
Cell surface TFPI activity.
Lp(a) decreases endothelial cell surface TFPI activity in a dose-dependent manner in vitro in the presence (shade bars) or absence (filled bars) of L-PLG (2 μM). TFPI activity on human endothelial cells was measured following incubation for 1 hour at 37°C in the presence of varying concentrations of Lp(a). Following incubation the cells were washed with TBS buffer and cell surface TFPI activity assessed. All data presented are the means of 3 separate experiments and the asterisk indicates P < .01.
Apo(a) and TFPI immunostaining in coronary plaque
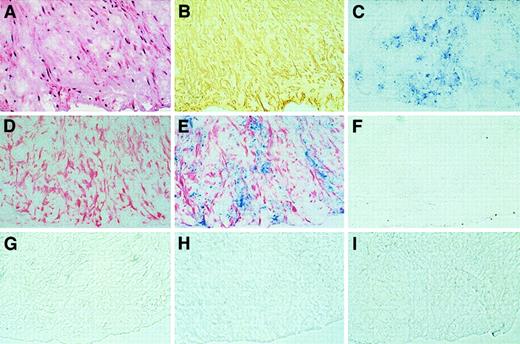
Coronary atherectomy specimens (n = 4) consisted of portions of intima and a smaller portion of media in each case. TFPI staining localized predominantly to VSMCs within the plaque (Figure9A,B,D). Apo(a) staining was distributed in a spotlike pattern predominantly in the intima immediately subjacent to the lumen of the plaque (Figure 9C). Double immunolabeling showed TFPI and apo(a) immunostaining in SMC-rich areas of the intima (Figure9E). No immunostaining in the plaques was noted with use of a monoclonal antibody to human PLG (Figure 9F). Isotyped-matched mouse, rabbit, and goat IgG control antibodies showed no background staining (Figure 9G,H).
TFPI and apo(a) immunostaining coexist in human coronary atherosclerotic plaque.
(A) Photomicrograph showing hematoxylin and eosin staining of human fibrocellular plaque obtained by coronary atherectomy (objective × 20). (B) Adjacent section showing SMC α-actin staining (peroxidase with DAB substrate, objective × 20). (C) Adjacent section showing apo(a) immunostaining (alkaline phosphatase with vector blue, objective × 20). (D) Adjacent section showing TFPI immunostaining (alkaline phosphatase with fast-red substrate, objective × 20). (E) Adjacent section showing double immunostaining of TFPI (red) and apo(a) (blue) (objective × 20). (F) Adjacent section showing lack of staining by mouse anti-PLG antibody (objective × 20). (G-I) Adjacent sections showing lack of staining by isotype-matched mouse, rabbit, and goat IgG control antibodies (objective × 20).
TFPI and apo(a) immunostaining coexist in human coronary atherosclerotic plaque.
(A) Photomicrograph showing hematoxylin and eosin staining of human fibrocellular plaque obtained by coronary atherectomy (objective × 20). (B) Adjacent section showing SMC α-actin staining (peroxidase with DAB substrate, objective × 20). (C) Adjacent section showing apo(a) immunostaining (alkaline phosphatase with vector blue, objective × 20). (D) Adjacent section showing TFPI immunostaining (alkaline phosphatase with fast-red substrate, objective × 20). (E) Adjacent section showing double immunostaining of TFPI (red) and apo(a) (blue) (objective × 20). (F) Adjacent section showing lack of staining by mouse anti-PLG antibody (objective × 20). (G-I) Adjacent sections showing lack of staining by isotype-matched mouse, rabbit, and goat IgG control antibodies (objective × 20).
Discussion
Lipoprotein (a) has been shown to be an independent risk factor for coronary disease in several studies,34,35 and its homology and putative competition with PLG in vivo has been suggested as a mechanism whereby this lipoprotein complex inhibits fibrinolysis. However, inhibition of fibrinolysis may not be the only mechanism whereby elevated Lp(a) levels promote acute thrombotic events.35 36 The current study is to our knowledge the first to explore the potential for direct prothrombotic effects of Lp(a) mediated through inactivation of TFPI, a central regulator of TF-mediated thrombosis.
