Abstract
Heterotypic interaction among tumor cells (TCs) and endothelial cells (ECs) may play a critical role during the vascular dissemination of neoplastic cells and during pathologic angiogenesis in tumors. To identify molecules involved in these processes, the distribution of vascular junctional proteins was first studied by immunofluorescence at sites of heterologous intercellular contact using TC-EC mosaic monolayers grown on 2-dimensional collagen. Several members of the tetraspanin superfamily, including CD9, CD81, and CD151, were found to localize at the TC-EC contact area. The localization of tetraspanins to the TC-EC heterologous contact area was also observed during the active transmigration of TCs across EC monolayers grown onto 3-dimensional collagen matrices. Dynamic studies by time-lapse immunofluorescence confocal microscopy showed an active redistribution of endothelial CD9 to points of melanoma insertion. Anti-CD9 monoclonal antibodies were found to specifically inhibit the transendothelial migration of melanoma cells; the inhibitory effect was likely caused by a strengthening of CD9-mediated heterotypic interactions of TCs to the EC monolayer. These data support a novel mechanism of tetraspanin-mediated regulation of TC transcellular migration independent of TC motility and growth during metastasis and a role for these molecules in the formation of TC-EC mosaic monolayers during tumor angiogenesis.
Introduction
The dissemination of cells from a primary tumor by invasion and metastasis represents the hallmark of malignance.1 Invasion is the local dissemination of neoplastic tissue, whereas metastasis is the distant dissemination to secondary places of growth involving the transport of neoplastic cells through the fluid spaces of the body (blood, lymph, cerebrospinal, or peritoneal fluid).2 Although tumor cell (TC) migration through the extracellular matrix (ECM) is required for invasion, success in metastasis requires migration through the ECM and across host cell layers (transcellular migration).3 Because most cancer cells reach distant sites by dissemination through blood or lymphatic circulation, transendothelial migration of TCs is a crucial event in vascular metastasis formation. In addition, 2 recent works in human melanoma4 and colon carcinoma5suggesting that tumor vessels are “mosaic” lined by endothelial cells (ECs) and malignant TCs extend the role of TC-EC interactions to the process of tumor angiogenesis. Thus, TC transendothelial invasion and molecules involved in TC-EC interactions could contribute to the formation of new blood vessels in tumors.
Tetraspanins comprise a numerous group of proteins that have 4 putative transmembrane domains and that have been implicated in the regulation of cell development, proliferation, activation, and motility.6,7 It has been suggested that tetraspanins may provide a bridge between β1 integrins and other proteins to generate functional complexes that regulate cell-cell and cell-ECM interactions.8 The expression of many members of the tetraspanin superfamily is altered in different types of human cancer.9 For the most part, tetraspanins become down-regulated in metastatic tumors,10-13 though CD151 has a positive effect in cell metastasis.14 Transfection of tetraspanins CD9, CD63, and CD82 reduces metastasis in vivo,15-17 and this effect has been related to the suppression of tumor cell growth and motility.
A large array of adhesion molecules has been described as mediating the adhesion and migration of TCs through ECM and in the initial attachment to EC monolayers, including integrins, selectins, cadherins, immunoglobulins, and proteoglycans.18,19 However, little is known about the mechanisms that regulate the passage of TCs through endothelial junctions. Earlier studies20-22 suggest that TCs disseminate by breaching the vasculature wall, and they point to several tumor-derived factors to induce EC retraction. In contrast, recent work23 indicates that the establishment of heterologous gap junctions between melanoma and ECs may contribute to in vivo metastasis formation. Furthermore, heterologous cell-cell interactions between TCs and other host cell layers can take place during TC dissemination through visceral cavities, and the cellular and molecular pathways implicated could be related to those acting during TC-EC interactions. In this regard, an essential role for the activation of the Rho-ROCK pathway has been described during transcellular invasion of a mesothelial cell monolayer by hepatoma TCs, and its inhibition reduced the peritoneal dissemination of TCs in an in vivo model.24 An attractive hypothesis is that TCs may establish intimate contact with ECs, and this TC-EC interaction could coordinate the opening of interendothelial junctions, facilitating their transmigration. In the current study, EC monolayers grown onto 3-dimensional collagen matrices were used to study the TC-EC interactions that take place during active transmigration of melanoma cells. We have identified several members of the tetraspanin superfamily that localize to the TC-EC contact region, and we have investigated the functional role of these molecules during TC transmigration.
Materials and methods
Cells and cell cultures
Human umbilical vein endothelial cells (HUVECs) were obtained and cultured as described previously.25 Briefly, umbilical veins were cannulated, washed, and incubated with 0.1% collagenase P (Boehringer Mannheim GmbH, Mannheim, Germany) for 20 minutes at 37°C. Cells were seeded on tissue culture flasks (Costar, Corning, NY) coated with 0.5% gelatin (Sigma Chemical, St Louis, MO) and grown in 199 medium (Gibco BRL Life Technologies, Paisley, Scotland) supplemented with 20% fetal calf serum (FCS; Gibco BRL), 50 IU/mL penicillin, 50 μg/mL streptomycin (ICN Biomedicals, Costa Mesa, CA), 250 μg/mL Fungizone (Squibb Industria Farmacéutica, Barcelona, Spain), 50 μg/mL EC growth supplement (prepared from bovine brain), and 100 μg/mL heparin (Sigma) and were used up to the third passage. Cells were split 1:3 and were detached with a solution of 0.05% trypsin and 0.02% EDTA (Sigma) before use. The A375 melanoma cell line26 was maintained in RPMI-1640 medium (Gibco BRL) supplemented with 10% FCS and 10 mM HEPES buffer (Biological Industries, Kibbutz Beit Haemek, Israel). TCs or ECs were labeled with fluorescent dye BCECF-am or SNARF-1 (Molecular Probes Europe BV, Leiden, The Netherlands) according to the manufacturer's instructions.
Antibodies
Monoclonal antibodies (mAbs) TS2/16 (anti–β1 integrin), VJ1/18 (anti-α3), VJ2/9 (anti-PECAM-1), W6/32 (anti-HLA class I), VJ1/10 and VJ1/20 (anti-CD9), Lia1/1 (anti-CD151), and TEA1/31 (anti-VE–cadherin) have been described.27-31 GR2110 (anti-CD9) was provided by Dr F. Garrido (Hospital Virgen de las Nieves, Granada, Spain), and I.33.22 (anti-CD81) was provided by Dr Vilella (Hospital Clinic, Barcelona, Spain). Anti-Zonula occludens antigen-1 (ZO-1) rabbit polyclonal antibody was purchased from Zymed Laboratories (San Francisco, CA), and anti–β-catenin was purchased from Transduction Laboratories (Lexington, KY). Fab fragments of VJ1/10 and VJ1/20 mAb, and F(ab′)2 fragments of VJ1/10 were prepared as previously reported32 and analyzed by SDS-PAGE. Briefly, mAb was incubated with immobilized papain and pepsin (Pierce Chemical, Rockford, IL). Undigested antibody and Fc fragments were removed by passage over protein A-Sepharose (Pharmacia).
