Abstract
Kaposi sarcoma–associated herpesvirus (KSHV)–related multicentric Castleman disease (MCD) is potentially lethal. Growing evidence indicates that, as in Epstein-Barr virus–driven lymphoproliferative disorders after transplantation, KSHV DNA burden in peripheral blood mononuclear cells (PBMCs) may represent the most accurate marker of disease activity. This report describes a patient with human immunodeficiency virus who was followed up clinically and by quantitative polymerase chain reaction for KSHV DNA sequences in PBMCs for more than 3 years following the diagnosis of KSHV-related MCD. Therapy with the antiherpesvirus agent cidofovir, antihuman interleukin-6 antibody BE-8, antiblastic chemotherapy, and combination antiretroviral agents did not achieve durable clinical or virologic remission of the disease. By contrast, administration of the anti-CD20 monoclonal antibody rituximab was well tolerated and allowed a 14-month remission of clinical symptoms and KSHV viremia. Rituximab should be added to the therapeutic armamentarium for KSHV-related MCD.
Introduction
Kaposi sarcoma–associated herpesvirus (KSHV) is implicated in the pathogenesis of 2 rare lymphoproliferative disorders, primary effusion lymphoma, and a subset of multicentric Castleman disease (MCD).1 The concomitant presence of human immunodeficiency virus (HIV) infection does not alter the clinical picture of KSHV-related MCD, which is characterized by a rapidly progressive and often fatal course.2 Kaposi sarcoma (KS) may precede, coexist with, or follow the diagnosis of MCD, and the development of aggressive non-Hodgkin lymphoma is not a rare outcome in patients with this disorder.3
KSHV is a lymphotropic γ2-herpesvirus homologous to the Epstein-Barr virus (EBV) that may be present in the CD19+peripheral blood mononuclear cells (PBMCs) of patients with KS.4 Lymph nodes of patients with KSHV-related MCD specifically harbor the virus in B cells located in the mantle zone,2,5 which stain positively for the CD20 surface antigen.6 The humanized anti-CD20 antibody Mabthera (rituximab) is increasingly used for the treatment of EBV-driven lymphoproliferative disorders after transplantation. In this context, single-dose administration of rituximab induces complete clinical remission of the disease.7 We treated an HIV-positive patient with KSHV-related MCD with a single dose of anti-CD20 antibody. This resulted in a 14-month remission of the disorder.
Study design
PBMCs were isolated by Ficoll-Hypaque density gradient centrifugation (Pharmacia, Uppsala, Sweden). DNA was extracted from PBMCs after digestion in a proteinase K–containing buffer by the phenol-chloroform method.8 One microgram PBMC DNA was subjected to polymerase chain reaction (PCR) using KSHV and human β-globin–specific primers.9 Quantitation of viral and human genomes was carried out using the limiting-dilution technique.10 Twenty replicates for each target were made around the end-point dilution using single-copy, sensitivity-nested PCR assays.11 Viral DNA load in PBMCs was expressed as the absolute number of viral genomes in 150 000 human diploid cells. PBMC samples that tested negative by PCR were considered to have less than 1 viral genome per 150 000 cells. Immunohistochemical demonstration of the KSHV protein viral interleukin-6 (v–IL-6) was performed using specific antisera, as previously described. 2,5
Case report
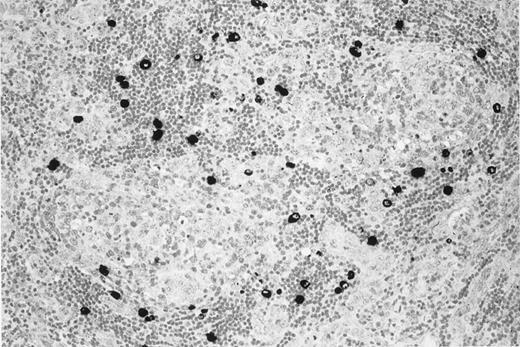
On November 26, 1997, a 52-year-old woman with HIV-1 infection came to the emergency department because of a 2-week history of progressive fatigue, high-grade fever (39°C), profuse sweating, and dyspnea. She had acquired HIV infection 4 years earlier through sexual contact. The absolute number of CD4+ T cells was 383 cells/μL, and HIV-1 viral load in plasma was less than 500 copies/mL. She was receiving antiretroviral therapy with d4T and 3TC. Generalized lymphadenopathy and splenomegaly were evident on physical examination. Chest radiography findings were normal. Her hemoglobin concentration was 7 g/dL. C-reactive protein (CRP) concentration was 4.8 mg/dL, and lactic dehydrogenase (LDH) level was 893 IU/L. She had hemolytic anemia, positive Coombs test findings, and evidence of cryoagglutinins. A latero-cervical lymph node was excised. Microscopic examination and immunohistochemistry for KSHV v–IL-6 revealed KSHV-related MCD (Figure1).
