Mutations of the RAG1 or RAG2 protein that eliminate their recombination activity result in T-B-severe combined immunodeficiency (SCID), whereas mutations retaining partial recombination activity lead to Omenn syndrome, a peculiar SCID characterized by increased host T cells and absence of circulating B cells. The prognosis of this disease is fatal, unless hematopoietic stem cell transplantation is performed. This study reports a case of atypical SCID, carrying RAG1 mutations. The patient survived for 6 years without hematopoietic stem cell transplantation. The missense mutation, tested by in vivo recombination assay, revealed residual recombination activity. By the age of 5 years, the patient developed host B cells, but not T cells, possibly due to engrafted maternal T cells. In addition, the host B cells were able to produce antibodies, including anti–herpes simplex virus–antibodies. The fact that host B cells could produce antibodies in this patient could explain not only the mild phenotype observed but also, at least in part, how patients with Omenn syndrome produce immunoglobulin E and sometimes immunoglobulin M, as the same missense mutation of RAG1 gene has been reported in a patient with Omenn syndrome.
Introduction
Two lymphoid specific genes, RAG1and RAG2, are essential for V(D)J recombination of the antigen receptors.1,2 Mutations of the RAG1 or RAG2 protein that eliminate the recombination activity result in T-B-severe combined immunodeficiency (SCID).3 Missense mutations of these proteins with partial recombination activity have been demonstrated to lead to Omenn syndrome, a SCID characterized by failure to thrive, diarrhea, diffuse erythrodermia, hepatosplenomegaly, lymphadenopathy, hypereosinophilia, elevated serum immunoglobulin E (IgE), increased host T cells that show oligoclonal expansion with an activated phenotype, and absence of circulating B cells.4,5 These diseases are fatal; the patients die early in life, unless treated by bone marrow transplantation, because of susceptibility to opportunistic organisms.6-8 Most recently, 41 patients carrying RAG mutations were analyzed and a subgroup, termed atypical SCID/OS, was identified. The patients in this subgroup were characterized by the presence of some T and occasionally B cells but did not fulfill the criteria of Omenn syndrome.9 However, the immunopathologic mechanism of this subgroup has not been well characterized. A similar phenotype to Omenn syndrome has also been reported due to engraftment of maternal T cells, leading to graft-versus-host disease (GVHD).10 11
In the present study, we report the case of a Japanese girl, bearing RAG1 mutations, who presented with peripheral host B cells in combination with maternal T cells, and who survived for 6 years without stem cell transplantation. Furthermore, at least a subset of her B cells was demonstrated to be functional, as the patient produced anti–herpes simplex virus (HSV)–antibodies.
Materials and methods
Cell lines
Peripheral blood mononuclear cells (PBMCs) from heparinized blood samples were isolated by Ficoll-Paque (Amersham Pharmacia, Uppsala, Sweden) density centrifugation. PBMCs from the patient were immortalized by Epstein-Barr virus (EBV) as described previously,12 and the resulting B-LCLs were maintained in RPMI1640 medium containing 10% fetal bovine serum (FBS; Gibco BRL, Rockville, MD) at 37°C in 5% CO2.
Lymphocyte activation
Proliferative responses were measured in triplicates of 200 μL containing 1 × 105 total PBMCs per well with various stimulation agents, including 100 ng/mL human interleukin 2 (IL-2; Ajinomoto, Kanagawa, Japan), 2000-fold diluted phytohemagglutinin-P (Difco, Detroit, MI), 5 ng/mL phorbol myristyl acetate (Sigma, St Louis, MO) plus 500 ng/mL ionomycin (Sigma) or 100-fold diluted poke weed mitogen (Seikagaku Kogyo, Tokyo, Japan) in RPMI1640 medium supplemented with 10% heat-inactivated FBS. After 72 hours of culture at 37°C in 5% CO2, the cells were pulsed for the last 4 hours with 1 μCi [3H]thymidine deoxyribose (TdR; Amersham, Buckinghamshire, England), and the incorporated [3H]TdR was counted by TopCountTM Microplate Scintillation Counter (Packard Instrument, Meriden, CT).
