The polarization of the immune response toward a Th2 or a Th1 profile can be mediated by dendritic cells (DCs) following antigen presentation and interaction with T cells. Costimulatory molecules such as CD80 and CD86 expressed by DCs, the polarizing cytokine environment during DC–T-cell interaction, and also the nature of the antigen are critical in the orientation of the immune response. In this study, the effect of the cysteine protease Der p 1, one of the major allergens of the house dust mite Dermatophagoides pteronyssinus, on these different parameters was evaluated comparatively on monocyte-derived DCs obtained from healthy donors, from pollen-sensitive patients, or from patients sensitive toDermatophagoides pteronyssinus. Results showed that Der p 1 induced an increase in CD86 expression only on DCs from house dust mite–sensitive patients. This was also associated with a higher capacity to induce T-cell proliferation, a rapid increase in the production of proinflammatory cytokines, tumor necrosis factor–α and interleukin (IL)-1β, and the type 2 cytokine IL-10. No changes in the release of IL-12 p70 were induced by Der p 1. Finally, purified T cells from house dust mite–sensitive patients stimulated by autologous Der p 1–pulsed DCs preferentially produced IL-4 rather than interferon-γ. These effects were abolished in the presence of the inactive precursor of Der p 1 (ProDer p 1). Taken together, these data suggest that DCs from house dust mite–sensitive patients, in contrast to DCs from healthy donors and from pollen-sensitive patients, exposed to Der p 1 play a pivotal role in the enhancement of the Th2 response associated with the allergic reaction developed in response to house dust mite exposure.
Introduction
Dendritic cells (DCs) are considered the most powerful antigen-presenting cells (APCs) and play a central role in the initiation of primary immune responses and in the enhancement of secondary immune responses. DCs differ from other APCs such as B cells, monocytes, or macrophages by their unique capacity to stimulate naive T cells in vivo1 and in vitro2 and to induce the polarization of the immune response toward a Th1 or a Th2 profile. These 2 types of T-lymphocyte responses differ on the basis of the cytokine production and also on the functional effects that Th1 and Th2 cells are able to exert after their contact with the antigen.3 Basically, Th1 cells are considered interferon (IFN)-γ–producing effector cells that can activate macrophages or cytotoxic cells. Th2 lymphocytes secrete interleukin (IL)-4 and IL-5, which induce humoral immune responses dominated by an enhanced immunoglobulin E (IgE) production such as in allergic diseases.
DCs can carry processed antigen from nonlymphoid tissues to the draining lymph node, where they become able to stimulate naive T cells and play a role in their instruction to become Th1 or Th2 cells.4,5 Many factors are important for the determination of the differentiation into Th1 and Th2 cells. In addition to the type and the dose of antigen, the route of exposure, and the genetic background of the host, the most critical factors remain (1) the type of DCs involved in the interaction with naive T cells, (2) the type of costimulatory molecules expressed on the surface of the DCs, and (3) the polarizing cytokine microenvironment during antigen presentation.
It is suggested that the type of costimulatory molecules expressed on DCs are essential for determining Th differentiation. CD86 seems to be more important than CD80 in the induction of a Th2 response.6 Indeed, in mice exposed to ovalbumin aerosols, treatment with anti-CD86 but not with anti-CD80 significantly inhibits the 3 features of the allergic reaction—namely, the production of ovalbumin-specific IgE and IgG1 antibodies, airway eosinophilia, and airway hyperresponsiveness.7 In atopic dermatitis, CD86 is predominantly expressed on Langerhans cells and seems to play an important role in the pathogenesis of this disease because anti-CD86 antibodies completely inhibit T-cell proliferation stimulated with crude extracts of Dermatophagoides pteronyssinus.8 In addition, B cells from patients with atopic dermatitis express CD86, and this expression is correlated with the total serum IgE level.9 Moreover, recent evidence demonstrates that B7-CD28 costimulation is required for the production of Th2 cytokines such as IL-5 and IL-13 by allergen-specific T cells from asthmatic patients.10
In addition, it has been reported that DCs can induce either Th1 or Th2 responses depending on their origin. In humans, DC1 derived from monocytes induces Th1 responses, whereas DC2 generated from plasmacytoid monocytes cultured in vitro with CD40L induces Th2 responses.11 However, it is also clear that myeloid DCs can give rise to either DC1 or DC2 depending on the nature of stimulus influencing the production of IL-12.12 IL-12, the critical Th1-polarizing cytokine,13 can be produced by DCs incubated with bacterial components such as lipopolysaccharide (LPS)14 or with CD40L.15 Lastly, IL-10 is able to down-regulate DC-derived IL-12 production,16leading to a type 2 response.
It has been postulated that some allergens may influence allergic responses by noncognate interactions. Thus, Der p 1, a major allergen of the house dust mite Dermatophagoides pteronyssinus, inhibits the secretion of IFN-γ, thus promoting a Th 2 response.17 Because DCs play a role in the orientation of the immune response, we wondered if Der p 1 could favor a type 2 response, first by modulating the CD80 and CD86 costimulatory molecules expressed on DCs and, secondly, by enhancing the production of the Th1- or the Th2-polarizing cytokines IL-12 and IL-10, respectively. Thus, in this report, the effect of Der p 1 on these parameters was evaluated on DCs from both healthy donors and allergic patients.
