Abstract
In the initial phase of an inflammatory response, leukocytes marginate and roll along the endothelial surface as a result of adhesive interactions between molecules on the endothelial cells and leukocytes. To evaluate the role of the 3 selectins (E, L, and P) in leukocyte rolling and emigration, a null mutation for L-selectin was introduced into previously described embryonic stem cells with null mutations in the genes for both E-selectin and P-selectin (E/P double mutants) to produce triple-selectin–null mice (E-selectin, L-selectin, and P-selectin [E/L/P] triple mutants). Triple-selectin homozygous mutant mice are viable and fertile and only rarely develop the severe mucocutaneous infections or pulmonary inflammation characteristic of E/P double-mutant mice. Surface expression of L-selectin was undetectable in triple-mutant mice on fluorescence-activated cell-sorter analysis of peripheral neutrophils. Pathological studies revealed moderate cervical lymphadenopathy and lymphoplasmacytic infiltrate, but these were less extensive than in E/P double-mutant mice. Neutrophil emigration during thioglycolate-induced peritonitis was significantly reduced at 4, 8, and 24 hours (35%, 65%, and 46% of wild-type values, respectively). Intravital microscopy of the cremaster muscle revealed almost no rolling at times up to 6 hours after exteriorization, with or without addition of tumor necrosis factor α. The small amount of residual rolling was dependent on α4-integrin. The occurrence of skin and pulmonary disease in E/P double-mutant mice but not E/L/P triple-mutant mice suggests that deficiency of L-selectin alters the inflammatory response in E/P mutants.
Introduction
Leukocyte emigration into the tissues after an inflammatory stimulus is a process consisting of several distinct steps. These have been described as (1) leukocyte rolling along activated endothelium, (2) leukocyte activation, (3) firm adhesion to the endothelium, and (4) transendothelial emigration.1,2The selectins (E, L, and P) and their ligands are primarily responsible for the initial leukocyte tethering and rolling events, and each selectin has been shown to support rolling in vitro and in vivo.3-9 L-selectin is expressed constitutively on most leukocytes. E-selectin is expressed on endothelium, and up-regulation requires de novo synthesis. P-selectin is expressed on endothelium and platelets, and up-regulation occurs by rapid mobilization from α-granules of platelets and from Weibel-Palade bodies of endothelial cells. The firm attachment of leukocytes after rolling depends largely on interactions between immunoglobulin superfamily members (eg, intercellular adhesion molecule 1 [ICAM-1]) and the β2-integrins (eg, lymphocyte function antigen 1 and Mac-1).
Mice with null mutations in the individual selectin genes have been described previously. P-selectin–deficient mice10and L-selectin–deficient4 mice both have defects in leukocyte rolling, and neutrophil emigration into the peritoneal cavity is delayed during thioglycollate-induced peritonitis. L-selectin–deficient mice also have severe defects in lymphocyte homing to the lymphoid tissues. E-selectin–null mice, though originally thought to have no defects in the inflammatory response,11 have been found to have defects in slow leukocyte rolling12 and have increased mortality compared with wild-type mice after intraperitoneal infection withStreptococcus pneumoniae.13 All 3 of the single-selectin–deficient strains of mice remain healthy under specific-pathogen–free (SPF) conditions. The differences in the phenotypes of individual-selectin knockout mice suggest distinct roles for the selectins in inflammatory processes.
E-selectin and P-selectin (E/P) double-mutant mice have been described by 2 research groups.10,14 These mice have profound leukocytosis, elevated levels of inflammatory cytokines, hypergammaglobinemia, and severe defects in neutrophil emigration during both chemically and bacterially induced peritonitis.10,14 The mice also develop and eventually die of spontaneous mucocutaneous infections. No leukocyte rolling was observed in these mice after trauma or short-term treatment with tumor necrosis factor α (TNF-α),15 but minimal rolling was found 6 hours after administration of TNF-α.16 These mice illustrate the synergistic roles of E-selectin and P-selectin in leukocyte rolling and emigration, since the 2 mutations together produce a much more severe phenotype than does either individually.
Generation of mice that mimic an E-selectin, L-selectin, and P-selectin (E/L/P) triple-mutant phenotype by transplantation of bone marrow from L-selectin–deficient mice into irradiated E/P double mutants17 showed a severe defect in leukocyte rolling in these pseudo–triple-selectin–null mice. These observations were confirmed when Robinson et al18 generated triple-selectin knockout mice genetically. Studies of these mice indicated a dominant role for P-selectin in leukocyte recruitment from the vasculature. The mice also had severe defects in leukocyte emigration.
To further investigate the roles of the selectin molecules in vivo, we independently generated mice deficient in all 3 selectin molecules. These mice had defects in leukocyte rolling and emigration at least as severe as those in double-selectin mutants, but surprisingly, they appeared to be healthier, with a near absence of mucocutaneous and pulmonary disease and greatly reduced leukocytosis compared with E/P double-mutant mice.
Materials and methods
L-selectin–gene targeting in embryonic stem cells
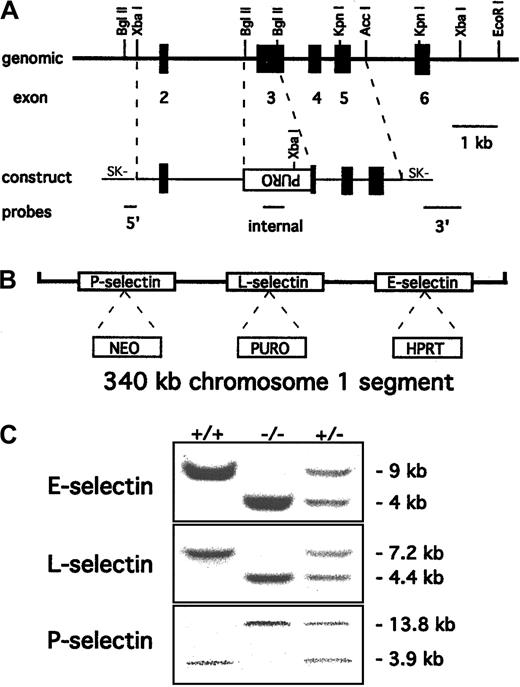
Polymerase chain reaction primers for exon 3 of the mouse L-selectin gene (sense, 5′-ACTTCCTGATACACCATGGA-3′; and antisense, 5′-CTGTGTAGCAGAGAGCTGC-3′) were used to generate a probe from tail DNA of C57BL/6 mice. A 129/SvEv genomic DNA library (Stratagene, La Jolla, CA) was screened with the probe, and several positive clones were isolated and characterized, confirming a previously described restriction map.4 A targeting construct similar to one used previously to create a null mutation for L-selectin4was prepared as shown in Figure 1A. This construct was electroporated into AB2.1 embryonic stem (ES) cells19 already containing the E-selectin and P-selectin mutations described previously.10 Digestion withXbaI followed by Southern blotting and probing with the 3′ probe shown in Figure 1A identified several homologous recombinant clones.
Targeting of the L-selectin gene to produce triple-null ES cells.
