Abstract
Recent data suggest that mast cells (MCs) in patients with systemic mastocytosis or mast cell leukemia express a CD2-reactive antigen. To explore the biochemical nature and function of this antigen, primary MCs as well as the MC line HMC-1 derived from a patient with mast cell leukemia were examined. Northern blot experiments revealed expression of CD2 messenger RNA in HMC-1, whereas primary nonneoplastic MCs did not express transcripts for CD2. In cell surface staining experiments, bone marrow (BM) MCs in systemic mastocytosis (n = 12) as well as HMC-1 cells (30%-80%) were found to express the T11-1 and T11-2 (but not T11-3) epitopes of CD2. By contrast, BM MCs in myelodysplastic syndromes and nonhematologic disorders (bronchiogenic carcinoma, foreskin phimosis, uterine myeomata ) were consistently CD2−. All MC species analyzed including HMC-1 were found to express LFA-3 (CD58), the natural ligand of CD2. To study the functional role of CD2 on neoplastic MCs, CD2+ and CD2− HMC-1 cells were separated by cell sorting. CD2+ HMC-1 cells were found to form spontaneous aggregates and rosettes with sheep erythrocytes in excess over CD2−cells, and a T11-1 antibody inhibited both the aggregation and rosette formation. Moreover, exposure of CD2+ HMC-1 cells to T11-1 or T11-2 antibody was followed by expression of T11-3. In addition, stimulation of neoplastic MCs through T11-3 and a second CD2 epitope resulted in histamine release. These data show that neoplastic MCs express functionally active CD2. It is hypothesized that expression of CD2 is associated with pathologic accumulation and function of MCs in systemic mastocytosis.
Introduction
Mastocytosis is a term collectively used for a heterogeneous group of disorders characterized by abnormal proliferation and accumulation of mast cells (MCs) in one or multiple organs.1-5 Cutaneous and systemic variants of the disease have been described.1-8 Cutaneous mastocytosis is usually detected in childhood and shows a benign course with spontaneous regression in many cases.8 Systemic mastocytosis (SM) may develop at any age throughout lifetime and is characterized by persistent disease and multiorgan involvement.1-7 The formation of multifocal dense aggregates of MCs in the bone marrow (BM) is the pathologic hallmark of SM.9 In a small group of patients, a malignant course or even mast cell leukemia (MCL) develops.1-5 9-12 Little is known, however, about the mechanisms that contribute to abnormal growth and accumulation of MCs or disease progression in patients with mastocytosis.
Recent data suggest that distinct gain-of-function mutations inC-KIT, a gene that encodes the tyrosine kinase receptor for stem cell factor (SCF, a major growth factor for MCs), are involved in the pathophysiology of mastocytosis. Such point mutations are associated with ligand-independent receptor activity and autonomous growth of mast cells.13,14 One of these mutations,Asp816Val (C-KIT), has been detected in most patients with SM and MCL15-17 as well as in the human MCL-derived cell line HMC-1.13 The emerging hypothesis is that the mutation-induced activation of the SCF receptor (KIT) is directly involved in the abnormal growth of MCs in patients with SM. The notion that patients with SM frequently exhibit C-KIT mutations also supports the concept that SM is a clonal neoplastic disease.15-17
A number of attempts have been made in the past to establish the surface receptor profile of MCs in patients with mastocytosis. In common with normal MCs, MCs in patients with SM or MCL express substantial amounts of KIT.12,18-23 Other surface receptors are also coexpressed on normal and neoplastic MCs.18-23 However, recent data suggest that several surface molecules are differentially expressed on neoplastic versus normal MCs.20-23 The observation that MCs in patients with SM or MCL react with antibodies against leukocyte function–associated antigen-2 (LFA-2, or CD2) is a remarkable finding. In fact, LFA-2 is expressed physiologically on thymocytes, activated T cells, and a subset of natural killer cells,24-32 whereas normal MCs and other normal myeloid cells apparently are CD2−.
The CD2 antigen (LFA-2) was originally characterized as a T-cell molecule that mediates sheep erythrocyte rosette formation.24 However, the CD2 molecule on T lymphocytes contains several distinct epitopes with specific functional properties.24-32 Moreover, these epitopes are variably expressed on T cells. Thus, the T11-1 and T11-2 epitopes of CD2 are expressed on both resting and activated T cells, whereas the T11-3 epitope is only expressed when T cells are activated by antigen or other stimuli.24-32 The T11-1 epitope on T cells is primarily involved in adhesive cell functions (including sheep erythrocyte binding), whereas the T11-3 epitope appears to be associated with cell activation and related biochemical processes.24-32
The aims of the present study were to analyze expression of CD2 in normal and neoplastic human MCs at the messenger RNA (mRNA) and protein level, to study the epitope composition of CD2 on MCs, and to examine the regulation and functional role of CD2 epitopes on MCs.
Materials and methods
Monoclonal antibodies
The following unconjugated monoclonal antibodies (mAbs) were used: 39C1.5 (CD2), 6F10.3 (CD2), B1.49.9 (CD25), and 1C3 (CD58) from Immunotech (Marseille, France); AB75 (CD2) from Novocastra (Newcastle, United Kingdom); RPA-2.10 (CD2), 7G3 (CD123), 9F5 (CD123), and YB5.B8 (CD117) from PharMingen (San Diego, CA); T11 (CD2) from Coulter (Miami, FL); SK3 (CD4), L17F12 (CD5), L178 (CD44), and S5.2 (CD2) from Becton Dickinson (San Jose, CA); and G3 (antitryptase) from Chemicon (Temecula, CA). The fluorescein isothiocyanate (FITC)–conjugated mAbs S5.2 (CD2) and 2A3 (CD25), phycoerythrin (PE)–labeled mAbs S5.2 (CD2), 8G12 (CD34), and 104D2 (CD117) as well as the peridinin chlorophyll protein–labeled mAb 2D1 (CD45) were from Becton Dickinson; FITC-conjugated mAb 39C1.5 (CD2) from Immunotech; PE-labeled YB5.B8 (CD117) and RPA-2.10 (CD2) from Pharmingen; and allophycocyanin (APC)–labeled mAb 57A5 (CD117) from Alexis (Läufelingen, Switzerland). The mAb VIT13 was produced at the Institute of Immunology (Vienna University).
Cytokines, buffers, and other reagents
Recombinant human (rh) SCF was purchased from Strathmann Biotech (Hannover, Germany); rh granulocyte colony-stimulating factor (G-CSF) from Hoffmann–La Roche (Basel, Switzerland); rh macrophage CSF (M-CSF) and rh interleukin-12 (rhIL-12) from R&D Systems (Minneapolis, MN); and rhIL-1α, rhIL-2 through rhIL-11, rhIL-13, and rhIL-15 from Peprotech (Rocky Hill, NJ). RhIL-1β was purchased from Pharma Biotechnology (Hannover, Germany); rhIL-16, rhIL-17, and rhIL-18 from Antigenix America (New York, NY); and rhGM-CSF was provided by Sandoz Vienna (Vienna, Austria). Collagenase type II was purchased from Sebak (Suben, Austria), RPMI 1640 medium and fetal calf serum (FCS) from PAA Laboratories (Linz, Austria), and Iscoves modified Dulbecco medium (IMDM), l-glutamine, penicillin, and streptomycin from Gibco Life Technologies (Gaithersburg, MD).
