Abstract
The treatment of older patients with acute myeloid leukemia (AML) remains unsatisfactory, with complete remission (CR) achieved in only approximately 50% and long-term disease-free survival in 10% to 20%. Three hundred eighty-eight patients (60 years of age and older) with newly diagnosed de novo AML were randomly assigned to receive placebo (P) or granulocyte-macrophage colony-stimulating factor (GM-CSF) or GM in a double-blind manner, beginning 1 day after the completion of 3 days of daunorubicin and 7 days of cytarabine therapy. No differences were found in the rates of leukemic regrowth, CR, or infectious complications in either arm. Of 205 patients who achieved CR, 169 were medically well and were randomized to receive cytarabine alone or a combination of cytarabine and mitoxantrone. With a median follow-up of 7.7 years, the median disease-free survival times were 11 months and 10 months for those randomized to cytarabine or cytarabine/mitoxantrone, respectively. Rates of relapse, excluding deaths in CR, were 77% for cytarabine and 82% for cytarabine/mitoxantrone. Induction randomization had no effect on leukemic relapse rate or remission duration in either postremission arm. Because cytarabine/mitoxantrone was more toxic and no more effective than cytarabine, it was concluded that this higher-dose therapy had no benefit in the postremission management of older patients with de novo AML. These results suggest the need to develop novel therapeutic strategies for these patients.
Introduction
Although acute myeloid leukemia (AML) is generally responsive to chemotherapy, most patients with this disease are older than 60 years1 and have relatively resistant neoplasms. AML in the older patient is distinctive, both biologically and clinically, from that occurring in patients younger than 60. The intrinsic resistance of AML in the older patient is exemplified by the higher incidence of chromosomal abnormalities suggesting deranged pluripotent hematopoietic stem cells such as loss of all or part of chromosomes 5 and 7,2 the same abnormalities that frequently develop in patients with myelodysplasia3 and in AML induced by alkylating agents3 and environmental exposure.4 Many older patients with AML have had a known or presumed myelodysplastic prodrome, suggesting derivation of the leukemic clone from a stem cell that is proximal in the hematopoietic hierarchy. Furthermore, blasts obtained in elderly patients with AML are more likely to express proteins that mediate multidrug resistance, such as the p-glycoprotein encoded by the MDR1gene.5
In addition to the inherent chemotherapy resistance exhibited by their AML cells, older patients are generally less tolerant of complications associated with the administration of myelosuppressive chemotherapy. A reduced or qualitatively defective pool of hematopoietic stem cells associated with aging could result in more prolonged myelosuppression after chemotherapy, accounting for the higher (10%-30%) likelihood of hypoplasia-related mortality.5,6 Although hematopoietic growth factors, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) or granulocyte-colony-stimulating factor (G-CSF) administered after induction chemotherapy in older patients with AML, reduce the duration of neutropenia,6-12 these agents have not been associated with a reduction in treatment-related mortality. In part, because the use of such growth factors has not reduced treatment failure rates attributed to death during hypoplasia, the complete remission (CR) rate in this cohort of patients with AML remains disappointing. For example, in the previously reported induction phase of the current study, we randomly assigned 388 patients 60 years of age and older with newly diagnosed de novo AML to receive placebo or GM-CSF in a double-blind fashion at the conclusion of 7 days of induction chemotherapy.6 The CR rate was 51% among the 193 patients assigned to GM-CSF and 54% among the 195 patients assigned to placebo. Although the median durations of neutropenia were 15 days in those who received GM-CSF and 17 days in those who received placebo, the growth factor had no effect on the rate of treatment-related mortality or the incidence of severe or lethal infection. Of 7 large randomized, double-blind trials6-12 in which a hematopoietic growth factor was compared with placebo in the induction-treatment supportive care of elderly patients with AML, there was an improvement in CR rates in only 2.7 12
Based on the intrinsic disease resistance and the problems of supportive care, the CR rate for elderly adults with AML is approximately 50%; in comparison, it is approximately 70% in adults younger than 60.13,14 Once a patient with AML achieves CR, postremission therapy is required to reduce the burden of leukemia cells to a level at which cure may be possible. From a historical standpoint, after the benefit of postremission chemotherapy was established,15,16 increasingly intensive chemotherapeutic regimens have been used. Two large randomized studies comparing high-dose cytarabine to lower doses of this agent have shown that an intensified approach results in improved disease-free and overall survival.13,17 However, the disease-free and overall survival benefit of 4 cycles of high-dose cytarabine administered after remission and compared with less intense schedules of this drug was demonstrated only for patients 60 years of age or younger.13
To determine whether a novel intensive therapy administered to older patients with AML in remission was superior to lower doses of cytarabine, we conducted a randomized trial in which patients in remission were randomly assigned to receive either a conventional regimen of cytarabine alone for 4 cycles or an experimental combination of intermediate-dose cytarabine and mitoxantrone for 2 cycles 60 days apart. This sequential regimen, based on in vitro evidence of time-dependent leukemia cell killing, was derived from a pilot study conducted at the Mount Sinai Division of Neoplastic Diseases in 47 patients, up to age 75, with refractory or relapsed AML. The regimen was well tolerated, and a 32% CR rate was achieved.18However, our findings demonstrate the inability of this type of intensive postremission chemotherapy to alter the poor natural history of AML in the elderly.