(A) Partial restriction map of the mouse L-selectin locus together with the targeting construct. Exons 2 to 6 are indicated as solid boxes. Most of the lectin domain (exon 3) is deleted and replaced with a puromycin resistance cassette in the orientation opposite to that of the L-selectin gene. The 5′, 3′, and internal probes used to detect the desired homologous recombination event are shown. (B) Schematic representation (not to scale) of the 3 selectin genes with the selectable markers inserted after the L-selectin gene was targeted incis to the original E- and P-selectin mutations. (C) Probing of Southern blots identified wild-type +/+, −/−, and +/− mouse genotypes for all 3 selectin mutations. The E-selectin genotype was identified by 4.3-kb mutant and 7.5-kb wild-type EcoRI fragments respectively10 and the P-selectin genotype by 13.8-kb wild-type and 3.9-kb mutant EcoRV fragments.15 The introduced L-selectin mutation was identified by a 7.2-kb wild-type XbaI fragment and a 4.4-kb mutant fragment. The blots show that the mutations segregate together, creating triple-selectin–null mice.
Targeting of the L-selectin gene to produce triple-null ES cells.
(A) Partial restriction map of the mouse L-selectin locus together with the targeting construct. Exons 2 to 6 are indicated as solid boxes. Most of the lectin domain (exon 3) is deleted and replaced with a puromycin resistance cassette in the orientation opposite to that of the L-selectin gene. The 5′, 3′, and internal probes used to detect the desired homologous recombination event are shown. (B) Schematic representation (not to scale) of the 3 selectin genes with the selectable markers inserted after the L-selectin gene was targeted incis to the original E- and P-selectin mutations. (C) Probing of Southern blots identified wild-type +/+, −/−, and +/− mouse genotypes for all 3 selectin mutations. The E-selectin genotype was identified by 4.3-kb mutant and 7.5-kb wild-type EcoRI fragments respectively10 and the P-selectin genotype by 13.8-kb wild-type and 3.9-kb mutant EcoRV fragments.15 The introduced L-selectin mutation was identified by a 7.2-kb wild-type XbaI fragment and a 4.4-kb mutant fragment. The blots show that the mutations segregate together, creating triple-selectin–null mice.
A scheme of negative selection followed by Southern blotting was used to identify clones in which recombination had occurred incis with the previous mutations. Clones were selected for loss of the chromosome containing the targeted E-selectin locus by using 6-thioguanine to select against the hypoxanthine phosphoribosyltransferase (HPRT) cassette in the E-selectin gene. DNA was prepared from the colonies surviving the selection, digested withXbaI, and probed for concurrent loss of the L-selectin mutation by Southern blot analysis. Clones that had lost both the E-selectin and L-selectin mutations were judged to have the mutations in cis. Injection of 2 clones into C57BL/6 embryos resulted in germline transmission of the chromosome carrying the 3 mutations, and no differences were observed for the 2 clones. The mice used in the experiments were of a mixed C57BL/6 and 129/SvEv background and were housed in a SPF barrier facility.
The mice prepared by our laboratory and those described by Hynes and colleagues are referred to in the discussion section of this paper as the Baylor (Bay) and Hynes (Hyn) mutations, respectively, in agreement with the nomenclature of the Induced Mutant Resource of the Jackson Laboratory (http://jaxmice.jax.org/html/infosearch/searchDB_index.html). The technical nomenclature for the genotypes so designated is as follows:
Pathological assessments and blood counts
Necropsies were performed on 4 E/L/P triple-selectin–null mice, 4 L-selectin–null mice, 12 E/P double-selectin–null mice, and 3 control mice at 12 weeks of age. Tissue was obtained from all major organ systems, including lymph nodes, skin, and bone. The tissue was fixed in 10% buffered formalin, embedded in paraffin, and prepared serially as 3-μm tissue sections stained with hematoxylin and eosin. Lungs were examined both inflated and uninflated. Gram stains and methenamine silver stains were done on selected tissue sections for detection of bacteria and fungi. A histopathological comparison was made of alterations in the organ systems in selectin-deficient and selectin-competent mice.
Blood was obtained from 8-week-old mice by means of bleeding of the retro-orbital venous plexus, collected in heparin-coated capillary tubes, and transferred to heparin-coated plastic tubes. All mice appeared healthy on gross examination at the time of complete blood counts done by using an automatic cell counter (Beckman Coulter, Fullerton, CA). Differential counts were performed in smears stained with Wright stain. Ten wild-type, 10 E/L/P triple-mutant, and 5 E/P double-mutant male mice were analyzed.
Intravital microscopy
Mice were anesthetized with an intraperitoneal injection of ketamine hydrochloride (Ketalar [100 mg/kg]; Parke-Davis, Morris Plains, NJ) after pretreatment with sodium phenobarbital (Nembutal [30 mg/kg given intraperitoneally]; Abbott Laboratories, North Chicago, IL) and atropine (0.1 mg/kg given intraperitoneally; Elkins-Sinn, Cherry Hill, NJ). A tube was inserted into the trachea and one jugular vein was cannulated for administration of anesthetic throughout the intravital microscopical experiment. One carotid artery was cannulated for blood pressure monitoring, blood samples, and systemic injections of monoclonal antibodies (mAbs). Mice were kept at a constant temperature of 37°C with use of a thermo-controlled heating lamp (Physitemp, Clifton, NJ) and received diluted phenobarbital in saline (0.2 mL/hour) intravenously to maintain anesthesia and a neutral fluid balance.
The cremaster muscle was prepared for intravital microscopy as described previously20 and was superfused with thermo-controlled (35°C) bicarbonate-buffered saline. Blood samples (10 ul each) were obtained from the carotid catheter throughout the experiment at approximately 45-minute intervals to analyze systemic leukocyte concentrations. Differential leukocyte counts were obtained by evaluating blood samples stained with Kimura stain in a hemocytometer. Microscopical observations were made by using a Zeiss intravital microscope (Axioskop, Thornwood, NY) with a saline immersion objective (SW 40/0.75 numerical aperture). Venules with diameters between 20 and 80 fm were observed and the results recorded by using a CCD camera system (VE-1000CD; Dage-MTI, Michigan City, IN) on a Panasonic S-VHS recorder. Center-line red blood cell velocity was measured with a dual photodiode and a digital on-line cross-correlation program.21 Center-line velocities were converted to mean blood flow velocities by multiplying by an empirical factor of 0.625.22 Wall shear rates (γw) were estimated as 2.12 (8Vb/d), where Vb is the mean blood flow velocity, d is the diameter of the vessel, and 2.12 is a median empirical correction factor obtained from velocity profiles determined by assessing microvessels in vivo.23
Rolling and adhesion variables were determined as follows. Each rolling leukocyte passing a line perpendicular to the vessel axis was counted, and leukocyte rolling flux was expressed as leukocytes per minute. Rolling flux fraction was calculated as described previously20 by dividing leukocyte rolling flux by total leukocyte flux estimated as white blood cell count (WBC) vbπ (d/2),2 where WBC is the systemic leukocyte count, vb is the mean blood flow velocity, and d is the venule diameter. A mAb (PS/2) and an isotype-matched control were used to evaluate the effect of blocking the adhesive function of α4-integrin.