Coculture experiments
Collagen gels were used for 3-dimensional assays. Type I collagen (Cellagen solution AC-3; ICN Biomedicals) was mixed with 10 × medium 199 according to the manufacturer's directions. Appropriate volumes of this solution were allowed to gel at 37°C (1 hour) in each well of a 24-well tissue culture plate or on glass coverslips (12-mm diameter). Dehydrated collagen gels were made by allowing the gels on glass coverslips to dry in a laminar flow hood. Collagen gels were used as culture substrata for HUVEC cells. For 2-dimensional assays, glass coverslips were incubated with phosphate-buffered saline (PBS) containing 2% gelatin (Difco, Detroit, MI) for 30 minutes at 37°C, fixed with PBS containing 0.5% glutaraldehyde (Sigma) for 25 minutes at room temperature, washed 3 times in PBS, incubated with aqueous 0.1 M glycine (to block free aldehydes), washed 3 times in PBS, and stored for up to 1 week in HUVEC medium. BCECF-labeled A375 cells were added to the EC monolayer as indicated. This 2-dimensional model system did not allow the complete migration of the TCs underneath the endothelium.
Silver nitrate staining
Cell monolayers were fixed in 0.05% glutaraldehyde in PBS for 15 minutes at room temperature, washed twice with 5% glucose (Braun, Barcelona, Spain), and incubated for 30 seconds with 5% glucose containing 0.25% AgNO3 (Sigma). Cells were washed again with 5% glucose and covered with glycerol (87%) (Merck, Darmstadt, Germany). Silver lines were developed after the exposure of cells to UV light.
Immunofluorescence analysis and confocal microscopy
Cells were fixed for 15 minutes in 4% formaldehyde in PBS at room temperature. When necessary, cells were permeabilized with 0.2% Triton X-100 in Tris-buffered saline (TBS; 50 mM Tris-HCl, 150 mM NaCl, 0.1% NaN3, pH 7.6) for 3 minutes. After blocking nonspecific binding sites by incubation with TNB (0.1 M Tris-HCl, 0.15 M NaCl, 0.5% blocking reagent; Boehringer Mannheim GmbH), cells were sequentially incubated with specific mAb or polyclonal antibodies and appropriate Cy3-labeled secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA). Cells were visualized using a Nikon Eclipse E800 photomicroscope (Nikon, Tokyo, Japan) with 100 × oil immersion objective, 1.4 NA. Preparations were photographed on either Ektachrome or TMAX 400 ASA film (Eastman Kodak, Rochester, NY). Fluorescence quantification was performed using Optimas 5.2 (Bioscan, Edmonds, WA). Series of optical sections distanced 0.5 μm on the z-axis were obtained with a confocal scanning laser microscope (MRC 1024; Bio-Rad, Hercules, CA) mounted on a Zeiss Axiovert 135 inverted microscope (Carl Zeiss, Oberkochen, Germany).
Migration assays
Transendothelial migration assays for A375 melanoma cells were performed in polycarbonate transwell inserts (8-μm pore; Costar, Corning, NY) coated with HUVECs and grown as a monolayer for 24 hours. BCECF-labeled A375 cells were resuspended in 10% FCS–RPMI-1640 and were preincubated with mAbs for 20 minutes before plating. HUVEC cells were also incubated with the same mAbs at this time. Typically, 1.5 × 105 BCECF-labeled A375 were seeded in the upper compartment (100 μL), and 10% FCS–RPMI-1640 was placed in the lower compartment (600 μL). After 16 hours at 37°C, the number of fluorescence-labeled A375 cells that had migrated through the monolayer was determined by direct counting on a FACScan (Becton Dickinson Immunocytometry Systems, Mountain View, CA) using CellQuest software. Alternatively, migration assays were performed either with uncoated polycarbonate filters or after coating of the top side of the filter with fibronectin or collagen (10 μg/mL).
Adhesion and aggregation assays
For cellular adhesion assays, HUVECs were cultured on 96-microwell plates (Corning Glass Works) as a monolayer for 24 hours. BCECF-labeled A375 cells were resuspended in RPMI containing 0.4% bovine serum albumin (BSA) and the distinct mAbs for 15 minutes. HUVECs also were incubated with the same mAbs at this time. A375 cells (6 × 104) were allowed to adhere to each well for 5 minutes at 37°C. Unbound cells were removed by 3 washes with warm RPMI. The number of cells adhered to the wells was obtained by fluorescence intensity measurement in a microplate fluorescence reader (Victor Wallak, Turku, Finland). All assays were run in triplicate.
Cell aggregation assays were performed as previously described33 with slight modifications. BCECF-A375 cells or SNARF-ECs were resuspended at 8 × 105 cells/mL in RPMI–1% BSA containing 10 μg/mL of either CD9 or control W6/32 mAb, mixed, and allowed to aggregate on BSA-coated plates for 30 minutes at 37°C and rotation (100 rpm). Numbers of particles before and after aggregation were quantified in a FACScan flow cytometer. After fixation, cell aggregates were photographed for either red or green fluorescence.
Transient transfection and in vivo time-lapse confocal microscopy
Cells were trypsinized and resuspended in RPMI 10% FCS medium supplemented with 5 μL 1.5 M NaCl, 20 μg Bluescript, and 5 μg CD9–green fluorescent protein (GFP)–DNA construct. Cells were electroporated at 975 μF/200 V in a Gene Pulser II (Bio-Rad) and were used for the time-lapse videomicroscopy experiments 24 hours after transfection. CD9-GFP fusion protein construct was obtained by polymerase chain reaction amplification of the CD9 cDNA (a kind gift of Dr E. Rubinstein, INSERM, Villejuif Cedex, France) and cloned in pEGFP-N1 Vector (Clontech Laboratories, Palo Alto, CA) inEcoRI sites of the cloning site.
A375 cells were layered on a confluent HUVEC monolayer initially seeded on a collagen gel. Preparations were maintained at 37°C and 5% CO2 on an incubator coupled to a Leica TCS-SP confocal microscope (Leica Microsystems, Heidelberg, Germany). Series of images distanced 2 μm on the z-axis were acquired every 3 or 5 minutes. Fluorescence and DIC acquisition was performed simultaneously using a 63 ×, 1.4 NA oil-immersion objective.
Statistical analysis
Data were presented as the mean ± SD and were compared using Student t test or one-way analysis of variance.
Results
Tumor cells transmigrate at the lateral borders of endothelial cells
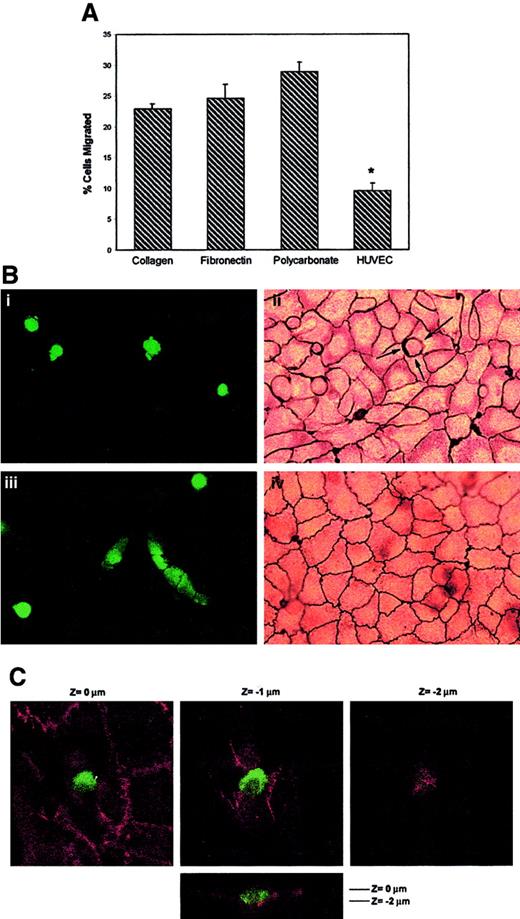
To investigate the transendothelial migration of TCs, EC monolayers were established on transwell inserts, and transendothelial migration (TEM) assays were performed using A375 melanoma cells labeled with the fluorescence dye BCECF (Figure1A). In the absence of an endothelial monolayer, a significant number of A375 cells migrated after 16 hours, through the 8-μm pore polycarbonate filter, to the lower transwell compartment (28.9% ± 1.6% migrated cells). A slight reduction in the percentage of migrated A375 cells was observed using membrane inserts coated with ECM proteins, such as collagen or fibronectin (22.9% ± 0.9% and 24.6% ± 2.3% migrated cells, respectively). The presence of an endothelial cell monolayer coating the membrane filter resulted in an approximately 67% reduction in melanoma cell migration to the lower compartment (9.6% ± 1.2% migrated cells;P < .001).