Materials and methods
PBMC preparation and Der p 1 preparation
Blood was collected from donors sensitive (n = 18) or not (n = 11) to the house dust mite. All allergic patients presented the usual features of house dust mite sensitization: specific IgE antibodies and positive skin prick tests toward Dermatophagoides pteronyssinus. Total IgE concentrations were above 0.36 mg/L (0.36-3.84 mg/L). A third class of patients (n = 4) who are allergic to aeroallergens but are not sensitive to house dust mite was also included in this work. After platelet-rich plasma depletion, blood cells were then diluted in RPMI 1640 (Life Technologies, Paisley, Scotland) and layered over a Ficoll gradient (Pharmacia, Uppsala, Sweden). After centrifugation (400g, 30 minutes), peripheral blood mononuclear cells (PBMCs) were harvested and washed. Der p 1 was isolated from spent growth medium as previously described.11
Differentiation and antigen loading of DCs
Purified PBMCs were incubated with magnetic beads conjugated with monoclonal mouse antihuman CD14 antibodies (Miltenyi Biotec, Auburn, CA) at 4°C for 30 minutes. After washing, the cells were applied onto a column placed in the magnetic field of a MACS separator (Miltenyi Biotec). After elimination of negative cells, the column was removed from the separator and the CD14+ cells (monocytes) were collected and washed twice in RPMI 1640 medium. Cells were cultured (2 × 106 cells/well) into 6-well flat-bottomed culture plates in RPMI 1640 supplemented with 1% antibiotics and 10% fetal calf serum (Life Technologies) for 7 days in the presence of 20 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Peprotech, London, United Kingdom) and 200 IU/mL IL-4 (R&D Systems, Oxon, United Kingdom).
For experiments carried out in the presence of Der p 1, generated DCs were pulsed for 24 hours with 0, 10, and 100 ng/mL Der p 1; with the proenzymatic and inactive form of Der p 1, ProDer p 1 (provided by SmithKline Beecham Biologicals, Rixensart, Belgium),18 using the same protocol; or with 1 μg/mL LPS for 24 hours as a control of DC maturation.
DC surface marker analysis
Cells were collected, washed in phosphate-buffered saline, and incubated for 30 minutes at 4°C with different monoclonal antibodies (mAbs) (all from Becton Dickinson, Mountain View, CA): fluorescein isothiocyanate (FITC)-conjugated anti-CD80 or anti-CD1a; phycoerythrin (PE)-conjugated anti-CD86, anti-CD11c, or anti-CD83; Cy-chrome–conjugated anti–HLA-DR; or an irrelevant mAb of the same isotype (Becton Dickinson). After washing, the cells were fixed in phosphate-buffered saline–1% paraformaldehyde and analyzed using a FACSCalibur (Becton Dickinson).
Cytokine assay
Supernatants of 1 × 106 generated DCs pulsed or not with Der p 1, ProDer p 1, or LPS were harvested 6, 24, and 48 hours following stimulation, centrifuged (400g for 7 minutes), and assayed for the presence of tumor necrosis factor (TNF)-α, IL-1β, IL-6, IL-10, and IL-12 p70 by specific enzyme-linked immunosorbent assay (ELISA) using Eli-pairs (R&D Systems). The sensitivity of detection of TNF-α, IL-1β, and IL-6 was 10 pg/mL; the sensitivity of detection of IL-10 and IL-12 was 12.5 pg/mL and 6.5 ng/mL, respectively.
Allogeneic proliferation assay
Isolation of T cells from PBMCs was performed by depletion of B cells, monocytes, natural killer cells, DCs, early erythroid cells, platelets, and basophils by an indirect magnetic label using a cocktail of hapten-conjugated CD11b, CD16, CD19, CD36, and CD56 antibodies and MACS microbeads coupled to an antihapten mAb (Miltenyi Biotec). The negative cells were collected, washed, and plated at a concentration of 105 cells per well in RPMI 1640–fetal calf serum 10% into 96-well round-bottomed culture plates. Increasing numbers of DCs pulsed or not with Der p 1, ProDer p 1, or LPS were added into the wells containing the allogeneic T cells. Three days later, 3H thymidine (18.5 KBq/well) was added for 16 hours before measurement of the incorporated radioactivity.
DC–T-cell cocultures
CD4+ T cells were purified from PBMCs after negative depletion of CD14+, CD16+, CD19+, CD56+, and CD8+ cells using MACS microbeads and a MACS column. After 7 days of culture with GM-CSF and IL-4, generated DCs from allergic patients or from healthy donors previously pulsed or not with 100 ng/mL Der p 1 or ProDer p 1 for 24 hours were cocultured with the autologous purified CD4+ T cells at a ratio of 1:10. After 24 or 48 hours of coculture, supernatants were harvested and assayed for IL-4 and IFN-γ production using a specific ELISA (Diaclone).
Statistical analysis
Parametric statistical analysis of cell surface molecule expression and analysis of the cytokine production by DCs were performed using the Student t test. Values ofP ≤ .05 were considered statistically significant.
Results
Phenotype of monocyte-derived DCs
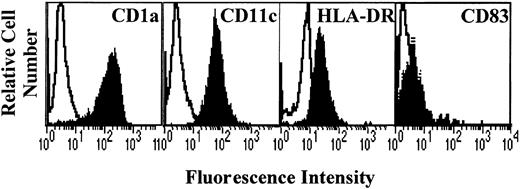
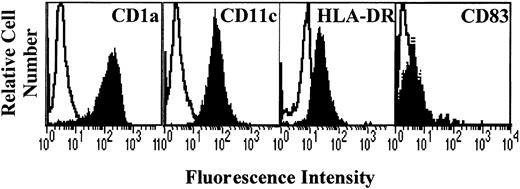
After 7 days of culture with GM-CSF and IL-4, generated cells were analyzed for the expression of DC markers CD1a, CD11c, and HLA-DR. Results show that the cells obtained displayed a high expression of CD1a and CD11c and a low expression of HLA-DR (Figure1), the costimulatory molecules CD80 and CD86 (Figure 2), and CD83 (Figure3). This profile is specific for immature DCs.