Sequencing analysis of genomic DNA
Genomic DNAs were isolated from fractionated PBMCs or the EBV-transformed B-LCLs by using the QIAamp Blood Kit (QIAGEN, Chatsworth, CA). The RAG1 gene was amplified in 4 segments (Table 1). Sequencing was performed directly on the polymerase chain reaction (PCR) products purified by QIAquick PCR Purification Kit (QIAGEN). Mutations were confirmed by analysis of several clones from PCR amplification products cloned into the TA vector (Invitrogen, Carlsbad, CA) and sequenced by ABI Taq DyeDeoxy Terminator Cycle Sequencing kit on an automated Applied Biosystems DNA sequencer, model 373A (Perkin-Elmer, Foster City, CA) as previously described.12
Genomic DNAs from the patient and healthy individuals were obtained after informed consent according to the Declaration of Helsinki.
Expression plasmid construction and transfection
The mutant complementary DNAs (cDNAs) were generated by site-directed mutagenesis, and they were then cloned into an expression vector, pCDM8. The wild-type human RAG1 vector was a generous gift from Dr K. Schwarz (University Ulm, Germany). Sequences of the mutant cDNAs were confirmed by sequencing. The expression vectors were transiently transfected into 293T cells by using FuGene 6 (Boehringer Mannheim, Mannheim, Germany). RAG1 protein from the transfected 293T cells was detected by immunoblotting with rabbit polyclonal anti–human RAG1 antibodies (Santa Cruz, Santa Cruz, CA) as previously described.13
Magnetic cell sorting
PBMCs were resuspended in phosphate-buffered saline (PBS) supplemented with 0.5% bovine serum albumin and 2 mM EDTA and incubated with anti-CD4, anti-CD8, anti-CD19, and anti-CD56 microbeads at 4°C for 30 minutes. The cells were purified by positive magnetic immunoselection by using automated magnetic cell sorting (autoMACS; Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The selected fractions contained 98.7% CD4+ cells, 95.1% CD8+ cells, 91.8% CD19+ cells, and 96.1% CD56+ cells (data not shown).
In vivo recombination assay
PCR products bearing the RAG1 mutations found in the patients were cloned in pEBG vector containing aa 330-1043 of human RAG1 (RAG1 active core14 15).
The mutant proteins were tested for recombination activity in 293T cells. Human RAG1 active core (aa 330-1043) carrying the mutations were cotransfected with mouse wild-type RAG2 active core (aa 1-388) and with the recombination substrate pJH200.16 Cells were transfected by calcium phosphate precipitation and harvested 48 hours later.
Recombined products were isolated and analyzed for recombination frequency by PCR analysis by using primers OOP2 and CR3.17The reactions incorporated 32P dCTP. Reaction products were analyzed on a 5% polyacrylamide gel and visualized by autoradiography.
Detection of anti-HSV antibody–producing cells
Anti-HSV antibody–producing cells were screened by an enzyme-linked immunosorbent assay (ELISA) kit (Herpes Simplex IgG detection kit; Denka Seiken, Tokyo, Japan) according to the manufacturer's protocol. Briefly, the assay was performed by incubating 1:1 dilutions of culture supernatants from the patient's EBV-transformed B-LCLs for 1 hour at room temperature in 96-well microtiter plates coated with HSV antigen. After rinsing with 0.05% Tween 20 in PBS, bound human antibody was detected with goat anti–human IgG antibody labeled with horseradish peroxidase and 3,3′,5,5′-tetramethylbentidine in H2O2. The reaction was measured at 450 nm with an ELISA reader (Kokusai Shiyaku, Kobe, Japan). The antibody-producing cells were isolated by 2 rounds of limiting dilution. Limiting dilution was performed by co-culturing, in 96-well plates, 0.3 cells per well of an EBV-transformed B-LCL established from the patient with 1 × 104EBV-transformed B-LCL, irradiated at 30 Gy, established from an X-SCID patient who lacks antibody production.