We establish in this study several lines of evidence that Lp(a) binds and inactivates rTFPI and cell-associated TFPI in vitro. Using different binding assays, we showed Lp(a) and L-PLG bound rTFPI protein in vitro. Lp(a) also bound TFPI overexpressed on the surface of CHO cells with a much higher binding affinity than L-PLG. Binding of PLG to TFPI was also inhibited by nanomolar concentrations of apo(a). The physiologic relevance of Lp(a)-TFPI interaction was highlighted by concurrent dose-dependent inactivation of TFPI activity by Lp(a) in vitro and on endothelial cell surfaces. PLG alone had no such effect on TFPI activity and physiologic concentrations of L-PLG had no effect on the ability of Lp(a) to inhibit TFPI activity either in fluid phase or on the endothelial cell surface. These data suggest that under conditions where Lp(a) and PLG coexist, Lp(a) is likely to have a significant inhibitory effect on TFPI function, which is not altered by PLG. Finally we show that Lp(a), via its apo(a) moiety, may bind the c-terminal region of TFPI and present evidence for the coexistence of both apo(a) and TFPI in similar regions of human atherosclerotic plaque. Taken together, these data suggest a biologic interaction between Lp(a) and TFPI, which may have implications for TFPI activity and hence thrombotic events.
In this study, Lp(a) but not Lp(a−) saturably bound to TFPI in solid-phase conditions. Recombinant apo(a) bound TFPI in a similar concentration-dependent saturable manner. These data are consistent with the hypothesis that the apo(a) portion of Lp(a) is most likely involved in TFPI binding. Further data showing that apo(a) and Lp(a) bind the K1K2C (lacking the K3 domain and the intervening sequence between K2 and K3) and a synthesized 37-mer c-terminus of TFPI but not TFPI161 (lacking K3 and c-terminus) would support the concept that the c-terminus of TFPI is important for Lp(a) binding. Although similar binding of Lp(a) to a normal and scrambled sequence c-terminal TFPI was seen in solid-phase assays, the normal sequence TFPI peptide attenuated Lp(a) inhibitory effects on TFPI to a significantly greater extent than the scrambled peptide in fluid phase. The difference observed between solid- and fluid-phase assays may reflect different TFPI binding site access for Lp(a) under these 2 experimental conditions. However, we cannot make definitive conclusions from these data and the possibility of conformational changes in other domains of the mutated TFPI proteins accounting for differences in Lp(a) binding cannot be excluded.
An important aspect of Lp(a) biology is thought to be determined by its interaction with PLG with which apo(a) shares significant homology. In this study L-PLG bound to immobilized TFPI in a solid-phase assay and was inhibited by EACA suggesting lysine-dependent binding. However, L-PLG binding to TFPI was almost completely inhibited by nanomolar concentrations of apo(a). Radiolabeled L-PLG also bound TFPI with much lower affinity than Lp(a). Together these data suggest a limited role for the PLG-TFPI binding in the presence of elevated concentrations of Lp(a).
To explore the biologic significance of the interaction between Lp(a), PLG, and TFPI, a series of TFPI activity assays were performed in the presence of one or both ligands. Lp(a) caused a concentration-dependent decrease in rTFPI activity in vitro with an IC50 in the 20- to 30-nM range. This IC50 value was lower than the binding dissociation constant of Lp(a) for TFPI (150 nM) and may be due to the multiple washes used in binding studies causing underestimation of Lp(a) binding affinity. Plasminogen at a much higher physiologic concentration (2 μM) had no such effect on rTFPI activity. Furthermore, the coexistence of a similar micromolar concentration of PLG (2 μM) had no effect on the concentration-dependent inhibition of rTFPI activity induced by Lp(a) [similar IC50 to Lp(a) alone]. These data are consistent with the results from earlier binding experiments and suggest that Lp(a) but not PLG is a potent inhibitor of rTFPI activity in vitro. This is further supported by the potent inhibitory effects of Lp(a) on endothelial cell surface TFPI activity in the presence or absence of PLG and lack of any inhibitory effect using similar concentrations of LDL or PLG alone.