Transendothelial migration of A375 melanoma cells.
(A) Migration and TEM assays. Labeled A375 melanoma cells were allowed to migrate for 16 hours in transwell inserts coated with collagen, fibronectin, uncoated polycarbonate, or an EC monolayer–coated membrane, as indicated. Bars represent the mean ± SD of the percentage of migrated A375 in relation to the total number of cells added to migrate, from 2 parallel experiments in duplicate of 12 independent experiments performed (n = 24). *Statistically significant at P < .001 compared with the other conditions. (B) A375 cells transmigrated across EC monolayers at lateral junctions. BCECF-labeled A375 cells were added to confluent HUVECs grown on collagen gels. After 2 hours (i, ii) and 16 hours (iii, iv), the cocultures were fixed and stained with silver nitrate as described. Green conventional fluorescence microscopy (i, iii) and corresponding light microscopy (ii, iv) micrographs are shown. An actively migrating melanoma cell is indicated by arrows in panel Bii. Interestingly, in the fluorescence image of transmigrated melanoma in panel Biii, silver staining is also visible of the intercellular junctions of the endothelial monolayer above because of its opacity, confirming restoration of the integrity of the EC monolayer after TC passage. (C) Confocal microscopy analysis of the distribution of VE-cadherin during melanoma cell transmigration. BCECF-labeled A375 cells were added to confluent HUVECs on dehydrated collagen gels and allowed to migrate for 2 hours. After fixation, coculture was stained for VE-cadherin. Serial horizontal (xy) sections distanced 1 μm apart, and a vertical (xz) section (lower panel) are shown.Z = 0 corresponds to the level of EC-EC junctions.
Transendothelial migration of A375 melanoma cells.
(A) Migration and TEM assays. Labeled A375 melanoma cells were allowed to migrate for 16 hours in transwell inserts coated with collagen, fibronectin, uncoated polycarbonate, or an EC monolayer–coated membrane, as indicated. Bars represent the mean ± SD of the percentage of migrated A375 in relation to the total number of cells added to migrate, from 2 parallel experiments in duplicate of 12 independent experiments performed (n = 24). *Statistically significant at P < .001 compared with the other conditions. (B) A375 cells transmigrated across EC monolayers at lateral junctions. BCECF-labeled A375 cells were added to confluent HUVECs grown on collagen gels. After 2 hours (i, ii) and 16 hours (iii, iv), the cocultures were fixed and stained with silver nitrate as described. Green conventional fluorescence microscopy (i, iii) and corresponding light microscopy (ii, iv) micrographs are shown. An actively migrating melanoma cell is indicated by arrows in panel Bii. Interestingly, in the fluorescence image of transmigrated melanoma in panel Biii, silver staining is also visible of the intercellular junctions of the endothelial monolayer above because of its opacity, confirming restoration of the integrity of the EC monolayer after TC passage. (C) Confocal microscopy analysis of the distribution of VE-cadherin during melanoma cell transmigration. BCECF-labeled A375 cells were added to confluent HUVECs on dehydrated collagen gels and allowed to migrate for 2 hours. After fixation, coculture was stained for VE-cadherin. Serial horizontal (xy) sections distanced 1 μm apart, and a vertical (xz) section (lower panel) are shown.Z = 0 corresponds to the level of EC-EC junctions.
To assess the interactions between TCs and ECs, endothelial monolayers were grown at confluence on collagen gels and were used to model the tightly apposed ECs that lined the vascular intima in vivo. Under these conditions, the EC junctions stained with silver nitrate and had well-developed adherens and tight junctions (not shown). BCECF-labeled A375 melanoma cells were added to confluent endothelial monolayer cultures and were observed for 16 hours. To monitor the integrity of the EC monolayer and the location of the TC-EC attachment in relation to the EC borders, TC-EC cocultures were fixed every 30 minutes and were stained with silver nitrate (Figure 1B) and with anti-VE–cadherin (Figure 1C). Initially, most TCs were observed above the EC monolayer and appeared round, with no silver deposition (data not shown). After 2 hours of coculture, most tumor cells were observed in direct contact with the silver-stained EC borders, showing a preferential localization where multiple ECs joined together (tricellular corners) (Figure 1B, panel ii, arrows). At this time, some TCs were observed in the same focal plane as the silver-stained vascular junctions, displaying pseudopods into the subendothelial matrix (Figure 1B, panels i-ii), and the silver reaction product stained the TC-EC contact area (Figure 1B, panel ii, arrows). VE-cadherin staining was observed to be continuous in the vicinity and under the TCs (Figure 1C). Only a focal loss of VE-cadherin staining was seen at the bottom of the EC-junction depression induced by the attached TCs, without a significant disruption of the neighboring EC monolayer (Figure 1C, right panel). At 16 hours, some TCs displayed a spread morphology and were observed under the plane of the silver-stained vascular junctions (Figure 1B, panel iii). Continuous lines of silver stain were observed overlying the TCs that appeared to have completed their passage through the monolayer to the abluminal surface (Figure 1B, panels iii-iv), indicating that EC cell-cell junctions were re-established after TC passage.