Results
Clinical findings
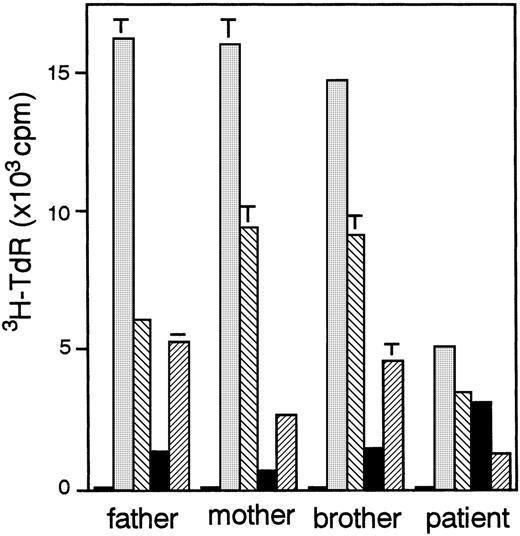
A 4-month-old girl from non-consanguinous parents was referred to our hospital with diffuse papular scaling rash with lymphadenopathy. The patient had experienced recurrent infections, including otitis media and impetigo since the age of 2 months. Laboratory evaluation at onset revealed elevated serum levels of IgM (3.47 g/L [347 mg/dL]) and IgE (611 U/mL), hypereosinophilia (9.308 × 109/L [9308/μL]), increased numbers of T cells (6.1 × 109/L [6100/μL]) with activated phenotype, and markedly reduced B cells because of a developmental block in the bone marrow (Table 2). With corticosteroid therapy, the skin lesions and lymphadenopathy were improved, although the skin was secondarily infected withStaphylococcus aureus resistant to methicillin. At 18 months, she experienced periodic thrombocytopenia followed by spontaneous recovery and a transient neutropenia with antineutrophil antibodies at the age of 4 years. Since then, the patient had recurrent episodes of sino-pulmonary infections caused by Streptococcus pneumoniae resistant to penicillin, and antibiotic therapy was required to control the infection. HLA analysis of the patient and her family revealed maternal T-cell engraftment in the patient's peripheral blood (Table 3). The paternal HLA was not detected in her CD4+ and CD8+T-cell population. In addition, all the CD4+ T cells were CD4+CD45RO+ memory phenotype, and CD4+CD45RA+ naive T cells were not detected in her periphery (data not shown). Responses of PBMCs to various mitogens such as phytohemagglutinin and poke weed mitogen were reduced, whereas response to IL-2 was increased (Figure1). The result indicated that T cells of this patient were in an anergic state. As shown in Table 2, the patient had few circulating B cells at age 4 months, but the number increased with age. The blood group of the patient was A, and isoagglutinin was positive in her serum. In addition, specific antibodies against diphtheria toxin and HSV were detected after diphtheria toxin vaccination and HSV infection, respectively, although monoclonal κ gammopathy was present.
Proliferative response of PBMCs to various stimuli.
PBMCs were cultured in medium alone (□), containing 2000-fold diluted phytohemagglutinin-P (░), 5 ng/mL phorbol myristyl acetate plus 500 ng/mL ionomycin (▧), 50 ng/mL human IL-2 (▪), or 100-fold diluted poke weed mitogen (▨). Results represent the mean ± SD of triplicate wells.
Proliferative response of PBMCs to various stimuli.
PBMCs were cultured in medium alone (□), containing 2000-fold diluted phytohemagglutinin-P (░), 5 ng/mL phorbol myristyl acetate plus 500 ng/mL ionomycin (▧), 50 ng/mL human IL-2 (▪), or 100-fold diluted poke weed mitogen (▨). Results represent the mean ± SD of triplicate wells.
Identification of RAG1 mutations in the patient
Because the patient did not have circulating B cells and her own T cells at her first appearance, we sequenced her RAG1 genes. For this purpose, we used genomic DNA isolated from a B-LCL of the patient, having demonstrated that the B-LCL was of host origin by HLA analysis.
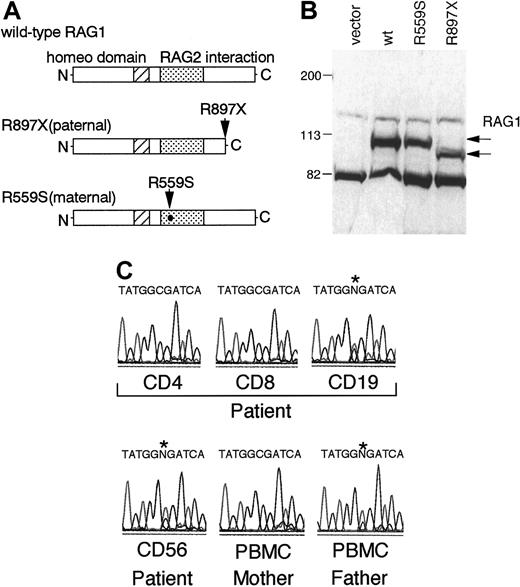
The patient was a compound heterozygote bearing a G1789T transition causing a missense mutation, R559S, located to the RAG2 binding domain, and a C2801T mutation leading to a nonsense mutation, R897X, mapping to the core domain responsible for the catalytic activity14 15(Figure 2A). The former mutation comes from her mother and the latter from her father. To rule out a possible polymorphism of the G1789T transition, we sequenced 100 chromosomes from Japanese controls and verified that the mutation is not a representative polymorphism (data not shown).
