Abstract
The solubility of deoxygenated hemoglobin S (HbS), which is the concentration of fully deoxygenated HbS in equilibrium with polymer (CSAT), is a factor that determines in vivo polymer formation. However, measurement of CSAT is usually performed under conditions that are far from physiological. In solution studies of HbS by Benesch et al, it was demonstrated that p50, the point at which hemoglobin is half-saturated with oxygen, is proportional to the amount of polymer formed and that it may be used to measure CSAT. This method has been extended to measure CSAT in intact red cells by varying extracellular osmolarity, which, in turn, varies intracellular hemoglobin concentration. This method measures intracellular CSAT under physiological conditions with intact red cell contents and can be applied to human and transgenic mouse red cells. The principle is demonstrated by measuring p50 as a function of extracellular osmolarity for AA, SS, and AS red cells.
Introduction
Sickle cell disease is the result of a single amino acid mutation in hemoglobin (β6 Glu Val) that results in intracellular formation of rigid polymers of deoxygenated hemoglobin (HbS). When deoxygenated HbS concentration exceeds CSAT, polymer formation begins. Because CSAT is a measure of the onset of polymer formation, it may be correlated with the probability of vaso-occlusion in humans and transgenic mice. However, not only is CSAT usually measured under nonphysiological conditions, these measurements are also not sensitive to other important in vivo factors such as red cell intracellular hemoglobin concentration (MCHC) and 2,3-DPG content.
Several methods for measuring polymer formation in solutions of deoxygenated HbS have been developed. The measurement, usually referred to as CSAT, is regarded as the criterion standard.1,2 Relatively large amounts of hemoglobin are required, the final pH is low (approximately 6.8), and the measurement is made under fully deoxygenated conditions in the presence of dithionite. Intracellular components that either do or may affect polymerization are generally not present because they have been removed by dialysis. They can, of course, be added back, but accurate replication of intracellular conditions is difficult. Other methods requiring smaller amounts of hemoglobin are also in use, such as the high-phosphate method developed by Adachi and Asakura3,4and the dextran method developed by Bookchin et al.5Although these methods have the advantage of consuming smaller amounts of hemoglobin, they deviate even further from physiological conditions and, particularly in the high-phosphate method, may systematically cause deviation from values obtained with the CSAT method. Another method requiring relatively small amounts of hemoglobin was developed by Benesch et al.6
In studies of HbS solutions, it was shown by Benesch et al6 that the p50 is proportional to the amount of polymer formed because polymer has a much lower oxygen affinity than HbS tetramer. In the Benesch method, the hemoglobin concentration at which polymer formation begins can be detected by the onset of rapidly-linearly increasing p50 in a plot of p50 versus hemoglobin concentration. The increase in p50 is linear because, under conditions in which polymer is formed, the concentration of hemoglobin in both the solution and the polymer phases is constant, and the variable is the proportion of hemoglobin in these phases.
To apply the same technique to red cells, MCHC can be varied by changing the extracellular osmolarity. The MCHC at which onset of polymer formation occurs (the intracellular CSAT) is the point at which p50 begins to increase rapidly in a plot of p50 versus osmolarity. This is an estimate of the polymer-forming tendency of hemoglobins in the red cell with all red cell components (such as 2,3-DPG, antisickling hemoglobins, or added antisickling agents) present. We demonstrate the principle here by measuring p50 as a function of extracellular osmolarity for AA, SS, and AS red cells.