Isolation and purification of primary MCs
Primary MCs were isolated from BM, lung, foreskin, and uterus. In each case, informed consent was obtained from patients or parents (circumcision of juvenile foreskin) before surgery or BM biopsy. BM was obtained from the iliac crest of 13 patients with indolent SM and 8 with myelodysplastic syndromes (MDSs; subtypes: refractory anemia [RA], n = 2; RA with ringed sideroblasts [RARS], n = 1; RA with excess of blasts [RAEB], n = 2; RAEB in transformation [RAEB-T], n = 3). Diagnoses were established according to published criteria.5,33 None of the MDS patients exhibited focal dense infiltrates of BM MCs excluding a coexisting SM. BM was also obtained from 4 donors with reactive MC hyperplasia and 1 with normal BM. Aspirated BM was collected in preservative-free heparin, diluted 1:1 (vol/vol) in phosphate-buffered saline, and prepared for flow cytometry as described.20,21 BM mononuclear cells (MNCs) were isolated using Ficoll. Surgical specimens of lung (lobectomy; 15 patients with bronchiogenic carcinoma), uterus (uterine myomata, n = 5), and juvenile foreskin (n = 5) were collected and subjected to MC isolation as described.34 In brief, tissue was chopped, washed in Mg2+/Ca++-free Tyrode buffer, and then incubated in collagenase type II (2 mg/mL) at 37°C for 1 to 3 hours. Dispersed cells were recovered by filtration through Nytex cloth and washed. Cell suspensions were cultured at 37°C in RPMI 1640 medium with 10% FCS and antibiotics for at least 24 hours before analysis. In stimulation experiments, lung and uterus MCs were incubated in rhSCF (100 ng/mL) or control medium for up to 48 hours. In 7 donors, lung cells were further enriched for MC by elutriation as described.34 In brief, cells were loaded at a flow rate of 12 mL/min into a Beckman elutriator equipped with a JE-6B rotor (Beckman Instruments, Palo Alto, CA). Fractions were recovered at increasing flow rates (14, 18, 20, 30 mL/min). A selection was performed based on the content of MCs. In one donor, 2 fractions contained more than 90% MCs (total cell number 6 × 107). These MCs were cultured overnight (37°C, 5% CO2), washed, split, exposed to either rhSCF (100 ng/mL) or control medium for 2 hours (37°C), and then subjected to RNA isolation. In the other 6 donors, elutriated MCs were further enriched by cell sorting using anti-KIT mAb YB5.B8.34 MCs were sorted as KITbright cells and were more than 98% pure after isolation. The 6 lung MC preparations were pooled (total cell number 7 × 106) and used for Northern blot experiments.
Culture of HMC-1 cells and sorting of CD2+ and CD2− subpopulations
The human MC line HMC-1 35 was a kind gift from J. H. Butterfield (Mayo Clinic, Rochester, MN). HMC-1 cells were cultured in Iscoves modified Dulbecco medium containing 10% FCS, l-glutamine, and antibiotics at 37°C. Because initial experiments revealed the presence of 2 subsets of HMC-1—namely, a CD2+ and a CD2−subpopulation—sorting experiments were conducted to separate these 2 cell subsets using the PE-conjugated mAb RPA-2.10 (CD2) and a FACStar Plus (Becton Dickinson). In stimulation experiments, HMC-1 cells (subpopulations and unseparated) were exposed to CD2 antibodies (39C1.5, 6F10.3, VIT13; 3-6 μg/mL), rh cytokines (SCF, 10-1000 ng/mL; other cytokines, 100 ng/mL), or control medium for 1 to 48 hours (37°C).
Culture of human MCs from cord blood cell–derived progenitors
To analyze expression of CD2 on immature “nonneoplastic” MCs, we cultured MCs from their CD34+ cord blood progenitors. Cord blood was obtained from full-term deliveries (n = 11) after informed consent was given by mothers. MNCs were obtained using Ficoll and enriched for CD34+ cells by mAb QBEND/10 and magnetic cell sorting (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) as described.36 After MACS, CD34+ cells were further enriched by fluorescence-activated cell sorting (FACS) using mAb 8G12 (CD34) and a FACS Vantage SE (Becton Dickinson). The resulting purity of CD34+ cord blood MNCs was more than 98%. The MC differentiation assay was performed according to published techniques.37-39 In brief, cells were cultured in RPMI 1640 medium containing 10% FCS,l-glutamine, antibiotics, and rhSCF (100 ng/mL) for up to 63 days (37°C, 5% CO2). During the first 7 days, SCF was used as the single growth factor. Thereafter, cells were divided: One part was maintained in rhSCF (100 ng/mL) and rhIL-6 (100 ng/mL) and the other part in rhSCF (100 ng/mL), rhIL-4 (10 U/mL), rhIL-6 (100 ng/mL), and rhIL-10 (10 ng/mL). Cultures were fed in 2-week intervals. On days 21, 42, and 63, cultured cells were examined for viability, cell number, morphology, and expression of CD2 and other antigens.
Cell surface staining techniques
HMC-1 and cultured cord blood MNC-derived MCs were analyzed by single-color flow cytometry. Surface marker expression on lung and uterine MCs was analyzed by 2-color staining using unconjugated “first-step” mAb, FITC-conjugated “second-step” antibody, and PE-labeled CD117 mAb as described.40 Expression of surface antigens on BM MCs was determined by 3- or 4-color staining using conjugated mAb (FITC: CD2, CD25; PE: CD2, CD117; peridinin chlorophyll protein: CD45; APC: CD117) as reported.20-23,41 In brief, 1.5 × 106 cells were incubated with combinations of mAb (CD2/CD117/CD45 or CD25/CD117/CD45) for 10 minutes. Erythrocytes were then lysed in FACS lysing solution (Becton Dickinson). After washing, cells were examined by flow cytometry on a FACSCalibur or FACScan (Becton Dickinson). Staining reactions were controlled using isotype-matched mAb. To analyze surface marker expression on single metachromatic MCs (lung, n = 7; foreskin, n = 5; uterus, n = 4; mastocytosis-BM, n = 3; cultured MCs), a toluidine blue/immunofluorescence (TB/IF) double-staining technique was applied.42 43 Briefly, cells were incubated with mAb for 30 minutes (4°C), washed, and exposed to FITC-conjugated goat antimouse antibody. After washing, cells were fixed in glutaraldehyde (0.025%) for 1 minute, washed, and stained with TB (0.0125%) for 8 minutes. Then, cells were again washed and examined under a fluorescence microscope.