Materials and methods
Eligibility
Eligibility for this trial was limited to patients 60 years of age or older with the diagnosis of de novo AML, as defined morphologically by the French-American-British (FAB) classification system.19 To support the diagnosis, leukemia cells were required to demonstrate at least one of the following characteristics: Auer rods; peroxidase or Sudan black B cytochemical staining; chloracetate esterase or nonspecific-esterase staining, or, in acute megakaryocytic leukemia (FAB M7), platelet peroxidase detected by electron microscopy or platelet antigens detected by appropriate monoclonal antibodies. Bone marrow aspirate had to show 30% or greater replacement of nonerythroid elements by myeloblasts. Patients were not entered in this study if they had a history of myelodysplasia or other antecedent hematologic malignancies, such as polycythemia vera, pre-existing liver disease or alcohol abuse, myocardial infarction within the previous year, or uncontrolled infection, or if they received nonsteroidal cytotoxic chemotherapy (except hydroxyurea administered for the current diagnosis of AML) or radiation therapy. Appropriate measures were initiated to control any systemic infection, hydration and allopurinol were administered, and written, informed consent was obtained.
The study was open to patient accrual between February 1990 and November 1993. Beginning in March 1991, patients with AML-M0 (myeloperoxidase-negative blasts in which the presence of myeloid, but not lymphoid, antigens could be demonstrated through immunophenotypic analysis20) were included. After October 1992, patients with acute promyelocytic leukemia were no longer eligible for this study because of the activation of another study focusing on those patients.
As part of the quality assurance program of the CALGB, members of the Data Audit Committee visited all participating institutions at least once every 3 years. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, toxic effects, tumor response, and outcome in a sample of protocols at each institution. Such on-site review of medical records was performed for a randomly selected subgroup of 73 patients (19%) of the 388 patients treated in this study.
Treatment design
Induction chemotherapy consisted of daunorubicin (45 mg/m2 per day on days 1-3) in combination with cytarabine (200 mg/m2 per day by continuous intravenous infusion on days 1-7). Study infusion (double-blinded randomization) consisted of placebo or GM-CSF (5 μg/kg given intravenously at a minimum concentration of 15 μg/kg in sterile water over 6 hours at 8:00 am daily beginning on the day after the cytarabine infusion was completed). The study drug was stopped in the event of perceived drug toxicity, leukemic regrowth, or marrow recovery. Patients were eligible for re-treatment with a second induction course (2 days of daunorubicin and 5 days of cytarabine at the above doses) if the bone marrow examination 22 days after the start of the chemotherapy revealed residual leukemic cells.
Patients in whom bone marrow remission was documented underwent lumbar puncture. If leukemia cells were identified in the spinal fluid, the patient was removed from the study and counted as having had resistant disease. Another bone marrow examination was mandated 2 weeks after the initial remission was documented. If stable remission was confirmed and if the physician felt that highly myelosuppressive therapy could be tolerated, the patient was randomized a second time to receive one of 2 postremission regimens—single-agent cytarabine (100 mg/m2per day by continuous intravenous infusion for 5 days) for 4 monthly courses or cytarabine (500 mg/m2 every 12 hours [given as 250 mg/m2 over 15 minutes, followed by 250 mg/m2 over 3 hours]) in combination with mitoxantrone (5 mg/m2 every 12 hours, administered 6 hours after the start of each cytarabine infusion) for 6 doses. Two courses of this combination were given 60 days apart. GM-CSF was not given after courses of postremission therapy.