Cytokine measurements and flow cytometry
Enzyme-linked immunosorbent assays (ELISAs) for granulocyte-monocyte colony-stimulating factor (GM-CSF) and TNF-α were done according to the instructions of the manufacturer (R & D Systems, Minneapolis, MN). Expression of L-selectin on leukocyte populations was determined by flow cytometry analysis in 8-week-old mice. Whole blood was collected and incubated with Fc Block (1:50; Pharmingen, San Diego, CA) on ice for 5 minutes. Fluorescein isothiocyanate (FITC)–labeled mAb to L-selectin (MEL-14 [1:50]; Pharmingen) or FITC-labeled isotope control was added, and the mixture was incubated for 30 minutes. The granulocyte-specific, isotope-matched, phycoerythrin-labeled mAb to Ly-6G (Gr-1; Pharmingen) was used to identify the granulocyte population in the experiments shown in Figure 2. After centrifugation and red blood cell lysis, flow cytometric analysis of L-selectin expression was done by using a Coulter Electronics XL-MCL flow cytometer (Beckman Coulter).
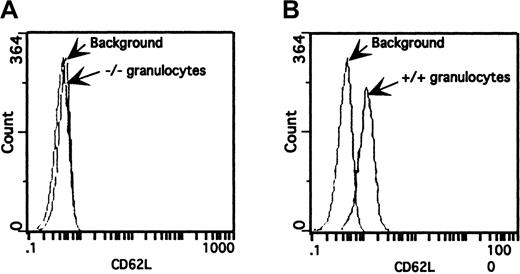
Surface expression of L-selectin on granulocytes from wild-type (+/+) and triple-null (−/−) mice.
Peripheral blood was incubated with FITC-conjugated MEL-14 mAb and analyzed by flow cytometry. The fluorescence histogram is representative of those from several mice (n = 6) from different litters on a 4-decade log scale. (A) Granulocytes from triple-null (−/−) mice overlaid on a granulocyte population incubated with an isotope-matched mAb. (B) Granulocytes from wild-type (+/+) mice stained with FITC-labeled MEL-14 mAb produced a pronounced shift in fluorescence over background fluorescence.
Surface expression of L-selectin on granulocytes from wild-type (+/+) and triple-null (−/−) mice.
Peripheral blood was incubated with FITC-conjugated MEL-14 mAb and analyzed by flow cytometry. The fluorescence histogram is representative of those from several mice (n = 6) from different litters on a 4-decade log scale. (A) Granulocytes from triple-null (−/−) mice overlaid on a granulocyte population incubated with an isotope-matched mAb. (B) Granulocytes from wild-type (+/+) mice stained with FITC-labeled MEL-14 mAb produced a pronounced shift in fluorescence over background fluorescence.
Peritonitis
One milliliter of 3% thioglycolate (Difco, Mountain View, CA) was injected into the peritoneal cavity to induce peritonitis. At 4, 8, and 24 hours after injection, the mice were killed and peritoneal cells were collected by injecting 5 mL phosphate-buffered saline containing 0.1% bovine serum albumin, 0.54 mM EDTA, and 10 U/mL heparin (Sigma Chemical, St Louis, MO) and removing the peritoneal lavage. The total number of cells collected was determined with a hemocytometer. Cytospin preparations of the lavage were neat stained (Fisher Scientific, Pittsburgh, PA), and differential counts were used to determine the total number of neutrophils (differential percentage times total cells). For the peritonitis experiments with blocking of α4-integrin, 50 μg mAb PS/2 was injected into the tail vein 1 hour before and 6 hours after thioglycolate injection. Lavage was collected and neutrophils were counted as described above.
Results
Targeting of L-selectin in ES cells
Triple-selectin–null mice were generated by targeting the L-selectin gene in ES cells containing previously introducedcis mutations in the genes for E-selectin and P-selectin.10 Because the 3 selectin genes in mice are closely linked on chromosome 1, triple-mutant mice could not be obtained readily by breeding of single–L-selectin– null mice with E/P double-selectin–null mice. The targeting construct (LSCON2-2) was designed to replace most of exon 3 with a puromycin resistance cassette by homologous recombination (Figure 1A). Exon 3 codes for the lectin-binding domain of L-selectin, and a similar deletion was used to produce a single– L-selectin–null mouse previously.4The linearized construct was electroporated into double-mutant ES cells and selected for puromycin resistance. Genomic DNA from the surviving colonies was digested with XbaI, and Southern blotting with the 3′ probe identified several clones with the desired 4.4-kb fragment expected for a replacement mutation. Further analysis usingBglII and the 5′ probe confirmed homologous integration of the construct, with a frequency of 1 in 30 of the puromycin-resistant colonies.
A selection scheme was used to identify clones that had the integration event in cis or trans orientation to the 2 previous mutations. Cultures of individual clones were selected in 6-thioguanine to obtain subclones that had lost the chromosome with the HPRT cassette in the E-selectin locus. Genomic DNA from clones that had lost the E-selectin mutation was analyzed for the concurrent loss of the L-selectin mutation by Southern blotting. Loss of both the E-selectin and L-selectin mutations indicated that the integration had occurred in cis to the previous mutations (Figure 1B), resulting in cosegregation of the mutations that would generate the triple-null mouse when homozygous.
Clones containing the triple-selectin mutation and the single–L-selectin mutation were injected into C57BL/6 blastocysts. This resulted in a high percentage of male chimeras that transmitted the mutations to the mouse germline. Intercrossing the progeny produced homozygous mice for both genotypes: triple-selectin–null mice and single–L-selectin–null mice. Southern blot analysis of tail DNA from the pups obtained from the mating of heterozygous triple-null mice demonstrated cosegregation of the mutations and generation of triple-null mice (Figure 1C). No evidence of prenatal or early postnatal death was found in either the triple-null or L-selectin–null mice, thereby confirming that the selectin genes are not essential for development or survival after birth. Surprisingly, the triple-null mice had less skin disease than the E/P double-null mice.
Verification of null alleles
Verification that the P-selectin and E-selectin mutations produce null alleles was reported previously.10 15 Eight-week-old wild-type (+/+) and homozygous null (−/−) littermates from both the triple- and single–L-selectin mutants were tested for surface expression of L-selectin. Leukocytes from peripheral blood and bone marrow of 8-week-old mice were incubated with FITC-labeled MEL-14 mAb and analyzed by flow cytometry. Although wild-type mice had a pronounced shift in fluorescence compared with the background level, granulocytes from the triple-null mice did not show increased fluorescence (Figure 2). This observation was made with all leukocyte populations present in peripheral blood and bone marrow from both the E/L/P triple-mutant mice and the single–L-selectin −/− mutant mice (data for single mutant not shown).
White blood cell counts
As shown in Table 1, introduction of the L-selectin mutation into E/P double-mutant mice resulted in partial reduction of the severe leukocytosis observed in double-null mice. The severe leukocytosis in the E/P double-mutant mice was consistent with previous observations in E/P double mutants generated from the ES cells used to make these triple-mutant mice10 and in other previously described E/P double-mutant mice.14 Leukocyte counts in triple-mutant mice were elevated over those in wild-type mice, including the counts of total WBCs (P = .0006), neutrophils (P < .0001), monocytes (P < .0001), and eosinophils (P < .05). There were no significant differences in lymphocyte counts between +/+ and triple-null mice (P = .519). However, addition of the L-selectin mutation resulted in a significant reduction in leukocytosis compared with findings in E/P double mutants. Total leukocyte counts were significantly lower in the E/L/P triple-mutant mice compared with the E/P double-mutant mice, including the counts of WBCs (P < .0001), neutrophils (P = .0097), lymphocytes (P = .0006), monocytes (P = .0002), and basophils (P = .0002). The reduction in leukocytosis observed when the L-selectin mutation was added to mice with mutations in E-selectin and P-selectin suggested decreased rather than increased inflammatory disease and was consistent with the decreased skin disease in the triple null mice.