In situ staining experiments
To confirm expression of CD2 in neoplastic MCs (focal BM infiltrates in SM), in situ staining was performed on BM sections (SM, n = 8; RAEB, n = 2; MC hyperplasia, n = 3). BM biopsy specimens were fixed in formalin, decalcified in ethylenediaminetetraacetic acid, and embedded in paraffin. Immunohistochemistry was performed on serial BM sections (2 μm) by avidin-biotin-immunoperoxidase staining technique.44-46 Endogenous peroxidase was blocked with 0.3% H2O2. The mAbs AB75 (CD2) and G3 (antitryptase) were diluted (AB75: 1:20; G3: 1:5000) in TBS with 1% bovine serum albumin (Behring, Marburg, Germany) and applied for 60 to 90 minutes. For CD2 staining, sections were heated in an autoclave prior to antibody exposure. Isotype-matched mAbs were used as negative control. After exposure to first-step mAbs, slides were washed and incubated with biotinylated horse antimouse immunoglobulin G (IgG) (Vector Laboratories, Burlingame, CA) for 30 minutes. After washing, slides were exposed to avidin-biotin-peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories) for 30 minutes. DAB (CD2) or AEC (antitryptase) was used as chromogen. Slides were counterstained in Mayer hemalum and mounted. Double immunohistochemistry was also performed. For this purpose, CD2-stained slides were photographed, remounted, washed, and then stained with antitryptase mAb G3 (1:5000). In a separate set of experiments, serial sections of lesional skin obtained from 3 patients with cutaneous mastocytosis and 3 with SM were examined. Immunocytochemistry was performed on HMC-1, lung MCs, and cultured MCs. HMC-1 and lung MCs were exposed to control medium or rhSCF (100 ng/mL) for 1 to 48 hours. After incubation, cells were spun on cytospin slides, fixed in acetone, washed, and incubated with CD2 mAb 6F10.3 (1:20) or antitryptase mAb G3 (1:5000) for 60 minutes. Slides were then washed and incubated with biotinylated horse antimouse IgG. Slides were again washed and exposed to streptavidin-biotin-alkaline phosphatase complex (Dako, Glostrup, Denmark) for 30 minutes. Antibody reactivity was made visible with vector red alkaline phosphatase substrate.
Northern blot analysis
Total RNA was extracted from untreated and SCF-treated lung MCs and HMC-1 cells by the guanidinium isothiocyanate/cesium chloride extraction technique.47 Northern blot analysis was performed as described.48,49 In brief, 10 μg RNA was size-fractionated on 1.2% agarose gels, transferred to synthetic membranes (Hybond N, Amersham, Little Chalfont, United Kingdom) with 20 × SSC (1 × SSC = 150 mM NaCl and 15 mM sodium citrate, pH 7.0) overnight, and cross-linked to membranes by UV irradiation (UV Stratalinker 1800, Stratagene, CA). RNA was prehybridized at 65°C for 4 hours in 5 × SSC, 7% sodium dodecyl sulfate (SDS), 10 × Denhardt solution (1 × Denhardt solution = 0.02% bovine serum albumin, 0.02% polyvinyl pyrolidone, and 0.02% Ficoll), 10% dextran sulfate, 20 mM sodium phosphate (pH 7.0), sonicated salmon sperm DNA (100 μg/mL), and poly(A)+ (100 μg/mL). Hybridization was performed with a 32P-labeled synthetic oligonucleotide probe specific for CD2 (29-mer: 5′-GCTGACAGGCTCGACACTGGATTCCTTGC-3′, gene position: base pairs 601-629).50 After stripping the blot, a probe specific for GAPDH (29-mer: 5′-CCATGGTGGTGAAGACGCCAGTGGACTCC-3′)51 was applied. All probes were from MWG Biotech (Ebersberg, Germany). Blots were washed once in 3 × SSC, 5% SDS, 10 × Denhardt solution, and 20 mM sodium phosphate, pH 7.0, for 30 minutes at 65°C, and once in 1 × SSC and 1% SDS for 30 minutes at 65°C. Bound radioactivity was visualized by exposure to XAR-5 films at −70°C using intensifying screens (Eastman Kodak, Rochester, NY).
Aggregation assay and sheep erythrocyte rosette formation
To demonstrate functional significance of CD2 expression, a sheep erythrocyte rosette formation assay was applied. For this purpose, primary BM MCs (SM, n = 2; reactive BM, n = 1) were highly purified (> 98% purity) by cell sorting using a CD117 mAb as described.34 In addition, HMC-1 subsets (CD2+and CD2−) were examined. Cells were incubated with sheep erythrocytes (Behring) at 37°C for 15 minutes. Cells were then centrifuged at 200g and incubated at 4°C for 18 hours. In blocking experiments, CD2+ HMC-1 cells were preincubated with the T11-1 antibody 39C1.5 (100 μg/mL), isotype-matched control antibody, or control medium for 30 minutes (4°C) and then washed prior to incubation with sheep erythrocytes. Sheep erythrocyte rosette formation and formation of aggregates was analyzed using an inverted microscope (Olympus, Vienna, Austria).
Histamine release experiments
Histamine release experiments were conducted using BM MNCs (SM, n = 2; reactive BM, n = 2), unseparated HMC-1, as well as sorted HMC-1 subsets (CD2+ vs CD2− subset). Histamine release was performed essentially as described.52 Cells were resuspended in histamine release buffer (Immunotech) and adjusted to a final cell concentration of 0.5 × 106/mL. Cells were incubated with mAb 39C1.5 (T11-1) (3 μg/mL), 6F10.3 (T11-2) (3 μg/mL), or VIT13 (T11-3) (3 μg/mL), a combination of mAbs (3 or 6 μg/mL of each mAb), or control buffer for 3 hours at 37°C. After incubation, cells were centrifuged and the cell-free supernatants recovered. Liberated histamine was measured by radioimmunoassay (Immunotech). Total histamine was quantified in cell lysates. Histamine release was expressed as percentage of total histamine.
Statistical analysis
To determine the levels of significance, appropriate statistical tests including the paired Student t test and ANOVA were applied. Results were considered to be statistically significant atP < .05.
Results
Evaluation of CD2 expression on BM MCs
Three different staining techniques were applied to investigate CD2 expression in BM MCs in patients with SM, MDSs, and normal or reactive BM. As assessed by multicolor flow cytometry, the KIT+ BM MCs in patients with SM (n = 12) stained positive with CD2 mAb S5.2 and CD25 mAb 2A3 (Figure1 and Table1). Moreover, BM MCs in SM were found to react with the epitope-specific CD2 mAbs 39C1.5 (T11-1) and 6F10.3 (T11-2) but did not react with the T11-3–specific mAb VIT13. BM MCs in MDSs (n = 6) or normal or reactive BM (n = 3) were not recognized by any of the CD2 mAbs applied. Expression of CD2 on BM MCs in SM was confirmed by combined TB/IF staining. In these experiments, TB+ BM MCs were recognized by mAbs against KIT, CD25, and CD58 as well as by the CD2 mAbs 39C1.5, 6F10.3, S5.2, and T11 but not by mAb VIT13 (T11-3). These data show that BM MCs in SM express T11-1 and T11-2 but not T11-3 epitopes of CD2. In a next step, CD2 expression in BM MCs was analyzed by double immunohistochemistry. These experiments revealed expression of CD2 in spindle-shaped neoplastic tryptase-positive BM MCs (Figure 2). Serial section staining confirmed expression of CD2 in BM MCs in SM. In MDSs and normal or reactive BM, MCs did not stain positive for CD2. These results suggest that CD2 (T11-1/T11-2) is selectively expressed in BM MCs in patients with SM. A summary of staining results is shown in Table 1.