Tetraspanins CD9, CD81, and CD151 localize at the heterotypic TC-EC intercellular junctions
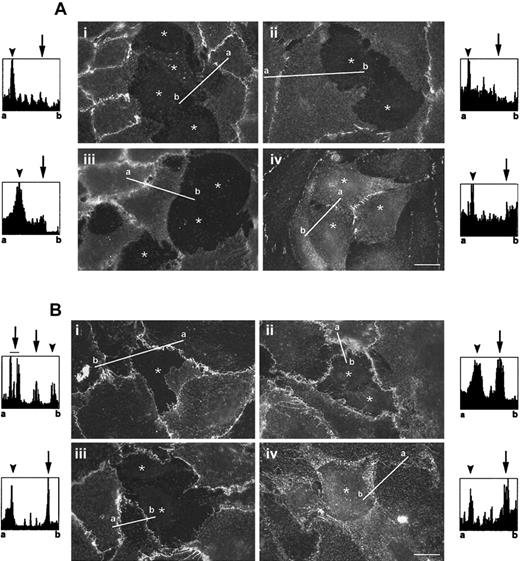
To identify EC molecules involved in the interaction with TCs, mAbs against several vascular junction proteins were studied for their ability to stain the TC-EC heterotypic contact areas by immunofluorescence. To study these interactions, a mosaic monolayer of TCs and ECs was prepared by seeding fluorescently labeled TCs over a confluent EC monolayer grown onto cross-linked gelatin. After 16 hours of coculture, the A375 cells were observed inserted between the ECs, forming a mosaic TC-EC monolayer. VE-cadherin staining completely disappeared from the edges of the ECs in contact with the TCs (Figure2Ai), as previously described.34 Similarly, a lack of staining at sites of TC-EC contact was observed for β-catenin (Figure 2Aii), a cytoplasmic protein that links cadherins with the actin cytoskeleton, and for PECAM-1 (Figure 2Aiii), a receptor involved in the transmigration of leukocytes. The tight junction protein ZO-1 was localized at EC-EC and TC-TC junctions, but it was absent from heterologous TC-EC contacts (Figure 2Aiv). Interestingly, the tetraspanin superfamily members (TM4SF) CD9, CD81, and CD151, which have been previously described to localize at the lateral junctions of endothelial and epithelial cells,6,30 were concentrated at TC-EC contact areas and at EC-EC junctions (Figure 2B, panels i-iii). The α3integrin that associates with members of the TM4SF30 was also observed to be partially redistributed to the TC-EC contact area (Figure 2Biv). In addition, the intensity of the fluorescence signal in the images was quantified along lines traced over TC-EC contact regions and over homotypic (EC-EC or TC-TC) junctions (Figure 2, histograms). Although cadherin/β-catenin, ZO-1, and PECAM-1 showed a sharp increment in the intensity of the fluorescence signal only at homotypic junctions, the quantification of the stainings of CD9, CD81, CD151, and α3 integrin peaked at sites of TC-EC contact and at EC-EC contacts, indicating that these molecules are indeed concentrated at heterotypic intercellular contact regions. The concentration of CD9, CD81, and CD151 was only observed at sites of intercellular contact (heterologous or homologous), and no accumulation was observed in the periphery of TC or EC when cell-cell contact was lost (data not shown).
Distribution of junctional proteins in TC-EC monolayers.
BCECF-labeled A375 cells were added to confluent ECs grown onto cross-linked gelatin and were allowed to spread for 16 hours. The mosaic TC-EC monolayer was fixed and stained in red fluorescence for (A) EC junctional proteins—(i) VE-cadherin, (ii) β-catenin, (iii) PECAM-1, and (iv) ZO-1—or for (B) some members of the tetraspanin family (i) CD9, (ii) CD81, (iii) CD151, or (iv) the associated α3 integrin. The locations of melanoma cells are indicated (*) (originally labeled in green fluorescence). Bars, 15 μm. Histograms show the fluorescence intensity quantification along the white line traced on the immunofluorescence images; a and b correspond to start and end points, respectively. TC-EC heterotypic junctions are indicated by arrows, and EC-EC and TC-TC homotypic junctions are indicated by arrowheads in the fluorescence histograms.
Distribution of junctional proteins in TC-EC monolayers.
BCECF-labeled A375 cells were added to confluent ECs grown onto cross-linked gelatin and were allowed to spread for 16 hours. The mosaic TC-EC monolayer was fixed and stained in red fluorescence for (A) EC junctional proteins—(i) VE-cadherin, (ii) β-catenin, (iii) PECAM-1, and (iv) ZO-1—or for (B) some members of the tetraspanin family (i) CD9, (ii) CD81, (iii) CD151, or (iv) the associated α3 integrin. The locations of melanoma cells are indicated (*) (originally labeled in green fluorescence). Bars, 15 μm. Histograms show the fluorescence intensity quantification along the white line traced on the immunofluorescence images; a and b correspond to start and end points, respectively. TC-EC heterotypic junctions are indicated by arrows, and EC-EC and TC-TC homotypic junctions are indicated by arrowheads in the fluorescence histograms.
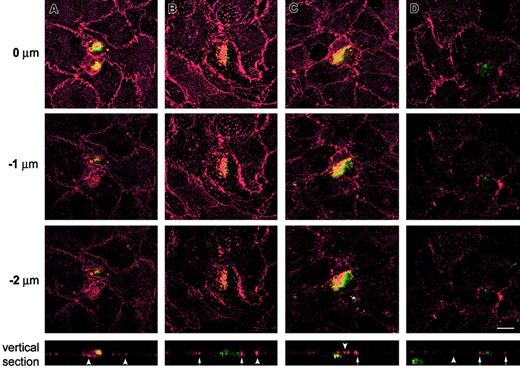
The distribution of the tetraspanins CD9, CD81, and CD151 was also studied during the active transmigration of TCs across ECs grown on 3-dimensional collagen matrix and analyzed by laser scanning confocal microscopy. TCs above the endothelium (yet to transmigrate) were observed in proximity with EC junctions, where CD9, CD81, and CD151 staining was highly concentrated at TC-EC contact areas (Figure3A and data not shown). Transmigrating TCs were observed inserted between ECs along several focal planes. Staining for CD9 (Figure 3B), CD81 (Figure 3D), and CD151 (data not shown) revealed a strong concentration along the TC-EC contact regions. This was further demonstrated when vertical confocal sections were analyzed (Figure 3B, D, lower panels, arrows). Transmigrated TCs were observed in the subendothelial matrix in proximity with the overlying endothelium (Figure 3C) and deep in the collagen matrix (Figure 3D, lower panel). In both cases, the integrity of the EC monolayer overlying the migrated TCs was restored (arrowheads). Thus, during the active migration of TCs across the lateral borders of the ECs, the TM4SF members CD9, CD81, and CD151 were redistributed around the transmigrating TCs. These data support a possible role for tetraspanins CD9, CD81, and CD151 in the molecular interactions required for TC transendothelial invasion.
Confocal microscopy analysis of the localization of CD9 and CD81 tetraspanins during active TEM in 3-dimensional collagen gels.
BCECF-labeled A375 cells were added to confluent ECs grown onto dehydrated collagen gels and allowed to migrate for 2 to 6 hours. After fixation the cocultures were stained in red fluorescence for CD9 (A-C) and for CD81 (D). Each series of panels (A-D) depicts horizontal (xy) confocal sections distanced 1 μm on the z-axis. Z = 0 corresponds to the level of EC-EC junction. A representative vertical (xz) section is shown at the bottom of each corresponding series. Note that green shows the position of melanoma cells but did not completely stain the periphery of spread tumor cells. In the vertical sections, TC-EC heterotypic junctions are indicated by arrows, and EC-EC homotypic junctions are indicated by arrowheads. Bar, 10 μm.
Confocal microscopy analysis of the localization of CD9 and CD81 tetraspanins during active TEM in 3-dimensional collagen gels.
BCECF-labeled A375 cells were added to confluent ECs grown onto dehydrated collagen gels and allowed to migrate for 2 to 6 hours. After fixation the cocultures were stained in red fluorescence for CD9 (A-C) and for CD81 (D). Each series of panels (A-D) depicts horizontal (xy) confocal sections distanced 1 μm on the z-axis. Z = 0 corresponds to the level of EC-EC junction. A representative vertical (xz) section is shown at the bottom of each corresponding series. Note that green shows the position of melanoma cells but did not completely stain the periphery of spread tumor cells. In the vertical sections, TC-EC heterotypic junctions are indicated by arrows, and EC-EC homotypic junctions are indicated by arrowheads. Bar, 10 μm.