Leukocyte rolling in triple null mice
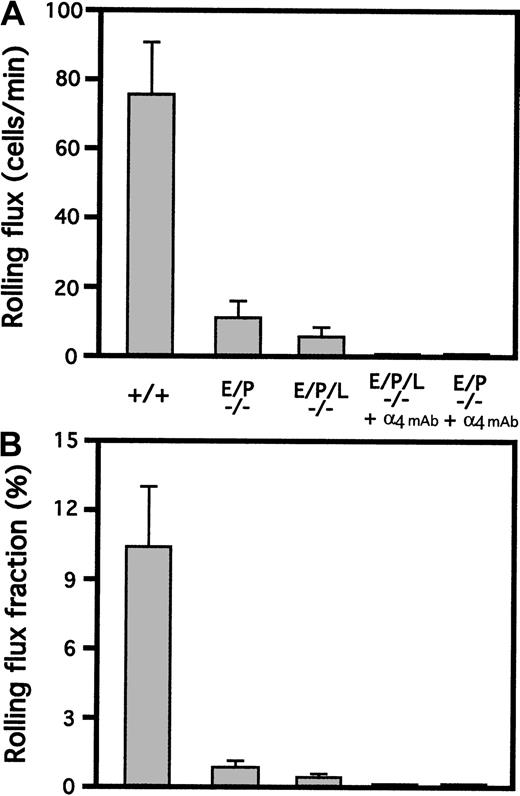
As expected, leukocyte rolling in the triple-selectin–null mice was severely compromised. Leukocyte rolling was completely absent at time points up to 5 hours after exteriorization of the cremaster muscle both with and without pretreatment with TNF-α. Both rolling flux (Figure 3A) and rolling flux fraction (Figure 3B) were more reduced than in the E/P double-mutant mice, again demonstrating the importance of L-selectin in rolling at a time long after the initial inflammatory stimulation (5 hours). The small amount of rolling observed 5 hours after exteriorization was completely eliminated by administration of mAb PS/2, which blocks the adhesive function of α4-integrin (Figure 3); these results indicate that this residual rolling is mediated by α4-integrin. The physiologic importance of this α4-integrin–mediated rolling is unclear.
Leukocyte rolling in the cremaster muscle of triple-selectin–null mice 5 hours after exteriorization.
Leukocyte rolling in the postcapillary venules of the cremaster muscle was severely inhibited (P < .001) compared with the wild type in mice null for all 3 selectin genes. This was reflected by both the rolling flux (A) and rolling flux fraction (B) in mutant and control (+/+) mice. Rolling was completely absent at time points up to 5 hours, with or without pretreatment with TNF-α. A small amount of residual rolling was present at this time, with or without the pretreatment. These results are similar to those in E/P double mutants. The residual rolling observed at 5 hours in both double-mutant and triple-mutant mice was eliminated completely by intravenous administration of a mAb (PS/2) blocking α4-integrin, indicating that the residual rolling is dependent on α4-integrin.
Leukocyte rolling in the cremaster muscle of triple-selectin–null mice 5 hours after exteriorization.
Leukocyte rolling in the postcapillary venules of the cremaster muscle was severely inhibited (P < .001) compared with the wild type in mice null for all 3 selectin genes. This was reflected by both the rolling flux (A) and rolling flux fraction (B) in mutant and control (+/+) mice. Rolling was completely absent at time points up to 5 hours, with or without pretreatment with TNF-α. A small amount of residual rolling was present at this time, with or without the pretreatment. These results are similar to those in E/P double mutants. The residual rolling observed at 5 hours in both double-mutant and triple-mutant mice was eliminated completely by intravenous administration of a mAb (PS/2) blocking α4-integrin, indicating that the residual rolling is dependent on α4-integrin.
Chemically induced peritonitis
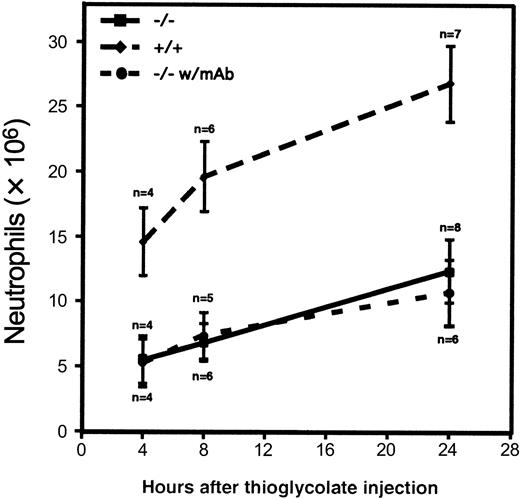
Neutrophil influx into the peritoneal cavity in the triple-null mice was compromised during thioglycolate-induced peritonitis. Significant inhibition of neutrophil emigration was present at all time points tested (Figure 4). The small amount of emigration observed indicated either that emigration may occur to some extent in the absence of rolling or that other molecules may act redundantly to mediate enough rolling in vivo for firm attachment followed by emigration. This was especially evident at the 24-hour time point, when more than 10 × 106 neutrophils were recovered from the peritoneal lavage. Because rolling mediated by α4-integrin was revealed at later time points by intravital microscopy, we hypothesized that blocking the action of α4-integrin might further reduce the number of emigrating neutrophils, especially at the 24-hour time point. Figure 4 shows that administration of mAb PS/2, which blocks function of α4-integrin, did not reduce neutrophil emigration at any time point to a greater extent than did the deficiency of the 3 selectin molecules alone.
Neutrophil influx into the peritoneal cavity during thioglycolate-induced peritonitis.
Thioglycolate was injected into the peritoneal cavity of 4 to 8 wild-type mice (diamonds) or triple-null mice (squares). Values represent the mean ± SD number of neutrophils recovered from peritoneal lavage at the indicated times. The differences between the triple-selectin–null mice and the wild-type mice were significant (P < .001) at all times studied. Solid circles represent the number of neutrophils collected after intravenous administration of the α4-integrin–blocking mAb PS/2 to triple-null mice 1 hour before and 6 hours after thioglycolate injection. Although we hypothesized that this blocking antibody might reduce neutrophil emigration into the peritoneal cavity, we observed no effect in this model.
Neutrophil influx into the peritoneal cavity during thioglycolate-induced peritonitis.
Thioglycolate was injected into the peritoneal cavity of 4 to 8 wild-type mice (diamonds) or triple-null mice (squares). Values represent the mean ± SD number of neutrophils recovered from peritoneal lavage at the indicated times. The differences between the triple-selectin–null mice and the wild-type mice were significant (P < .001) at all times studied. Solid circles represent the number of neutrophils collected after intravenous administration of the α4-integrin–blocking mAb PS/2 to triple-null mice 1 hour before and 6 hours after thioglycolate injection. Although we hypothesized that this blocking antibody might reduce neutrophil emigration into the peritoneal cavity, we observed no effect in this model.
