Extracorporeal photochemotherapy (ECP) has been associated with clinical improvement in several patients with acute and chronic graft-versus-host disease (cGVHD) after allogeneic bone marrow transplantation, but the mechanism of action is unknown. This study tested the hypothesis that in patients with cGVHD, ECP modulates alloreactivity by affecting activated lymphocyte populations or by altering the interaction between effector lymphocytes and antigen-presenting cells (APCs). Ten patients who had refractory cGVHD were treated with ECP, and the clinical response to and immunologic effects of this therapy were assessed. Seven patients had a response and 3 had no change in clinical manifestations of cGVHD. One patient died from catheter-related sepsis. Immunologic effects observed after ECP included normalization of inverted ratios of CD4 to CD8 cells, an increase in the number of CD3-CD56+ natural killer (NK) cells, and a decrease in CD80+ and CD123+ circulating dendritic cells. The results suggest that ECP modulates both NK cells and APC populations in patients with cGVHD.
Introduction
Chronic graft-versus-host disease (cGVHD) occurs in 30% to 60% of patients after allogeneic bone marrow transplantation (BMT).1 Minor antigen mismatches cause donor T-cell activation against recipient tissues,2 and antigen-presenting cells (APCs) are essential in initiating this process.3 4 Natural killer (NK) cells have been shown to be suppressed in most patients with active cGVHD. Clinical management of cGVHD includes the use of steroids, cyclosporine, and FK506 (tacrolimus).
Extracorporeal photochemotherapy (ECP) has been shown to be effective in the treatment of cutaneous T-cell lymphoma (CTCL), some autoimmune diseases, and rejection of solid-organ grafts. Several small studies found improvement in the skin and visceral manifestations of cGVHD after ECP.5,6 One proposed reason for the effects of ECP in CTCL and autoimmune diseases is that exposure to ultraviolet light and 8-methoxypsoralen induces apoptosis in a small subset of circulating clonal tumor or autoreactive T lymphocytes, thus stimulating a cytotoxic T-cell (CTL) response against the clone.7 8 We analyzed the clinical and immunologic effects of ECP in patients with steroid-refractory cGVHD and found that response correlated with normalization of the ratio of CD4 to CD8 cells, an increase in CD3-CD56+ natural killer (NK) cells, and a decrease in circulating CD80+ and CD123+APCs. These results suggest that in patients with ongoing alloreactivity and cGVHD, ECP may interfere with the presentation of alloantigens by altering both effectors and APCs, resulting in establishment of immune tolerance.
Study design
All patients had a diagnosis of symptomatic, extensive cGVHD refractory to standard therapy (at least 4 weeks of prednisone [1 mg/kg of body weight or an equivalent], with therapeutic levels of cyclosporine). In 5 patients, there had been no response to mycophenolic acid therapy in an institutional phase II study; and in 2, there had been no response to tacrolimus. All patients had extensive sclerodermatous skin changes and a performance status of 3 or lower according to Eastern Cooperative Oncology Group criteria. Patients with a history of photosensitive disease, allergy to psoralen, or active uncontrolled infection were ineligible for the study. All patients provided written informed consent in accordance with a protocol approved by our institutional review board.
ECP was done on 2 consecutive days every 2 weeks as described previously.9 10 Toxicity and response characteristics were assessed before ECP was begun and at the beginning of each 2-week treatment cycle by using the common toxicity criteria of the National Cancer Institute. Patients continued to take immunosuppressive agents, according to regimens adjusted for the activity of their cGVHD.
Clinical assessment
Clinical response was graded by using standard cGVHD criteria. A complete response represented resolution of all manifestations of cGVHD; a partial response, a 50% improvement in the sum total of all disease; and progressive disease, development of a new skin lesion or worsening of existing lesions, or new visceral disease.
Biologic studies
Peripheral blood mononuclear cells were isolated before ECP began and monthly during therapy. They were labeled with either phycoerythrin or fluorescein isothiocyanate–conjugated monoclonal antibodies (Becton Dickinson, Mountain View, CA) to CD3, CD4, CD8, CD28, CD56, CD69, CD80, and CD123 and measured by using a fluorescence-activated, cell-sorter scanner flow cytometer.