Chromosomal analysis of bone marrow was performed in institutional CALGB cytogenetics laboratories, and karyotypes were centrally reviewed biannually by an expert panel of CALGB cancer cytogeneticists as part of a prospective study of cytogenetics in acute leukemia, CALGB 8461. Specimens were obtained at diagnosis and were processed using direct methods and unstimulated short-term (24-, 48-, and 72-hour) cultures. G banding was typically performed, though Q banding was also acceptable for inclusion in this series. The criteria used to describe a cytogenetic clone and the description of karyotypes followed the recommendations of the International System for Human Cytogenetic Nomenclature.21
Immunophenotyping was performed by multiparameter flow cytometry, as previously described.22 CD56 expression was studied with NKH1 antibody (Coulter, Hialeah, FL) labeled with phycoerythrin/Cy5 in a 3-antibody panel, including anti-CD15 and anti-CD34. Samples were considered CD56+ if CD56 was expressed on more than 10% of the cells in the leukemia gate and if CD56 was coexpressed with other antigens on the surfaces of the leukemia cells.22
Registration and randomization procedures
Patients were registered and simultaneously randomized to one of the 2 postremission treatment groups by a telephone call to the CALGB Statistical Center. Direct registrations were allowed only from CALGB main member institutions; registrations from affiliates of the main members were made through the appropriate main member. Randomization was a stratified permuted-block design, with stratification by the induction regimen received by the patient and a preassigned block size of 8.
Outcome measures
The definition of CR followed accepted criteria,23which required that patients undergo bone marrow examination demonstrating more than 20% cellularity with evidence of normal erythropoiesis, granulopoiesis, and megakaryocytopoiesis, and containing no more than 5% blasts. In addition, at least 1500 granulocytes per microliter and 100 000 platelets per microliter in the peripheral blood were required for at least 4 weeks, in the absence of intervening chemotherapy. Relapse was defined as marrow infiltration by more than 5% leukemia cells in previously normal bone marrow or evidence of extramedullary leukemia.
Disease-free survival was defined as the interval from the date of second randomization to the date of relapse (bone marrow or extramedullary), date of death from any cause, or date the patient was last known to be in remission. Patients still in remission were censored in the statistical analyses at the time of last follow-up. Overall survival was measured as the time from second randomization in the study to death from any cause.
Statistical design and analysis
Primary design considerations concerned the induction therapy regimens, which called for 384 patients to be registered. Based on previous results, it was anticipated that the overall CR rate would be approximately 50% and that 85% of the CR patients would be randomized. This would yield approximately 163 patients randomized to one of the 2 post-CR regimens (close to the actual outcome; see “Results” for details). This number of patients would provide approximately 80% power to detect a failure rate ratio between treatments of 1.67 with 1.5 years of follow-up (2-sided log-rank test, (P = .05).
Disease-free survival and overall survival, the main end points in this study, were estimated using the Kaplan-Meier method for right-censored data.24 Differences in these end points between the randomized arms were tested using the log-rank statistic.25 Medians for these end points were calculated by the method of Simon and Lee, and 95% confidence intervals for the medians were calculated by the method of Brookmeyer and Crowley.26,27 Analysis of prognostic factors and of treatment by factor interactions was carried out using the Cox regression model.28 Fisher exact 2-sided test was used to compare the treatment arms with respect to toxicity.29Results were based on follow-up data as of August 2000.
Results
Three hundred eighty-eight patients were enrolled at 25 main-member institutions of the CALGB and their affiliated hospitals. Patient characteristics at study entry and results of induction therapy according to treatment with either GM-CSF or placebo have been reported.6 Among the 193 patients randomized to receive GM-CSF, 99 (51%) achieved CR compared to 106 (54%) of the 195 patients who received placebo during induction therapy (P = .61). There was no difference in the CR rate between those who were 70 years of age or older and those who were between the ages of 60 and 69 (P = .54).
Of the 205 patients achieving CR, 169 were randomized to receive one of the 2 postremission therapies. The remaining 36 patients were not randomized to postremission therapy because of the reasons listed in Table 1. Primary reasons were patient refusal and a conclusion on the part of a physician that a patient was too ill to receive intensive postremission chemotherapy. Randomized patients with various prognostic factors were equally distributed in the 2 postremission arms (Table 2).