Numerous mechanisms can be speculated to explain the loss of TFPI activity following interaction with Lp(a) or apo(a) in vitro. We have shown in this study that the apo(a) portion but not the LDL portion of Lp(a) binds rTFPI and that recombinant apo(a) binds the c-terminus of TFPI, a region known to determine TFPI function.37-39 The mere binding of apo(a) to the c-terminus of TFPI may be sufficient to alter stoichiometric relationships between protease substrates such as factor VIIa and Xa and their inhibitor TFPI. Furthermore, binding of Lp(a) to the c-terminus of TFPI may reduce (by steric inhibition) access of these protease substrates to either the K1 or K2 domains of TFPI. The reduction in endothelial cell surface TFPI activity by Lp(a) but not by LDL or plasminogen again supports an interaction between the apo(a) portion of Lp(a) and TFPI. The mechanism of cell surface TFPI inactivation may again involve the stoichiometric interactions outlined above. Alternatively we speculate that reduced cell surface TFPI activity may involve receptor-mediated internalization and degradation of TFPI by Lp(a). In this regard it is interesting to note that the very low-density lipoprotein receptor on endothelial cells, which facilitates Lp(a) internalization,40 also interacts with and internalizes TFPI.41
In this study, we showed that the c-terminus region of TFPI bound the apo(a) moiety of Lp(a) in a lysine-dependent manner. It is possible that a series of lysine residues on the c-terminus of TFPI may bind the kringle IV–like lysine-binding domains of apo(a).38,42TFPI therefore may share a characteristic with other binding proteins possessing a series of carboxy-terminal lysine residues.43,44 Apo(a)-lysine binding has previously been well described for a range of extracellular matrix proteins including fibrin45 and fibronectin,30 and it is conceivable the extracellular matrix– or cell surface–associated TFPI may bind apo(a) in a similar manner.
The potential sites for interaction between Lp(a) and TFPI in vivo may include the bloodstream where TFPI activity has already been described in Lp(a)-rich lipoprotein fractions46 and the vessel wall. Finally in this study we show that apo(a) and TFPI immunostaining coexist in SMC-rich areas within the subendothelial intima of plaque, suggesting the potential for in vivo interaction in the vessel wall.
We conclude that Lp(a) binds and inactivates TFPI in vitro in a cellular and noncellular environment. Lp(a) binds TFPI with much higher affinity than PLG with which it shares homology and inactivates TFPI activity in the presence or absence of physiologic concentrations of PLG. Based on these data we propose that Lp(a) elevation may have prothrombotic implications for TFPI biology. This prothrombotic potential of Lp(a) will need to be examined in future in vivo studies.
We gratefully acknowledge Paul Stalboerger for his technical assistance, Andre Terzic for his valuable suggestions, and Traci Paulson for preparation of the manuscript.
Supported in part by grants from the Bruce and Ruth Rappaport Program in Vascular Biology at the Mayo Clinic, the National Institutes of Health (HL-03473), the Miami Heart Institute, and the Minnesota Affiliate of the American Heart Association. Portions of this work were presented at the 71st Scientific Sessions of the American Heart Association, Orlando,FL, November 1998.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Noel M. Caplice, Mayo Clinic, GU 1801, 200 First St SW, Rochester, MN 55905; e-mail: caplice.noel@mayo.edu.

![Fig. 6. Apo(a) binds the c-terminus of TFPI and apo(a) binding is inhibited by EACA (0.2 M). / (A) Binding of recombinant apo(a) to cTFPI37 in the presence (⋄) or absence of EACA (■). (B) Dose-dependent inhibition of apo(a) binding to rTFPI with EACA. (C) Binding of varying concentrations of Lp(a) to cTFPI37 (▪]) or cTFPIs (⋄). Data presented are the means values of 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/10/10.1182_blood.v98.10.2980/5/m_h82211756006.jpeg?Expires=1769342629&Signature=ZFOoFKNUIeGEZj-zojfm5kXvhTKWFX7KwV5Rzf48znXgAljrpwKLF-bAMGxUBS0hnbBmzVKR7ypWCodRFRnNqK6qpYcjBIMc1sPqTLIlqJNu93CGN2W-GcmOJB7AXQdgX~I6rtoT-q1VgU18FweYmROyKklx8aBjXBx-eP5dVMyhDac7eOMlrdEBl6rT8gWNGvQDx3tVLjytoRCvw28Jn~9N9maXCnZGWUVc8R3Yx303OOTbwSzJU~goB-hR8NQnGLL-MZzU31A9CKBTHzHyfyzJpVhGCp0OEtE0VrJwhxzy9wVakwUFmTNYcImmp-QhN0nUh2EIMkSNukFaTI2Fiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)