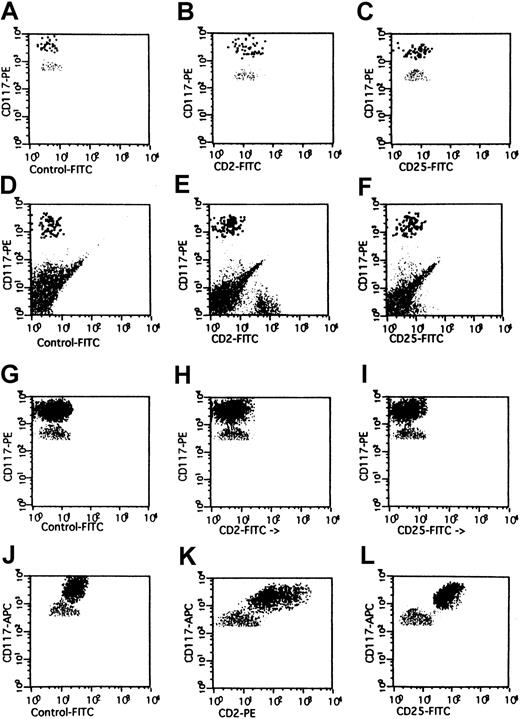
Expression of CD2 on BM MCs in mastocytosis.
Multicolor flow cytometry analysis of expression of CD2 on human BM MCs. BM MCs were obtained from a healthy donor (A-C), one with reactive MC hyperplasia (D-F), one with MDS/RA (G-I), and one with SM (J-L). Expression of CD2 and CD25 on KITbright MCs was analyzed by multicolor staining. Isotype-matched control mAbs (A,D,G,J), the CD2 mAb S5.2 (B,E,H,K), and the CD25 mAb 2A3 (C,F,I,L) were applied. In the patient with SM, BM MCs were found to react with mAbs against CD2 (K) and CD25 (L). By contrast, BM MCs in RA as well as in the control cases were not recognized by CD2 mAb or CD25 mAb.
Expression of CD2 on BM MCs in mastocytosis.
Multicolor flow cytometry analysis of expression of CD2 on human BM MCs. BM MCs were obtained from a healthy donor (A-C), one with reactive MC hyperplasia (D-F), one with MDS/RA (G-I), and one with SM (J-L). Expression of CD2 and CD25 on KITbright MCs was analyzed by multicolor staining. Isotype-matched control mAbs (A,D,G,J), the CD2 mAb S5.2 (B,E,H,K), and the CD25 mAb 2A3 (C,F,I,L) were applied. In the patient with SM, BM MCs were found to react with mAbs against CD2 (K) and CD25 (L). By contrast, BM MCs in RA as well as in the control cases were not recognized by CD2 mAb or CD25 mAb.
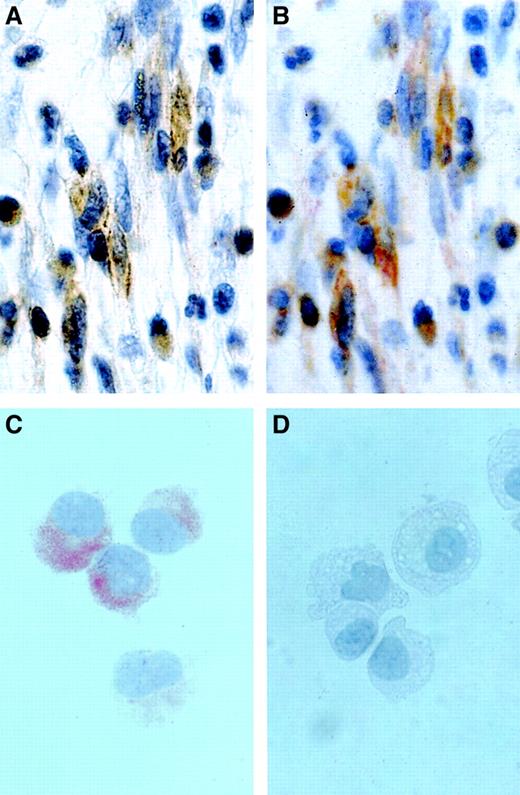
Detection of CD2 in neoplastic MCs by in situ immunostaining.
(A,B) Immunohistochemical detection of CD2 in BM MCs in a patient with SM. The BM biopsy section was first stained with CD2 mAb AB75 (A), demounted, and then stained with mAb G3 against tryptase (B). As visible, the spindle-shaped tryptase-positive MCs were found to be immunoreactive for CD2. Original magnification approximately × 500. (C,D) Immunocytochemistry of HMC-1 cells. Sorted HMC-1 subsets, ie, CD2+ HMC-1 cells (C) and CD2− HMC-1 cells (D), were spun on cytospin slides and stained with CD2 mAb 6F10.3 as described in the text. Immunocytochemistry confirmed expression of the CD2 protein in the cytoplasm of CD2+ HMC-1 cells and absence of (cytoplasmic) CD2 in the surface CD2− HMC-1 cells. Note granular staining pattern of CD2+ HMC-1 cells. Original magnification approximately × 250.
Detection of CD2 in neoplastic MCs by in situ immunostaining.
(A,B) Immunohistochemical detection of CD2 in BM MCs in a patient with SM. The BM biopsy section was first stained with CD2 mAb AB75 (A), demounted, and then stained with mAb G3 against tryptase (B). As visible, the spindle-shaped tryptase-positive MCs were found to be immunoreactive for CD2. Original magnification approximately × 500. (C,D) Immunocytochemistry of HMC-1 cells. Sorted HMC-1 subsets, ie, CD2+ HMC-1 cells (C) and CD2− HMC-1 cells (D), were spun on cytospin slides and stained with CD2 mAb 6F10.3 as described in the text. Immunocytochemistry confirmed expression of the CD2 protein in the cytoplasm of CD2+ HMC-1 cells and absence of (cytoplasmic) CD2 in the surface CD2− HMC-1 cells. Note granular staining pattern of CD2+ HMC-1 cells. Original magnification approximately × 250.
Evaluation of CD2 expression on primary MCs obtained from extramedullary organs
The phenotype of MCs may depend on the organ/tissue environment. We therefore asked if CD2 could be a marker of organ-specific phenotypic heterogeneity of MCs. To address this question, we analyzed expression of CD2 in primary nonneoplastic MCs in extramedullary organs. MCs were isolated from lung, uterine tissue, and juvenile foreskin. As assessed by multicolor flow cytometry, KIT+MCs in various organs expressed CD58 but did not express detectable amounts of CD2 (Table 2). Identical results were obtained by combined TB/IF staining. In fact, MCs from lung, uterus, and foreskin reacted with mAbs against KIT and CD58 but did not react with CD2 mAbs (Table 2). In a separate set of experiments, CD2 expression in MC in lesional skin in patients with SM (n = 3) or cutaneous mastocytosis (n = 3) was analyzed by immunohistochemistry. In all patients, skin MCs stained positive for tryptase. In SM, skin MCs were also found to react with CD2 antibody AB75, whereas skin MCs in cutaneous mastocytosis were found to be CD2−. Thus, expression of CD2 in MC seems to be a disease-related phenomenon but not determined by a specific environment.