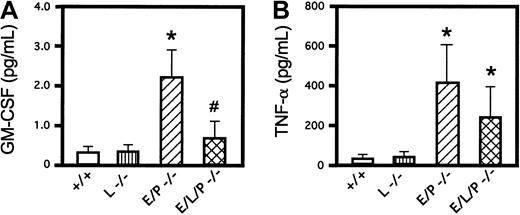
Cytokine assays
ELISAs of 2 proinflammatory cytokines, GM-CSF and TNF-α, were done in wild-type mice, homozygous L-selectin mutant mice, E/P double-mutant mice, and E/L/P triple-mutant mice. GM-CSF levels were increased in E/P double mutants compared with +/+ mice as expected (Figure 5A) and reported previously.14 Mice null for L-selectin had GM-CSF levels similar to those in +/+ mice. Consistent with the decreased leukocytosis in triple-null mice compared with E/P double-null mice, levels of GM-CSF in triple-null mice were substantially lower than those in E/P double-null mice and were not significantly different from those in +/+ or L-selectin −/− mice (0.67 ± 0.46 pg/mL in E/L/P triple mutants versus 0.34 ± 0.16 pg/mL and 0.37 ± 0.16 pg/mL in +/+ and L-selectin −/− mice, respectively; P < .16 for both). Introducing the L-selectin mutation into the E/P double mutants prevented the 6- to 7-fold elevation of levels of GM-CSF observed in the double mutants. Serum levels of TNF-α in the E/L/P triple-mutant mice were significantly higher than those in the +/+ or L-selectin −/− mice (Figure 5B). Although the difference was not statistically significant (P = .1647), levels of circulating TNF-α were somewhat lower in the triple mutants compared with the double mutants, similar to the findings for GM-CSF. The cytokine elevations associated with the severe inflammatory disease of skin and lungs in the E/P double mutants10 14 were reduced rather than aggravated with introduction of a null mutation for the third selectin.
Levels of the proinflammatory cytokines GM-CSF and TNF-α.
Introduction of the L-selectin mutation into the E/P-selectin double-mutant mice lowered the levels of GM-CSF and TNF-α in triple-selectin–null mice. (A) GM-CSF levels were elevated by a factor of 6 in E/P double-selectin–null mice over values in wild-type and L-selectin–deficient mice (P < .001 for both; mice 22-24 weeks of age; n = 6 for all). GM-CSF was also significantly increased (3 fold) in E/P −/− mice compared with triple-mutant mice (P < .0013; n = 6). GM-CSF levels in triple-mutant mice were not significantly higher than those in wild-type mice (P < .159) or L-selectin −/− mice (P < .156). (B) TNF-α levels in E/P double-null mice were significantly higher than those in wild-type or L-selectin–deficient mice (P < .0001 andP < .0016, respectively). Serum TNF-α levels in triple-null mice, though lower than those in E/P double mutants, were also significantly higher than those in either wild-type or L-selectin–deficient mice (P < .02 for both). Although the levels of TNF-α in E/P double-null mice were nearly twice those in triple-null mice, the difference was not significant (P < .16). The error bars in the histograms indicate means ± SD. The asterisk indicates a significant difference from wild-type and L-selectin–deficient mice (P < .001), and the number sign indicates a significant difference from E/P double-selectin mutant mice (P < .002).
Levels of the proinflammatory cytokines GM-CSF and TNF-α.
Introduction of the L-selectin mutation into the E/P-selectin double-mutant mice lowered the levels of GM-CSF and TNF-α in triple-selectin–null mice. (A) GM-CSF levels were elevated by a factor of 6 in E/P double-selectin–null mice over values in wild-type and L-selectin–deficient mice (P < .001 for both; mice 22-24 weeks of age; n = 6 for all). GM-CSF was also significantly increased (3 fold) in E/P −/− mice compared with triple-mutant mice (P < .0013; n = 6). GM-CSF levels in triple-mutant mice were not significantly higher than those in wild-type mice (P < .159) or L-selectin −/− mice (P < .156). (B) TNF-α levels in E/P double-null mice were significantly higher than those in wild-type or L-selectin–deficient mice (P < .0001 andP < .0016, respectively). Serum TNF-α levels in triple-null mice, though lower than those in E/P double mutants, were also significantly higher than those in either wild-type or L-selectin–deficient mice (P < .02 for both). Although the levels of TNF-α in E/P double-null mice were nearly twice those in triple-null mice, the difference was not significant (P < .16). The error bars in the histograms indicate means ± SD. The asterisk indicates a significant difference from wild-type and L-selectin–deficient mice (P < .001), and the number sign indicates a significant difference from E/P double-selectin mutant mice (P < .002).
Pathological findings
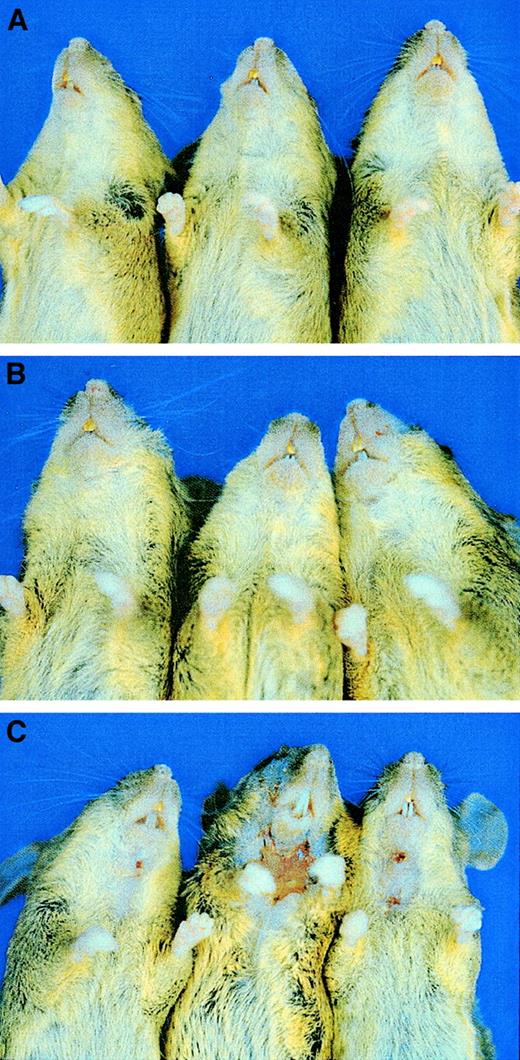
As reported previously, mice null for E/P double mutations develop mucocutaneous infections or ulcerative dermatitis10,14 and progress to poor general health. Surprisingly, in our study, addition of L-selectin deficiency to the E/P null genotype virtually eliminated the development of skin disease and returned the mice to a much healthier appearance. Figure 6 shows 3 15-month-old littermate control mice (Figure 6A) and 3 E/L/P triple-mutant mice of the same age (Figure 6B); the mice were second filial generation C57BL/6 mice crossed with 129/SvEv mice. None of 34 triple-selectin–null mice that were housed in a SPF barrier facility for at least 15 months developed the severe skin disease observed at a much younger age in the E/P double mutants in the same mouse room.10 14 Only 3 of 156 triple-selectin–null mice (1.9%) had any dermatitis by the age of 6 months. These 3 mice (Figure6C) were littermates, and the dermatitis observed was not as severe as that in the double-null mice. The E/L/P triple-mutant mice mated normally until at least 12 to 15 months of age and had much better overall health than the E/P double-null mice in the same mouse room, which only mated normally until 2 to 5 months of age.