Phenotype of monocyte-derived DCs.
Monocytes from allergic patients or from healthy donors were cultured in the presence of GM-CSF and IL-4 for 7 days. The cells were then collected and analyzed by flow cytometry for CD1a, CD11c, HLA-DR, and CD83 expression (filled histograms). Open histograms represent the reactivity of fluorochrome-matched isotype control mAbs.
Phenotype of monocyte-derived DCs.
Monocytes from allergic patients or from healthy donors were cultured in the presence of GM-CSF and IL-4 for 7 days. The cells were then collected and analyzed by flow cytometry for CD1a, CD11c, HLA-DR, and CD83 expression (filled histograms). Open histograms represent the reactivity of fluorochrome-matched isotype control mAbs.
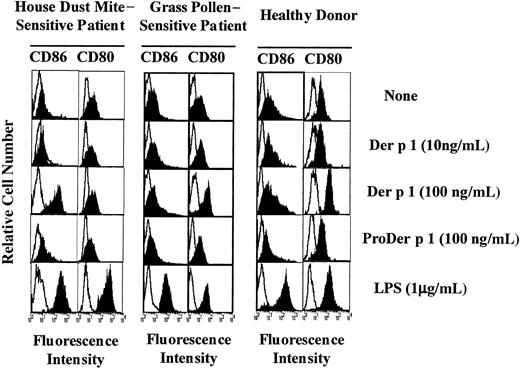
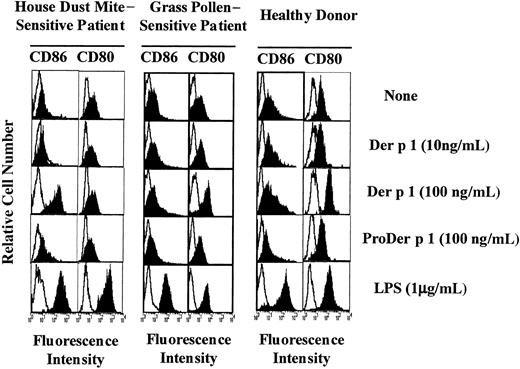
Effect of Der p 1 on CD86 and CD80 expression on DCs.
Monocyte-derived DCs from house dust mite–sensitive patients (left panels), from grass pollen–sensitive patients (middle panels), and from healthy donors (right panels) were exposed or not to Der p 1, ProDer p 1, or LPS for 24 hours. DCs were then incubated with PE-conjugated anti-CD86 mAbs or with FITC-conjugated anti-CD80 mAbs, electronically gated, and analyzed for CD86 and CD80 expression (filled histograms). Open histograms represent reactivities of fluorochrome-matched isotype control mAbs. One representative experiment of 7 is shown.
Effect of Der p 1 on CD86 and CD80 expression on DCs.
Monocyte-derived DCs from house dust mite–sensitive patients (left panels), from grass pollen–sensitive patients (middle panels), and from healthy donors (right panels) were exposed or not to Der p 1, ProDer p 1, or LPS for 24 hours. DCs were then incubated with PE-conjugated anti-CD86 mAbs or with FITC-conjugated anti-CD80 mAbs, electronically gated, and analyzed for CD86 and CD80 expression (filled histograms). Open histograms represent reactivities of fluorochrome-matched isotype control mAbs. One representative experiment of 7 is shown.
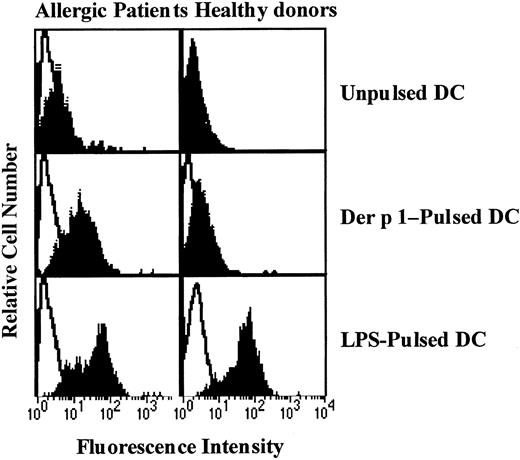
Effect of Der p 1 on CD83 expression by DCs.
Monocyte-derived DCs from house dust mite–sensitive patients (left panels) and from healthy donors (right panels) were exposed or not to Der p 1 or LPS for 24 hours. DCs were incubated with PE-conjugated anti-CD83 mAbs and analyzed for CD83 expression (filled histograms). Open histograms represent fluorochrome-matched isotype control mAbs. One representative experiment of 3 is shown.
Effect of Der p 1 on CD83 expression by DCs.
Monocyte-derived DCs from house dust mite–sensitive patients (left panels) and from healthy donors (right panels) were exposed or not to Der p 1 or LPS for 24 hours. DCs were incubated with PE-conjugated anti-CD83 mAbs and analyzed for CD83 expression (filled histograms). Open histograms represent fluorochrome-matched isotype control mAbs. One representative experiment of 3 is shown.
Effect of Der p 1 on CD80, CD86, and CD83 expression
Monocyte-derived DCs from both allergic patients and healthy donors were incubated with 0, 10, or 100 ng/mL Der p 1 for 24 hours. As shown in Figure 2 (1 representative experiment of 7), in the presence of Der p 1 at 100 ng/mL an exclusive increase in CD86 expression was observed on DCs from house dust mite–sensitive allergic patients. In contrast, on DCs from healthy donors and from patients allergic to grass pollen, at the same dose, Der p 1 induced an up-regulation of CD80 expression but not of CD86 expression. In the presence of LPS, DCs from both allergic patients and healthy donors showed an increase in both CD80 and CD86 expression, indicating that the effect observed with Der p 1 was specific to the allergen itself. The effects induced with Der p 1 were not detected at a lower dose.