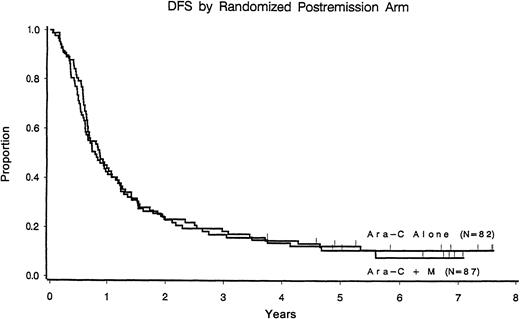
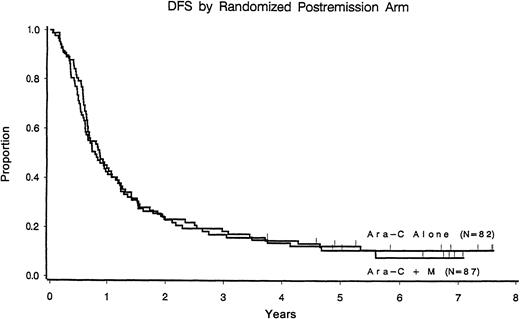
With a median follow-up of 7.7 years among patients still at risk, the median disease-free survival time was 11 months (95% confidence interval, 9-13 months) among those who received cytarabine alone compared with 10 months (95% confidence interval, 8-14 months) for those randomized to receive cytarabine plus mitoxantrone (P = .67) (Figure 1). Relapse rates were 77% and 82% in each arm, respectively. Induction randomization had no effect on leukemic relapse rate or disease-free survival in either postremission arm (Table3).
Disease-free survival according to the postremission randomization arm.
CR patients only.
Disease-free survival according to the postremission randomization arm.
CR patients only.
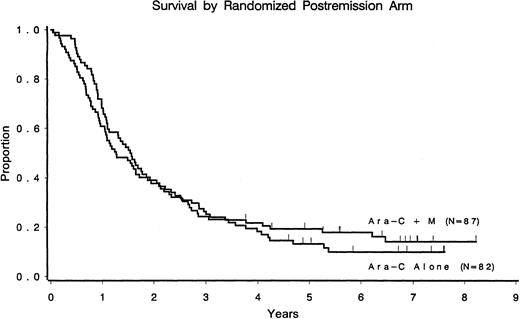
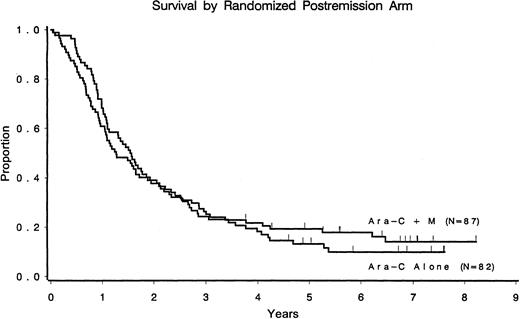
Only 16 of 169 (9%) patients randomized to undergo postremission therapy remain alive and free of disease (9 in the cytarabine arm and 7 in the cytarabine/mitoxantrone arm). Median overall survival times from the second randomization were 1.6 years for the cytarabine arm and 1.3 years for the cytarabine/mitoxantrone arm (Figure2). Of the 388 patients registered in the study, 27 were alive at the last follow-up, with survival times ranging from 5.4 to 9.9 years from study entry.
Overall survival according to the postremission randomized arm.
CR patients only.
Patients who received the cytarabine/mitoxantrone combination after remission experienced more severe toxicities than those who received cytarabine alone—in particular, grade IV hemorrhage, infection, diarrhea, dysrhythmias, and malaise (Table4). Fifty-eight (71%) patients received all 4 cycles of cytarabine; 68 (78%) patients received both cycles of cytarabine/mitoxantrone.
To determine the influence of prognostic factors on outcome, we analyzed disease-free and overall survival according to disease features at study entry, including cytogenetics (Table5). Only the initial white blood cell count and immunophenotype were strongly related to outcome. Those patients whose blasts expressed the neural adhesion molecule CD56 had inferior disease-free and overall survival times compared with those whose blasts did not express this antigen, though the number of patients (6 patients) in this category was small. Using a proportional hazards regression model with treatment by factor interactions, we were unable to identify any patient subgroup that benefited from intensive postremission chemotherapy.