KSHV v–IL-6 expression in a lymph node of the patient with multicentric Castleman disease.
MCD lymph node section showing KSHV v–IL-6 expression (black) restricted to lymphocytes in the mantle zone of lymphoid follicles.
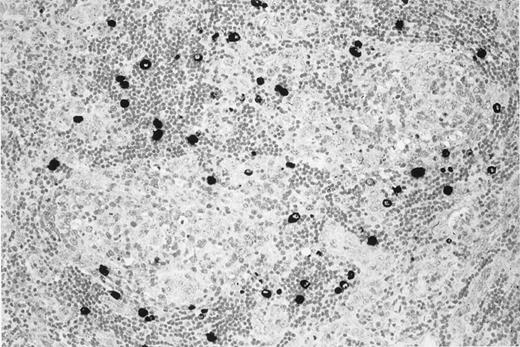
KSHV v–IL-6 expression in a lymph node of the patient with multicentric Castleman disease.
MCD lymph node section showing KSHV v–IL-6 expression (black) restricted to lymphocytes in the mantle zone of lymphoid follicles.
Treatment
With the approval of the Institutional Review Board and the informed consent of the patient, cidofovir was administered intravenously for 18 weeks beginning on December 17, 1997. Murine antihuman IL-6 monoclonal antibody BE-8 was administered to the patient as described by Beck et al12 for 12 weeks beginning the next day, December 18. Fever and CRP levels dramatically abated within 48 hours of BE-8 administration. However, in the next 12 days hemolytic anemia, lymphadenopathy, and splenomegaly were not affected by combined antiherpesvirus and antihuman IL-6 therapy.
On December 30, the patient's hemoglobin concentration was 3.9 g/dL. That day she underwent chemotherapy with doxorubicin (20 mg/m2), vincristine (2 mg), and bleomycin (15 mg). Her condition improved rapidly with this cytotoxic therapy. On January 28, 1998 she was referred to our outpatient clinic after 2 cycles of the therapy; she had a hemoglobin concentration of 9.8 g/dL and no signs of ongoing hemolysis. Combination antiretroviral therapy with d4T, nevirapine, and indinavir was introduced on February 16, when tests showed 104 CD4+ T cells/μL and 14 690 copies of HIV-1 RNA/mL plasma. After 4 months, HIV-1 RNA reached plasma values lower than 80 copies/mL, and it has since remained below this threshold.