To evaluate the effect of Der p 1 on DC maturation, CD83 expression on unpulsed, Der p 1-pulsed, or LPS-pulsed DCs was measured. As shown in Figure 3, Der p 1 at the dose of 100 ng/mL increased CD83 expression on DCs from only house dust mite–sensitive patients. In response to LPS, DCs from both house dust mite–sensitive patients and healthy donors showed an increase in CD83 expression.
Because a difference in the response of DCs issued from allergic patients and healthy donors on costimulatory molecule expression was observed with 100 ng/mL Der p 1, the following experiments were carried out by using only the 100 ng/mL dose.
The effect on the costimulatory molecules is dependent on Der p 1 enzymatic activity
To evaluate the potential role of Der p 1 enzymatic activity in the modulation of CD80 and CD86 expression, DCs were incubated either with Der p 1 or with the inactive precursor of Der p 1 (ProDer p 1) at 100 ng/mL, the allergen concentration previously shown to efficiently modulate CD80 on DCs from healthy donors and CD86 on DCs from allergic patients. As shown in Figure 2, the up-regulation of CD80 and CD86 observed in Der p 1–pulsed DCs from healthy donors and allergic patients, respectively, was not induced by ProDer p 1.
These results suggest that the proteolytic activity of Der p 1 might be involved in the allergen-induced increase in CD80 expression and CD86 expression on DCs from healthy donors or patients allergic to grass pollen and allergic patients, respectively.
Proliferation of T cells by DCs
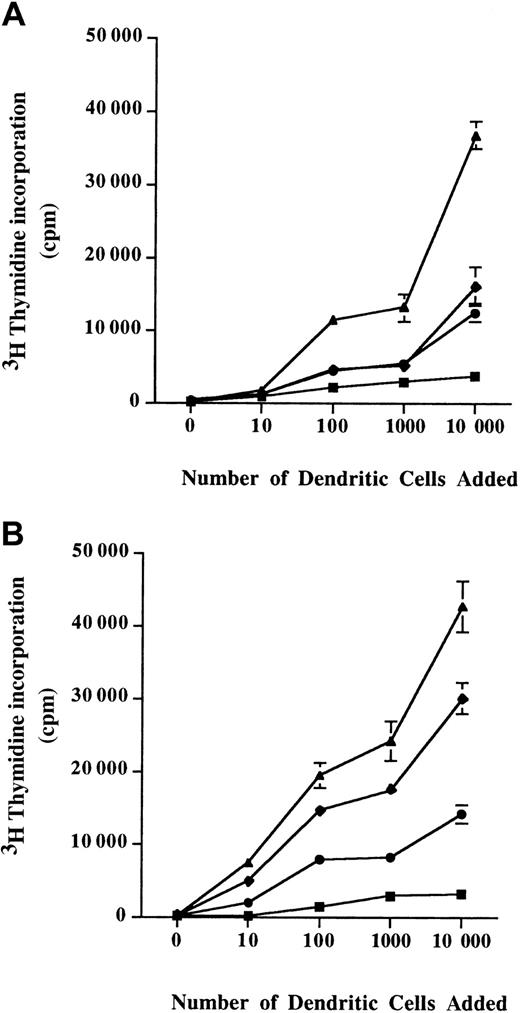
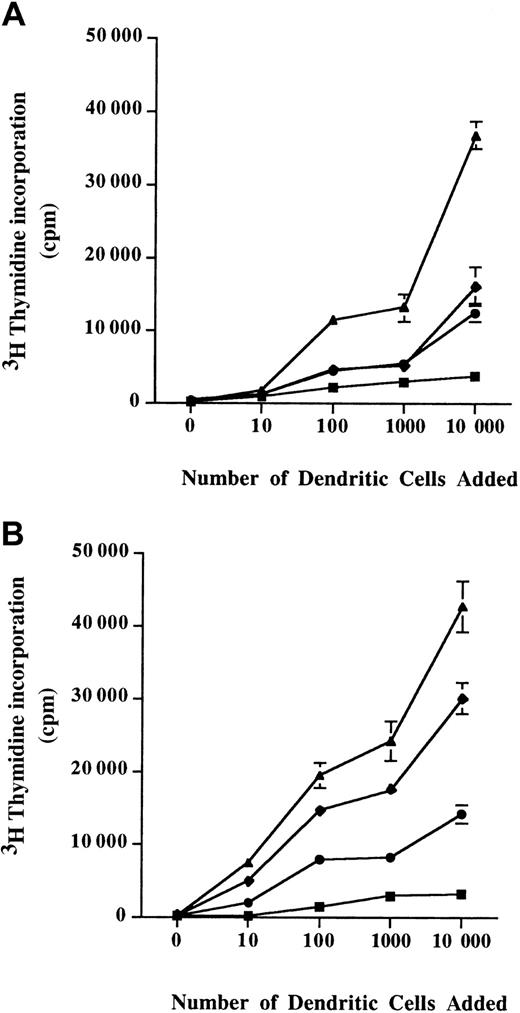
When T cells were incubated with unpulsed DCs from either allergic patients or from healthy donors, a low proliferation of T cells was observed. Interestingly, the T-cell proliferative response induced by Der p 1–pulsed DCs from allergic patients (Figure4A) was higher than the one observed with Der p 1–pulsed DCs from healthy donors (Figure 4B).
Allogeneic stimulation of T cells.
A total of 105 T cells were coincubated with monocyte-derived DCs from healthy donors (A) or allergic patients (B) for 3 days. Diagrams represent the incorporated radioactivity measured 16 hours after adding 3H thymidine in the cell culture medium. ▪, unpulsed cells; ♦, Der p 1–pulsed cells; ●, ProDer p 1–pulsed cells; ▴, LPS-pulsed cells. Mean ± SEM values are shown.