Reduction in skin disease in E/L/P triple-mutant mice compared with E/P double-mutant mice.
Introduction of the L-selectin mutation virtually eliminated the severe skin lesions that were observed in nearly all the E/P double mutants. (A) Three 15-month-old +/+ control mice (second filial generation of mixed C57BL/6 and 129/SvEv background). (B) Three 15-month-old E/L/P triple-mutant mice of mixed background. None of the more than 30 triple-null mice observed until 15 months of age showed any sign of skin lesions and all appeared generally healthy, as shown here. (C) The 3 mice in which lesions developed at 5 to 6 months of age. The few lesions observed in the triple-null mice (3 of 156 mice) were not as severe grossly or histopathologically as those in the E/P double-null mice.
Reduction in skin disease in E/L/P triple-mutant mice compared with E/P double-mutant mice.
Introduction of the L-selectin mutation virtually eliminated the severe skin lesions that were observed in nearly all the E/P double mutants. (A) Three 15-month-old +/+ control mice (second filial generation of mixed C57BL/6 and 129/SvEv background). (B) Three 15-month-old E/L/P triple-mutant mice of mixed background. None of the more than 30 triple-null mice observed until 15 months of age showed any sign of skin lesions and all appeared generally healthy, as shown here. (C) The 3 mice in which lesions developed at 5 to 6 months of age. The few lesions observed in the triple-null mice (3 of 156 mice) were not as severe grossly or histopathologically as those in the E/P double-null mice.
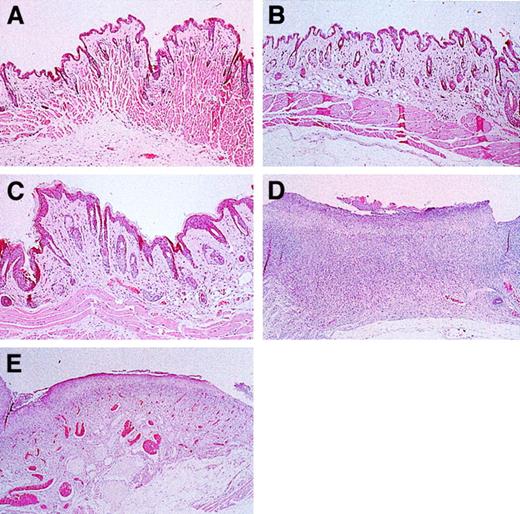
On gross examination, cervical skin from the control, L-selectin–deficient, and nearly all the E/L/P-selectin–deficient mice was intact. In contrast, skin from the ventral portion of the neck from most of the E/P double-mutant mice showed large areas of ulceration. Representative histological sections of cervical skin from these mice are shown in Figure 7. Cervical skin from control, L-selectin–deficient, and E/L/P-selectin–deficient mice (Figure 7A, 7B, and 7C, respectively) had no excoriative lesions and was intact. Tissue sections of cervical skin from these mice lacked a dermal infiltrate, and the surface had no bacterial colonization. In contrast, histopathological examinations of skin from E/P double-mutant mice (Figure 7D) revealed diffuse excoriation and loss of the epidermis, with focal retention of the basal cell layer of the epidermis. A mixture of gram-positive and gram-negative bacteria colonized the surface of these denuded areas. An intense mixture of granulocytes and mononuclear cells disrupted the underlying pannicular muscle layer and infiltrated the underlying dermis. No fungi or viral inclusions were observed. One of the E/L/P triple-mutant mice (middle, Figure 6C) did have diffuse ulceration similar to that in the E/P group. This mouse was one of the 3 of 156 triple-mutant mice mentioned above. However, less granulocytic and mononuclear infiltration and less disruption of the underlying dermis was observed in these mice (Figure 7E) than in the E/P double-mutant mice.
Skin histological studies of triple-mutant, E/P double-mutant, and control mice.
Skin sections were obtained from the area of the neck where lesions formed (Figure 6). Cervical skin from wild-type (A) and L-selectin–deficient (B) mice was normal and without lesions. (C) Skin from 15-month-old E/L/P triple mutants was essentially normal. (D) Cervical skin from E/P double-mutant mice was diffusely ulcerated, with the surface colonized by bacteria and a mixed acute and chronic inflammatory infiltrate that disrupted the underlying dermis and pannicular skeletal muscle layer. (E) Section of an excoriative skin lesion representative of the lesions that developed in the 3 most severely affected triple-null mice. Lesions that formed in the few triple-null mice affected were much less severe than those common in the E/P double-mutant mice, with the underlying structural layers remaining intact (hematoxylin and eosin, original magnification × 200).
Skin histological studies of triple-mutant, E/P double-mutant, and control mice.
Skin sections were obtained from the area of the neck where lesions formed (Figure 6). Cervical skin from wild-type (A) and L-selectin–deficient (B) mice was normal and without lesions. (C) Skin from 15-month-old E/L/P triple mutants was essentially normal. (D) Cervical skin from E/P double-mutant mice was diffusely ulcerated, with the surface colonized by bacteria and a mixed acute and chronic inflammatory infiltrate that disrupted the underlying dermis and pannicular skeletal muscle layer. (E) Section of an excoriative skin lesion representative of the lesions that developed in the 3 most severely affected triple-null mice. Lesions that formed in the few triple-null mice affected were much less severe than those common in the E/P double-mutant mice, with the underlying structural layers remaining intact (hematoxylin and eosin, original magnification × 200).
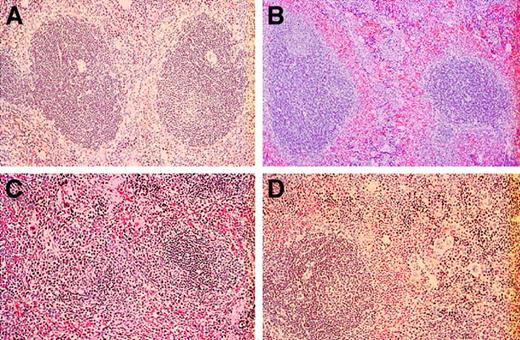
On gross examination during necropsy, both the younger and older E/P-selectin–deficient and E/L/P-selectin–deficient mice had marked cervical lymphadenopathy, whereas L-selectin–deficient and control mice had cervical lymph nodes closer to normal in size. Control and L-selectin–deficient mice had relatively small and inconspicuous cervical lymph nodes, with preservation of typical nodal architecture in the wild-type mice, whereas the L-selectin– null mice had smaller nodes with disturbed architecture, as reported previously.4 Cervical lymphadenopathy was massive in E/P-selectin–deficient mice and was less extensive but still moderate to severe in E/L/P-selectin–deficient mice. In both the E/P double- and the E/L/P triple-mutant mice, the lymph nodes were replaced by a reactive lymphoplasmacytic infiltrate that effaced the typical corticomedullary architecture of the lymph nodes (data not shown). In the E/L/P triple-mutant mice, residual lymphoid follicles with germinal centers were observed occasionally, whereas these structures were not recognized in the E/P double mutants.