Among the patients randomized to postremission therapy, 103 had adequate cytogenetic study results. Six patients (3 on each arm), had core binding factor leukemia, one with t(8;21) (q22;q22) and 5 with inv16(p13q22) or t(16;16)(p13q22). All have had relapses, including the 3 who received the more intensive consolidation. Forty had other cytogenetic abnormalities, including 2 with t(15;17)(q22;q12) and 17 with sole numerical abnormalities. Only 3 of these patients remain disease free; all are in the single-agent cytarabine arm. The remaining 57 patients had normal karyotypes; 5 of them (one in the single-agent arm and 4 in the combination arm) remain disease free.
Discussion
The results of this study emphasize the problems inherent in the treatment of older patients with AML. Median survival time in this trial (9 months) is similar to that reported in other cohorts of patients with AML in this age group.30 Patients in this trial might not be fully representative of all older patients with AML. Patients with a known history of myelodysplasia or exposure to chemotherapy and those with active infections were excluded because of concerns that they would be less tolerant of aggressive induction therapy. Thus, results in all other patients with AML would likely be even worse. As was the case in the older cohort in a previous CALGB study, which proved a cytarabine dose effect in younger patients,13 we were unable to demonstrate an improvement in disease-free or overall survival time in older patients randomized to an intensive postremission chemotherapy regimen. Moreover, there was no clear benefit to the use of more intensive therapy, even in the few patients with favorable cytogenetic abnormalities, who in the younger age cohort31 appeared to benefit from high-dose cytarabine. The findings in this study pertained to both the 216 patients who were 60 to 69 years of age (54% CR rate) and the 172 patients 70 or older (52% CR rate). Although based on only 6 patients, our results confirm those of a preliminary study in suggesting that CD56 expression confers a negative prognostic impact.32The reason for the adverse effect is unclear. Association between CD56 expression in AML and extramedullary disease has been variable.33,34 However, CD56 expression in patients whose blasts display the t(8;21) cytogenetic clone defines a poor prognostic subcategory.22
Although high-dose cytarabine has been useful in those with therapy-related myeloid leukemia and secondary leukemia, the remissions achieved in these patients are brief.4 As such, it is perhaps not surprising that this trial and the prior CALGB trial13 failed to demonstrate a benefit for dose-intensive therapy in the older cohort. A significant number of patients achieving remission, those aged 60 to 69 and those older than 69, were randomized to postremission therapy (83% and 82%, respectively). Part of the problem in the prior CALGB trial was that few older patients were able to receive all 4 cycles of planned postremission, high-dose cytarabine. In this trial, approximately 75% of the patients received both cycles of the modified high-dose cytarabine/mitoxantrone combination. These results suggest that acute leukemias in older patients are intrinsically resistant. We did not observe a significantly increased incidence of severe neurologic toxicity in patients receiving this modified (6-fold lower) conventional high-dose cytarabine regimen. Although older age is a risk factor for high-dose cytarabine-associated cerebellar toxicity, other factors, including hepatic and renal excretory function, may be equally important.35
Based on our results, it is reasonable to question the value of postremission therapy in older patients with AML. Given the certain relapse associated with no postremission therapy in mainly younger patients,15,16 most clinicians have been unwilling to conduct further clinical trials in older patients that include a no-treatment arm. The EORTC/HOVON group conducted studies9 36 in which older patients with AML in remission after one cycle of intensive consolidation therapy were randomized to receive 8 cycles of postremission therapy or observation. The results demonstrated a modest benefit in reducing the risk of relapse, but there was no effect on overall survival time (less than 10 months). Nonetheless, though postremission therapy may have no effect on median survival in this age cohort, a few patients experienced prolonged disease-free survival; such good outcomes are only associated with postremission chemotherapy. Additional studies might include a no-treatment control arm or explore quality-of-life issues that could help assess the value of variably intensive induction and postremission strategies in this age cohort.
The ability to intensify chemotherapy in the postremission setting is limited by hematopoietic stem cell and end-organ tolerance. Although the use of hematopoietic growth factors has not yet been explored in as much depth in the postremission setting11,37 as induction chemotherapy, it seems unlikely that such agents will have a major effect on disease-free survival because relatively few patients die in remission. Improving the outcome in older patients will likely remain a daunting task because of the heterogeneity of the patient population with regard to both end-organ reserve and leukemia pathophysiology. Mechanisms of resistance may well be multiple and pleiotropic. New approaches, such as those involving the modulation of drug resistance38 or immunomodulatory strategies,39 are under evaluation to try to address the problem of drug resistance in the older patient with AML.