Allogeneic stimulation of T cells.
A total of 105 T cells were coincubated with monocyte-derived DCs from healthy donors (A) or allergic patients (B) for 3 days. Diagrams represent the incorporated radioactivity measured 16 hours after adding 3H thymidine in the cell culture medium. ▪, unpulsed cells; ♦, Der p 1–pulsed cells; ●, ProDer p 1–pulsed cells; ▴, LPS-pulsed cells. Mean ± SEM values are shown.
DCs from healthy donors pulsed with Der p 1 or with ProDer p 1 were able to induce a similar T-cell proliferation (Figure 4A). Moreover, Der p 1–pulsed DCs from allergic patients showed a better allostimulatory capacity than ProDer p 1–pulsed DCs from allergic patients.
Production of cytokines by Der p 1–stimulated DCs
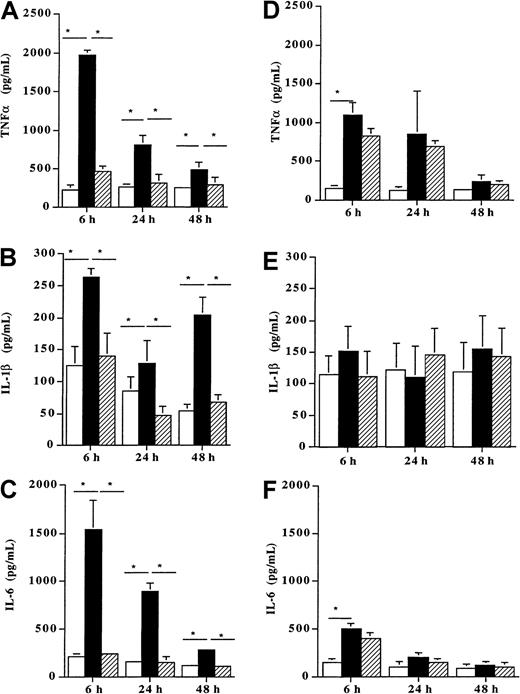
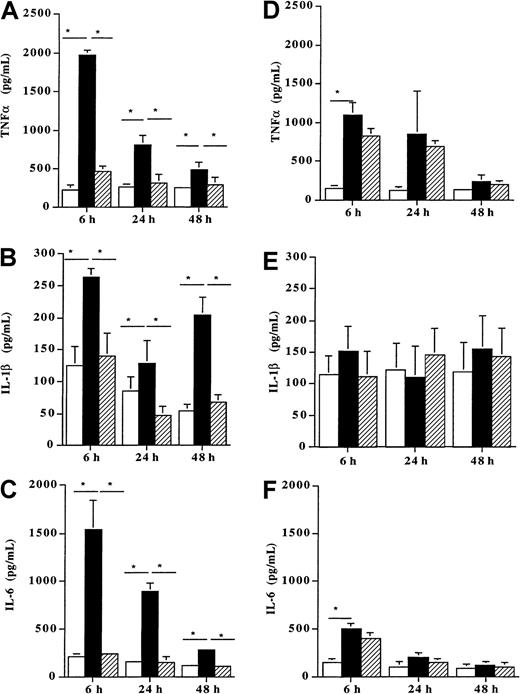
When incubated with 100 ng/mL Der p 1, DCs from allergic patients (n = 8) produced high amounts of the proinflammatory cytokines TNF-α (Figure 5A), IL-1β, (Figure 5B) and IL-6 (Figure 5C) as compared with unpulsed cells (P < .05). This enhancing effect of Der p 1 on the release of these 3 cytokines already observed after 6 hours remained significant up to 48 hours (P < .05). In contrast, when DCs from healthy donors (n = 7) were incubated with the same dose of Der p 1 (100 ng/mL), a significant increase was observed for TNFα after 6 hours (Figure 5D). This stimulatory effect was transient because at 24 and 48 hours no further increase was detected. In addition, after 6, 24, and 48 hours, the production of IL-1β and IL-6 was not modified by the allergen exposure as compared with unpulsed cells (Figure 5E and 5F, respectively).
Effect of Der p 1 on TNFα, IL-1β, and IL-6 production by DCs.
Monocyte-derived DCs from house dust mite–sensitive patients (A-C) and from healthy donors (D-F) were incubated with Der p 1 or with ProDer p 1 for 6, 24, or 48 hours. Supernatants were then collected, and the amounts of TNFα, IL-1β, and IL-6 were evaluated by ELISA. ■, unpulsed DCs; ▪, Der p 1–pulsed DCs; ▨, ProDer p 1–pulsed DCs. Results are expressed as the mean ± SEM. *P < .05.
Effect of Der p 1 on TNFα, IL-1β, and IL-6 production by DCs.
Monocyte-derived DCs from house dust mite–sensitive patients (A-C) and from healthy donors (D-F) were incubated with Der p 1 or with ProDer p 1 for 6, 24, or 48 hours. Supernatants were then collected, and the amounts of TNFα, IL-1β, and IL-6 were evaluated by ELISA. ■, unpulsed DCs; ▪, Der p 1–pulsed DCs; ▨, ProDer p 1–pulsed DCs. Results are expressed as the mean ± SEM. *P < .05.