Evaluation of CD2 expression on immature cultured MCs
We next asked whether CD2 is expressed at an early stage of MC development. To address this question, cultured immature (nonneoplastic) MCs derived from their CD34+ cord blood progenitors were examined. MC progenitors were cultured in the presence of SCF+IL-6 or SCF+IL-6+IL-4+IL-10 and analyzed on days 21, 42, and 63. As assessed by flow cytometry, cultured MCs were found to react with mAb against KIT and CD58 (Table 3). However, independent of the culture condition applied, MCs failed to react with CD2 mAbs (Table 3). Cultured MCs were also found to be negative for CD25 (IL-2Rα) and CD123 (IL-3Rα).
Detection of CD2 mRNA in neoplastic MCs
To study CD2 expression in normal and neoplastic MC at the mRNA level, Northern blot experiments were performed using a CD2-specific oligonucleotide probe and RNA of unstimulated or SCF-stimulated lung MCs and unstimulated or SCF-stimulated HMC-1 cells. HMC-1 cells were found to express CD2 mRNA in a constitutive manner (Figure3). By contrast, lung MCs did not express detectable transcripts for CD2 (Figure 3). Incubation of HMC-1 cells with rhSCF (100 ng/mL, 2 hours) did not result in a change of expression of CD2 mRNA. SCF also failed to induce expression of CD2 mRNA in lung MCs.
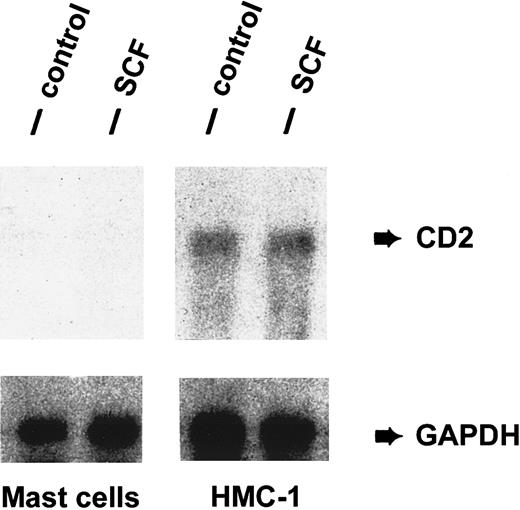
Detection of CD2 mRNA in HMC-1 cells.
Expression of CD2 mRNA in purified human lung MCs and HMC-1 cells was analyzed by Northern blot experiments using an oligonucleotide probe specific for CD2. Prior to RNA extraction, cells were cultured in the presence (SCF) or absence (control) of rhSCF, 100 ng/mL (37°C, 2 hours). Cells were then harvested and RNA prepared for Northern blotting. As visible, HMC-1 cells expressed CD2 mRNA in a constitutive manner, whereas primary lung MCs did not express detectable amounts of CD2 mRNA. SCF failed to induce expression of CD2 mRNA in lung MCs and did not modulate expression of CD2 mRNA in HMC-1 cells. The GAPDH control is also shown.
Detection of CD2 mRNA in HMC-1 cells.
Expression of CD2 mRNA in purified human lung MCs and HMC-1 cells was analyzed by Northern blot experiments using an oligonucleotide probe specific for CD2. Prior to RNA extraction, cells were cultured in the presence (SCF) or absence (control) of rhSCF, 100 ng/mL (37°C, 2 hours). Cells were then harvested and RNA prepared for Northern blotting. As visible, HMC-1 cells expressed CD2 mRNA in a constitutive manner, whereas primary lung MCs did not express detectable amounts of CD2 mRNA. SCF failed to induce expression of CD2 mRNA in lung MCs and did not modulate expression of CD2 mRNA in HMC-1 cells. The GAPDH control is also shown.
Detection of CD2 epitopes on HMC-1 cells
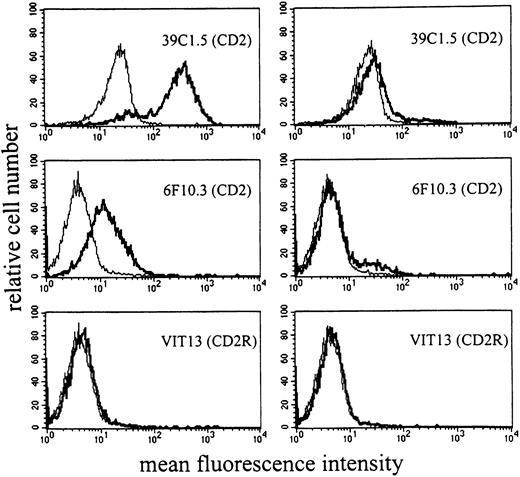
Six different CD2 mAbs were applied to detect the CD2 antigen on HMC-1 cells. As assessed by flow cytometry, unstimulated HMC-1 cells were recognized by all CD2 mAbs applied except VIT13 (T11-3) (Table 2). These data suggest that HMC-1 expresses T11-1 and T11-2 epitopes of CD2 but does not express T11-3. Interestingly, 2 distinct subpopulations of HMC-1 were detected by flow cytometry—namely, a distinctively CD2+ and a distinctively CD2− cell fraction. These 2 populations were seen with all CD2 mAbs applied (except VIT13) (Figure 4). The CD2+ fraction accounted for 30% to 80% of all HMC-1 cells.
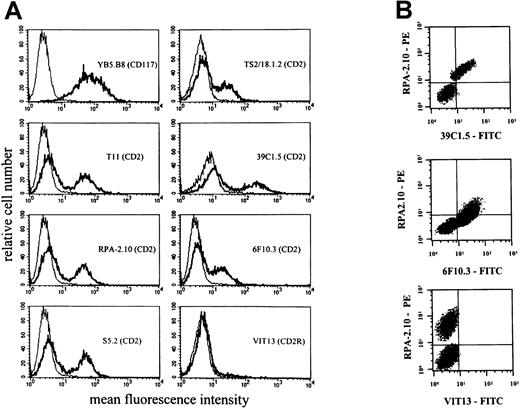
Expression of CD2 epitopes on HMC-1 cells.
HMC-1 cells were incubated with mAbs against different epitopes of CD2. (A) A single-color flow cytometry experiment. As visible, HMC-1 reacted with various CD2 mAbs, including those directed against T11-1 (39C1.5) and T11-2 epitopes (6F10.3). However, HMC-1 cells did not react with mAb VIT13 directed against the T11-3 epitope of CD2. (B) A multicolor staining experiment performed with the PE-labeled mAb RPA-2.10, the FITC-labeled mAb 39C1.5, FITC-labeled mAb 6F10.3, and FITC-labeled mAb VIT13. Multicolor staining confirmed that the CD2 epitopes T11-1 (39C1.5) and T11-2 (6F10.3) are coexpressed on the same (identical) subset of HMC-1.
Expression of CD2 epitopes on HMC-1 cells.