Anti-CD9 monoclonal antibodies impair the transendothelial migration of tumor cells
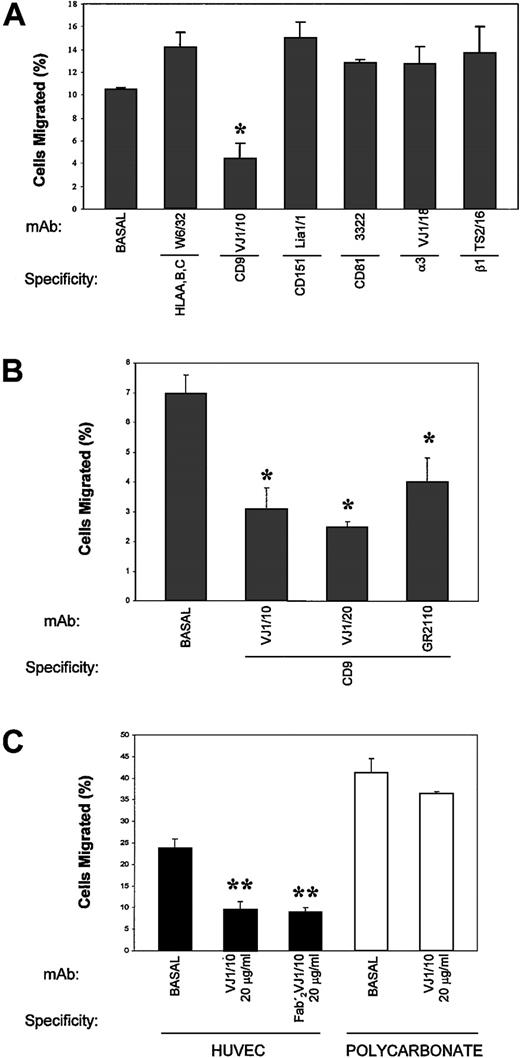
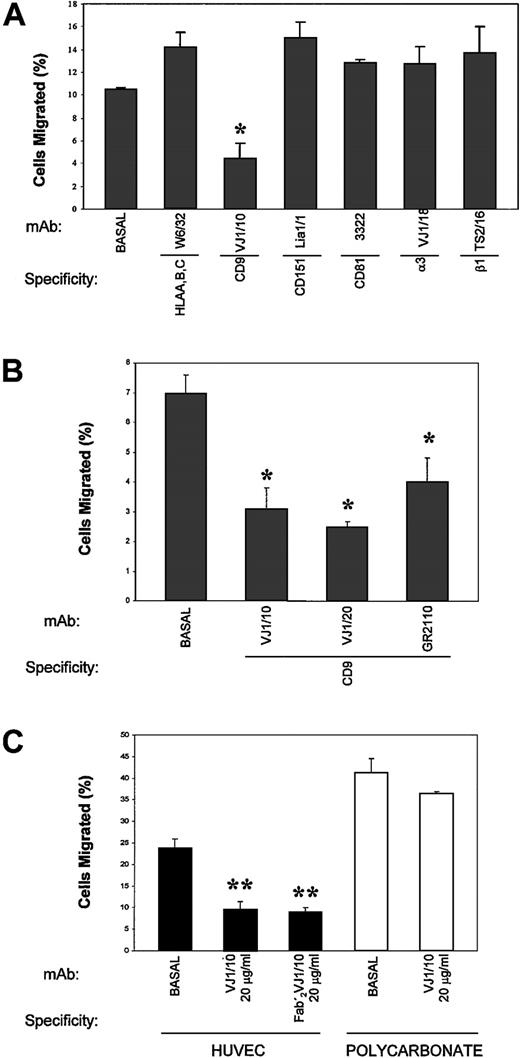
To determine the possible functional involvement of members of the TM4SF in the passage of TCs across an EC monolayer, TEM assays of A375 cells were performed in the presence of different mAbs to these molecules. As shown in Figure 4A, the anti-CD9 VJ1/10 mAb markedly inhibited (approximately 69%;P < .005) the transendothelial migration of TCs (4.4% ± 1.4% vs 14.2% ± 1.3% control anti-HLA–class I W6/32). In contrast, mAbs to either the tetraspanins CD81 and CD151 or to the integrins β1 and α3 did not affect TEM findings of TCs. When the ability of several other anti-CD9 mAbs to inhibit TC transmigration was assessed, we found that VJ1/10, VJ1/20, and GR2110 mAbs also blocked TC transmigration (Figure 4B). Moreover, the divalent F(ab′)2 fragment of VJ1/10 was inhibitory, thus ruling out any Fc-mediated effect (Figure 4C). The inhibition of TC transmigration was dependent on the mAb dose, with a maximal induction in the range of 1 to 20 μg/mL for VJ1/10 mAb (data not shown).
Effect of anti-CD9 mAb in TEM assays.
EC monolayers were established in transwell inserts, and TEM assays were carried out with BCECF-labeled A375 cells in the presence of the indicated purified mAb (10 μg/mL) for 24 hours, as described. (A) TEM in the presence of different mAbs against tetraspanin and integrins (B) TEM in the presence of different anti-CD9 mAbs. (C) TEM and bare migration in the presence of anti-CD9 VJ1/10 mAb or its F(ab′)2 fragment. Bars represent the mean ± SD of the percentage migrated TCs in relation to the total number of TCs added to migrate, from 2 parallel experiments in duplicate of 12 independent experiments performed (n = 24) with anti-CD9 VJ1/10 and VJ1/20 and anti-HLA class I W6/32 and of 3 experiments (n = 6) with the other mAbs. *P < .05; **P < .001 compared with medium control or with mAb W6/32.
Effect of anti-CD9 mAb in TEM assays.
EC monolayers were established in transwell inserts, and TEM assays were carried out with BCECF-labeled A375 cells in the presence of the indicated purified mAb (10 μg/mL) for 24 hours, as described. (A) TEM in the presence of different mAbs against tetraspanin and integrins (B) TEM in the presence of different anti-CD9 mAbs. (C) TEM and bare migration in the presence of anti-CD9 VJ1/10 mAb or its F(ab′)2 fragment. Bars represent the mean ± SD of the percentage migrated TCs in relation to the total number of TCs added to migrate, from 2 parallel experiments in duplicate of 12 independent experiments performed (n = 24) with anti-CD9 VJ1/10 and VJ1/20 and anti-HLA class I W6/32 and of 3 experiments (n = 6) with the other mAbs. *P < .05; **P < .001 compared with medium control or with mAb W6/32.
Previous reports14,15 30 have addressed the roles of CD9, CD81, and CD151 in cell migration, describing the inhibition of cell motility by several mAbs against these molecules. Thus, a general impairment of TC motility induced by the anti-CD9 mAb could be the mechanism involved in the prevention of TC transendothelial migration. To ascertain the effect of the anti-CD9 mAb in TC migration versus TC transendothelial migration, parallel assays were carried out either in the absence or in the presence of an EC monolayer coating the transwell filter (Figure 4C). Consistent with previous data, a mild reduction of approximately 5% (36.4% ± 0.4% vs 41.1% ± 3.4%) in TC migration was induced by the anti-CD9 VJ1/10 mAb in the absence of endothelium. In contrast, when the transwell filter was coated with an EC monolayer, the inhibitory effect of the anti-CD9 mAb was markedly increased by more than 60% (9.4% ± 2% vs 23.6% ± 2.3%;P < .001).