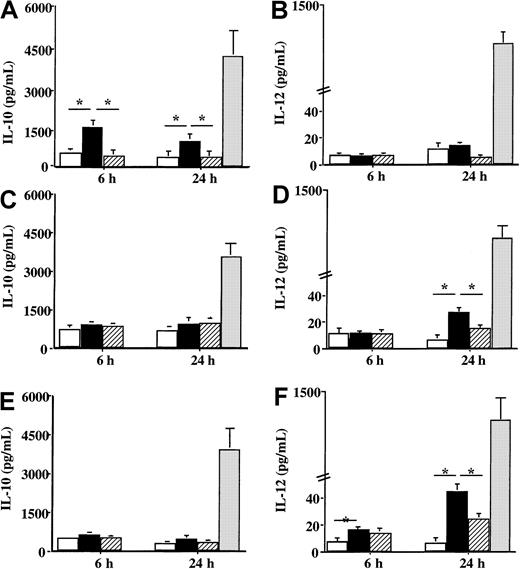
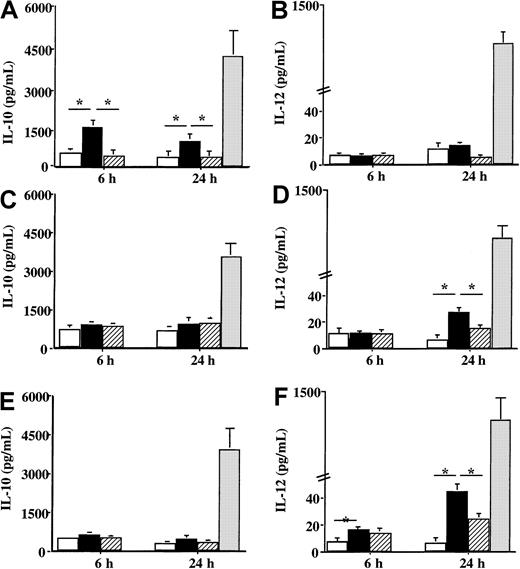
Concerning the production of cytokines involved in the orientation of the immune response, a difference of reactivity toward Der p 1 was also noticed between DCs from house dust mite–allergic patients and DCs from healthy donors or patients sensitive to grass pollen. When DCs from patients sensitive to the house dust mite were incubated with Der p 1, a significant increase in IL-10 production was observed 6 hours after the contact with the allergen (P < .05) (Figure6A), whereas IL-12 production was not modified by Der p 1 as compared with unpulsed cells (Figure 6B). However, Der p 1 had a different effect on the production of IL-10 and IL-12 by DCs issued from both grass pollen–sensitive patients and from healthy donors. Following the incubation with Der p 1, only a very slight effect was detected on IL-10 production (Figure 6C and 6E, respectively), whereas a significant increase in IL-12 production was observed (Figure 6D and 6F, respectively) (P < .05 as compared with unpulsed cells). Stimulation with LPS showed an increase in both IL-10 and IL-12 by DCs from both allergic patients and healthy donors without any statistical difference between allergic and healthy donors.
Effect of Der p 1 on the production of IL-10 and IL-12 by DCs.
Monocyte-derived DCs from house dust mite–sensitive patients (A-B), from grass pollen–sensitive patients (C-D), and from healthy donors (E-F) were incubated with 100 ng/mL Der p 1 or 100 ng/mL ProDer p 1 for 6 or 24 hours. Supernatants were collected, and the amounts of IL-10 and IL-12 were measured by specific ELISA. ■, unpulsed cells; ▪, Der p 1–pulsed cells; ▨, ProDer p 1–pulsed cells; ░, LPS-pulsed cells. Results are expressed as the mean ± SEM. *P < .05.
Effect of Der p 1 on the production of IL-10 and IL-12 by DCs.
Monocyte-derived DCs from house dust mite–sensitive patients (A-B), from grass pollen–sensitive patients (C-D), and from healthy donors (E-F) were incubated with 100 ng/mL Der p 1 or 100 ng/mL ProDer p 1 for 6 or 24 hours. Supernatants were collected, and the amounts of IL-10 and IL-12 were measured by specific ELISA. ■, unpulsed cells; ▪, Der p 1–pulsed cells; ▨, ProDer p 1–pulsed cells; ░, LPS-pulsed cells. Results are expressed as the mean ± SEM. *P < .05.
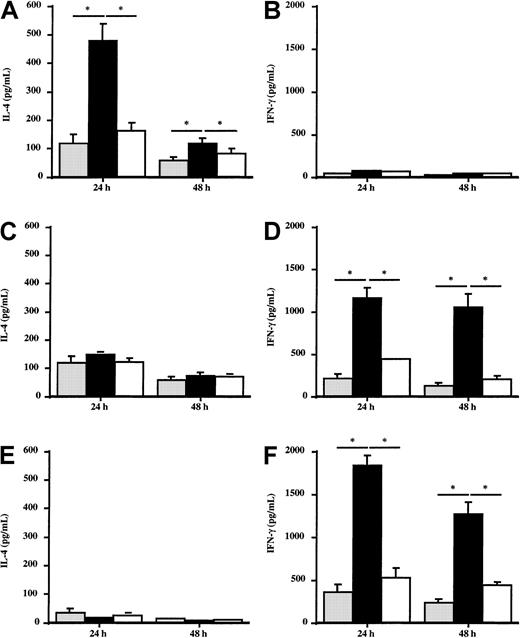
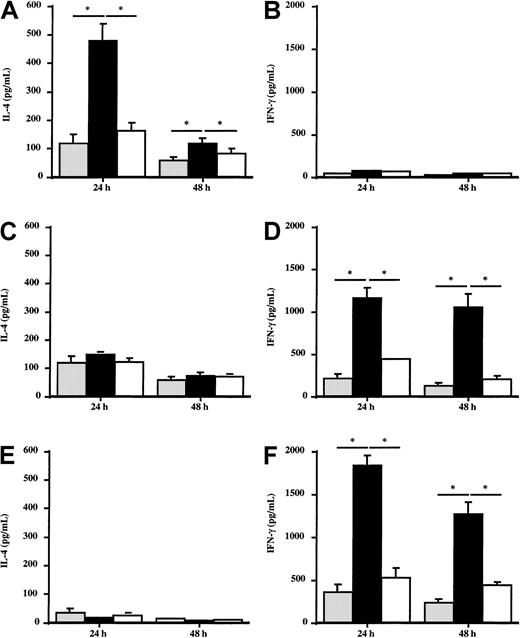
Effect of Der p 1–pulsed DCs on the in vitro production of IL-4 and IFN-γ by autologous T cells
To evaluate the potential effect of DCs from allergic patients or healthy donors on the Th1/Th2 response, the production of IFN-γ and IL-4 by autologous T cells stimulated by Der p 1–pulsed DCs was analyzed. T cells coincubated with Der p 1–pulsed DCs from house dust mite–sensitive patients (n = 5) produced large amounts of IL-4 (Figure 7A), whereas IFN-γ production was not modified (Figure 7B) as compared with T cells stimulated with unpulsed DCs. The effect induced by Der p 1 is dependent on its enzymatic activity because ProDer p 1–pulsed DCs did not modulate IL-4 or IFN-γ production by T cells. This suggests that DCs need to be activated by Der p 1 to induce the amplification of the cytokine production by autologous T cells. In contrast, after incubation with autologous Der p 1–pulsed DCs, T cells from healthy donors and from grass pollen–sensitive patients (n = 5) did not modify their production of IL-4 (Figure 7C and 7E, respectively) but significantly increased IFN-γ production up to 48 hours (Figure 7D and 7F, respectively).