The splenic tissue differed considerably among selectin-deficient and control mice. Control mice had typical splenic architecture, with well-defined lymphoid follicles with central arterioles separated distinctly by the sinusoids of the red pulp, which lacked extramedullary hematopoiesis (Figure 8A). The white pulp was relatively normal in spleens from L-selectin–deficient mice, with only a mild degree of extramedullary hematopoiesis in the sinusoids of the red pulp (Figure 8B). The definition between red pulp and white pulp areas was not as distinct as that in the normal spleens. Again, the E/P double mutants had the greatest alteration in splenic parenchyma. In these mice (Figure 8C), there was massive extramedullary hematopoiesis, with myeloid and megakaryocytic lineages readily identifiable and a lesser erythroid component. Lymphoid follicles forming the white pulp of the spleen were effaced and observed infrequently because of expansion of the red pulp by extensive extramedullary hematopoiesis. Spleens from younger and older E/L/P-selectin–deficient mice had moderate disruption of the splenic architecture by extramedullary hematopoiesis in the sinusoids of the red pulp; however, lymphoid follicles of the white pulp were identified occasionally and these had irregular sizes and shapes (Figure 8D).
Histopathological studies of spleens from wild-type, L-selectin–deficient, E/P double-selectin–null, and E/L/P triple-selectin–null mice.
(A) The normal red and white pulp architecture of the spleen was present in control mice (n = 6). (B) Splenic architecture was distorted by extramedullary hematopoiesis in L-selectin–deficient mice (n = 6); however, this was to a much lesser extent than in the E/P-selectin– and E/L/P-selectin–null mice. Extramedullary hematopoiesis in spleens was markedly increased in the E/P-selectin (C) and E/L/P-selectin (D) null mice. The architectural disorganization was much more pronounced in the E/P double-mutant mice (n = 12) than in the triple-selectin–null mice (n = 6; hematoxylin and eosin, original magnification × 200).
Histopathological studies of spleens from wild-type, L-selectin–deficient, E/P double-selectin–null, and E/L/P triple-selectin–null mice.
(A) The normal red and white pulp architecture of the spleen was present in control mice (n = 6). (B) Splenic architecture was distorted by extramedullary hematopoiesis in L-selectin–deficient mice (n = 6); however, this was to a much lesser extent than in the E/P-selectin– and E/L/P-selectin–null mice. Extramedullary hematopoiesis in spleens was markedly increased in the E/P-selectin (C) and E/L/P-selectin (D) null mice. The architectural disorganization was much more pronounced in the E/P double-mutant mice (n = 12) than in the triple-selectin–null mice (n = 6; hematoxylin and eosin, original magnification × 200).
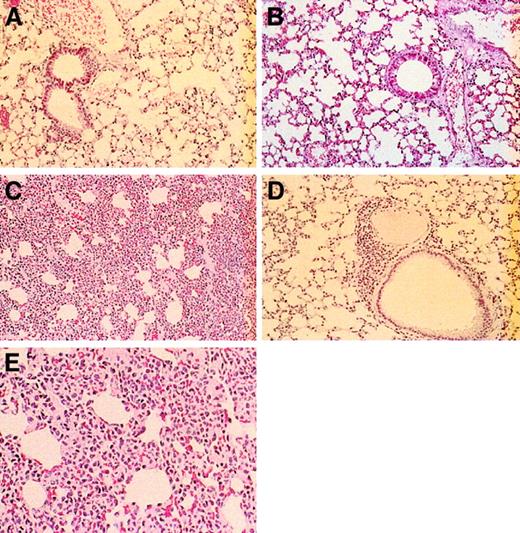
Lung tissue from the selectin-deficient mice had a gradient of decreasing cellularity within the alveolocapillary walls, with the most severely affected interstitial areas observed in the E/P double mutants. Control (Figure 9A) and L-selectin–deficient (Figure 9B) mice had no pulmonary alterations. These groups of mice had lung parenchyma with typical architecture of small airways and alveoli and lacked the bronchial lymphoid aggregates observed in the younger and older E/L/P-selectin–deficient mice. The 12 alveolocapillary walls examined in the E/P double mutants were engorged by leukocytes to various degrees; however, these leukocytes were confined to the small vascular spaces and did not infiltrate the interstitial supporting tissue or the alveolar and airway spaces (Figure 9C). No organisms or viral inclusions were observed. There was only a small increase in leukocytes in the alveolocapillary walls in both the younger and older E/L/P-selectin–deficient mice, and occasional small bronchial lymphoid aggregates were also present (Figure 9D). The appearances of alveolar and airway spaces in the lung were moderately distorted but readily recognized. Again, no organisms or viral inclusions were observed. Although there was some distortion of lung architecture in the triple-null mice, it was never as dramatic as that in the E/P double-mutant mice. Figure 9E shows a higher-power magnification of lungs from an E/P double-mutant mouse; the cells present are granulocytic.
Histopathological studies of lungs from wild-type, L-selectin–deficient, E/P double-selectin–null, and E/L/P triple-selectin–null mice.
Control mice (A) and L-selectin–deficient mice (B) had normal-appearing lung parenchyma. (C) In E/P double-selectin–null mice, alveolocapillary walls in tissue from inflated lungs were engorged with inflammatory cells, resulting in greatly expanded interstitial regions. (D) In contrast, the interstitial regions were remarkably more normal in the triple-null mice. Occasionally, these mice had bronchial lymphoid aggregates and a small increase in leukocytes in the alveolocapillary walls (hematoxylin and eosin, original magnification × 200). (E) Higher-power magnification (× 400) of the alveolocapillary walls from lungs of E/P double-selectin–null mice. Most of the inflammatory cells are neutrophils.
Histopathological studies of lungs from wild-type, L-selectin–deficient, E/P double-selectin–null, and E/L/P triple-selectin–null mice.
Control mice (A) and L-selectin–deficient mice (B) had normal-appearing lung parenchyma. (C) In E/P double-selectin–null mice, alveolocapillary walls in tissue from inflated lungs were engorged with inflammatory cells, resulting in greatly expanded interstitial regions. (D) In contrast, the interstitial regions were remarkably more normal in the triple-null mice. Occasionally, these mice had bronchial lymphoid aggregates and a small increase in leukocytes in the alveolocapillary walls (hematoxylin and eosin, original magnification × 200). (E) Higher-power magnification (× 400) of the alveolocapillary walls from lungs of E/P double-selectin–null mice. Most of the inflammatory cells are neutrophils.
Discussion
We prepared mice with homozygous deficiency for all 3 selectin molecules. These E/L/P triple-null mutants had remarkably less inflammatory disease than we observed previously in E/P double-null mice. There are now 2 independently prepared mutant strains for E/L/P triple-null mice and E/P double-null mice: the Bay and Hyn mutations. The original reports on the E/PBay and E/PHynmutants were similar in their descriptions of prominent skin disease, but they differed with respect to the lungs, which had no obvious abnormalities in E/PHyn mice but increased cellularity of the alveolocapillary walls in E/PBay mice.10Although the findings in the lungs were not emphasized in the original report on E/PBay mice, they were usually quite prominent (Figure 9).