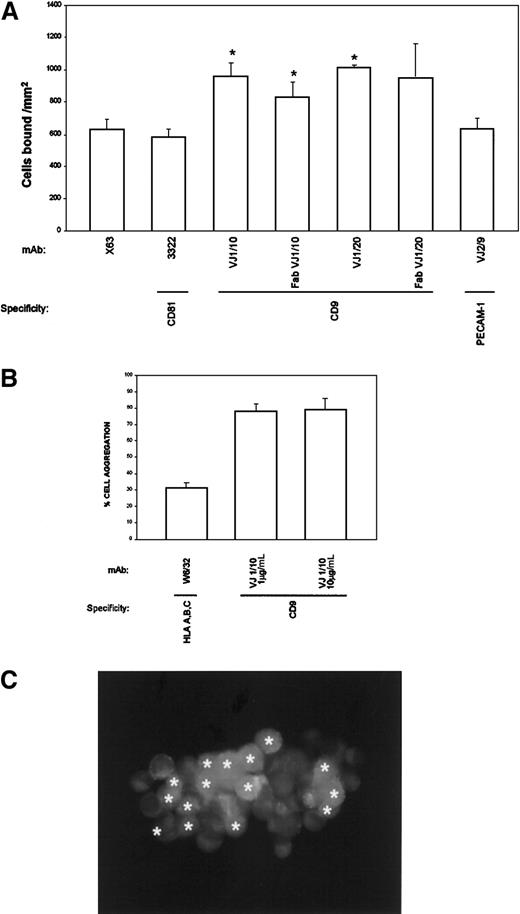
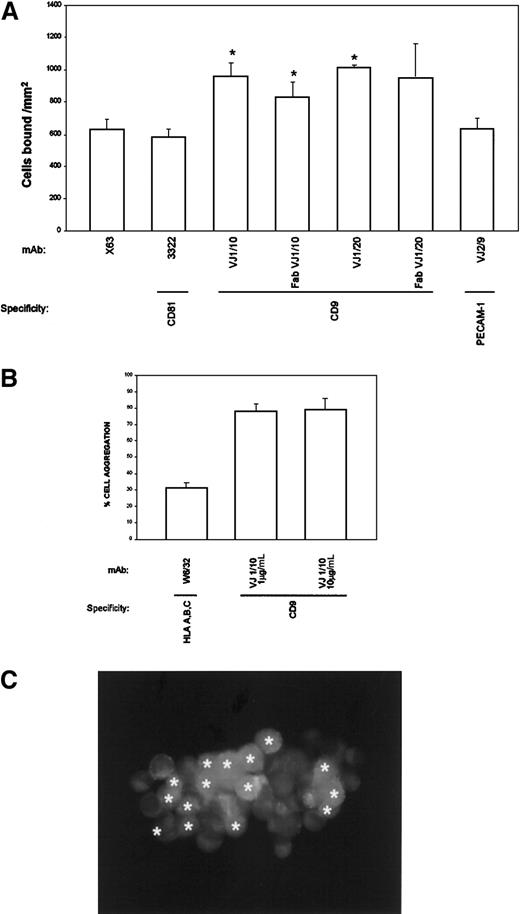
To explore the mechanism responsible for the inhibition of TC transmigration, the ability of anti-CD9 mAbs to modify TC adhesion to an EC monolayer was analyzed. BCECF-labeled A375 cells were allowed to attach for 5 minutes to an EC monolayer in the presence of the different mAbs. Anti-CD9 mAbs consistently enhanced the adhesion of A375 cells to endothelium (Figure 5A). Because CD9 is highly expressed by TCs and ECs, experiments were performed to rule out the possibility that CD9 mAb influenced cell adhesion by cross-linking TCs to ECs. As shown in Figure 5A, monovalent Fab fragments of anti-CD9 VJ1/10 and VJ1/20 mAbs induced TC adhesion to EC monolayers, thus ruling out any antibody-mediated cross-linking effect. Furthermore, other mAbs against a different tetraspanin molecule, CD81/TAPA-1, also expressed by both cell types, did not enhance TC adhesion to EC monolayers (Figure 5A). The CD9-mediated enhancement of TC attachment to EC occurred rapidly, at a maximum at 5 to 10 minutes, and was dependent on mAb dose, with induction of the proadhesive effect in the range of 1 to 10 μg/mL VJ1/10 that correlated well with inhibitory doses in the transmigration assays (data not shown). These data suggest that the mechanism responsible for the inhibition of transendothelial migration might be related to an increase in heterotypic TC-EC adhesion mediated by CD9.
Effect of anti-CD9 in TC-EC intercellular interactions.
(A) BCECF-labeled A375 cells were allowed to adhere to EC-coated plates for 5 minutes in the presence of the indicated purified mAb (10 μg/mL). Adhesion data represent the mean ± SD of triplicate samples from 1 of 4 independent experiments. *Statistically significant at P < .05 compared with control mAb X63 and with anti-CD81. (B) BCECF-labeled A375 cells (green) and SNARF-labeled ECs (red) were mixed in the presence of anti-CD9 VJ1/10 or anti-HLA class I W6/32 mAb and allowed to aggregate for 30 minutes, under rotation, as described in “Materials and methods.” Cell aggregation was quantified by flow cytometry and fluorescence microscopy. (C) One representative aggregate is shown. ECs are marked with asterisks (originally labeled in red fluorescence).
Effect of anti-CD9 in TC-EC intercellular interactions.
(A) BCECF-labeled A375 cells were allowed to adhere to EC-coated plates for 5 minutes in the presence of the indicated purified mAb (10 μg/mL). Adhesion data represent the mean ± SD of triplicate samples from 1 of 4 independent experiments. *Statistically significant at P < .05 compared with control mAb X63 and with anti-CD81. (B) BCECF-labeled A375 cells (green) and SNARF-labeled ECs (red) were mixed in the presence of anti-CD9 VJ1/10 or anti-HLA class I W6/32 mAb and allowed to aggregate for 30 minutes, under rotation, as described in “Materials and methods.” Cell aggregation was quantified by flow cytometry and fluorescence microscopy. (C) One representative aggregate is shown. ECs are marked with asterisks (originally labeled in red fluorescence).
To assess the functional role of CD9 in heterotypic TC-EC adhesion, cell aggregation assays were performed with TCs and ECs labeled with BCECF (green) or SNARF (red), respectively. VJ1/10 anti-CD9 mAb, at doses of 1 to 10 μg/mL, was able to induce the formation of heterotypic cell aggregates (Figure 5B). CD9-mediated TC-EC aggregation was strong, involving most of the cells, and most aggregates contained both TC and EC cell types (98% of TC-EC heterotypic aggregates). A representative TC-EC aggregate is shown in Figure 5C. These data point to a role for CD9 in the heterotypic adhesion between TCs and ECs that may have profound implications for metastasis and development of mosaic blood vessels in tumors.