Effect of Der p 1–pulsed DCs on IL-4 and IFN-γ production by T cells.
Monocyte-derived DCs from house dust mite–sensitive patients (A,B), from grass pollen–sensitive patients (C,D), and from healthy donors (E,F) were pulsed or not with 100 ng/mL Der p 1 or ProDer p 1 for 24 hours. Then, Der p 1–pulsed or unpulsed DCs were cocultured with autologous T cells for 24 and 48 hours. Supernatants were harvested, and the amounts of IL-4 and IFN-γ were measured by specific ELISA. ░, without Der p 1; ▪, with Der p 1; ■, with ProDer p 1. Results are expressed as the mean ± SEM. *P < .05.
Effect of Der p 1–pulsed DCs on IL-4 and IFN-γ production by T cells.
Monocyte-derived DCs from house dust mite–sensitive patients (A,B), from grass pollen–sensitive patients (C,D), and from healthy donors (E,F) were pulsed or not with 100 ng/mL Der p 1 or ProDer p 1 for 24 hours. Then, Der p 1–pulsed or unpulsed DCs were cocultured with autologous T cells for 24 and 48 hours. Supernatants were harvested, and the amounts of IL-4 and IFN-γ were measured by specific ELISA. ░, without Der p 1; ▪, with Der p 1; ■, with ProDer p 1. Results are expressed as the mean ± SEM. *P < .05.
If Der p 1–pulsed DCs from allergic patients were preincubated with blocking anti-CD80 or anti-CD86 for 2 hours before coculture with T cells, IL-4 production was blocked preferentially in the presence of anti-CD86 antibodies. The blockade of both molecules did not completely abolish Der p 1–induced IL-4 production (Table1).
Discussion
Costimulation through the CD28/CD80-CD86 pathway is one critical point in the establishment of antigen-driven immune responses. Antigen-specific immune responses are initiated after interaction between T cells and APCs such as DCs, and an efficient activation of T cells requires 2 signals. The first signal is delivered after interaction between T-cell receptor and major histocompatibility complex class II molecules; the second involves costimulatory molecules such as CD80 and CD86. The relevance of the CD28/CD80-CD86 pathway in allergic responses is supported by experimental studies demonstrating the role of CD28 in IgE responses.19 The issue of a potential dichotomy in the ability of CD80 and CD86 to generate a Th1 versus a Th2 response remains to be solved.
Until now, the effects of allergens on DC function were not well described. In this study, we demonstrate that in DCs from allergic patients incubated with Der p 1, the CD86:CD80 ratio was higher than in DCs from healthy donors that preferentially showed an up-regulation of CD80 expression. CD86 molecule plays an important role in allergic diseases and has been shown to be up-regulated on B cells from allergic patients.20 In contrast, CD80 seems to be preferentially associated with Th1-type T-cell responses. For example, DCs interacting with Mycobacterium tuberculosis antigens are activated and highly express CD80.21 In leishmaniasis, an antigen homolog to the eukaryotic initiation factor 4A described as an inducer of Th1-type responses was shown to increase CD80 (B7.1) expression on normal human monocyte-derived DCs.22 In this study, we also show that Der p 1 induces an increase in CD86 on DCs from allergic patients that is dependent on the enzymatic activity of Der p 1 because the up-regulation of CD86 is abolished using the cysteine protease inhibitor E64 (data not shown) or the proenzymatic form of Der p 1, ProDer p 1. This dependency on the catalytic activity has already been shown to be critical in other noncognate interactions reported for the allergen, such as the inhibition of IFN-γ messenger RNA and the decrease in the number of IFN-γ–secreting cells.17 Moreover, cysteine proteases are known to play a role in the induction of type 2 responses. Thus, in a murine model of leishmaniasis, the infection with mutant strains lacking cysteine protease genes preferentially induced a shift from the type 2 phenotype associated with the infection using wild-type strains to a type 1–associated response.23 Interestingly, the stimulation of DCs from allergic patients or from healthy donors with LPS leads to an up-regulation of both CD80 and CD86. LPS is a very potent inducer of DC maturation.24 Very recently, Langenkamp et al showed that monocyte-derived DCs stimulated with LPS rapidly produce IL-12, leading to a Th1 response, and that after 24 hours they lose their capacity to produce this pro-Th1 cytokine and acquire the capacity to favor a Th2 response associated with an increase in CD86:CD80 ratio.2 Thus, the effect induced by Der p 1 seems to be specific to the allergen and the origin of the cells.
