Abstract
The feasibility and efficacy of a combination of thalidomide, cyclophosphamide, etoposide, and dexamethasone were studied in 56 patients with poor-prognosis multiple myeloma. Of 50 patients evaluable for response, 4% achieved complete response (CR), 64% partial response (PR), 18% minimal response (MR), 6% stable disease (SD), and 8% progressive disease (PD), resulting in an objective response rate (≥ MR) of 86.0% (76.7% overall objective response rate in intent-to-treat analysis; n = 56). Subsequent to successful remission induction, 18 patients received autologous or allogeneic stem cell transplantation. The median progression-free survival in all patients was 16 months. The median overall survival time could not be calculated, since the last observed death occurred after 16 months of follow-up (median follow-up of 14 months) with a corresponding estimated survival probability of 55%. Severe adverse effects (World Health Organization III/IV) included infectious complications (35.7%) and cardiovascular events (7.1%). The data suggest that Thal improves antitumor activity of salvage chemotherapy regimens in poor-prognosis multiple myeloma.
Introduction
Despite modern treatment modalities, including high-dose chemotherapy with stem cell support, multiple myeloma (MM) remains incurable in most cases. The majority of patients suffer from recurrent disease and ultimately succumb to sequelae of this disease.1-4 Allogeneic stem cell transplantation might induce long-term remission in some patients but is associated with relatively high morbidity and mortality.2 The antimyeloma effect of thalidomide (Thal) alone has been demonstrated in several clinical trials.5-7 Recent data indicate that Thal can increase the therapeutic effect of chemotherapy and might be able to overcome drug resistance.8-11 We report our results of a clinical phase 2 trial using a combination of cyclophosphamide, etoposide, and dexamethasone simultaneously with Thal. The aim was to improve the outcome of patients with MM and we have observed a very high response rate in a group of patients with poor prognosis.
Study design
Fifty-six patients with poor-prognosis MM were included in a phase 2 clinical protocol (thalidomide/cyclophosphamide/etoposide/dexamethasone [TCED] protocol; Table 1). The study protocol was approved by the institutional review board. Thal treatment (400 mg taken daily per os [po]) was continued until toxic side effects, progression, or another event occurred that led to the termination of the patient from the study. CED chemotherapy (400 mg/m2 per day cyclophosphamide intravenously [iv] and 40 mg/m2 per day etoposide iv, both as continuous infusion days 1-4; 40 mg dexamethasone po days 1-4; repeat after 28 days) was given for 3 to a maximum of 6 cycles until best response. Patients received daily antibiotic prophylaxis.
To reduce the number of leukopenic days after chemotherapy, subcutaneous administration of granulocyte colony-stimulating factor (G-CSF; Amgen, Thousand Oaks, CA) was recommended in an absolute dose of 300 μg or 480 μg depending on the patient's body weight.
The primary end point of the study was response to TCED therapy. Response criteria were used according to guidelines of the EBMT/IBMTR (European/International Bone Marrow Transplantation Registry).12 All patients, irrespective of the duration of therapy, were included in the evaluation of adverse effects. The system of classification of the World Health Organization (WHO) was used.
Events defining for end of progression-free survival (PFS) were death from any cause and progressive disease. Estimates of PFS and overall survival (OAS) distributions were calculated according to the method of Kaplan and Meier.13 Statistical computations were performed using the software package S-PLUS (MathSoft, Seattle, WA).