Dynamic assessment of CD9-mediated heterotypic TC-EC interaction
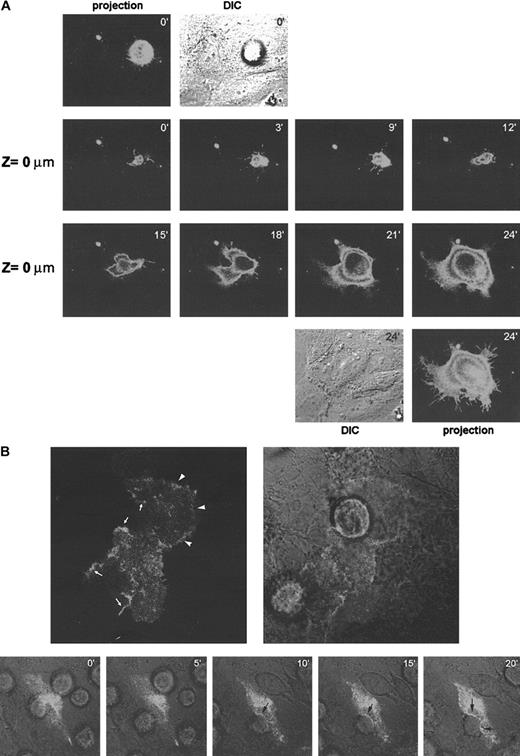
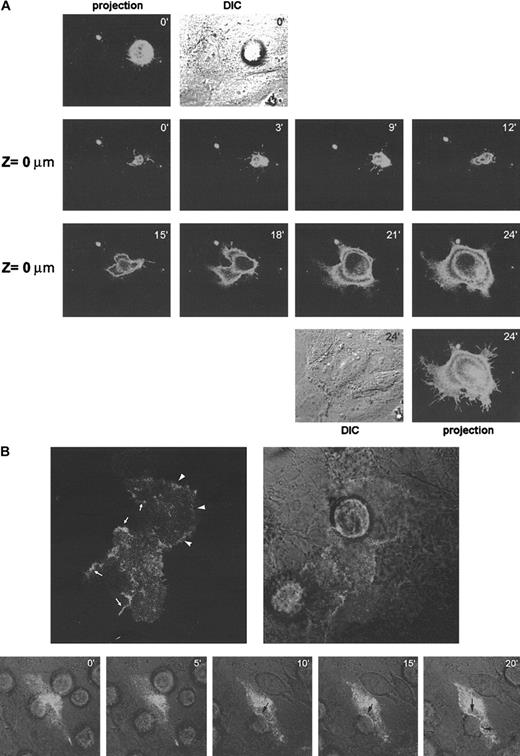
To assess directly the possible role of CD9 in heterotypic TC-EC cell-cell interaction, a CD9-GFP DNA construct was transfected in either A375 or EC cells. First, A375 cells were transfected with GFP-tagged CD9 and were seeded over an endothelial cell monolayer. At the focal plane of TC contact with the endothelium (Z = 0 μm), CD9 was present in filopodia extensions that seemed to survey the endothelial surface (Figure 6A, panels 0-9 minutes). A reconstruction of the signal in different confocal planes showed that there was no clear relocalization of melanoma CD9 to the TC-EC contact (Figure 6A, upper panels, 0 minutes). Once the melanoma cell found an appropriate insertion site, it spread in less than 15 minutes forming a mosaic monolayer (Figure 6A, panels 15-24 minutes).
Dynamic distribution of CD9 during TC-EC interactions.
(A) A375 melanoma cells were transfected with GFP-tagged CD9 and were allowed to invade a confluent EC monolayer. Cellular distribution of CD9-GFP was assessed by time-lapse confocal microscopy every 3 minutes. The focal plane corresponding to TC-EC contact is shown (Z = 0 μm). At 0 and 24 minutes, a projection of the fluorescence signal of the different sections and of the DIC image is shown. (B). ECs were transfected with GFP-tagged CD9 and allowed to grow onto collagen gels to confluence. A375 melanoma cells were allowed to invade the EC monolayer, and the distribution of endothelial CD9-GFP was observed by confocal microscopy. Upper panels show a representative EC-TC interaction confocal section. Reinforcement of the signal because of CD9-GFP relocalization is indicated by arrowheads at EC-EC contact sites by arrows at EC-TC heterotypic junctions. Lower panels show a time-lapse experiment of the TC insertion. Merging of fluorescence and DIC images is shown. Note that no redistribution of CD9 is seen at initial time points where TC adheres to luminal surfaces of ECs but only at the time of insertion (10-20 minutes, arrows). The processes in panels A and B are also depicted in 2 video sequences on the Blood website (see the Supplemental Videos link at the top of the online article).
Dynamic distribution of CD9 during TC-EC interactions.
(A) A375 melanoma cells were transfected with GFP-tagged CD9 and were allowed to invade a confluent EC monolayer. Cellular distribution of CD9-GFP was assessed by time-lapse confocal microscopy every 3 minutes. The focal plane corresponding to TC-EC contact is shown (Z = 0 μm). At 0 and 24 minutes, a projection of the fluorescence signal of the different sections and of the DIC image is shown. (B). ECs were transfected with GFP-tagged CD9 and allowed to grow onto collagen gels to confluence. A375 melanoma cells were allowed to invade the EC monolayer, and the distribution of endothelial CD9-GFP was observed by confocal microscopy. Upper panels show a representative EC-TC interaction confocal section. Reinforcement of the signal because of CD9-GFP relocalization is indicated by arrowheads at EC-EC contact sites by arrows at EC-TC heterotypic junctions. Lower panels show a time-lapse experiment of the TC insertion. Merging of fluorescence and DIC images is shown. Note that no redistribution of CD9 is seen at initial time points where TC adheres to luminal surfaces of ECs but only at the time of insertion (10-20 minutes, arrows). The processes in panels A and B are also depicted in 2 video sequences on the Blood website (see the Supplemental Videos link at the top of the online article).
In parallel studies, EC cells were transfected with the CD9-GFP construction (Figure 6B). CD9 appeared diffusely on the endothelial cell surface, and only in some fields did it concentrate at EC-EC contact sites (upper panel arrowheads). When melanoma cells were layered on top, endothelial CD9 clearly redistributed to TC-EC contacts at the time of insertion (Figure 6B, upper and lower panels 10-20 minutes, arrows). These data support a role for endothelial CD9 in EC heterotypic adhesion with melanoma cells during transmigration.
Discussion
The spread of cancer cells from a primary tumor to a site of metastasis formation involves multiple steps, including migration of TCs through the surrounding stroma, entry into the circulatory system, and arrest, extravasation, and growth at a distant secondary site.2 Metastasizing TCs accomplish 2 rounds of vessel wall invasion. First, during intravasation, TCs invade the basal lamina and migrate across ECs lining the capillaries that service the tumor to the vascular space. Second, during extravasation, blood-borne TCs bind a specific ligand on the surfaces of ECs and transmigrate across ECs and the basal lamina into the different tissues.3 TC-EC interactions may participate in the metastatic process during TC intravasation into the vascular space and during TC extravasation into tissues. Either distinct or common molecular mechanisms could be involved in direct (luminal to abluminal) and the reverse (abluminal to luminal) TC transendothelial invasion.
Because little is known about the molecular interactions between TCs and ECs during transendothelial migration of cancer cells, we established several model systems that allow the in vitro characterization of this process. We used either a 3-dimensional system that allows the complete migration of TCs under the EC monolayer into the subendothelial collagen matrix or a 2-dimensional system that prevents the complete migration of the TCs and creates a TC-EC mosaic monolayer. These cocultures were used to analyze, by immunofluorescence staining and confocal microscopy, the subcellular distribution of vascular junctional molecules at sites of TC-EC contact. Using this approach, several members of the tetraspanin family—including CD9, CD81, and CD151—were found to be concentrated at the TC-EC contact regions. When TC partially disrupted the EC monolayer, these molecules did not concentrate at EC cell borders around the gap; thus, the redistribution of CD9, CD81, and CD151 at sites of TC insertion into the EC monolayer appears to be dependent on TC-EC intercellular contact. Moreover, when CD9 GFP was expressed by the ECs, a redistribution of the fluorescence molecule was observed at sites of TC-EC contact during tumor cell transmigration.