Characterization of RAG1 mutations of the patient.
(A) Schematic representations of mutant RAG1 proteins. Arrows indicate the parental mutations. (B) Steady-state levels of the mutant and wild-type RAG-1 proteins in transfected 293T cells. Protein from transfected 293T cells was detected by immunoblotting with polyclonal anti-RAG1 antibodies. (C) The paternal mutation was not detected in CD4+ and CD8+ T cells from the patient. Cells were purified by positive magnetic immunoselection by using anti-CD4, anti-CD8, anti-CD19, and anti-CD56 microbeads, and direct sequencing was performed on the PCR products by using genomic DNAs isolated from the fractionated PBMCs. An asterisk indicates the position of the paternal mutation.
Characterization of RAG1 mutations of the patient.
(A) Schematic representations of mutant RAG1 proteins. Arrows indicate the parental mutations. (B) Steady-state levels of the mutant and wild-type RAG-1 proteins in transfected 293T cells. Protein from transfected 293T cells was detected by immunoblotting with polyclonal anti-RAG1 antibodies. (C) The paternal mutation was not detected in CD4+ and CD8+ T cells from the patient. Cells were purified by positive magnetic immunoselection by using anti-CD4, anti-CD8, anti-CD19, and anti-CD56 microbeads, and direct sequencing was performed on the PCR products by using genomic DNAs isolated from the fractionated PBMCs. An asterisk indicates the position of the paternal mutation.
To determine whether the RAG mutations of this patient affect steady-state RAG protein levels, an immunoblot for RAG1 protein was performed. The mutant RAG1 cDNAs were generated by site-directed mutagenesis, and expression vectors containing the cDNAs were transiently transfected into 293T cells. Protein from the transfected 293T cells was detected by immunoblotting with polyclonal anti-RAG1 antibodies. The size and expression level of the RAG1 mutant containing the R559S substitution is similar to that of the wild type, whereas the size of the RAG1 mutant containing R897X is shorter as predicted (Figure 2B).
From the results of HLA analysis, T cells of the patient were of maternal origin, whereas B cells were of host origin. To confirm the result of HLA analysis in each cell population, RAG1 genes were sequenced by using fractionated CD4, CD8, CD19, and CD56 cells. In accordance with the HLA analysis, the paternal mutation (C2801T) was detected in CD19+ cells but not in CD4+ or CD8+ cells (Figure 2C). In addition, CD56+cells were demonstrated to be of host origin. These results demonstrated that maternal T cells were present with host B and natural killer cells in her periphery.
Recombination activity of the RAG1 mutants on an extrachromosomal substrate
To test the ability of the RAG1 mutants to mediate recombination of extrachromosomal substrates in vivo, RAG1, RAG2, and the recombination substrate, pJH200 (deletional), were cotransfected into 293T cells. Recombination products were determined by PCR analysis. The nonsense mutant, R897X, abolished the ability of the mutant protein to mediate recombination of the substrate, whereas the missense mutant, R559S, shows a weaker but clearly detectable signal compared with the wild-type RAG1 (Figure 3).
In vivo recombination activity of the RAG1 mutants.
Harvested plasmids were analyzed for coding joint (CJ) formation by PCR. The PCR products were run on a 5% polyacrylamide gel and autoradiographed. Full-length wild-type (wt) and mutant alleles (R559S, R897X) were transfected into 293T cells with pEBG and deletion substrate pJ200. Lane 1: negative control; Lane 2: negative control; Lane 3: positive control (wild-type RAG1 cotransfected with wild-type RAG2); Lane 4: mutant R559S cotransfected with wild-type RAG2; Lane 5: mutant R897X cotransfected with wild-type RAG2.