Study design
Blood samples
Heparinized venous blood was obtained with informed consent from patients with SS and AS who were enrolled in Heredity Clinic of the General Clinical Research Center. Genotype was confirmed by hemoglobin electrophoresis and isoelectric focusing. None of the patients with sickle cell disease had been transfused within the prior 3 months. AA blood was drawn from hematologically normal donors after informed consent.
p50 measurements
High- and low-osmolarity buffers containing 10 mM HEPES, 5 mM KCl, 5 mM glucose and NaCl, which was added to adjust the osmolarity to 160 or 450 mOsm, were prepared. The buffers had a pH of 7.4 at 37°C, and intermediate osmolarities were prepared by mixing the 2 buffers and measuring osmolarity with a MicroOsmette (Precision Systems, Natick, MA). Cells were washed 3 times with buffer and allowed to equilibrate for a standardized period, and aliquots were removed for measurement of p50 using a Hemoscan (Aminoco, Silver Spring, MD) on the slowest change in percentage oxygen program to allow the equilibration of polymer and solution phases. Hemoscan (Aminoco) measurements were made by deoxygenating the sample, waiting 7 minutes, and slowly reoxygenating the sample; this protocol minimized the kinetic effects associated with polymerization. Three aliquots were also removed for determination of MCHC by measurement of hematocrit using a microhematocrit centrifuge (MicroHematocrit, Damon/IEF Division, Needham Heights, MA) and hemoglobin concentration with Drabkins reagent (Sigma Diagnostics, St Louis, MO). Supernatant of the remaining cells was removed, and osmolarity was measured.
The approximate point at which p50 began to increase rapidly was estimated visually. Data below this point were fitted by linear least-squares regression, and data above this point were similarly fitted using Statgraphics Plus (Manugistics, Rockville, MD). The intersection of the 2 lines was the osmolarity at which the onset of polymer formation occurred. This osmolarity was correlated with MCHC measured on cells equilibrated in the same buffer.
Results and discussion
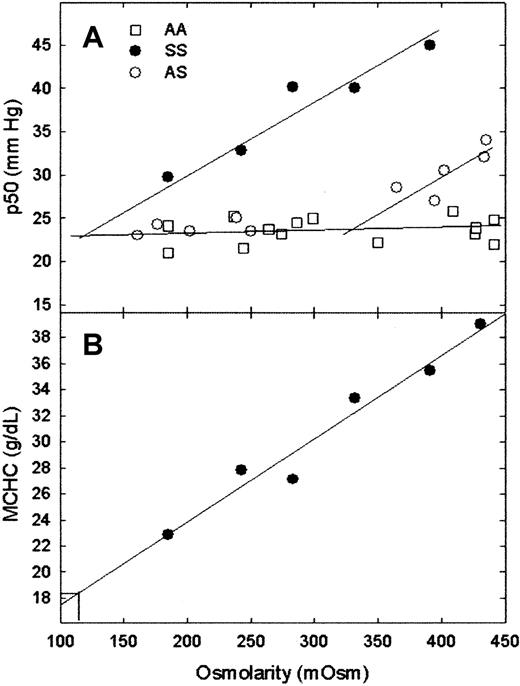
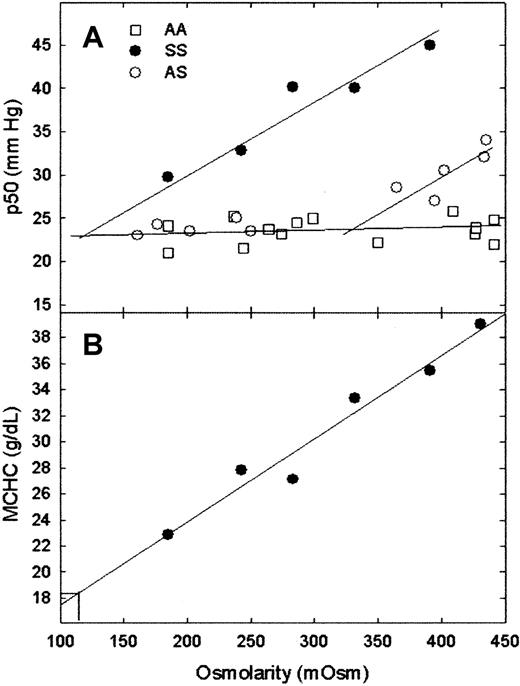
The transition from polymer-free red cells to red cells with polymer occurs within the range of osmolarities tested for AS red cells (Figure 1A). Within the limits of error, a straight line with a slope of nearly 0 and a p50 of 23 mm Hg was found for AA red cells and for AS red cells at osmolarities less than 280 mOsm. This is expected because the p50s for HbA and HbS are the same in dilute Hb solutions in physiological buffers. A straight line with a slope of 0.089 ± 0.01 mm Hg/mOsm, withr2 = 0.94, was found for SS red cells. For SS cells, p50 versus osmolarity is linear to the lowest osmolarity tested; the line intercepts the low-osmolarity plots for AA and AS red cells at 124 mOsm, which corresponds to an MCHC of 18.2 g/dL (Figure 1B), with a maximum value of 23 g/dL at the 95% confidence interval. The intersection of the low-osmolarity and the high-osmolarity lines for AS cells occurs at 330 mOsm; MCHC at this osmolarity is 32.2 g/dL. These values are higher than those found in solution CSATmeasurements (Figure 1), which may be attributed in part to higher pH (pH 7.4 vs pH 6.8) and, in the case of SS cells, the presence of HbF in these measurements. Patient-to-patient variation is expected and observed.