We thank Dr Richard A. Larson for critical reading of the manuscript, Cynthia L. Curti and Marcella E. Hussey for secretarial support, the physicians, nurses, and data managers at each of the CALGB institutions, the data coordinators at the CALGB Data Management Center, and, most of all, the patients. The Schering Corporation (Rahway, NJ) provided the GM-CSF used in this trial.
The following institutions participated in the study: Christiana Care Health Services, CCOP, Wilmington, DE, Irving M. Berkowitz, DO; supported by CA45418; Community Hospital Syracuse CCOP, Syracuse, NY, Jeffrey Kirshner, MD, supported by CA45389; Dana-Farber Cancer Institute, Boston, MA, George P. Canellos, MD, supported by CA32291; Dartmouth Medical School–Norris Cotton Cancer Center, Lebanon, NH, L. Herbert Maurer, MD, supported by CA04326; Duke University Medical Center, Durham, NC, Jeffrey Crawford, MD, supported by CA47577; Eastern Maine Medical Center CCOP, Bangor, ME, Philip L. Brooks, MD, supported by CA35406; Kaiser Permanente CCOP, San Diego, CA, Johnathan A. Polikoff, MD, supported by CA45374; Long Island Jewish Medical Center, Lake Success, NY, Marc Citron, MD, supported by CA11028; Massachusetts General Hospital, Boston, MA, Michael L. Grossbard, MD, supported by CA12449; McGill Department of Oncology, Montreal, QC, Brian Leyland-Jones, MD, supported by CA31809; Milwaukee CCOP, Milwaukee, WI, Ronald Hart, MD, supported by CA45400; Mount Sinai Medical Center CCOP, Miami, Miami Beach, FL, Enrique Davila, MD, supported by CA45564; Mount Sinai School of Medicine, New York, NY, James F. Holland, MD, supported by CA04457; North Shore University Hospital, Manhasset, NY, Daniel R. Budman, MD, supported by CA35279; Rhode Island Hospital, Providence, Louis A. Leone, MD, supported by CA08025; Roswell Park Cancer Institute, Buffalo, NY, Ellis Levine, MD, supported by CA02599; Southeast Cancer Control Consortium CCOP, Goldsboro, NC, James N. Atkins, MD, supported by CA45808; Southern Nevada Cancer Research Foundation CCOP, Las Vegas, John Ellerton, MD, supported by CA35421; SUNY Health Science Center at Syracuse, NY, Stephen L. Graziano, MD, supported by CA21060; University of Alabama Birmingham, Robert Diasio, MD, supported by CA47545; University of California at San Diego, Stephen L. Seagren, MD, supported by CA11789; University of Chicago Medical Center, IL, Nicholas J. Vogelzang, MD, supported by CA47642; University of Maryland Cancer Center, Baltimore, David Van Echo, MD, supported by CA31983; University of Massachusetts Medical Center, Worcester, F. Marc Stewart, MD, supported by CA37135; University of Minnesota, Minneapolis, Bruce A. Peterson, MD, supported by CA16450; University of Missouri/Ellis Fischel Cancer Center, Columbia, Michael C. Perry, MD, supported by CA47559; University of Tennessee, Memphis, Harvey B. Niell, MD, supported by CA47555; Virginia Commonwealth University MB CCOP, Richmond, John D. Roberts, MD, supported by CA52784; Wake Forest University School of Medicine, Winston-Salem, NC, David D. Hurd, MD, supported by CA03927; Walter Reed Army Medical Center, Washington, DC, John C. Byrd, MD, supported by CA26806; Washington University School of Medicine, St Louis, MO, Nancy L. Bartlett, MD, supported by CA77440; Weill Medical College of Cornell University, New York, NY, Ted P. Szatrowski, MD, supported by CA07968.
Supported by National Cancer Institute (NCI) grants CA03927 and CA77658; CA 77658; the Roswell Park Cancer Institute, Buffalo, NY; the Ohio State University Medical Center, Columbus; and Wayne State University School of Medicine, Detroit, MI. Supported in part by NCI grants CA31946 and CA33601 (S.L.G.). Additional grant support for participating institutions is listed in the .
The contents of this article are solely the responsibility of the authors and do not represent the official views of the National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Richard M. Stone, Dana-Farber Cancer Institute, Rm D-840, 44 Binney St, Boston, MA 02115; e-mail: rstone@partners.org.