There are also substantial similarities and some differences between the findings in the E/L/PBay mice reported here and those in observed E/L/PHyn mice.18 Both types of triple-null mice had leukocytosis, impaired neutrophil emigration, and a severe deficit in leukocyte rolling. The important differences were that E/L/PBay mice had minimal or no skin disease, whereas E/L/PHyn mice had skin disease as severe as that in E/PHyn double-mutant mice. The degree of leukocytosis correlated with the presence of severe skin disease; thus, leukocyte counts were lower in E/L/PBay triple-mutant mice than in the double mutants but higher in E/L/PHyn triple-mutant mice than in the double mutants. Because of the nature of the mutations introduced into the mice, it appears likely that all those mutations provide functional null alleles for all 3 selectins. It seems more likely that differences in observations are related to the genetic background of the mutant strains and differences in husbandry and environment. Exposure to opportunistic normal flora is likely to be an important variable in the development of skin disease, although extensive screening for pathogenic bacteria, fungi, and parasites yielded negative results in both mutant and sentinel mice. Organisms cultured from the severe infections in E/PBay mutant mice were opportunistic normal flora. Normal flora were also found in E/PHyn double-mutant mice.14 Although little or no skin disease was observed in the E/L/PBaytriple-mutant mice during our experiments at Baylor or at the University of Virginia, a collaborator using these mice observed a higher incidence of skin infections in experiments conducted in Seattle (T. Papayannopoulou, personal communication, November 2000). Because our triple-mutant mice at another location had skin disease, we believe that the presence or absence of skin disease is related more to environment and microbiologic flora than to any difference between the E/L/PBay and E/L/PHyn mutations. It would be of interest to evaluate the effect of various microbes on the phenotypic differences with all mutations backcrossed onto a similar genetic background and all mutant mice kept in the same room.
Results of studies of leukocyte rolling using intravital microscopy were quite similar for the E/L/PBay and E/L/PHyn triple-mutant mice and the E/PBaydouble-mutant mice made additionally deficient for L-selectin by means of bone marrow transplantation.17 In all 3 types of mice, the rolling was more severely reduced than in mice with the double- or single-selectin deficiencies. Rolling mediated by α4-integrin at later time points (> 5 hours) was demonstrated in both E/L/PBay mice (Figure 3) and E/PBay mice in which L-selectin–deficient bone marrow was transplanted.17
The phenotype of the triple-selectin–null mice shows some similarity to that of mice lacking the α1,3 fucosyltransferase, Fuc-TVII.24 This fucosyltransferase has been implicated in the addition of fucose to the terminal galactosamine needed to form the ligands for selectin binding. Mice deficient in Fuc-TVII have a severe defect in leukocyte rolling in the cremaster muscle, but some rolling occurs in the ear, indicating that molecules other than selectins are involved or the existence of ligands that do not require Fuc-TVII for binding of selectins, such as glycoprotein Ibα.25Fuc-TVII–deficient mice also have a defect in thioglycolate-induced extravasation of neutrophils into the peritoneal cavity and a leukocytosis similar to that observed in triple-selectin–null mice. Fuc-TVII–deficient mice are reportedly healthy and do not have the skin lesions observed in E/P double- or E/L/P triple-mutant mice. The pathophysiologic findings in Fuc-TVII–deficient mice may be somewhat similar to those in triple-selectin–null mice.
The amelioration of skin disease in the E/L/PBay mice compared with the E/PBay mice, although unexpected at the time of their generation, is not surprising when compared with the phenotypic improvement in E/PBay mice made additionally deficient for ICAM-1.26 Introduction of deficiency for ICAM-1 resulted in a complete rescue from the severe skin disease observed in the E/PBay double mutants, much like the amelioration observed in the E/L/PBay triple mutants. The types of skin lesions that occurred in most E/P double-mutant mice were not observed in 193 E/PBay mice that were also deficient for ICAM-1, including 14 mice older than 1 year. The amelioration in skin disease observed when a deficiency of either ICAM-1 or L-selectin was added to the E/PBay genotype may be explained by some functional similarities between ICAM-1 and L-selectin. Both L-selectin and ICAM-1 have roles in the development of T-cell responses and in emigration of neutrophils and other leukocytes. In specific immunity, ICAM-1 was found to be an important costimulatory molecule needed on splenic stimulator cells, as shown by the greatly diminished T-cell–stimulating activity in these cells in mice lacking ICAM-1.27 L-selectin is required for entry of lymphocytes into peripheral lymph nodes4 and for maintenance of normal lymphocyte recirculation through the body.28 It was also reported that mice deficient in L-selectin have impaired T-cell responses.29 Thus, both ICAM-1 and L-selectin have prominent roles in specific immunity. ICAM-1 and L-selectin have long been recognized as important components of the inflammatory response and leukocyte emigration.1,30 L-selectin is important in the early rolling of leukocytes along the endothelial surface at sites of inflammation, and ICAM-1 is important in firm adhesion of these same leukocytes. It was also shown that ICAM-1 and L-selectin have roles dependent on and overlapping each other for their proper function in leukocyte rolling and emigration.31 32 These observations are compatible with the finding that a deficiency of either ICAM-1 or L-selectin ameliorates skin disease in E/PBay mice.
In our studies comparing E/PBay and E/L/PBaymice, we focused substantially greater attention on the prominent accumulation of leukocytes within the alveolar capillaries. Electron microscopy studies previously confirmed that this accumulation of leukocytes in the lung was retained within the vascular space.10 The pulmonary accumulation of leukocytes in the E/PBay mice but not the E/L/PBay triple-mutant mice may primarily reflect the substantially increased leukocytosis and some associated increase in proinflammatory cytokines. We support the interpretation that establishment (or not) of skin disease is the primary phenotypic variable, with changes in cytokine levels, blood count, and pulmonary leukocytes being secondary. Alternatively, the lung involvement may reflect a form of undetected infectious pneumonitis, but this seems unlikely given the extensive effort to identify microorganisms and the fact that leukocytes in the lung remain entirely within the vascular space. Microcirculatory obstruction in the lung with extreme hyperleukocytosis has been reported.33 34 There is a high frequency of respiratory distress in hyperleukocytic granulocytic leukemias, and similar pathophysiologic characteristics in E/PBay mice may provide a mouse model for this syndrome.
In conclusion, there are important similarities and differences between E/L/PBay and E/L/PHyn triple-mutant mice, as well as marked differences between these mice and the corresponding E/P double-mutant mice. In particular, severe compromise of the alveolar space was observed in E/PBay mice but not in mice with any other genotype. The severe skin disease and pulmonary disease in E/PBay mice was greatly ameliorated by introduction of deficiency of either L-selectin or ICAM-1 in addition to the E/P double deficiency; this milder phenotype is similar to that observed in Fuc-TVII −/− mice lacking most selectin ligands. In contrast, the skin disease in E/PHyn double-mutant mice was not ameliorated by introduction of deficiency of L-selectin. Both L-selectin and ICAM-1 have important functions in the immune response; thus, modulating their response to normal flora may ameliorate the inflammatory disease in E/PBay mice. Overall, our data suggest that important differences in the genetic backgrounds of the mice, animal husbandry, and the presence of opportunistic or pathogenic infectious agents in the environment remain to be delineated.
We thank D. Bulnes, P. Grennan, M. Idunoba, T. Mayer, F. Nails, S. Rodgers, J. Smalls, and E. Walker for histotechnology expertise.
Supported by National Institutes of Health grants AI-32177 to A.L.B. and HL-54136 to K.L. and HL-42550 and ES-06091 to C.W.S.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Arthur L. Beaudet, Department of Molecular and Human Genetics, Baylor College of Medicine, One Baylor Plaza, Room T619, Houston, TX 77030; e-mail: abeaudet@bcm.tmc.edu.