HMC-1 cells were incubated with mAbs against different epitopes of CD2. (A) A single-color flow cytometry experiment. As visible, HMC-1 reacted with various CD2 mAbs, including those directed against T11-1 (39C1.5) and T11-2 epitopes (6F10.3). However, HMC-1 cells did not react with mAb VIT13 directed against the T11-3 epitope of CD2. (B) A multicolor staining experiment performed with the PE-labeled mAb RPA-2.10, the FITC-labeled mAb 39C1.5, FITC-labeled mAb 6F10.3, and FITC-labeled mAb VIT13. Multicolor staining confirmed that the CD2 epitopes T11-1 (39C1.5) and T11-2 (6F10.3) are coexpressed on the same (identical) subset of HMC-1.
Separation and properties of CD2+ and CD2−HMC-1 cells
In 3 independent experiments, CD2+ and CD2− HMC-1 cells were separated from each other by cell sorting and cultured separately for 140 days. In all 3 experiments, both cell populations showed comparable survival and growth characteristics as well as comparable morphologic features. After sorting, most of the surface CD2+ HMC-1 cells appeared to be CD2+ by immunocytochemistry, whereas the surface CD2− cells were all CD2− in their cytoplasm (Figure 2C,D). During the entire culture period (140 days), HMC-1 sorted for CD2 remained all CD2+, and the sorted CD2− cells all remained CD2− (Figure5).
Antibody reactivity of sorted HMC-1 fractions.
After sorting for CD2+ and CD2− cells, the HMC-1 subpopulations were kept in culture for 140 days. On day 42, cells were incubated with mAbs directed against the T11-1 epitope (39C1.5), T11-2 epitope (6F10.3), and T11-3 epitope (VIT-13) of CD2 and analyzed by flow cytometry. As visible, the cells retained their phenotype after long-term culture. In fact, the HMC-1 cell subset sorted for CD2 (left panel) remained T11-1+ and T11-2+, whereas the CD2− HMC-1 fraction still was T11-1− and T11-2− on day 42. The same result was obtained after keeping HMC-1 cell subpopulations in culture for 140 days.
Antibody reactivity of sorted HMC-1 fractions.
After sorting for CD2+ and CD2− cells, the HMC-1 subpopulations were kept in culture for 140 days. On day 42, cells were incubated with mAbs directed against the T11-1 epitope (39C1.5), T11-2 epitope (6F10.3), and T11-3 epitope (VIT-13) of CD2 and analyzed by flow cytometry. As visible, the cells retained their phenotype after long-term culture. In fact, the HMC-1 cell subset sorted for CD2 (left panel) remained T11-1+ and T11-2+, whereas the CD2− HMC-1 fraction still was T11-1− and T11-2− on day 42. The same result was obtained after keeping HMC-1 cell subpopulations in culture for 140 days.
Sheep erythrocyte rosette formation of CD2+MCs
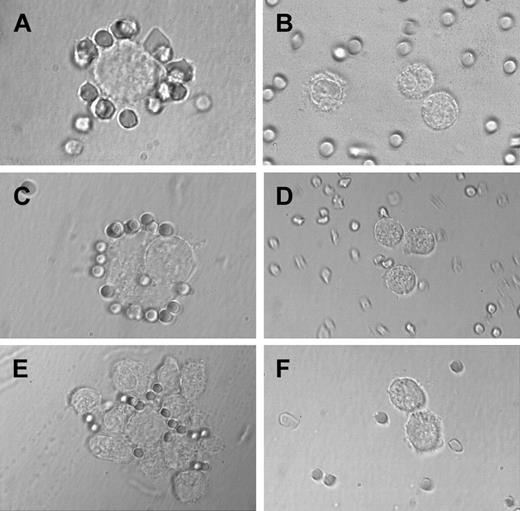
The CD2 antigen was originally described as sheep erythrocyte receptor. In the present study, primary BM MCs and HMC-1 cells were examined for their capacity to bind sheep erythrocytes in a specific manner. For this purpose, BM MCs of patients with SM (n = 2) and reactive marrow (n = 1) as well as HMC-1 cells (CD2+versus CD2− subset) were examined. In both patients with SM, BM MCs (50% and 70%, respectively) were found to form rosettes with sheep erythrocytes (Figure 6A), whereas no rosette formation was seen on MCs in the reactive marrow (< 10% of MC formed rosettes) (Figure 6B). Corresponding results were obtained with HMC-1 cells. In particular, when cultured in the presence of sheep erythrocytes, CD2+ HMC-1 cells were found to form significantly more rosettes than the CD2− subset (P < .05) (Figure 6C,D and Table4). In addition, the CD2+HMC-1 cells showed an increase in spontaneous formation of aggregates compared with CD2− cells (10%-30% increase). The increased sheep erythrocyte rosette and aggregate formation of CD2+ HMC-1 cells was inhibited by preincubation with mAb 39C1.5 directed against the T11-1 epitope of CD2, whereas no inhibition was seen with antibodies against T11-2 or T11-3.
Sheep erythrocyte rosette formation.
Highly purified BM MCs and HMC-1 cells (CD2+ and CD2− subsets) were incubated with sheep erythrocytes as described in the text. In the patient with SM, MCs were found to form rosettes with sheep erythrocytes (A), whereas no rosette formation was seen in a patient with reactive marrow (B). (C) A typical rosette of sheep erythrocytes on a CD2+ HMC-1 cell. In fact, in the CD2+ HMC-1 cell fraction, many sheep erythrocyte rosettes were found, whereas only few, if any, rosettes were found in the CD2− HMC-1 cell fraction (D). (E) A cluster of HMC-1 cells binding to sheep erythrocytes. In the same experiment, cluster and rosette formation were both inhibited by preincubating HMC-1 cells with the mAb 39C1.5 recognizing the T11-1 epitope of CD2 (F).
Sheep erythrocyte rosette formation.
Highly purified BM MCs and HMC-1 cells (CD2+ and CD2− subsets) were incubated with sheep erythrocytes as described in the text. In the patient with SM, MCs were found to form rosettes with sheep erythrocytes (A), whereas no rosette formation was seen in a patient with reactive marrow (B). (C) A typical rosette of sheep erythrocytes on a CD2+ HMC-1 cell. In fact, in the CD2+ HMC-1 cell fraction, many sheep erythrocyte rosettes were found, whereas only few, if any, rosettes were found in the CD2− HMC-1 cell fraction (D). (E) A cluster of HMC-1 cells binding to sheep erythrocytes. In the same experiment, cluster and rosette formation were both inhibited by preincubating HMC-1 cells with the mAb 39C1.5 recognizing the T11-1 epitope of CD2 (F).