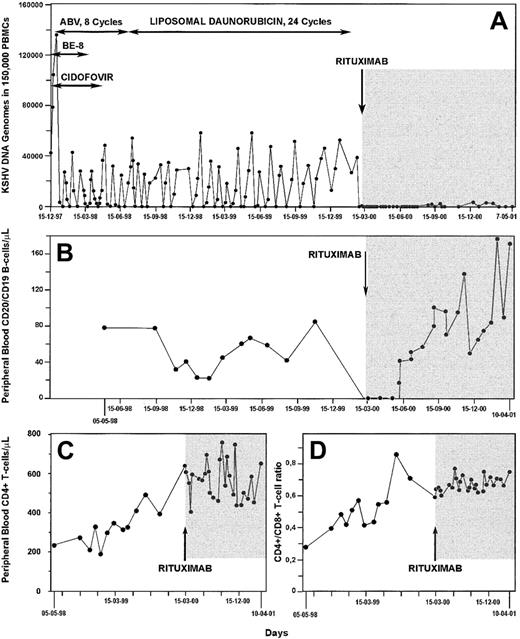
Chemotherapy was continued for 8 cycles at intervals of 3 weeks. On June 16, 1998, treatment with intravenous liposomal daunorubicin (40 mg/m2) administered every 2 to 4 weeks was started and was continued until February 24, 2000. On October 19, 1998, total body computed tomography documented complete remission of lymphadenopathy and splenomegaly. However, constitutional symptoms (ie, profuse night sweating and asthenia) monotonously re-emerged after each successive cycle with the antineoplastic agents and were regularly paralleled by increasing KSHV levels in the circulation (Figure2A). Furthermore, the intervals with undetectable virus in the blood were never longer than few days, despite 60 weeks of antiblastic therapy (Figure 2A).
Kinetics of circulating KSHV DNA burden and lymphocyte subsets during a 3-year follow-up period in the patient with multicentric Castleman disease.
Changes in KSHV DNA load in PBMCs (A), peripheral blood CD19/CD20+ B cell counts (B), CD4+ T-cell counts (C), and CD4+/CD8+ T-cell ratio (D) in an HIV-1– infected patient with multicentric Castleman disease during treatment with cidofovir, antihuman IL-6 monoclonal antibody (BE-8), and cytotoxic therapy (horizontal arrows) and after a single dose of anti-CD20 monoclonal antibody (shaded area). Throughout most of the study period, the patient was under effective, highly active antiretroviral therapy.
Kinetics of circulating KSHV DNA burden and lymphocyte subsets during a 3-year follow-up period in the patient with multicentric Castleman disease.
Changes in KSHV DNA load in PBMCs (A), peripheral blood CD19/CD20+ B cell counts (B), CD4+ T-cell counts (C), and CD4+/CD8+ T-cell ratio (D) in an HIV-1– infected patient with multicentric Castleman disease during treatment with cidofovir, antihuman IL-6 monoclonal antibody (BE-8), and cytotoxic therapy (horizontal arrows) and after a single dose of anti-CD20 monoclonal antibody (shaded area). Throughout most of the study period, the patient was under effective, highly active antiretroviral therapy.
On March 8, 2000, with the approval of the Institutional Review Board and the informed consent of the patient, the anti-CD20 chimeric monoclonal antibody Mabthera (Roche, Basel, Switzerland) was administered intravenously at the standard dose of 375 mg/m2 according to the instructions of the manufacturer. No adverse effects were encountered during infusion of the drug. Circulating CD19+ B cells were undetectable 48 hours from Mabthera administration and remained so for 14 weeks (Figure 2B). During this time, KSHV DNA in PBMCs was always negative by the PCR (Figure 2A). This finding indirectly argues for a restricted tropism of KSHV for the CD20+ circulating B cells in MCD. B cells returned to preimmunotherapy absolute counts 6 months after Mabthera administration (Figure 2B). During the 11-week phase of B-cell reconstitution and in the next 8 months with restored B-lymphocyte counts, the patient remained asymptomatic; KSHV viremia was present only episodically and always at low titers (Figure 2A). This was in striking contrast with the crescendo of constitutional symptoms and circulating KSHV burden shown within days of each successive cycle of chemotherapy. Finally, therapy with rituximab did not have detrimental effects on the immunologic and virologic markers of HIV-1 infection. Under antiretroviral therapy, CD4+ T cell levels kept rising, whereas the CD4+/CD8+ T-cell ratio did not significantly change when compared with the pre-Mabthera period of observation (Figure 2C-D).