MCHC and p50 versus osmolarity.
(A) Measurement of intracellular CSAT by plotting p50 versus extracellular osmolarity for AA, SS, and AS red cells. (B) Determination of MCHC at the onset of polymer formation for SS red cells by plotting MCHC versus extracellular osmolarity. MCHC and p50 for representative patients with AA, AS, and SS. As expected, patient-to-patient variation was observed (data not shown). The CSAT for SS red cells can also be estimated from the intersection of the SS p50 line with the horizontal line because the p50 values of HbA and HbS were the same in the absence of polymer formation. Intracellular CSAT for this patient, who had 9.7% HbF, was 18.2 g/dL. The patient with AS did not have the α-thalassemia trait and had an estimated CSAT of 32.2 g/dL. Under fully deoxygenated conditions, the traditional method of measuring CSAT yielded values of 15.8 g/dL for purified SS.5
MCHC and p50 versus osmolarity.
(A) Measurement of intracellular CSAT by plotting p50 versus extracellular osmolarity for AA, SS, and AS red cells. (B) Determination of MCHC at the onset of polymer formation for SS red cells by plotting MCHC versus extracellular osmolarity. MCHC and p50 for representative patients with AA, AS, and SS. As expected, patient-to-patient variation was observed (data not shown). The CSAT for SS red cells can also be estimated from the intersection of the SS p50 line with the horizontal line because the p50 values of HbA and HbS were the same in the absence of polymer formation. Intracellular CSAT for this patient, who had 9.7% HbF, was 18.2 g/dL. The patient with AS did not have the α-thalassemia trait and had an estimated CSAT of 32.2 g/dL. Under fully deoxygenated conditions, the traditional method of measuring CSAT yielded values of 15.8 g/dL for purified SS.5
These measurements yield 2 values—the osmolarity and the MCHC at which polymer formation begins. The latter is a measure of the effect of cell contents on polymer formation, whereas the former relates both whole animal physiology (plasma and renal osmolarity) and red cell physiology (factors affecting red cell density/MCHC) to polymer formation. The onset of polymer formation in AS cells occurs at an osmolarity (330 mOsm) that is higher than physiological (280-300 mOsm), but within the range found in kidney. This is consistent with the relative clinical severity of AS (AS has only a urine-concentrating defect).
Red cell heterogeneity is a potential complication for these measurements; however, because each cell acts like an independent container of well-defined solution, the effective p50 in the presence of red cell heterogeneity (MCHC or HbF) is a linear combination all cell types present. This technique may be particularly suitable for measuring relative CSAT for evaluating antisickling agents with multiple effects and for comparing different strains of transgenic mice that may express complex mixtures of hemoglobins.
Supported by grants NIH P01HL55435, NIH 1M01RR12248, and NIH P60HL38655.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mary E. Fabry, Department of Medicine, Ullmann Rm 921, Albert Einstein College of Medicine, 1300 Morris Park Ave, Bronx, NY 10461; e-mail: fabry@aecom.yu.edu.