In the present study, DCs from allergic patients exposed to Der p 1 were shown to increase the production of the proinflammatory cytokines TNFα, IL-1β, and IL-6. It is known that monocytes cultured with GM-CSF and IL-4 acquire immature DC morphology and functions. One common way to obtain mature DCs is to expose them to proinflammatory cytokines such as TNF-α and IL-1β.25Moreover, the incubation of DCs with antigens is often associated with an overproduction of proinflammatory cytokines such as TNFα, IL-1β, and IL-6. DCs cultured with GM-CSF and IL-4 and incubated withMycobacterium tuberculosis increase their production of both TNFα and IL-1β.21 This increase in the production of proinflammatory cytokines is also observed for other antigens such asSalmonella26 and haptens.27 The Der p 1–induced production of cytokines observed in our work was more sustained and long-lasting in DCs from allergic patients rather than in DCs from healthy donors. This could probably be responsible for an allergen-associated maturation of DCs, but this hypothesis remains to be further investigated. Interestingly, an elegant study showed that maturation factors such as TNF-α associated with IL-1β led to a DC exhaustion responsible for the development of a Th2 response associated with a production of IL-10 and a down-regulation of IL-12 secretion.2 Our results demonstrate a high release of the maturing factors TNF-α and IL-1β in response to Der p 1 by DCs from allergic patients. This suggests that monocyte-derived DCs from allergic patients might reproduce the model described by Langenkamp et al, explaining their lower IL-12 production and the promotion of a Th2 response.
The orientation toward a Th1 or a Th2 profile is also dependent on the cytokines present when the interaction of DCs with T cells occurs. Many cytokines are very efficient in focusing the immune response toward a Th1 or a Th2 profile. IL-10 is known to be a cytokine that inhibits type 1 responses, thus promoting a type 2 response.28 In contrast, IL-12 is an inducer of IFN-γ production by T lymphocytes and so might favor Th1 responses.28 Both IL-10 and IL-12 are produced by DCs,29 suggesting that DCs could have a key role in the establishment of an immune response. IL-10 is produced by DCs in response to different antigens such as bacteria22 and their derived products. DCs cultured in the presence of recombinant IL-10 showed a decreased expression of CD86 responsible for an inhibitory effect on alloreactive reactions.30 However, another study indicated that DCs that have undergone maturation in the presence of IL-10 down-regulated their own production of IL-12 and were unable to induce the synthesis of IFN-γ by T cells.16 Finally, in IL-10 knockout mice, DCs rapidly and efficiently promote Th1 responses.31 These data support the idea that IL-10 is essential for mounting Th2 responses. IL-12 is constitutively produced by DCs14 and is enhanced in response to a broad spectrum of stimuli such as microbial stimulation,32 parasites,33 and CD40 ligation through CD40L on activated T cells.15 IL-12 production is inhibited by IL-4 and IL-10 34,35 or after exposure with agents inducing type 2 responses.36Interestingly, in our study, only DCs from healthy donors exposed to Der p 1 could up-regulate CD80 expression, whereas DCs from allergic patients up-regulated CD86. This increase in CD80 expression by DCs from healthy donors was associated with an overproduction of IL-12 in response to the allergen. This Der p 1–induced IL-12 could then act in an autocrine manner, which could probably explain the up-regulation of CD80 only on DCs from healthy donors. DCs from allergic patients up-regulate IL-10 production rather than IL-12 in response to Der p 1. All of these data suggest that DCs from allergic patients exposed to Der p 1 could be able to promote type 2 responses through an overproduction of IL-10, whereas DCs from healthy donors could favor a type 1 response through an up-regulation of IL-12 release. This was confirmed when Der p 1–pulsed DCs were cocultured with T cells. T cells from healthy donors could increase their production of IFN-γ without any effect on IL-4 production, indicating a capacity of such DCs to orientate the response toward a Th1 response. In contrast, T cells from allergic patients stimulated by Der p 1–pulsed DCs preferentially increased their production of IL-4 without modifying IFN-γ production. Interestingly, in some other experiments, the IL-4 production induced by Der p 1–pulsed DCs from allergic patients was highly reduced by a treatment of DCs with anti-CD86 antibodies (reduction of around 70%). Anti-CD80 expression only decreased the IL-4 production of 30%. The IL-4 production by T cells from allergic patients following the interaction with Der p 1–pulsed DCs was then much more dependent on CD86 than on the CD80 molecule, indicating that DCs from allergic patients are able to favor a type 2 response especially through the CD86 costimulatory molecule.
Taken together, these data suggest that in the presence of the same antigen, the polarized immune response is highly dependent on the immune status of the donor. Indeed, DCs from allergic patients, in contrast with DCs from healthy donors, exposed to Der p 1 play a pivotal role in the amplification of a Th2 response because, in our study, T cells issued from the donors are already primed. Thus, a potential dysregulation at the level of the DCs in allergic patients might be responsible for the establishment of the allergic status.
The authors thank Prof B. Wallaert, Dr C. Lamblin-Degros, and the personnel of the Calmette Hospital (Service et Consultations d'Immunoallergologie) for the selection of patients and the blood collection involved in this study. A.-S. Charbonnier is an HMRGIP recipient.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joël Pestel, INSERM U416-Institut Pasteur de Lille, 1, rue du Professeur Calmette, B.P.245, 59019 Lille Cedex, France; e-mail: joel.pestel@pasteur-lille.fr.