Results and discussion
KSHV-related MCD is a life-threatening disorder. Detection of the virus in secondary lymphoid organs is essential for diagnosis because histologic findings do not allow distinction between KSHV-negative and KSHV-positive MCD.2,5,6,13,14 Elevated viral burden in PBMC may be a characteristic feature of this disorder,15 and blood viral monitoring may be the most accurate marker of disease activity and response to therapy15,16—similar to circulating EBV DNA levels in lymphoproliferative disorders after transplantation.17
Antiherpesvirus therapy has been advocated in KSHV-related MCD.16 Cidofovir is the most potent inhibitor of KSHV productive infection.18 Clinical and viral evidence presented here demonstrates that 4 months of therapy with cidofovir had no effect on KSHV-related MCD. Other mechanisms beyond productive viral replication are thus operational in perpetuating the high viremic levels typical of the disease.
Alleviation of the systemic manifestations of MCD may be achieved by use of an anti–IL-6 antibody.14,19 Our patient did not improve despite 3 months of daily anti–huIL-6 immunotherapy. Nevertheless, the normalization of serum CRP levels observed within hours of BE-8 administration suggests efficient neutralization of systemic huIL-6.20 However, BE-8 may not affect viral IL-6, the functional homologue of huIL-6 encoded by KSHV.
KSHV-MCD responds to the administration of cytotoxic agents.21 Clinical remission may be achieved by chemotherapy, but the periods free of systemic symptoms and KSHV viremia are typically short-lived. In addition, patients with KSHV-MCD are at risk for the development of non-Hodgkin lymphoma and Kaposi sarcoma.1 In a recent report, Dupin et al22demonstrate that KSHV-infected B cells in secondary lymphoid organs of patients with MCD are positive for the CD20 surface antigen and display monotypic rearrangements of the immunoglobulin λ-chain. These cells may represent microscopic foci of in situ neoplastic clones that may progress to overt plasmablastic, KSHV-related, non-Hodgkin lymphoma.22 Furthermore, expression of the CD20 antigen has been recently demonstrated in 1 patient with acquired immunodeficiency syndrome (AIDS)–associated primary effusion lymphoma in vivo and in vitro.23 Long-term ablation of KSHV viremia should thus be viewed a therapeutic goal to prevent the periodic exacerbations of MCD and the subsequent development of lymphoma.
In contrast to highly active antiretroviral therapy (HAART)–induced remission of early-stage AIDS-KS,24 the resolution of immune suppression as a result of antiretroviral therapy may not per se affect HIV-1–related MCD. In our patient, 21 months of HAART had no effect on disease activity, as judged clinically and by the kinetics of KSHV viremia. We cannot, however, exclude that after Mabthera-induced purging of KSHV-infected neoplastic B cells and the concomitant interruption of cytotoxic chemotherapy, control of KSHV viremia was achieved through a HAART-mediated, reconstituted antiviral response.
In conclusion, Mabthera is an effective agent for KSHV-related MCD. Of note, rituximab was introduced in this patient after chemotherapy-induced remission of her generalized lymphadenopathy, splenomegaly, and hemolytic anemia. Open issues thus remain concerning the role of Mabthera, alone or in combination with cytotoxic drugs, as a first-line agent in this disorder.
We are profoundly indebted to our patient for the admirable compliance shown throughout the course of her disease. We thank Dr John Wijdenes (Diaclone, Besançon, France) for the kind gift of the antihuman IL-6 antibody BE-8. Finally, we thank Fabio Franzetti, Andrea Gori, Laura Galimberti, Annalisa Ridolfo, and Laura Milazzo for their skillful care of our patient.
Supported by grants from Istituto Superiore di Sanità AIDS Research Program, Rome, and ANLAIDS, Sezione Lombarda, Milan, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mario Corbellino, Institute of Infectious Diseases and Tropical Medicine, University of Milan, Luigi Sacco Hospital, Via G.B. Grassi, 74 20157, Milan, Italy; e-mail:mcorbell@mailserver.unimi.it.