Results and discussion
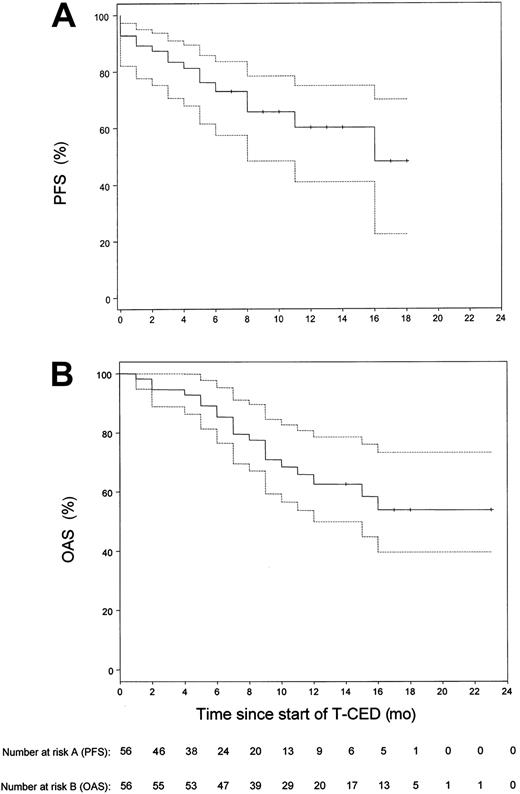
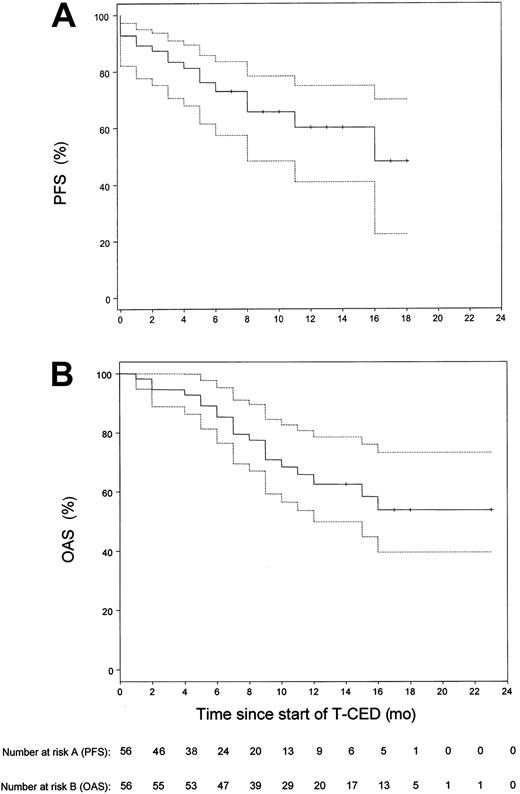
Our study focused on the treatment of pretreated patients with adverse prognostic factors (Table 1). Of these patients, 87.5% had stage III disease; in 76.7% of patients, β2-microglobulin levels were above the upper limit of normal range of 2.5 mg/L. Six patients were not evaluable for response as therapy could not be continued after the first cycle of chemotherapy for the following reasons: intolerance to Thal (4 patients), sudden cardiac death (1 patient at day 36 after start of Thal with a previous history of ischemic heart disease and tachyarrhythmias), septic death (1 patient). In the 50 remaining patients, a median number of 3 cycles (range, 3 to 6 cycles) was necessary to achieve maximal response to treatment. We recorded 4% (n = 2) complete responses (CR), 64% (n = 32) partial responses (PR), 18% (n = 9) minimal responses (MR), 6% (n = 3) stable disease (SD), 8% (n = 4) progressive disease (PD), resulting in a response rate (≥ MR) of 86.0% (n = 50). According to an intent-to-treat analysis, the overall objective response rate (n = 56) was 76.7%. The response to TCED treatment was consolidated in 18 patients by allogeneic blood stem cell transplantation (ABSCT) (n = 9) or allogeneic stem cell transplantation (n = 9). The observation time of these 18 patients was censored at the time of transplantation for calculation of PFS. Survival time estimation was done on an intent-to-treat basis for all 56 patients. The estimated median follow-up duration was 14 months. The median PFS was 16 months (Figure 1). The estimated one-year PFS was 60.2% (95% confidence interval [CI], 0.41 to 0.75). The median OAS time could not be calculated. The last observed death was after 16 months of follow-up with a corresponding estimated survival probability of 55%. The estimated one-year OAS for all 56 patients was 62.6% (95% CI, 46.8% to 75%). Using the Cox proportional hazards model to analyze the relationship of response and overall survival, the estimated hazard ratio of nonresponders (n = 7) compared with responders (n = 43) was 6.9, showing a significant survival benefit for responding patients (95% CI, 2.2 to 21.8;P = .003).