In vivo recombination activity of the RAG1 mutants.
Harvested plasmids were analyzed for coding joint (CJ) formation by PCR. The PCR products were run on a 5% polyacrylamide gel and autoradiographed. Full-length wild-type (wt) and mutant alleles (R559S, R897X) were transfected into 293T cells with pEBG and deletion substrate pJ200. Lane 1: negative control; Lane 2: negative control; Lane 3: positive control (wild-type RAG1 cotransfected with wild-type RAG2); Lane 4: mutant R559S cotransfected with wild-type RAG2; Lane 5: mutant R897X cotransfected with wild-type RAG2.
Identification of B cells producing anti-HSV antibodies
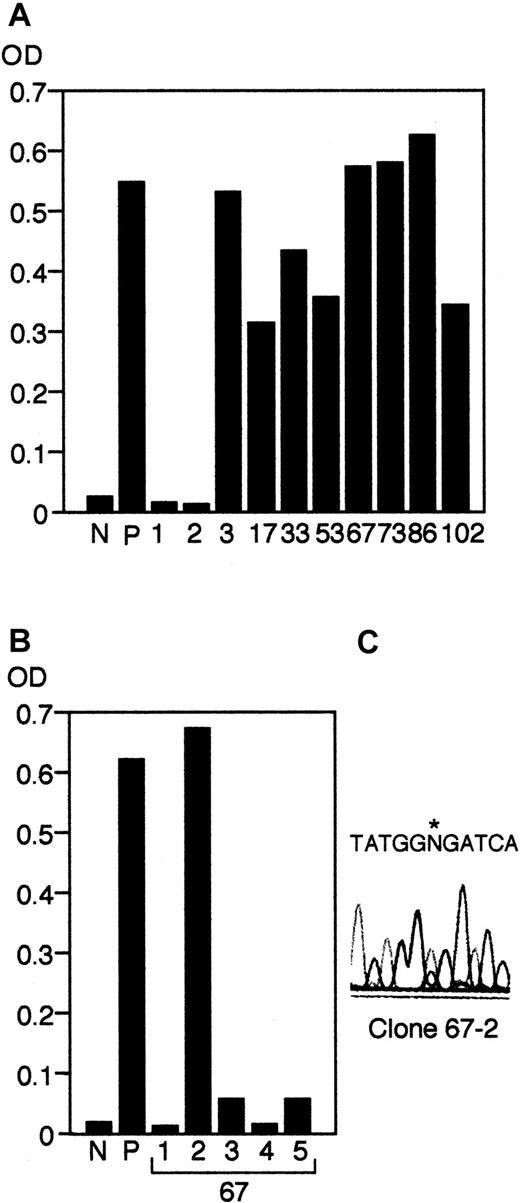
The patient had complications with a self-limited HSV infection. After the episode, anti-HSV antibodies were readily detectable in her serum. Therefore, we established EBV-transformed B-LCL clones from the patient and analyzed the anti-HSV titer of their supernatants by using the same ELISA kit that had been used for detection of anti-HSV antibodies in her serum. The anti–HSV antibody–producing cells were isolated by 2 rounds of limiting dilution. As shown in Figure 4A, supernatants from 5 independent clones of 150 clones of the first round of limiting dilution were positive for HSV-antibodies. The positive clones were then selected in a second round of limiting dilution. Although the growth of the clones was gradually impaired during the second round of cloning, we could recover 3 positive second round clones from one of the initial clones (clone 67; Figure 4B). To determine the origin of the B cells, we performed direct sequencing of the RAG1 gene and demonstrated that the clone was of host origin (Figure 4C, and data not shown). Furthermore, we examinedRAG1 genes in 40 independent clones after the first round of cloning and demonstrated that all of the B-cell clones were of host origin regardless of their production of anti-HSV antibodies (data not shown).
Screening of anti–HSV antibody–producing cells.
Supernatants from the first round (A) and the second round (B) of limiting dilution using EBV-transformed LCLs established from the patient were collected and screened by a Herpes Simplex IgG detection kit as described in “Materials and methods.” OD at 450 nm was measured in both experiments. N, negative control; P, positive control. (C) Sequence analysis of the positive clone (clone 67-2) after the second round of limiting dilution, indicating that the clone was of host origin. An asterisk indicates the position of the paternal mutation.