Results and discussion
Ten patients (7 men and 3 women aged 25-59 years) were evaluable after at least 5 cycles of treatment. Patient characteristics are shown in Table 1. Indications for allogeneic BMT were chronic myelogenous leukemia (4 patients), acute myelogenous leukemia (3 patients), non-Hodgkin lymphoma (2 patients), and chronic lymphocytic leukemia (1 patient). The time from transplantation to enrollment in the study ranged from 101 to 2928 days. Patients were selected on the basis of presence of refractory, progressive cGVHD and willingness to participate in the study. The median time from onset of cGVHD to treatment was 667 days (range, 101-2928 days). All patients had sclerodermatous skin changes. The proportion of body surface affected by cGVHD was 50% to 75% in 7 patients, 26% to 50% in 2 patients, and more than 75% in 1 patient. Seven patients also had oral cGVHD, 8 had eye involvement, 5 had joint contractures, and 3 had visceral cGVHD, including 1 patient who had extensive myositis. The reduction in articular range of motion was 26% to 50% in 3 patients (2 joints) and 51% to 75% in 2 others (2 joints).
ECP was administered for a median time of 9 months (range, 4-16 months). Seven patients had a partial response in skin or oral mucosal GVHD, and 3 patients had no further disease progression. In one patient with skin involvement, a partial response occurred after only 2 months of ECP. Some improvement in joint contractures was observed in all 5 affected patients. Liver-function abnormalities resolved completely in 2 patients. In the one patient with lung involvement, no improvement in lung function had occurred after 7 cycles of ECP.
Immunosuppressive therapy was reduced with clinical improvement in 7 of the 10 patients before the end of the trial, including one whose condition did not meet our criteria for a partial response. Among the 5 patients who were receiving mycophenolate mofetil before initiation of ECP, use of this agent was discontinued in 4. Similarly, tacrolimus was discontinued in 2 other patients. The prednisone dosage was decreased at least 50% in 5 patients with a response. The cyclosporine dosage was decreased 50% to 75% in 3 patients, and use of the drug was discontinued in 1.
Despite the need to place central access catheters to administer ECP, the incidence of sepsis and serious infections was low. However, one patient who had a partial response died from catheter-related sepsis 2 months after initiation of ECP.
A 2- to 10-fold increase in the number of CD3-CD56+ NK cells was observed in the patients with a response (Table2). The low number of NK cells before ECP was begun was probably due to cGVHD, since the number and function of these cells usually return to normal within 2 months after allogeneic BMT.11 Participation of activated NK cells in the GVHD and graft-versus-leukemia process was previously demonstrated in animal models in which adoptive transfer of donor NK cells prevented acute GVHD (aGVHD) and promoted a graft-versus-tumor effect.12 13 The effects of NK cells on GVHD were found to be partly related to the secretion of the immunosuppressive cytokine transforming growth factor β. Interestingly, the 2 patients who had no response to ECP did not have a suppression of NK cells before therapy.
Normalization of a previously inverted ratio of CD4 to CD8 cells was observed in 3 patients. This was related predominantly to decreases in CD8+ T lymphocytes. Previous studies of ECP for treating cGVHD found a similar modulation of T-cell subsets. An overall reduction in CD8+ cells was reported in 1 pediatric and 4 adult patients undergoing ECP for cGVHD.14 15