Kaplan-Meier estimates of the distribution of progression-free survival and overall survival for all 56 patients included in the study.
(A) Progression-free survival (PFS). (B) Overall survival (OAS). The dotted lines show the 95% confidence limits of the estimated survival probabilities. The median PFS was 16 months. The median OAS time could not be calculated, since the observed death was after 16 months of follow-up with a corresponding estimated survival probability of 55%.
Kaplan-Meier estimates of the distribution of progression-free survival and overall survival for all 56 patients included in the study.
(A) Progression-free survival (PFS). (B) Overall survival (OAS). The dotted lines show the 95% confidence limits of the estimated survival probabilities. The median PFS was 16 months. The median OAS time could not be calculated, since the observed death was after 16 months of follow-up with a corresponding estimated survival probability of 55%.
Using the Cox proportional hazards model for further univariate analyses, overall survival was positively related to less than 50% plasma cell infiltration in the bone marrow (P = .04; n = 41) and duration of intake of full-dose Thal (P = .02; n = 56). Other parameters (β2-microglobulin level, age, and previous therapy) did not show a statistically significant relation to PFS, OAS, or response to therapy.
Thal-associated WHO grade I and II adverse effects were in the same range as reported previously, including somnolence (57.1%), constipation (50.0%), tingling or numbness (41.0%), weakness (21.4%), tremors (19.6%), and dizziness (17.8%).5-7Thal-associated WHO grade III and IV adverse effects were tingling or numbness (5.2%), hearing disturbance (one patient), and constipation (one patient). Adverse effects attributed to Thal resulted in a dose reduction in 55% of patients and discontinuation of Thal in 19.6% of patients. WHO grade III and IV toxicities were leukocytopenia (75%), infections (35.7%), thrombocytopenia (20.4%), cardiovascular events (7.1%), and acute psychosis (one patient).
The rational to use the CED regimen in our study was to avoid potential cross resistance to anthracyclines or melphalan, often used in first-line therapy of MM, and usage of chemotherapeutic agents with established activity in relapsed patients with MM.1-3,14,15 The CED regimen has a lower incidence of neuralgic and renal toxicity than other second-line regimens.16-18 This is of importance, as we intended to avoid cumulative neurologic toxicity in combination with Thal. The lower dosage of chemotherapeutics in comparison with previous studies using the CE regimen was chosen and application of additional chemotherapeutic agents was avoided because our protocol included elderly and heavily pretreated patients with increased susceptibility to hematologic and infectious complications.14Consequently, dexamethasone was applied only in the first 4 days of each cycle and not between individual cycles as in other protocols.15 This was justified as the major benefit of our protocol was considered to result from the combination of CED with Thal.
Because of patient heterogeneity, it is difficult to directly compare the results of our trial with previous studies using conventional and high-dose therapy for poor-prognosis MM. Different treatment options for relapsed or refractory MM include cyclophosphamide- and etoposide-containing regimens. Up to now, best treatment results are reported for ABSCT, inducing overall response rates of 58% (CR and PR) with a median PFS of 11 months and an OAS of 19 months.3,14 19
Our study indicates an at-least-equivalent therapeutic efficacy of TCED as reported for conventional chemotherapy and ABSCT for this type of patient group. TCED is applicable to patients with MM eligible for autologous and allogeneic blood stem cell transplantation as remission-inducing treatment. Our results encourage future studies to evaluate the role of Thal in combination with chemotherapeutic regimen in MM and other oncologic entities.
We thank Dr U. Hegenbart (Leipzig, Germany) and Dr Sehlbach (Duisburg, Germany) for support in data accrual. We thank Dr K. Zwingenberger, Gruenenthal GmbH (Aachen, Germany) for providing thalidomide.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thomas M. Moehler, University of Heidelberg, Dept of Hematology/Oncology/Rheumatology, Hospitalstr 3, 69115 Heidelberg, Germany; e-mail: thomas_moehler@med.uni-heidelberg.de.