Screening of anti–HSV antibody–producing cells.
Supernatants from the first round (A) and the second round (B) of limiting dilution using EBV-transformed LCLs established from the patient were collected and screened by a Herpes Simplex IgG detection kit as described in “Materials and methods.” OD at 450 nm was measured in both experiments. N, negative control; P, positive control. (C) Sequence analysis of the positive clone (clone 67-2) after the second round of limiting dilution, indicating that the clone was of host origin. An asterisk indicates the position of the paternal mutation.
Discussion
We describe a girl with an attenuated form of SCID resulting from mutations in the RAG1 gene. Genetic analysis of the patient's B cells revealed a G1789T transition causing an R559S substitution on one allele and a C2801T mutation leading to an R897X change in the other one. The former mutant RAG1 protein, R559S, showed a reduced, but still present, ability to mediate recombination of the deletion substrate pJH200, whereas the latter R897X, which has already been reported in a patient with T-B-SCID, completely impaired the recombination activity.3 Therefore, these amino acids are critical for V(D)J recombination activity. A previous report demonstrated that mutations of the RAG1 or RAG2genes that eliminate the recombination activity result in T-B-SCID.3 Recently, Omenn syndrome has been demonstrated to arise from mutations of the RAG1 or RAG2 that decrease but do not abolish the efficiency of V(D)J recombination process.5Because one of the RAG1 mutations of our patient has partial recombination activity, and the same mutation was found in a patient with Omenn syndrome, the patient would be expected to have Omenn syndrome.9 However, our patient did not have any detectable levels of host T cells in her peripheral blood, as assayed by reverse transcriptase-PCR. This finding could be due to the presence of engrafted maternal T cells interfering with host T cells, or the host T cells might disappear with age.
One of the most prominent initial clinical symptoms of our patient was diffuse papular scalding rash with lymphadenopathy. Corticosteroid therapy was effective for this condition. Previous reports demonstrated that engraftment of maternal T cells lead to GVHD in some infants with SCID.10 11 Because CD4+ and CD8+ T cells from this patient were of maternal origin as assessed by HLA typing and RAG1 mutations, the initial clinical symptoms would have been caused by GVHD due to engrafted maternal T cells.
Patients with T-B-SCID carrying RAG1 or RAG2 mutations or with Omenn syndrome usually die within the first year of life because of severe infections unless hematopoietic stem cell transplantation is performed.6-8 Our patient is classified as a SCID with transplacentally transferred maternal T cells but not Omenn syndrome because of the lack of oligoclonal T cells, the presence of maternal T cells, her lengthy survival, and a skin rash responsive to steroids. In this regard, it is noteworthy that the allele bearing the missense RAG1 mutation showed a reduced but clearly detectable V(D)J recombination activity. She has not experienced serious viral infections, although she has had many episodes of bacterial infections. One of the reasons for this mild phenotype was that she had mature B cells in her periphery, and some of them could produce specific antibodies, although monoclonal κ gammopathy and autoreactive antibodies were present. The fact that host B cells could produce antibodies in this patient suggests how patients with Omenn syndrome produce IgE and sometimes IgM. In other words, some functional B cells may be present in such patients even though not detected in the circulation. Furthermore, it is possible that partially functional host B cells in combination with engrafted maternal T cells could have given rise to a partial B-T cell cooperation, protecting her against virus infections, and, as a result, she has been able to be treated on an outpatient basis without hematopoietic stem cell transplantation for 6 years. Similar observation was made in some patients with X-linked SCID, who had received HLA haploidentical T-cell–depleted bone marrow from related donors.18 In these cases, host B cells could cooperate with donor T cells to produce immunoglobulin. In contrast, SCID patients with engrafted maternal T cells usually could not produce immunoglobulin. Further analysis will be needed to clarify the discrepancy and to understand the mechanism of this B-T cell cooperation.
Supported in part by grants for Pediatric Research from the Ministry of Health, Labor, and Welfare, Japan, and E0917 from Telethon.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Satoru Kumaki, Department of Pediatric Oncology, Institute of Development, Aging and Cancer, Tohoku University, 4-1 Seiryo-Machi, Aoba-Ku, Sendai 980-8575, Japan; e-mail:kumakis@idac.tohoku.ac.jp.