Modulation of expression of CD2 epitopes on MCs
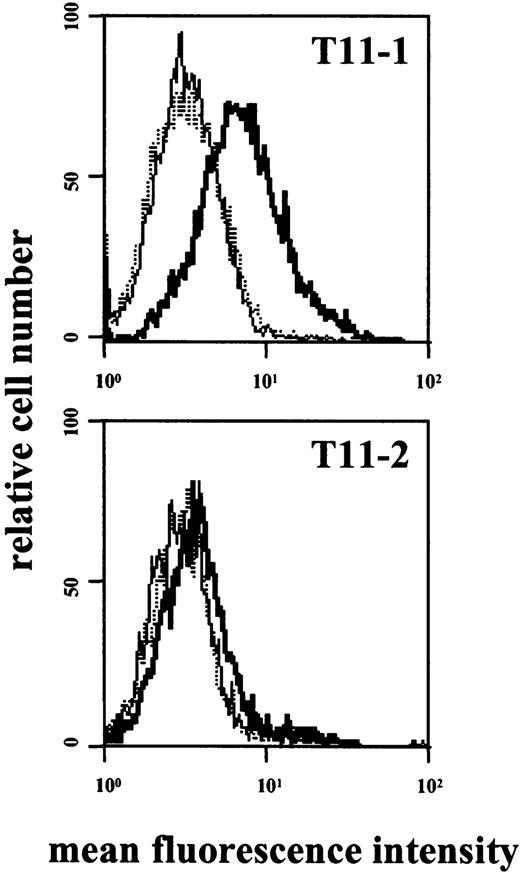
In line with previous observations,53 incubation of HMC-1 with rhSCF (100 ng/mL; 1-48 hours) resulted in decreased expression of KIT. In contrast, no difference in binding of CD2 mAbs to HMC-1 cells was seen after SCF incubation. The other cytokines tested (IL-1 through IL-18, GM-CSF, G-CSF, M-CSF) also failed to modulate expression of CD2 on HMC-1. We next asked whether expression of CD2 on tissue MCs is inducible by cytokines such as SCF. However, incubation of primary lung or uterine MCs with rhSCF was not followed by CD2 expression. In a next step, HMC-1 subsets were stimulated with epitope-specific mAbs. In these experiments exposure of CD2+ HMC-1 cells to mAb against T11-1 (39C1.5) or T11-2 (6F10.3) resulted in detectable expression of the T11-3 epitope (Figure7).
Expression of T11-3 on activated HMC-1 cells.
HMC-1 cells were incubated with mAb 39C1.5 directed against the T11-1 epitope of CD2 (top), with mAb 6F10.3 directed against the T11-2 epitope (bottom), or with control medium (dotted lines). Cells were incubated with mAb or control medium at 37°C for 6 hours. Cells were then washed and stained with FITC-conjugated T11-3 mAb VIT-13 (bold lines). As visible, incubation of HMC-1 cells with T11-1– or T11-2–specific mAbs was followed by expression of the T11-3 epitope. Interestingly, the amounts of expressed T11-3 were significantly higher in HMC-1 cells incubated with T11-1 mAb compared with stimulation with the T11-2 antibody. The dotted line indicates the background control (without antibodies).
Expression of T11-3 on activated HMC-1 cells.
HMC-1 cells were incubated with mAb 39C1.5 directed against the T11-1 epitope of CD2 (top), with mAb 6F10.3 directed against the T11-2 epitope (bottom), or with control medium (dotted lines). Cells were incubated with mAb or control medium at 37°C for 6 hours. Cells were then washed and stained with FITC-conjugated T11-3 mAb VIT-13 (bold lines). As visible, incubation of HMC-1 cells with T11-1– or T11-2–specific mAbs was followed by expression of the T11-3 epitope. Interestingly, the amounts of expressed T11-3 were significantly higher in HMC-1 cells incubated with T11-1 mAb compared with stimulation with the T11-2 antibody. The dotted line indicates the background control (without antibodies).
Effects of CD2 antibodies on histamine release
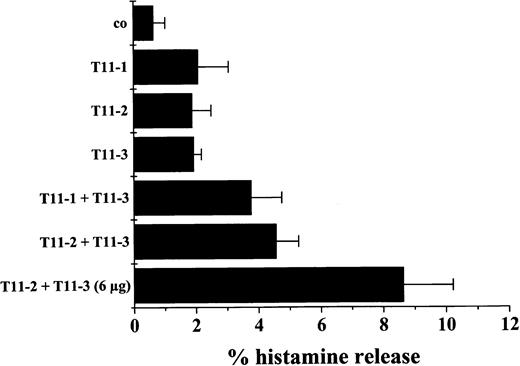
Activation of T cells through CD2 can be initiated by 2-epitope stimulation (T11-2, T11-3) using a mixture of specific mAbs. To examine whether activation of neoplastic MCs via CD2 is associated with cell activation, histamine release experiments were performed using BM MCs (SM, n = 2; control cases, n = 2) and HMC-1 subsets (CD2+ versus CD2−). Three different CD2 mAbs were applied. In 2 SM patients, exposure of BM MCs to a combination of CD2 mAbs against T11-3 and T11-1 or against T11-3 and T11-2 resulted in a substantial release of histamine above control (Figure8). A slight increase in release was also obtained by using only one CD2 mAb. By contrast, in the control cases (reactive BM), neither the mAb combinations nor the single mAbs applied were found to induced histamine release above control (not shown). Corresponding results were obtained with HMC-1 cells. In fact, incubation of CD2+ HMC-1 cells with a mixture of mAbs specific for T11-2 and T11-3 resulted in a significant increase in spontaneous histamine release (121% ± 32% of control, n = 6,P < .05), whereas no increase in histamine release in response to CD2 mAbs (alone or in combination) was seen when CD2− HMC-1 cells were examined (T11-2 plus T11-3: 89% ± 6% of control, n = 3, P > .05).
Induction of histamine release in MCs by CD2 stimulation.
MCs were isolated from the BM of a patient with SM and incubated with control medium (co), the T11-1–specific mAb 39C1.5 (3 μg/mL), T11-2–specific mAb 6F10.3 (3 μg/mL), the T11-3–specific mAb VIT13 (3 μg/mL), and combinations of mAbs as indicated. After 30 minutes of incubation (37°C), cells were centrifuged and the cell-free supernatants examined for the presence of histamine by radioimmunoassay. As visible, a combination of mAbs against T11-1 and T11-3 as well as a combination of T11-2– and T11-3–specific mAbs resulted in a marked increase in histamine release above control. A slight increase was also observed when cells were incubated with only one mAb. Corresponding results were obtained in a second mastocytosis patient. By contrast, in 2 reactive BMs, neither the antibody combinations nor the single mAb induced histamine release above control. Results represent the mean ± SD of triplicates.
Induction of histamine release in MCs by CD2 stimulation.
MCs were isolated from the BM of a patient with SM and incubated with control medium (co), the T11-1–specific mAb 39C1.5 (3 μg/mL), T11-2–specific mAb 6F10.3 (3 μg/mL), the T11-3–specific mAb VIT13 (3 μg/mL), and combinations of mAbs as indicated. After 30 minutes of incubation (37°C), cells were centrifuged and the cell-free supernatants examined for the presence of histamine by radioimmunoassay. As visible, a combination of mAbs against T11-1 and T11-3 as well as a combination of T11-2– and T11-3–specific mAbs resulted in a marked increase in histamine release above control. A slight increase was also observed when cells were incubated with only one mAb. Corresponding results were obtained in a second mastocytosis patient. By contrast, in 2 reactive BMs, neither the antibody combinations nor the single mAb induced histamine release above control. Results represent the mean ± SD of triplicates.