Our results show that TCs transmigrate through an EC monolayer by paracellular routes, spreading into the subendothelial matrix. During their passage, TC lateral borders were closely apposed to the adjacent edges of ECs, indicating tight association between TCs and ECs. TC-EC interactions appeared to be critical for vascular dissemination of TCs as assessed in the B16 melanoma mouse model.23 In this in vivo model, the ability of TCs to spontaneously metastasize was associated with the TC expression of connexin 26 and the establishment of heterologous gap junctions with ECs. Interestingly, the formation of TC-EC gap junctions was highly dependent on EC culture conditions, which was optimal on a vein segment23 or when ECs were cultured on collagen matrix (P.S.-M., unpublished observations, 1999). Similarly, EC junctions are stained with silver only when cells are grown on 3-dimensional matrix or cross-linked gelatin.35,36 Under these conditions, resembling those of the vascular intima found in vivo, TCs migrate through the EC lateral junctions interacting with the ECs; thus, it is an appropriate model to study TC-EC molecular interactions. Most works on the transendothelial migration of cancer cells show the formation of gaps in the EC monolayer that allow direct access of TCs to the exposed subendothelial matrix.21,22,37-39 In this regard, it has been reported that TC-EC interactions induce a rapid EC-EC dissociation, which correlates with a dramatic loss of VE-cadherin staining around the TCs.34 In contrast, only local changes in VE-cadherin staining at sites of TC-EC contact were reported using a 3-dimensional EC culture system on matrigel,40 in accordance with our results with collagen gels. These differences may be explained either by the EC culture conditions or by differences between TCs in their ability to interact with ECs. Reversible focal changes in VE-cadherin complex have recently been described during the transendothelial migration of monocytes.41 It is conceivable that TC transmigration shares some properties with leukocyte transmigration; hence, an active TC interaction with the EC may trigger the focal loss of VE-cadherin.
Notably, our data on the identification of several members of the tetraspanin superfamily, such as CD9, CD81, and CD151, as molecules that localize along the TC-EC contact area indicated that they can play a crucial role in TC transendothelial migration. Thus, during transmigration, TCs may establish dynamic heterologous junctional structures with ECs containing TM4SF/α3β1, whereas other molecules such as VE-cadherin/β-catenin, PECAM-1, and ZO-1 do not seem to participate in TC-EC intercellular junctions. Several reports have described the role of members of the tetraspanin family in homotypic intercellular adhesion.42-46 Our data further extend their role to heterotypic intercellular interactions. In this regard, tetraspanin proteins have been shown to localize at intercellular junctions independently of cadherin-mediated adhesion.6 In addition, it has been recently described that cancer cells are inserted between ECs in the vessel walls of some human tumors.4,5 Although the mechanisms involved in this phenomenon are unknown, our data indicate that TC invasion of an endothelial monolayer may produce a TC-EC mosaic monolayer. Apparently, these mosaic vessels are abundant in human tumors, involving 4% of the total vascular surface in some colon carcinomas.5 The dynamic of the TC-EC interactions in these mosaic vessels in vivo remains to be established, as does whether these TC-EC mosaic vessels may increase the frequency of metastasis, facilitating the release of exposed TCs from the vessel wall into the lumen. Thus, molecules such as CD9, CD81, and CD151, involved in TC-EC intercellular interactions, may participate during mosaic vessel formation in tumor angiogenesis and during TC transendothelial migration in metastasis. Interestingly, CD9 expression becomes down-regulated during melanoma progression.10 This lower expression of CD9 may diminish the adhesive strength between TCs and ECs, eventually facilitate the process of TC detachment, and contribute to enhancing the risk for melanoma metastatic spread.
Our data on the inhibitory effect on TC transmigration exerted by several anti-CD9 mAbs provide demonstrative evidence of the involvement of CD9 in the TC transmigration process. A possible mechanism accounting for the inhibition of TEM could reside in the anti-CD9–mediated enhancement of TC adhesion to EC monolayers and the promotion of a strong heterotypic TC-EC adhesion, without affecting adhesion to extracellular matrix proteins (P.S.-M. et al, unpublished observations, 1999). Interestingly, tetraspanins are associated with different β1 integrins and may regulate their function in an affinity-independent manner.6 Thus, our data suggest that anti-CD9 mAbs selectively block the transcellular migration step, likely by enhancing intercellular adhesion, that might involve associated αβ integrin. Furthermore, the CD9 inhibitory effect on transendothelial migration is much stronger than its inhibitory effect in cell migration in the absence of endothelium. Our data on time-lapse videomicroscopy support a predominant role for endothelial CD9 in TC-EC interactions. Overexpressed CD9 showed only a mild relocalization to EC-EC intercellular contacts, probably because of competition with the endogenous CD9. Nevertheless, it clearly marked heterotypic TC-EC intercellular contacts. However, overexpression of CD9 in melanoma cells neither affected migration through the endothelial monolayer (not shown) nor clearly relocalized to the contact sites with the endothelium. Again, because melanoma cells are high-expressing CD9 cells, competition with the endogenous antigen might mask the effect of CD9 overexpression in melanoma cells.
Members of the tetraspanin superfamily of proteins usually become down-regulated in metastatic tumors,9-13,46 and the transfection of CD9, CD63, or CD82 reduces metastasis in vivo.15-17 Inhibition in either bare cell migration or cell growth had been previously invoked to explain the reduction in metastatic potential of tetraspanin-overexpressing TCs, and a direct involvement in extravasation and colonization of secondary sites has only been suggested for CD151.14 Our data support a new mechanism for tetraspanin regulation of tumor cell metastasis independent of tumor cell growth or motility, and they highlight a role for endothelial CD9 in active recognition of TCs during insertion. At any rate, the contribution to the in vivo suppression of metastasis of the inhibitory effect of transcellular migration deserves further investigation.
We thank J. Villarejo and I. Treviño for their technical assistance, the members of Servicio de Obstetricia, Hospital General Universitario Gregorio Marañón for the material provided, and M. A. Olazcarizqueta for technical assistance with confocal microscopy. We also thank Dr José Luis Rodrı́guez for critical reading of the manuscript.
Supported by grants SAF96-0092 from Comisión Interministerial de Ciencia y Tecnologı́a and PM98-0016 from Dirección General de Enseñanza Superior e Investigación Cientı́fica (P.S.-M.); and SAF99-0034-CO2-01 from the Ministerio de Educación y Cultura and QLRT-1999-01036 from the European Community (F.S.-M.).
G.R. is the recipient of a fellowship from the Fundación Cientı́fica de la Asociación Española Contra el Cáncer.
N.L. and M.Y.-M. contributed equally to this paper.
The online version of the article contains supplemental videos.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paloma Sánchez-Mateos, Hospital Gregorio Marañón, Servicio de Inmunologı́a. C/Dr Esquerdo 46, 28007 Madrid, Spain; e-mail: inmunoonc@hispacom.es.