Because studies of cytokine expression by peripheral blood mononuclear cells in cGVHD found up-regulation of interferon γ and tumor necrosis factor α, a T-helper (TH)-1–driven mechanism has been proposed.16,17 Studies of ratios of TH1 to TH2 that used single-cell cytokine assays found no significant changes in cytokine profiles in patients with cGVHD undergoing ECP, suggesting an alternative mechanism of action of ECP in cGVHD compared with aGVHD, in which TH1 cytokine inhibition has been correlated with clinical response.18 19
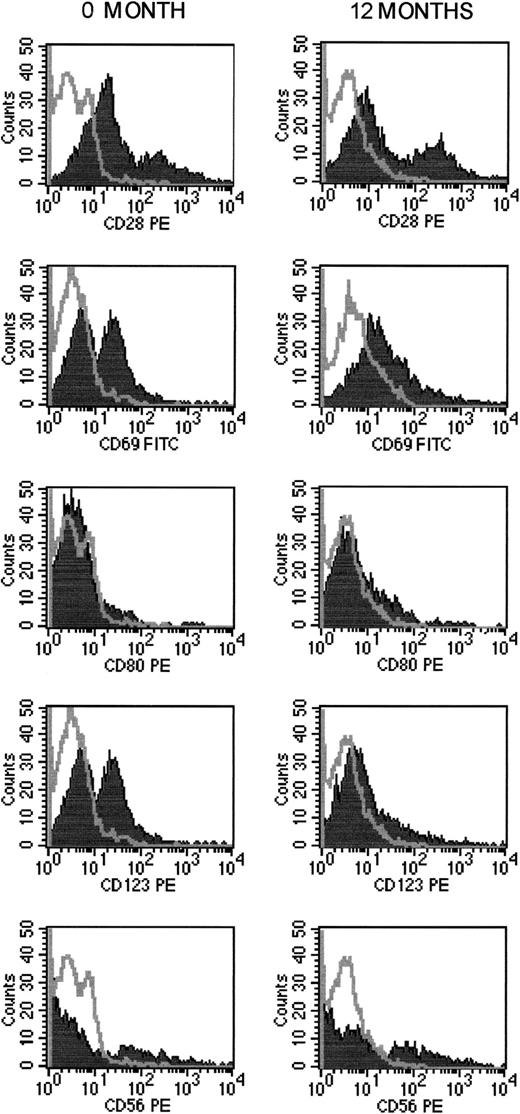
Our study is the first to demonstrate a modulation of peripheral blood dendritic cell populations (CD80+ and CD123+ cells) during ECP. We observed at least a 50% decrease in circulating CD80+ and CD123+dendritic cells (Figure 1), with no marked change in CD28 expression on lymphocytes, thus suggesting no change in class I major histocompatibility complex–restricted CTL function (Table 2). In studies in animals, DNA damage induced by photoactivation and 8-methoxypsoralen was shown to alter the idiotypes expressed by clones of autoreactive T cells by up-regulating class I expression, thus leading to induction of specific autoregulatory CD8+ T cells with immunosuppressive function.20 21 In our studies, we observed no modulation of CD8+ populations.
Changes in NK and dendritic cell populations during ECP.
Results are for patient 4 before ECP began and after 12 months of ECP.
Changes in NK and dendritic cell populations during ECP.
Results are for patient 4 before ECP began and after 12 months of ECP.
Other proposed mechanisms of ECP in the treatment of neoplastic or autoimmune disorders include induction of apoptosis in lymphocytes that leads to deletion of neoplastic or alloreactive T-cell clones.22-24 Similarly, ultraviolet irradiation has been shown to inhibit both the number and functional activity of dendritic cells in murine skin models, thereby leading to immune tolerance.25 Although coadministration of immunosuppressive agents might have affected the alterations in effector cells observed in these patients, the similarities in immune recovery despite the previous immunosuppressive regimens suggest that the observed effects on dendritic cells are mediated predominantly by the ECP.26-28
Our results indicate 2 possible but not mutually exclusive mechanisms of activity of ECP in cGVHD. One is a direct effect on dendritic cell number and function that leads to a decrease in the capacity to present alloantigens and to stimulate immune effector mechanisms. The other is a direct effect on populations of alloreactive T cells, similar to that observed in scleroderma and CTCL, that leads to a decrease in CD8+ effector cells and subsequently dendritic APCs. Our results support the importance of APCs in the initiation of cGVHD recently demonstrated by Shlomchik et al,29 who showed that initial target antigens for CD8+ T cells in GVHD are restricted to proteins expressed by host APCs and that persistence of host APCs may drive cGVHD. Interestingly, Greinix et al30reported promising results with the use of ECP in patients with acute, steroid-refractory GVHD, but changes in lymphocyte and dendritic cell populations were not measured in their study. Because the chimeric constitution of the APCs in both our patients and those of Greinix et al is unknown, it is unclear whether there is selectivity of the inhibitory effects of ECP on host or donor reconstituted APCs and whether the effects on APCs alone are sufficient to induce response without concomitant modulation of alloreactive T-cell populations. Additional studies are needed to address the functional effects of ECP on both dendritic cell function and T-cell activation in both aGVHD and cGVHD.
We acknowledge the nursing staff and bone marrow transplantation team for their dedicated care of the patients.
Supported by an Oncology Research Faculty Development Award from the National Cancer Institute (T.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Francine Marie Foss, Division of Hematology/Oncology, New England Medical Center, 750 Washington St, Box 542, Boston, MA 02111; e-mail: ffoss@lifespan.org.