Discussion
Recent data suggest that MCs in patients with SM express a CD2-reactive surface antigen.20-23 In the present study, we have examined the biochemical nature and function of this antigen using primary MCs as well as the MC line HMC-1. The results of our study show that MCs in patients with SM as well as HMC-1 express T11-1 and T11-2 epitopes of CD2. Expression of CD2 was confirmed at the mRNA and protein level as well as by functional studies. In fact, primary neoplastic MCs as well as HMC-1 formed rosettes with sheep erythrocytes. Moreover, incubation of neoplastic MCs with T11-1 or T11-2 antibodies resulted in exposure of the T11-3 epitope, and costimulation through T11-3 resulted in substantial release of histamine. These data point to a functional role of CD2 epitopes on neoplastic MCs.
To provide evidence for specific expression of CD2 on MCs in systemic MC disease, we analyzed patients with SM, MDS, as well as normal or reactive BM. However, the CD2 antigen was only detectable on BM MCs in SM but not in other disease states. Selective expression of CD2 in SM was also confirmed by immunohistochemistry. All in all, CD2 expression appears to be a specific marker of systemic MC disorders.
The phenotype of MCs may depend on diverse factors, including the maturation stage of MCs and the tissue environment.18,42 43 We therefore examined CD2 expression on MCs in various organs as well as in cultured immature MC progenitors. In a first step, MCs in various organs were analyzed. However, CD2 was not detected on lung MCs, uterine MCs, or foreskin MCs in patients without mastocytosis. By contrast, in patients with SM and cutaneous involvement, skin MCs were found to be CD2+. These data suggest that CD2 expression in MCs is determined by the disease rather than by the tissue environment. In a next step we asked if CD2 expression occurs in an early phase of MC development. To address this question, immature MCs cultured from their CD34+ progenitor cells were examined. However, at all stages of differentiation analyzed, MC progenitors failed to express CD2. These data further confirmed that CD2 expression on MCs is a disease-specific abnormality.
The HMC-1 cell line is derived from a patient suffering from MCL.35 Like primary neoplastic MCs in SM, HMC-1 cells contain the C-KIT mutationAsp816Val.13 HMC-1 cells are also known to stain positive for CD2.54 In the present study, the HMC-1 cell line was applied as a useful tool for the investigation of expression and function of CD2 epitopes on neoplastic MCs. Like primary MCs in patients with SM, resting HMC-1 cells were found to react with antibodies against T11-1 and T11-2 epitopes of CD2 but did not react with T11-3 antibody. A remarkable finding was that HMC-1 is composed of 2 distinct subsets—namely, a CD2+ subset and a CD2− subset. In sorting experiments we were able to separate these 2 cell fractions. Unexpectedly, when separated, both subsets remained viable for a prolonged time and did not change their phenotype (CD2+ vs CD2−) after multiple passage. This is of particular interest because in patients with SM, neoplastic MC populations may also be CD2+ or, rarely, CD2− or may even consist of 2 phenotypically different subsets (CD2+ and CD2− subclones). However, the reason for the formation of CD2+ and CD2−MC subsets in SM patients and HMC-1 remains unknown. One possibility could be that neoplastic MCs acquire distinct gene abnormalities that lead to expression or loss of CD2 during clone evolution. In this regard it is noteworthy that the separated HMC-1 subsets (CD2+ and CD2−) both exhibited theC-KIT mutation Asp816Val (unpublished data, February 2001).
The typical formation of MC aggregates in the BM of patients with SM is the histological hallmark of the disease.1-5,9Because normal and neoplastic MCs consistently express CD58, the natural ligand of CD2, we were interested to know whether the CD2 antigen on neoplastic MCs would fulfill adhesive functions. For this purpose, we analyzed the formation of sheep erythrocyte rosettes as well as aggregate formation in sorted HMC-1 subsets. In these experiments, the CD2+ HMC-1 cell subset formed sheep erythrocyte rosettes and aggregates, whereas only few, if any, rosettes were detectable in CD2− cells. Moreover, preincubation of CD2+ HMC-1 cells with T11-1 antibody resulted in a significant decrease in rosette and aggregate formation. These data suggest that the T11-1 epitope of CD2 is involved in adhesive cell functions of neoplastic MCs. A similar function has been reported for the T11-1 epitope exposed on activated T cells.26-32Because MCs in patients with SM typically form clusters (aggregates) in the tissues, an attractive hypothesis would be that CD58-CD2–mediated homotypic aggregation is involved in the pathologic accumulation of MCs. This hypothesis was confirmed by the demonstration that primary BM MCs in patients with SM can also form rosettes with sheep erythrocytes. In addition to CD2, however, neoplastic MCs may also express other critical adhesion receptors.41,48 54
Little is known about the regulation of expression of CD2 in MCs. We have addressed this issue by incubating primary normal MCs and HMC-1 cells with a number of cytokines and CD2 antibodies. So far, we have not been able to identify a cytokine that would induce expression of CD2 in normal MCs, MC progenitors, or CD2− HMC-1 cells. We also were not able to see any changes in expression of T11-1 or T11-2 epitopes on CD2+ HMC-1 cells in response to the cytokines tested. We then asked if the T11-3 epitope can be upregulated in HMC-1 cells by epitope stimulation as has been described for T cells.24-32 In these experiments, exposure of HMC-1 cells to T11-1 or T11-2 mAbs resulted in expression of T11-3. These data confirm expression of a functionally active CD2 on neoplastic MCs.
In T cells, CD2 has been characterized as a critical functional surface antigen that can mediate major activation signals.24-32 In previous years, the activation signals delivered by CD2 were thought to be restricted to T cells. In later years, however, it was found that CD2 is also expressed in other cells. In 1991, Arulanandam et al described that CD2-transfected mouse MCs release histamine in response to T11-2 plus T11-3 antibody.55 We therefore asked whether MCs in SM could be a potential target of CD2-dependent triggering and could release histamine in response to CD2 mAbs. For this purpose, we analyzed histamine release in resting and epitope-stimulated HMC-1 cells as well as primary BM MCs in patients with SM. Interestingly, in SM, incubation of isolated BM MCs with T11-2 plus T11-3 mAbs or T11-1 plus T11-3 mAbs resulted in release of histamine, whereas no release was obtained with MCs enriched from reactive BM. Corresponding results were obtained with HMC-1 cells. In fact, when CD2+ HMC-1 cells were exposed to T11-2 and T11-3 mAbs, a significant increase in spontaneous histamine release was observed, whereas no increase in histamine release was seen in CD2− HMC-1 cells. These data suggest that CD2 may play a role in secretory cell functions of neoplastic MCs in SM.
In conclusion, we show that neoplastic MCs in patients with SM express functionally active LFA-2 (CD2) epitopes on their surface. The notion that MCs in SM specifically express functional CD2 may have pathophysiologic and clinical implications.
We thank Yasamin Majlesi and Hans Semper for skillful technical assistance.
Supported by Fonds zur Förderung der Wissenschaftlichen Forschung in Österreich (FWF) grant no. P-12517, Fondo de Investigaciones Sanitarias de la Seguridad Social (FIS 01/0413), and Fundación Oftalmológica J. Cortés. R.N. is a recipient of a grant FPI from Comunidad de Madrid.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
P. Valent, Dept of Internal Medicine I, Div of Hematology & Hemostaseology, University of Vienna, Währinger Gürtel 18-20, A-1090 Vienna, Austria; e-mail:peter.valent@akh-wien.ac.at.